The Drosophila Polycomb Protein Interacts with Nucleosomal Core Particles In Vitro via Its Repression Domain (original) (raw)
Abstract
The proteins of the Polycomb group (PcG) are required for maintaining regulator genes, such as the homeotic selectors, stably and heritably repressed in appropriate developmental domains. It has been suggested that PcG proteins silence genes by creating higher-order chromatin structures at their chromosomal targets, thus preventing the interaction of components of the transcriptional machinery with their _cis_-regulatory elements. An unresolved issue is how higher order-structures are anchored at the chromatin base, the nucleosomal fiber. Here we show a direct biochemical interaction of a PcG protein—the Polycomb (PC) protein—with nucleosomal core particles in vitro. The main nucleosome-binding domain coincides with a region in the C-terminal part of PC previously identified as the repression domain. Our results suggest that PC, by binding to the core particle, recruits other PcG proteins to chromatin. This interaction could provide a key step in the establishment or regulation of higher-order chromatin structures.
Once segmental boundaries are established by the early patterning mechanisms, the products of the homeotic selector genes specify the characteristic features of each body segment of the developing Drosophila embryo. The genes that are required for the maintenance of the differential expression patterns of these key developmental regulators have been divided into two groups: the trithorax group (trxG) maintains the active state, while the Polycomb group (PcG) is responsible for keeping the target genes in a repressed state (reviewed in references 34 and 37). Indeed, maintaining cells in a determined state, as defined by the specific expression pattern of key regulatory factors, crucially depends on the appropriate action of the PcG and trxG. Embryos with mutant PcG genes show a general misexpression of target genes outside their usual expression boundaries (47, 53), leading to dramatic transformations of the body pattern. Conversely, mutations in trxG genes lead to a down-regulation of homeotic genes. Members of both groups were found to play equally important roles in mammalian development (reviewed in references 14 and 51).
Combinations of PcG alleles show synergistic effects, suggesting that their products interact in multimeric complexes. Indeed, the Polycomb (PC), Polyhomeotic (PH), Posterior sex combs (PSC), and other PcG proteins are parts of a complex consisting of about 20 proteins (12, 16, 19, 43, 49). PcG proteins share binding sites on Drosophila polytene chromosomes (9, 12, 22), indicating that many target genes are coregulated. Several PcG proteins carry conserved regions, such as the SET, WD-40, or SPM domains and RING- or PHD-finger motifs, which are thought to mediate protein-protein interactions. PC itself contains two regions that are conserved in the vertebrate homologues: the 50-amino-acid N-terminal chromodomain and a C-terminal region of 28 amino acids (see Fig. 1). Mutations in the chromodomain prevent the interaction of PC with PH (49) and abolish PC binding to its target sites on salivary-gland chromosomes (25). The C-terminal part of PC is necessary for the repression of reporter constructs in transgenic flies (28) or in mammalian tissue culture cells (8). Recently it has been shown that the C-terminal parts of the mouse PC homologue M33 and the human homologue hPc2 are necessary for the repressive action of these proteins (40, 42). The deletion of this portion has no effect on the recruitment of PC to the target genes (25). Thus, PC seems to be divided into at least two functionally distinct domains: the chromodomain, which is crucial for the integration of PC in the PcG complex and for its targeting, and the C-terminal part, which mediates regulative interactions that are necessary for the repressive function of the PcG complexes (8, 28).
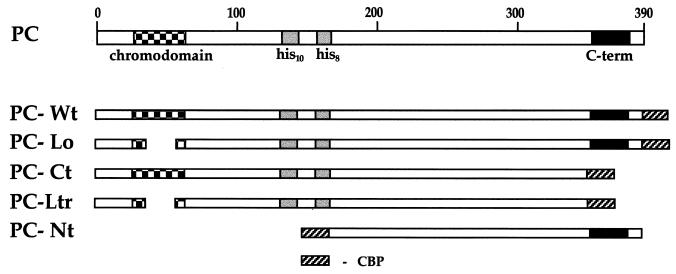
FIG. 1.
The conserved domain of the PC protein and the protein fusions used for the analysis. The three prominent regions of PC are indicated: the chromodomain, the two repeats of 10 and 8 histidines, and the C terminus, which is involved in repression. PC-Wt features the whole sequence of PC. PC-Lo carries a deletion of 24 amino acids in the chromodomain, PC-Ct has a C-terminal truncation of 42 amino acids, and PC-Ltr is a combination of both. PC-Nt has a large N-terminal truncation, deleting the chromodomain and the His repeats. All fusion proteins carry the 35 amino acids of CBP either as C-terminal fusions (PC-Wt, PC-Lo, PC-Ct, and PC-Ltr) or as N-terminal fusions (PC-Nt).
PcG proteins silence chromosomal regions by being targeted to DNA elements termed PREs (PcG response elements). PREs have been identified as _cis_-regulatory DNA elements, important for the PcG-dependent maintenance of the transcriptionally inactive state of homeotic genes. The integration of PREs into polytene chromosomes results in an ectopic binding of PcG proteins at the site of the transgene as well as in the silencing of an associated reporter gene (54). Recently, the product of the PcG gene pleiohomeotic (pho) was shown to encode a Drosophila YY1 homologue (7). Since PHO is a sequence-specific DNA binding protein, it is an excellent candidate for a targeting anchor for PcG complexes to PREs (7, 26). PREs apparently serve as nucleation sites that recruit PcG complexes and lead to a spreading of repressive chromatin structures into neighboring genes. This spreading may lead to packaging of regulatory units into structures that are inaccessible to the transcriptional machinery (54). Indeed, analysis of Drosophila tissue culture cells and embryonic chromatin by chromatin immunoprecipitation after cross-linking indicates that the PC protein associates with a broader chromatin domain of several kilobase pairs of DNA around PREs, in obvious contrast to the sequence-specific DNA-binding GAGA factor, which is strictly found at GAGA consensus sites in PREs (32, 48). This suggests that the PC protein interacts directly or via other subunits in the PcG complex with the nucleosomal backbone independently of the underlying DNA sequence to generate silenced higher-order structures. This structural model resembles that proposed for telomeric silencing and the repression of inactive mating type cassettes in yeast (15). SIR3 and SIR4, which both belong to a set of proteins that are required for these silencing processes, interact with the N-terminal domains of histones H3 and H4 (17). This interaction is thought to mediate the spreading of SIR complexes along the chromosome, creating a higher-order structure that is inaccessible to activators and the transcriptional machinery.
We tested the ability of the PC protein to interact with purified histones or reconstituted nucleosomes in vitro. Our data indicate that PC binds to nucleosomes via its conserved C-terminal domain and recruits other PcG proteins. Furthermore, we found a strong interaction of PC with cruciform (four-way junction) DNA (crDNA), suggesting an involvement of highly bent DNA in the PC-nucleosome interaction. This interaction of a PcG protein with nucleosomes may be important for the association of the PcG complex with extended chromosomal regions.
MATERIALS AND METHODS
Cloning, bacterial expression, and purification of PC-calmodulin-binding protein (CBP) fusion proteins.
The respective coding regions for the different PC derivatives (Fig. 1) were obtained from the PC cDNA by PCR amplification with Pfu polymerase (Stratagene) and the following primers: primer N (5′-CGAATTCGCCATGGCTATGACTGGTCGAGGCAAGG-3′), primer N-trunc (5′-CCATATCGGGATCCGAGTCCAAGCGTCAGCGCA-3′), primer C-trunc (5′-CGAATTCGGGATCCGCATTTGGCCGGCAGCCAG-3′), primer C (5′-CGAATTCGGGATCCAGCTACTGGCGACGAATCG-3′), and primer C-2 (5′-CCATATCGGAATTCCGAAGCTCAAGCTACTGGC-3′).
PC-Wt and PC-Lo were amplified by using primers N and C, PC-Ct and PC-Ltr were amplified by using primers N and C-trunc, and PC-Nt was amplified by using primers N-trunc and C-2. For the mutants PC-Lo and PC-Ltr, the cDNA p12c-PcΔ42-65 (25) was used for PCR amplification, and for the remaining constructs the full-length PC cDNA p12c-G1a (35) was used. Amplified fragments were cut with the appropriate restriction enzymes (_Nco_I for primer N, _Bam_HI for primers N-trunc, C, and C-trunc, and _Eco_RI for primer C-2), ligated into the pCal-c and pCal-n expression vectors (Stratagene), respectively, and verified by sequencing. Plasmids were finally transformed into the bacterial host BL21 (DE3)pLysS. The PC-CBP fusion proteins were expressed and purified as described in the protocols of the Affinity Protein Expression and Purification System (Stratagene).
Purification of Drosophila core histones.
The core histones were isolated essentially as described previously (44). To prepare the chromatin from nuclei, 50 g of early (0 to 90 min after egg laying) Drosophila embryos were homogenized in a Yamamoto homogenizer with six complete strokes at 1,000 rpm in 40 ml of glycine buffer (15 mM HEPES-KOH [pH 7.6], 10 mM KCl, 5 mM MgCl2, 0.05 mM EDTA, 0.25 mM EGTA, 1 mM dithiothreitol [DTT], 0.2 mM phenylmethylsulfonyl fluoride [PMSF], 10% [vol/vol] glycerol) including one tablet of Complete protease inhibitor (Boehringer). The suspension was centrifuged at 10,000 × g for 10 min. The nuclei were washed with 25 ml of sucrose buffer (15 mM HEPES-KOH [pH 7.6], 10 mM KCl, 5 mM MgCl2, 0.05 mM EDTA, 0.25 mM EGTA, 350 mM sucrose, plus Complete) and respun (this step was repeated once). The nuclei were resuspended in 40 ml of sucrose buffer including 3 mM CaCl2 and 300 U of micrococcal nuclease/ml and were incubated for 10 min at 26°C. The reaction was stopped by adding 800 μl of 0.5 M EDTA. The suspension was centrifuged 10 min at 10,000 × g, and the pelleted nuclei were resuspended in 6.0 ml of TE, pH 7.5 (10 mM Tris [pH 7.5], 1 mM EDTA, including 0.02 mM PMSF, 1 mM DTT, and Complete). The nuclear suspension was then homogenized in a Potter-Elvehjem homogenizer (six complete strokes) and centrifuged (for 30 min at 12,000 rpm in a Sorvall HB-4 at 4°C), and the supernatant was transferred to a fresh tube. This soluble chromatin solution was used for further purification of the histones by the procedure of Simon and Felsenfeld (44).
Trypsin digestion of Drosophila core histones.
Soluble chromatin was prepared as described above, except that no proteinase inhibitors were added to the buffers. To establish suitable digestion conditions, 10 μl of the soluble chromatin solution was adjusted to digestion buffer (10 mM Tris-HCl [pH 7.6], 70 mM NaCl, 0.1 mM EDTA) treated with 1, 2, 4, or 10 μg of trypsin (from a 10-mg/ml stock solution) in a final volume of 20 μl. The samples were incubated for 20 min at 26°C. The reactions were stopped by adding 100 μg of trypsin inhibitor (Boehringer), and the digested histones were analyzed on a sodium dodecyl sulfate (SDS)–15% polyacrylamide gel electrophoresis (PAGE) gel. These tailless histones form a stable digestion intermediate that is fairly resistant to further proteolysis. The optimal trypsin concentration for obtaining the typical pattern (see Fig. 3c or 6b) was used to digest the total chromatin solution under similar conditions. For partial trypsin treatment, an aliquot of the chromatin suspension was treated with the same amount of trypsin but at 4°C. The digested chromatin solution was then used for further purification of the histones by the procedure of Simon and Felsenfeld (44).
FIG. 3.
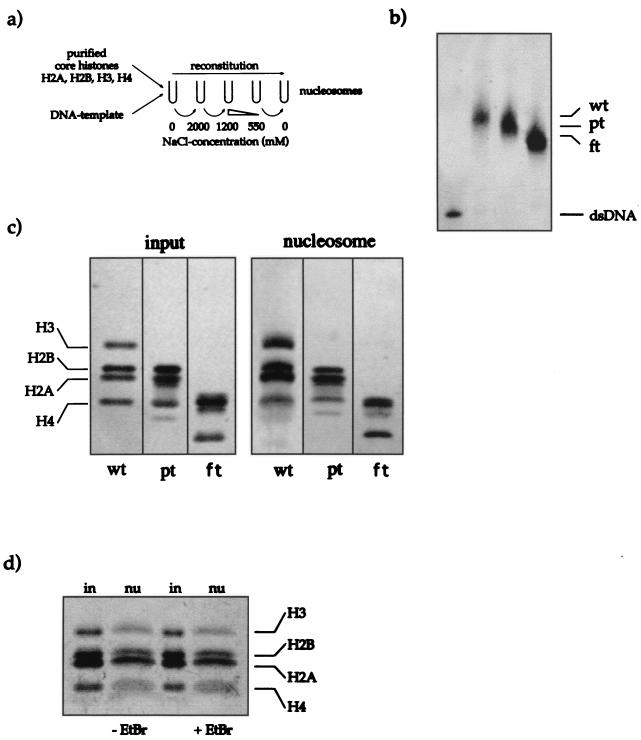
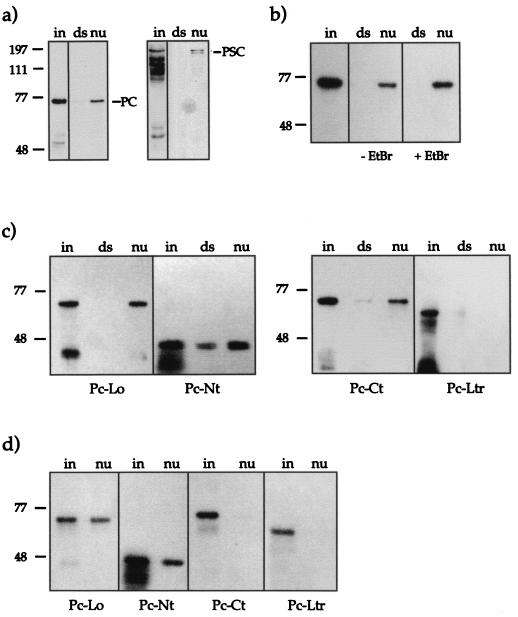
Reconstitution of nucleosomes. (a) Scheme illustrating the reconstitution protocol. Purified core histones (untreated, partially trypsinized, or fully trypsinized) and DNA template were mixed in a reconstitution buffer containing 2 M NaCl. During gradual reduction of the NaCl concentrations, histone octamers formed on the DNA fragment (see Materials and Methods). (b) The completeness of reconstitution was tested by analysis of the reconstituted nucleosomes on a native (band shift) gel. The 146-bp DNA fragment was radiolabeled with [γ-32P]ATP. The signals for the free DNA (dsDNA) and the nucleosomal DNA (wt, untreated; pt, partially trypsinized; ft, fully trypsinized) are indicated. Only nucleosome preparations that did not contain free DNA were coupled to paramagnetic beads and used for further experiments. An autoradiogram of the gel is shown. (c) Histone contents of the input material and the reconstituted nucleosomes. A silver-stained SDS–15% PAGE gel with 50 ng of input material (purified core histones) and 80 ng of coupled reconstituted nucleosomes is shown (abbreviations for the nucleosome preparations are as explained for panel b). (d) Reconstituted nucleosomes remain stable in the presence of EtBr. Two aliquots (80 ng) of nucleosomes (nu) on beads were incubated for 1 h in the presence of 50 μg of EtBr/ml (+EtBr) or without EtBr (−EtBr) in binding buffer. After a wash, both samples were resolved by SDS-PAGE and silver stained. For reference an aliquot of the input material (50 ng) was included (in).
FIG. 6.
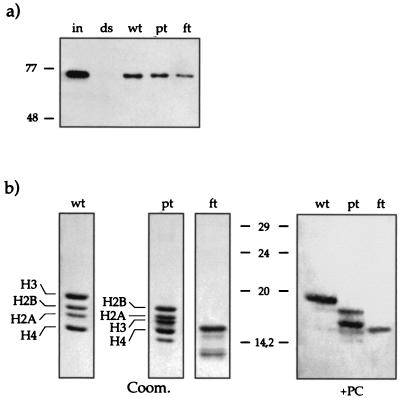
Interaction of recombinant PC with reconstituted nucleosomes by using trypsinized histones. (a) The assay was performed like those described for Fig. 4 except that PC-Wt was also incubated with trypsinized nucleosomes (wt, untreated; pt, partially trypsinized; ft, fully trypsinized). Portions (300 ng) of PC-Wt (input [in]) were incubated with 20 ng of nucleosome-wrapped DNA (wt, pt, ft) or a free DNA fragment (ds) coupled to paramagnetic beads in the presence of EtBr (50 μg/ml). (b) Far-Western blot analysis of the interaction of recombinant PC with trypsinized histones. Nontrypsinized (wt), partially trypsinized (pt), and fully trypsinized (ft) histones were separated on an SDS–15% PAGE gel, electroblotted, and renatured. Left three panels, Coomassie stain of the input material. Note the band of digested H3 in the pt fraction below H2A. Right panel, blot after incubation with PC-Wt. PC interacts mainly with H3 and weakly with H4 and H2B (as shown in Fig. 2) when full-length histones are present (wt). After partial trypsin treatment, PC still interacts with the shortened H3 and interacts more efficiently with H2B and H4 (pt). PC can still interact with fully trypsinized proteins, though it is no longer possible to determine with which of the histones it interacts (ft).
Far-Western blot analysis.
Far-Western blot analysis was carried out as described by Edmondson et al. (11). Portions (1 or 5 μg) of purified core histones were separated on an SDS–15% PAGE gel and electroblotted to polyvinylidene difluoride membranes (Immobilon-P; Millipore). After transfer the blots were stained with Ponceau red, and the positions of the four histones were marked. Renaturation of the blots took place in phosphate-buffered saline (PBS)–0.05% Tween 20 (PBST) for 2 h at room temperature, and blocking in PBS-bovine serum albumin (BSA) (PBS, 2% BSA, 0.5% NP-40, 0.01% NaN3) also took place for 2 h at room temperature. Blots were then incubated in a 50-ml conical tube with recombinant Pc-Wt (50 to 140 ng/cm2 of blot) in 2 ml of PBS-BSA (including 1% normal goat serum) for 2 h at room temperature. Membranes were then washed five times with PBS, and bound PC was detected by antibody staining using ECL (Amersham).
Preparation of the DNA template.
The fragments used for mononucleosome reconstitution were originally created for other purposes. Sixty base pairs of sequence from the Drosophila hsp26 promoter was engineered to contain two novel _Eco_RV restriction sites. The fragment was cloned between the _Eco_RI and _Hin_dIII sites of a modified pUC19 vector. Oligonucleotides were designed on the vector sequences to amplify 146- or 220-bp fragments containing the insert in the center. One biotinylated primer and one primer kinased with [γ-32P]ATP were used for PCR amplification. The PCR products were separated on a 1.3% agarose gel and purified with the Qiaquick gel extraction kit (Qiagen).
Reconstitution of nucleosomal core particles.
The nucleosomal templates were prepared by the procedures described in references 30 and 50. Each reconstitution mixture contained 2 to 10 μg of DNA (of which 200 ng was a biotinylated PCR fragment and the remainder was sheared herring sperm DNA), a slightly smaller amount (in mass) of purified Drosophila histones (intact or partially or fully trypsinized), 0.5 mg of chicken albumin (Sigma)/ml, and 0.05% NP-40. The reaction mixture was filled up to 50 μl with DB(2000) (2 M NaCl, 10 mM HEPES-KOH [pH 7.6], 1 mM EDTA, 1 mM β-mercaptoethanol, 0.05% NP-40). The samples were dialyzed in collodion bags (Sartorius) twice for 1 h each time against 1 liter of DB(2000) at 4°C and then for 1 h against 1 liter of DB(1200) (1.2 M NaCl). The bags were then transferred into 500 ml of fresh DB(1200), and the salt concentration was reduced gradually to about 550 mM NaCl by pumping DB(550) into the beaker while pumping a corresponding volume of the mixed dialysis buffer out (pump speed, 2.0 ml/min for 18 h). Finally, the samples were dialyzed for 3 h against DB (no NaCl). The samples were collected and stored in siliconized tubes on ice. The reconstituted nucleosomes were analyzed after dialysis by 4% nondenaturing PAGE (electrophoretic mobility shift assay). The gel was run at 10 to 12.5 V/cm, dried on Whatman DE-81 paper, and analyzed by autoradiography.
Coupling of the reconstituted nucleosomes to Dynabeads and binding assays.
For each 200 ng of biotinylated fragment, 40 μl of paramagnetic-bead solution (Dynabeads M-280; Dynal) was washed according to the specifications of the manufacturer. The beads were then carefully resuspended in the nucleosome solution derived from the salt gradient dialysis (in DB) and incubated overnight on a rotating wheel at 4°C. The beads were then washed three times with EX(120) (10 mM HEPES-KOH [pH 7.6], 10 mM KCl, 5 mM MgCl2, 0.5 mM EGTA, 10 mM glycerol-phosphate, 10% glycerol, 1 mM DTT, 0.2 mM PMSF) containing 0.05% NP-40 and 0.25 mg of chicken albumin/ml. The coupled nucleosomes were resuspended in EX(120) at a final concentration of 1 to 10 ng/μl. The histone stoichiometry was analyzed by SDS-PAGE and subsequent silver staining.
Nuclear extracts were prepared as previously described (12) with wild-type Drosophila embryos (0 to 20 h overnight egg lays) as starting material. Twenty microliters of nuclear extract or 300 ng of recombinant PC was incubated with 20 to 60 ng of coupled nucleosomes in 50 μl of EX(120) for 1 h at room temperature in siliconized tubes. For comparison the same amount of immobilized naked DNA template was used. In those experiments indicated, ethidium bromide (EtBr) was added up to concentrations of 50 μg/ml. After incubation the beads were washed three times with 100 μl of EX(120), and the pelleted beads were dissolved in SDS-PAGE sample buffer. Samples were resolved by SDS-PAGE and electroblotted, and proteins were detected by antibody staining using ECL (Amersham).
Binding assay with histone-GST fusion proteins and crDNA molecules.
Glutathione _S_-transferase (GST) and GST-histone fusions were expressed in Escherichia coli BL21 (DE3) as described elsewhere (45). GST or GST-histone fusion proteins bound to 20 μl of Glutathione Sepharose 4B (Pharmacia) were equilibrated for 10 min at room temperature in TNE(150) (20 mM Tris-HCl [pH 8], 150 mM NaCl, 1 mM EDTA, 0.1% Triton X-100, 1 mM DTT). After removal of the supernatant, 300 ng of recombinant PC was added in 50 μl of TNE(150) and the samples were incubated for an additional hour. Beads were then washed three times with 500 μl of TNE(150). Proteins were eluted twice in 100 μl of elution buffer (50 mM Tris-HCl [pH 8]–10 mM reduced glutathione), precipitated with methanol-chloroform (4:1), and redissolved in SDS-PAGE sample buffer. Samples were resolved by SDS-PAGE (8% polyacrylamide) and electroblotted, and bound PC was detected by antibody staining using ECL (Amersham).
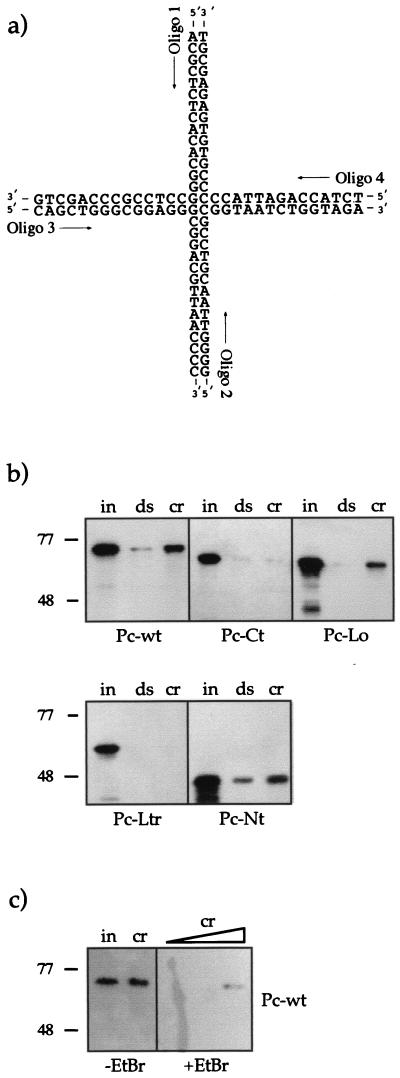
Stoichiometric amounts of the four oligonucleotides were incubated for 3 min in assembly buffer (50 mM Tris-HCl [pH 7.5]–10 mM MgCl2–100 mM NaCl) followed by cooling to room temperature within 2 h. Biotinylated crDNA or double-stranded DNA (dsDNA) was coupled to magnetic beads as described above at a final DNA concentration of 0.25 pmol/μl. Two 300-ng portions of each of the recombinant PC derivatives were incubated with 1.25 pmol of coupled crDNA and dsDNA, respectively, for 1 h in 20 μl of EX(120) (without BSA or NP-40) at room temperature. Magnetic beads were washed three times with 100 μl of EX(120), and the pelleted beads were resuspended in SDS-PAGE loading buffer. Bound PC was detected by Western blot analysis as described above.
RESULTS
PC protein interacts with core histones.
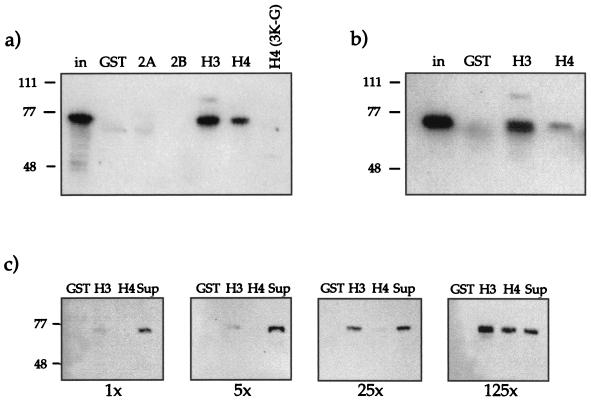
Potential PC-histone interactions were first assayed by Far-Western blot analysis. PC was expressed as a fusion protein with CBP (calmodulin binding protein, a C-terminal fragment of the myosin light chain kinase) in E. coli and was purified by using a calmodulin affinity resin. Pc-Wt features all 390 amino acids of the PC protein plus 35 additional amino acids of the CBP tag (Fig. 1). Core histones were purified from Drosophila embryos by hydroxyapatite chromatography (44). We observed strong binding of PC to histone H3 and a weaker interaction with histones H4 and H2B (Fig. 2a). No signal was obtained without the addition of PC, excluding any cross-reactions of histones with our antibody. By reducing the concentration of PC in the assay (50 μg/cm2 of filter), the binding was restricted mainly to H3 (Fig. 2b), whereas the binding to H4 and H2B was very weak. We never observed an interaction of PC with histone H2A. This indicates that the interaction of PC with histone H3 is preferred and most stable under the conditions of the assay. A general, nonspecific interaction of PC with the highly basic histones can be excluded. Furthermore, under the assay conditions we used, both the core histones and PC have a net positive charge (average isoelectric point [IEP] of all proteins, ≈9). Thus we conclude that the observed in vitro interaction of PC with histones is specific.
FIG. 2.
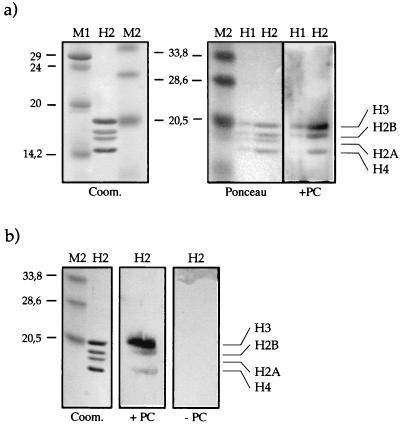
Far-Western blot analysis with Drosophila core histones and recombinant PC (Pc-Wt). (a) Histones (H1, 1 μg; H2, 5 μg) were separated on an SDS–15% PAGE gel, electroblotted, and renatured. The figure shows the resolved histones in a Coomassie blue-stained gel (Coom.), the blot stained with Ponceau red (Ponceau), and the blot after incubation with PC-Wt (150 ng/cm2) (+PC). After extensive washing, PC signals were detected with PC antiserum and visualized by ECL. (b) Purified core histones were treated as for panel a but were incubated with 50 ng of PC-Wt/cm2 (+PC) or without addition of protein (−PC). The positions of the four core histones and the sizes of the molecular weight standards (M1 and M2) in kilodaltons are indicated.
Evidence for the interaction of PC and a PcG complex with nucleosomes.
Since free histones are an unlikely target for PC in vivo, especially considering the presumptive role of this interaction in organizing chromatin, we examined in a second approach the ability of PC to interact with nucleosomal cores. Nucleosomes were reconstituted on a biotinylated 146-bp 32P-labeled DNA fragment by a salt dialysis protocol from purified core histones (50) (Fig. 3a). The quality of the resulting nucleosome preparations was checked by a band shift assay (Fig. 3b). Samples that did not contain free DNA were coupled to paramagnetic beads, and an aliquot was analyzed by SDS-PAGE (Fig. 3c). All four core histones were present in roughly equal amounts, as expected.
Binding assays were performed with these immobilized nucleosomes by using nuclear extracts from wild-type Drosophila embryos (0 to 20 h after egg laying) as a source of PC protein. In parallel, an equivalent amount of free DNA, coupled to paramagnetic beads, was assayed for PC interaction. Bound proteins were challenged by stringent washes and then analyzed by SDS-PAGE and Western blotting. Probing of the membranes with anti-PC antibodies revealed that PC interacted strongly with the reconstituted cores but not with the free DNA (146-bp fragment without reconstituted nucleosome) (Fig. 4a). Reprobing of the membrane with antiserum against PSC, another PcG group protein, showed that PSC also interacted with the nucleosome. This result suggests that the PcG complex present in Drosophila embryo extracts can interact with a mononucleosome.
FIG. 4.
PC binding to reconstituted nucleosomes. (a) PC and PSC are coisolated with reconstituted nucleosomes. Portions (20 μl) of nuclear extract were incubated with immobilized nucleosomes (nu) reconstituted on 60 ng of DNA or an equivalent amount of free DNA template (ds). After extensive washing, the bound material was analyzed by SDS-PAGE. Bound proteins were visualized by Western blot analyses with antisera against the indicated proteins. On the left, 5 μl of extract was loaded (in) to indicate the presence of the relevant proteins in the input material. (b) Interaction of recombinant PC (Pc-Wt) with reconstituted nucleosomes. Portions (300 ng) of PC-Wt (input) were incubated with 20 ng of nucleosome-carrying DNA (nu) or a free DNA fragment (ds) coupled to paramagnetic beads in the presence or absence of EtBr (50 μg/ml). The assay was performed as for panel a. (c) Interaction of mutant PC derivatives with reconstituted nucleosomes. The assay was performed as for panel b. A 300-ng portion of each mutant PC derivative was used. (d) Interaction of mutant PC derivatives as in panel c with reconstituted nucleosomes in the presence of EtBr (50 μg/ml).
The far-Western analysis had pointed to a role of PC itself in targeting the complex to the nucleosome. To further characterize this interaction, we repeated the binding assay with recombinant purified PC. When a 20-fold excess of PC-Wt over the nucleosome target was assayed for interaction (Fig. 4b), we found binding to the reconstituted nucleosomes and no or very weak interaction with free dsDNA (see also Fig. 7b). To assess the contribution of free DNA to the overall binding, we included EtBr in the assay, as this DNA intercalator disturbs protein-DNA interactions (20). The PC-nucleosome interaction could also be observed in the presence of 50 μg of EtBr/ml, whereas the faint binding of PC with free DNA was abolished under these conditions (Fig. 4b). Since nucleosomes may dissociate upon EtBr treatment in a time- and concentration-dependent manner (24), we treated nucleosomes with EtBr for 1 h and monitored their stability by analysis of the composition of the cores (Fig. 3d). Clearly, EtBr did not affect the integrity of nucleosomes within the short incubation times dictated by our experimental protocol.
FIG. 7.
Interaction of recombinant Pc (Pc-Wt) with crDNA. (a) The four-way junction DNA molecule shown in the open conformation. Under the conditions of our experiment (5 mM MgCl2), the molecule had a distorted, stacked X-like structure. crDNA was formed by annealing the four oligonucleotides indicated. Oligonucleotide 1 (Oligo 1) was biotinylated for coupling to paramagnetic beads. (b) Binding assay with crDNA and PC. Equal amounts of crDNA (cr) and dsDNA (ds) (1.25 pmol of each) immobilized on paramagnetic beads were incubated with 300 ng (input [in]) of the different PC derivatives (PC-Wt, PC-Ct, PC-Lo, PC-Ltr, and PC-Nt). After extensive washing, the bound material was analyzed by using SDS-PAGE and Western blotting as before. PC proteins were used at a fivefold molar excess in this experiment. (c) Influence of EtBr on the PC-crDNA interaction. Left two lanes (−EtBr), input of PC-Wt (300 ng) (in) and bound PC after incubation with 0.125 pmol of crDNA (cr). Right lanes (+EtBr), bound PC after incubation with 0.125, 0.3, and 1.5 pmol of crDNA in the presence of EtBr (50 μg/ml).
The PC C terminus mediates the nucleosome interaction.
All _Polycomb_− alleles examined so far show either point mutations in the chromodomain, deletions in the conserved region of the C terminus, or mutations that lead to frame shifts altering the C terminus (13). A third remarkable domain of PC, two stretches of histidines (see Fig. 1), has also been found conserved in the mouse homologue mPC2 (1). Deletions in the histidine repeats have little influence on PC binding on polytene chromosomes. Thus, it is thought that this region has a minor role in defining the PC target gene specificity (25) and might have other, not yet identified functions.
To identify a potential nucleosome interaction domain, we created PC derivatives that carry mutations in the conserved regions (Fig. 1). The mutant PC-Lo has a 24-amino-acid deletion in the chromodomain. This mutation was shown previously to prevent PC from binding to its target genes on polytene chromosomes (25), but it has no effect on the repression of a LexA-inducible reporter gene when present in a LexA-PC fusion protein in mammalian culture cells (8). PC-Ct has a 42-amino-acid truncation in the C terminus, deleting the entire repression domain. The mutant PC-Ltr combines the chromodomain deletion and the C-terminal truncation. Finally, PC-Nt has a deletion of a large N-terminal part of PC, including the chromodomain and both stretches of histidines. All four proteins were expressed and purified by using the CBP moiety and were tested in the nucleosome binding assay.
In the absence of EtBr, all mutants except the double mutant PC-Ltr bound to nucleosomes (Fig. 4c). The presence of EtBr (50 μg/ml) specifically affects the nucleosome interactions of C-terminal truncation mutants (Fig. 4d). While PC-Ct and PC-Ltr completely lost the ability to bind to nucleosomes the chromodomain deletion mutant PC-Lo and the large-N-terminal truncation mutant PC-Nt still retained the nucleosome interaction. These results show that PC can interact with nucleosomes via the chromodomain and the C terminus. However, binding via the chromodomain is DNA dependent, as shown by the fact that it cannot be detected after EtBr treatment. On the other hand, interaction via the C-terminal region of PC does occur in the presence of EtBr and thus most likely represents a protein-protein interaction. Thus, the C-terminal part of PC, which has been shown to be crucial for the repressive function of PC (8, 28), is responsible for the interaction with nucleosomal cores. The importance of this region is further supported by the fact that of the various degradation products of PC-Nt present in the input material, only the full-length form binds to nucleosomes (Fig. 4c and d). The degraded proteins must be C-terminally truncated, because the N-terminal CBP fusion (see Fig. 1) is used for purification. This additionally supports the notion that the C terminus is necessary for the interaction. A nonspecific interaction of the bacterially expressed PC-CBP proteins via the CBP tag can be excluded, since all PC variants carry the CBP portion.
PC interacts with the N-terminal domains of the core histones.
The N-terminal domains of the core histones protrude from the otherwise very compact histone octamer in the nucleosome and, therefore, are likely candidates for targets of interacting factors (23). Therefore we assayed the interaction of PC with GST fusion proteins of the N-terminal domains of the four core histones of yeast (17), comprising amino acids 1 to 35 (H4 and H2B), 1 to 34 (H2A), and 1 to 46 (H3). In addition, a derivative of the H4 peptide, where three lysines had been replaced by glycine [GST-H4(3K-G)], was assayed.
GST-histone fusion proteins were immobilized on Glutathione Sepharose and used as potential ligands for recombinant PC. Binding of PC to GST-H3 and GST-H4 was readily observed (Fig. 5a). This binding was also stable after EtBr treatment (Fig. 5b), indicating that the interaction was direct and not mediated by contaminating DNA. We could not detect interactions with GST-H2A, GST-H2B, or the mutant GST-H4(3K-G). In these assays GST fusion proteins were used at a roughly 125-fold excess. In order to test the concentration dependence of the interaction, we titrated GST, GST-H3, and GST-H4 into binding reactions at a 1-, 5-, 25-, or 125-fold molar excess compared to PC (Fig. 5c). This titration revealed that PC interacts more strongly with the H3 N terminus than with the H4 tail, since at a low input of histone tails, a preferential interaction of PC with the H3 N terminus was observed (Fig. 5c). The interaction with the H4 tail could be observed only when GST-H4 was present in a 125-fold excess.
FIG. 5.
Binding of PC to N-terminal “tail” domains of histones. (a) PC-Wt (300 ng) was incubated with GST or with different GST fusion proteins (GST-H2A, -H2B, -H3, and -H4) immobilized on Sepharose beads. GST fusion proteins were used at a 125-fold excess with respect to PC. For comparison the input is shown (in). After extensive washing, the bound material was analyzed by SDS-PAGE and Western blotting with antisera against PC. (b) Tail interaction assay with PC-Wt as in panel a, but in the presence of EtBr (50 μg/ml). PC-Wt (300 ng) was incubated with a 100-fold molar excess of GST, GST-H3, or GST-H4 immobilized on Sepharose beads. (c) PC interacts preferentially with the histone H3 N-terminal domain. PC-Wt was incubated with GST, GST-H3, or GST-H4 at a 1-, 5-, 25-, or 125-fold molar excess of fusion protein over PC (1×, 5×, 25×, and 125×, respectively). In the rightmost lanes, the supernatant (Sup) (unbound fraction) of the second sample (GST-H3) was loaded for comparison. After washing, the bound material was analyzed by SDS-PAGE and Western blotting as before.
Surprisingly, all mutant PC derivatives bound the H3 tail equally well (data not shown). We conclude that PC recognizes nucleosomal features in addition to the N-terminal domains and that the regions in PC that interact with isolated tail peptides are not deleted in our mutant proteins (PC-Lo, PC-Ct, PC-Ltr, and PC-Nt). Thus, we asked in the next set of experiments whether the N-terminal regions of the histones are necessary for the PC-nucleosome interaction.
PC interacts with tailless nucleosomes and trypsin-treated histones.
Proteolytic treatment of chromatin with trypsin leads to a shortening of the N-terminal domains of all four core histones and of the C-terminal domains of histones H3 and H2B, leaving the globular domains of the histones mainly intact (6). Mild trypsin treatment at 4°C (partial trypsinization) results mainly in digestion of the H3 tails (5) (see Fig. 3c and 6b). Stable nucleosomal core particles lacking the histone N termini can be reconstituted by using histones purified from trypsinized chromatin (2). We used fully and partially trypsinized purified histones for the nucleosome reconstitution as described above (Fig. 3a to c). With these “tailless” nucleosomes coupled to paramagnetic beads, we performed binding assays with recombinant PC. PC interacted with partially trypsinized core particles as well as with untreated core particles, whereas the interaction with fully trypsinized particles was slightly but significantly weaker (Fig. 6a). We also examined the binding of PC-Wt to isolated trypsinized histones in a far-Western analysis (Fig. 6b). PC showed a strong interaction with the globular domains of H3 and an increased interaction (compared to intact histones) with H2B and H4 after partial trypsin treatment (Fig. 6b). Finally, we also observed a significant interaction of PC with the globular domains of the fully trypsinized core histones (Fig. 6b). These results show that the N-terminal regions of the histones are apparently not necessary for the interaction of PC with the core particle, although PC is able to bind to isolated histone N termini.
PC interacts with crDNA.
For mammalian histone H1 and HMG1, the interaction with nucleosome core particles depends on accessible linker DNA (31). In our experiments we observed no difference in the binding of PC to nucleosomes reconstituted on 146- or 220 (containing linker DNA)-bp fragments (data not shown). If the interaction of PC with nucleosomes involved a DNA component, we would expect this DNA to be highly distorted due to the nucleosome association.
Linker histones or HMG proteins, which interact with nucleosomal DNA, usually also bind to crDNA (4, 52). crDNA molecules (four-way junction DNA) are thought to mimic Holliday junctions and the highly bent nucleosomal DNA (21). We therefore tested whether PC was able to interact with nucleosomal DNA. crDNA was assembled as described by Bianchi (4) (Fig. 7a), coupled to paramagnetic beads, and used in a binding assay with all bacterially expressed PC derivatives (Fig. 7b and c). Double-stranded 146-bp DNA was used as a control in the same molar amounts. PC-Wt, PC-Lo, and PC-Nt interacted with crDNA, and PC-Nt also interacted with dsDNA. Apparently, the extreme N-terminal truncation, exposing only the C-terminal interaction domain, leads to general nonspecific interaction with DNA molecules, thus supporting the DNA-binding properties of this region. The C-terminal truncation mutant PC-Ct and the double mutant PC-Ltr did not interact with the four-way junction. EtBr (Fig. 7c) substantially disturbed the PC-crDNA interaction, but binding was not completely abolished. The binding of PC to four-way junction DNA shows its affinity to distorted DNA such as the DNA segments wound around the histone octamere or the DNA that exits the nucleosomal core. The C-terminal region of PC thus has two functions: it mediates a protein-protein interaction with histones H3 and H4, and it is necessary for binding to the highly distorted nucleosomal DNA.
DISCUSSION
One of the models that try to explain the repressive function of the PcG complex assumes an effect on higher-order chromatin structures (33). A fundamental question arises of how such a chromatin modulator is targeted to specific chromosomal regions and how it is anchored at the nucleosomal fiber. In this report we show that PC itself interacts with the major chromatin components, the nucleosomes.
PcG proteins interact with nucleosomes mediated by the conserved C terminus of PC.
Using far-Western blot analysis, we demonstrated the ability of PC to interact with histones, observing that it bound most stably to histone H3. PC in nuclear extracts, where it resides in a complex with other PcG proteins, also interacted with reconstituted nucleosome core particles. The associated binding of PSC demonstrated the interaction of the entire PcG complex. Two regions in the PC protein could be identified as mediating the nucleosome interaction. Binding via the chromodomain was affected by EtBr, which identifies DNA as one target of PC. The structure of the chromodomain of the mouse chromatin modifier protein 1 (MoMOD1) was determined by nuclear magnetic resonance spectroscopy (3). Ball and colleagues found an unexpected homology to two archaebacterial DNA-binding proteins but suggest, judging from structural comparisons, that the chromodomain functions as a protein interaction motif. Consistent with this is our previous finding that the chromodomain is required for the PC-PH interaction in the complex (49). Thus, in vivo the task of the chromodomain appears to be to generate the target site specificity through the other factors of the PcG complex (38), though it seems to posses a cryptic nucleic acid binding ability. However, many DNA binding moieties, e.g., the homeodomains, are also the target of protein-protein interactions (36).
The C-terminal end of PC is the major determinant of nucleosome binding. We found the C terminus of PC to be crucial for direct interaction with the nucleosomal core. In addition the C-terminal region of PC showed a high affinity to crDNA. crDNA is known to be a substrate for proteins interacting with linker DNA, such as histone H1 and HMG proteins. Pöhler and coworkers (39) showed that the HMG box binds only to crDNA in the absence of divalent cations. Thus, HMG box-containing proteins seem to form specific complexes only with the square open form of the four-way junction (39). We also found PC binding to crDNA when divalent cations were present. Under these conditions, four-way junctions have a stacked X structure (10). We interpret this finding as an indication of a strong affinity of PC for highly distorted DNA molecules along the nucleosomal core. Since we found PC binding to trypsin-digested isolated histones and reconstituted nucleosomes, the N-terminal domains seem not to be essential for the PC-nucleosome interaction. Interestingly, Shao and coworkers recently found that the PC-containing PcG complex PRC1 can exert its anti-remodeling activity on chromatin that was assembled by using trypsinized histones (43). Thus, histone H3 and, to a lesser extent, histones H2B and H4, in conjunction with bent superhelical DNA structures, might form a specific binding motif which mediates the interaction with PC. Among the 42 deleted amino acids in the mutant PC-Ct, 28 amino acids are highly conserved in the mammalian PC homologues and have previously been described as the PC repression domain (1, 8, 28, 40). Expression of a mutant form of hPc2 (one of the two human homologues of PC identified to date) with a deleted C terminus in mammalian cell lines resulted in cellular transformations, altered marker gene expression, and anchorage-independent growth. Satijn et al., explain these defects by the inability of these cells to repress certain potential PcG target genes, especially the proto-oncogene c-myc (40). Thus, we propose that this C-terminal repression domain of PC interacts with the nucleosome core. This may not be the sole task of this region, however. Schoorlemmer and coworkers found that the mouse PC homologue M33 interacts via the C-terminal region with the transcriptional repressor RING1 in a yeast two-hybrid system (42). RING1 also interacts with several human PcG proteins, including hPC2, and is proposed to have an important role in the human PcG complex. Interestingly, its deregulation leads to oncogenic transformations due to a derepression of certain oncogenes (41). Thus, potential Drosophila RING1 homologues might participate in regulating PcG-nucleosome interaction in flies.
In addition to the nucleosome binding abilities of PC, we found that PC is also able to bind to isolated N-terminal histone tails in the form of GST fusion proteins. The interaction with GST-H3 turned out to be the most stable, while binding to GST-H4 was observed only with higher concentrations of the fusion protein. The replacement of three conserved lysine residues (lysines 5, 8, and 12) by glycine [GST-H4(3K-G)] completely abolished the interaction with GST-H4. However, the same mutations have no detrimental effect on the interaction of SIR3 and SIR4 with GST-H4 (17). This suggests a different mode of interaction for PC, which could be mediated by several of the evolutionarily conserved N-terminal lysine residues. Judging from our binding experiments, the PC domain responsible for this interaction is neither the chromodomain nor the conserved C terminus and has to be resolved in further experiments. This interaction with the histone tails might be independent of the nucleosome core interaction and leaves open the possibility that the PcG is involved in regulating the acetylation state of the histone tails. Since hypoacetylation of the core histones is often linked to inactive chromatin (for a review, see reference 27), a promotion of deacetylation of these sites by the PcG might induce the formation of repressive chromatin structures in the region of the PcG binding sites. The recent finding that dMi-2—a protein present in a complex containing a potential Drosophila deacetylase—interacts genetically with PC (18) suggests that histone acetylation might be involved in silencing by PcG proteins. Finally, it is also possible that different modes of PC binding to chromatin exist, analogous to the interactions of histone H1 with chromatin, which may occur either near its C terminus or via its globular domain (29).
Mechanism of PC-induced silencing.
For the repressive action of PcG complexes, several models are currently being discussed. PcG complexes might directly enhance the chromatin packaging of putative target genes and thus block the access of activators or the transcriptional machinery to DNA. Alternatively, the interaction of PcG complexes with several PREs might lead to a large DNA-protein complex, thus preventing direct promoter enhancer looping interactions. A third possibility, which does not necessarily exclude the other two, suggests that binding of the PC complexes to PREs could tether target genes to inactive nuclear compartments. Our data cannot discriminate between these different models. However, our finding that a major member of the PcG complex—PC—directly interacts with nucleosomes supports previous data showing that PC is not only concentrated at PREs but is also found associated with extended chromatin regions around PREs (32, 48). It is conceivable, therefore, that PcG complexes interact with nucleosomes over larger regions, thus immobilizing nucleosomal structures, or influencing the folding of the nucleosomal array into higher-order structures. This hypothesis is further substantiated by the recent isolation of a PC-containing complex in Drosophila (43). PRC1 (Polycomb repressive complex 1) blocks the ability of nucleosomal arrays to be remodeled by the SWI-SNF complex. This anti-remodeling activity could be observed only upon preincubation of the nucleosomal array with PCR1. This suggests that a direct interaction of components of the PCR1 with the nucleosomes has to take place, competing with SWI-SNF added subsequently (43). Our finding that PC interacts with the nucleosomal core and might also have an affinity to the nucleosomal DNA opens the possibility that PC locks nucleosomes in a structure that prevents any remodeling activity. Alternatively, PC may compete with SWI-SNF for essential interaction surfaces on the nucleosome.
Finally the results presented in this paper also allow speculations about the question of how the repressive chromatin structures created by the action of the PcG are transmitted through DNA replication. The most likely mechanism for the segregation of nucleosomes during replication is that of an equal distribution to both new DNA strands (46). If PC stayed connected to the nucleosomes during replication, it would be cosegregated with the core particles. After transfer to the daughter strands, the activity of the re-forming PcG complexes could lead to the re-formation of repressive chromatin structures.
ACKNOWLEDGMENTS
We thank the Grunstein laboratory (UCLA) for providing the histone tails.
This work was supported by a TMR Network fellowship to S.F. and by grants of the Human Frontier Science Program and the Deutsche Forschungsgemeinschaft to R.P.
REFERENCES
- 1.Alkema M J, Jacobs J, Voncken J W, Jenkins N A, Copeland N G, Satijn D P E, Otte A P, Berns A, van Lohuizen M. MPc2, a new murine homologue of the Drosophila Polycomb protein, is a member of the mouse Polycomb transcriptional repressor complex. J Mol Biol. 1997;273:993–1003. doi: 10.1006/jmbi.1997.1372. [DOI] [PubMed] [Google Scholar]
- 2.Ausio J, Dong F, van Holde K E. Use of selectively trypsinized nucleosome core particles to analyze the role of histone “tails” in the stabilization of the nucleosome. J Mol Biol. 1989;206:451–463. doi: 10.1016/0022-2836(89)90493-2. [DOI] [PubMed] [Google Scholar]
- 3.Ball L J, Murzina N V, Broadhurst R W, Raine A R C, Archer S J, Stott F J, Murzin A G, Singh P B, Domaille P J, Laue E D. Structure of the chromatin binding (chromo) domain from mouse modifier protein 1. EMBO J. 1997;16:2473–2481. doi: 10.1093/emboj/16.9.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi M E. Interaction of a protein from rat liver nuclei with cruciform DNA. EMBO J. 1988;7:843–849. doi: 10.1002/j.1460-2075.1988.tb02883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blank T A, Becker P B. Electrostatic mechanisms of nucleosome spacing. J Mol Biol. 1995;252:305–313. doi: 10.1006/jmbi.1995.0498. [DOI] [PubMed] [Google Scholar]
- 6.Böhm L, Crane-Robinson C. Proteases as structural probes for chromatin: the domain structure of histones. Biosci Rep. 1984;4:365–386. doi: 10.1007/BF01122502. [DOI] [PubMed] [Google Scholar]
- 7.Brown J L, Mucci D, Whiteley M, Dirksen M L, Kassis J A. The Drosophila Polycomb group gene pleiohomeotic encodes a DNA binding protein with homology to the transcription factor YY1. Mol Cell. 1998;1:1057–1064. doi: 10.1016/s1097-2765(00)80106-9. [DOI] [PubMed] [Google Scholar]
- 8.Bunker C A, Kingston R E. Transcriptional repression by Drosophila and mammalian Polycomb group proteins in transfected mammalian cells. Mol Cell Biol. 1994;14:1721–1732. doi: 10.1128/mcb.14.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrington E A, Jones R S. The Drosophila Enhancer of zeste gene encodes a chromosomal protein: examination of wild-type and mutant protein distribution. Development. 1996;122:4073–4083. doi: 10.1242/dev.122.12.4073. [DOI] [PubMed] [Google Scholar]
- 10.Duckett D R, Murchie A I H, Bhattacharyya A, Clegg R M, Diekmann S, von Kitzing E, Lilley D M. The structure of DNA junctions and their interaction with enzymes. Eur J Biochem. 1992;207:285–295. doi: 10.1111/j.1432-1033.1992.tb17049.x. [DOI] [PubMed] [Google Scholar]
- 11.Edmondson D G, Smith M M, Roth S Y. The repression domain of the yeast global repressor Tup1 interacts directly with histones H3 and H4. Genes Dev. 1996;10:1247–1259. doi: 10.1101/gad.10.10.1247. [DOI] [PubMed] [Google Scholar]
- 12.Franke A, Decamillis M, Zink D, Cheng N S, Brock H W, Paro R. Polycomb and Polyhomeotic are constituents of a multimeric protein complex in chromatin of Drosophila melanogaster. EMBO J. 1992;11:2941–2950. doi: 10.1002/j.1460-2075.1992.tb05364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franke A, Messmer S, Paro R. Mapping functional domains of the Polycomb protein of Drosophila melanogaster. Chrom Res. 1995;3:351–360. doi: 10.1007/BF00710016. [DOI] [PubMed] [Google Scholar]
- 14.Gould A. Functions of mammalian Polycomb group and trithorax group related genes. Curr Opin Genet Dev. 1997;7:488–494. doi: 10.1016/s0959-437x(97)80075-5. [DOI] [PubMed] [Google Scholar]
- 15.Grunstein M. Molecular model for telomeric heterochromatin in yeast. Curr Opin Cell Biol. 1997;9:383–387. doi: 10.1016/s0955-0674(97)80011-7. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto N, Brock H W, Nomura M, Kyba M, Hodgson J, Fujita Y, Takihara Y, Shimada K, Higashinakagawa T. RAE28, BMI1, and M33 are members of heterogeneous multimeric mammalian Polycomb group complexes. Biochem Biophys Res Commun. 1998;245:356–365. doi: 10.1006/bbrc.1998.8438. [DOI] [PubMed] [Google Scholar]
- 17.Hecht A, LaRoche T, Strahl-Bolsinger S, Gasser S M, Grunstein M. Histone H3 and H4 N-termini interact with SIR3 and SIR4 proteins: a molecular model for the formation of heterochromatin in yeast. Cell. 1995;80:583–592. doi: 10.1016/0092-8674(95)90512-x. [DOI] [PubMed] [Google Scholar]
- 18.Kehle J, Beuchle D, Treuheit S, Christen B, Kennison J A, Bienz M, Muller J. dMi-2, a hunchback-interacting protein that functions in Polycomb repression. Science. 1998;282:1897–1900. doi: 10.1126/science.282.5395.1897. [DOI] [PubMed] [Google Scholar]
- 19.Kyba M, Brock H W. The Drosophila Polycomb group protein PSC contacts PH and PC through specific conserved domains. Mol Cell Biol. 1998;18:2712–2720. doi: 10.1128/mcb.18.5.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai J-S, Herr W. Ethidium bromide provides a simple tool for identifying genuine DNA-independent protein associations. Proc Natl Acad Sci USA. 1992;89:6958–6962. doi: 10.1073/pnas.89.15.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lilley D M. DNA-protein interactions. HMG has DNA wrapped up. Nature. 1992;357:282–283. doi: 10.1038/357282a0. [DOI] [PubMed] [Google Scholar]
- 22.Lonie A, Dandrea R, Paro R, Saint R. Molecular characterisation of the Polycomblike gene of Drosophila melanogaster, a trans-acting negative regulator of homeotic gene expression. Development. 1994;120:2629–2636. doi: 10.1242/dev.120.9.2629. [DOI] [PubMed] [Google Scholar]
- 23.Luger K, Richmond T J. The histone tails of the nucleosome. Curr Opin Genet Dev. 1998;8:140–146. doi: 10.1016/s0959-437x(98)80134-2. [DOI] [PubMed] [Google Scholar]
- 24.McMurray C T, van Holde K E. Binding of ethidium to the nucleosome core particle. 1. Binding and dissociation reactions. Biochemistry. 1991;30:5631–5643. doi: 10.1021/bi00237a001. [DOI] [PubMed] [Google Scholar]
- 25.Messmer S, Franke A, Paro R. Analysis of the functional role of the Polycomb chromo domain in Drosophila melanogaster. Genes Dev. 1992;6:1241–1254. doi: 10.1101/gad.6.7.1241. [DOI] [PubMed] [Google Scholar]
- 26.Mihaly J, Mishra R K, Karch F. A conserved sequence motif in Polycomb-response elements. Mol Cell. 1998;1:1065–1066. doi: 10.1016/s1097-2765(00)80107-0. [DOI] [PubMed] [Google Scholar]
- 27.Mizzen C A, Allis C D. Linking histone acetylation to transcriptional regulation. Cell Mol Life Sci. 1998;54:6–20. doi: 10.1007/s000180050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller J. Transcriptional silencing by the Polycomb protein in Drosophila embryos. EMBO J. 1995;14:1209–1220. doi: 10.1002/j.1460-2075.1995.tb07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nacheva G A, Guschin D Y, Preobrazhenskaya O V, Karpov V L, Ebralidse K K, Mirzabekov A D. Change in the pattern of histone binding to DNA upon transcriptional activation. Cell. 1989;58:27–36. doi: 10.1016/0092-8674(89)90399-1. [DOI] [PubMed] [Google Scholar]
- 30.Neubauer B, Hörz W. Analysis of nucleosome positioning by in vitro reconstitution. Methods Enzymol. 1989;170:630–644. doi: 10.1016/0076-6879(89)70069-0. [DOI] [PubMed] [Google Scholar]
- 31.Nightingale K, Dimitrov S, Reeves R, Wolffe A P. Evidence for a shared structural role for HMG1 and linker histones B4 and H1 in organizing chromatin. EMBO J. 1996;15:548–561. [PMC free article] [PubMed] [Google Scholar]
- 32.Orlando V, Jane E P, Chinwalla V, Harte P J, Paro R. Binding of trithorax and Polycomb proteins to the bithorax complex: dynamic changes during early Drosophila embryogenesis. EMBO J. 1998;17:5141–5150. doi: 10.1093/emboj/17.17.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paro R. Propagating memory of transcriptional states. Trends Genet. 1995;11:295–297. doi: 10.1016/s0168-9525(00)89081-2. [DOI] [PubMed] [Google Scholar]
- 34.Paro R, Harte P J. The role of Polycomb group and trithorax group chromatin complexes in the maintenance of determined cell states. In: Russo V E A R, Martienssen A, Riggs A R, editors. Epigenetic mechanisms of gene regulation. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. [Google Scholar]
- 35.Paro R, Hogness D S. The Polycomb protein shares a homologous domain with a heterochromatin-associated protein of Drosophila. Proc Natl Acad Sci USA. 1991;88:263–267. doi: 10.1073/pnas.88.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Passner J M, Don Ryoo H, Shen L, Mann R S, Aggarwal A K. Structure of a DNA-bound Ultrabithorax-Extradenticle homeodomain complex. Nature. 1999;397:714–719. doi: 10.1038/17833. [DOI] [PubMed] [Google Scholar]
- 37.Pirrotta V. Polycombing the genome: PcG, trxG, and chromatin silencing. Cell. 1998;93:333–336. doi: 10.1016/s0092-8674(00)81162-9. [DOI] [PubMed] [Google Scholar]
- 38.Platero J S, Sharp E J, Adler P N, Eissenberg J C. In vivo assay for protein-protein interactions using Drosophila chromosomes. Chromosoma. 1996;104:393–404. doi: 10.1007/BF00352263. [DOI] [PubMed] [Google Scholar]
- 39.Pöhler J R G, Norman D G, Bramham J, Bianchi M E, Lilley D M. HMG box proteins bind to four-way junctions in their open conformation. EMBO J. 1998;17:817–826. doi: 10.1093/emboj/17.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satijn D P E, Olson D J, van der Vlag J, Hamer K M, Lambrechts C, Masselink H, Gunster M J, Sewalt R G A B, van Driel R, Otte A P. Interference with expression of a novel human Polycomb protein, hPc2, results in cellular transformation and apoptosis. Mol Cell Biol. 1997;17:6076–6086. doi: 10.1128/mcb.17.10.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satijn D P E, Otte A P. RING1 interacts with multiple Polycomb-group proteins and displays tumorigenic activity. Mol Cell Biol. 1999;19:57–68. doi: 10.1128/mcb.19.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schoorlemmer J, Marcos-Gutierrez C, Were F, Martinez R, Garcia E, Satijn D P, Otte A P, Vidal M. Ring1A is a transcriptional repressor that interacts with the Polycomb-M33 protein and is expressed at rhombomere boundaries in the mouse hindbrain. EMBO J. 1997;16:5930–5942. doi: 10.1093/emboj/16.19.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shao Z, Raible F, Mollaaghababa R, Guyon J R, Wu C, Bender W, Kingston R E. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell. 1999;98:1–20. doi: 10.1016/S0092-8674(00)80604-2. [DOI] [PubMed] [Google Scholar]
- 44.Simon R H, Felsenfeld G. A new procedure for purifying histone pairs H2A + H2B and H3 + H4 from chromatin using hydroxylapatite. Nucleic Acids Res. 1979;6:689–696. doi: 10.1093/nar/6.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with gluthathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 46.Sogo J M, Laskey R A. Chromatin replication and assembly. In: Elgin S C R, editor. Chromatin structure and gene expression. Oxford, United Kingdom: IRL Press; 1995. [Google Scholar]
- 47.Struhl G, Akam M. Altered distributions of Ultrabithorax transcripts in extra sex combs mutant embryos of Drosophila. EMBO J. 1985;4:3259–3264. doi: 10.1002/j.1460-2075.1985.tb04075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strutt H L, Cavalli G, Paro R. Co-localisation of Polycomb protein and GAGA factor on regulatory elements responsible for the maintenance of homeotic gene expression. EMBO J. 1997;16:3621–3632. doi: 10.1093/emboj/16.12.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strutt H, Paro R. The Polycomb group complex of Drosophila melanogaster has different compositions at different target genes. Mol Cell Biol. 1997;17:6773–6783. doi: 10.1128/mcb.17.12.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Studitsky V M, Clark D J, Felsenfeld G. Preparation of nucleosomal templates for transcription in vitro. Methods Enzymol. 1996;274:246–256. doi: 10.1016/s0076-6879(96)74021-1. [DOI] [PubMed] [Google Scholar]
- 51.van Lohuizen M. Functional analysis of mouse Polycomb group genes. Cell Mol Life Sci. 1998;54:71–79. doi: 10.1007/s000180050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varga-Weisz P, Zlatanova J, Leuba S H, Schroth G P, van Holde K. Binding of histones H1 and H5 and their globular domains to four-way junction DNA. Proc Natl Acad Sci USA. 1994;91:3525–3529. doi: 10.1073/pnas.91.9.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wedeen C, Harding K, Levine M. Spatial regulation of Antennapedia and bithorax gene expression by the Polycomb locus in Drosophila. Cell. 1986;44:739–748. doi: 10.1016/0092-8674(86)90840-8. [DOI] [PubMed] [Google Scholar]
- 54.Zink D, Paro R. Drosophila Polycomb-group regulated chromatin inhibits the accessibility of a trans-activator to its target DNA. EMBO J. 1995;14:5660–5671. doi: 10.1002/j.1460-2075.1995.tb00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]