Protein Kinase Cδ Targets Mitochondria, Alters Mitochondrial Membrane Potential, and Induces Apoptosis in Normal and Neoplastic Keratinocytes When Overexpressed by an Adenoviral Vector (original) (raw)
Abstract
Inactivation of protein kinase Cδ (PKCδ) is associated with resistance to terminal cell death in epidermal tumor cells, suggesting that activation of PKCδ in normal epidermis may be a component of a cell death pathway. To test this hypothesis, we constructed an adenovirus vector carrying an epitope-tagged PKCδ under a cytomegalovirus promoter to overexpress PKCδ in normal and neoplastic keratinocytes. While PKCδ overexpression was detected by immunoblotting in keratinocytes, the expression level of other PKC isozymes, including PKCα, PKCɛ, PKCζ, and PKCη, did not change. Calcium-independent PKC-specific kinase activity increased after infection of keratinocytes with the PKCδ adenovirus. Activation of PKCδ by 12-_O_-tetradecanoylphorbol-13-acetate (TPA) at a nanomolar concentration was lethal to normal and neoplastic mouse and human keratinocytes overexpressing PKCδ. Lethality was inhibited by PKC selective inhibitors, GF109203X and Ro-32-0432. TPA-induced cell death was apoptotic as evidenced by morphological criteria, TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assay, DNA fragmentation, and increased caspase activity. Subcellular fractionation indicated that PKCδ translocated to a mitochondrial enriched fraction after TPA activation, and this finding was confirmed by confocal microscopy of cells expressing a transfected PKCδ-green fluorescent protein fusion protein. Furthermore, activation of PKCδ in keratinocytes altered mitochondrial membrane potential, as indicated by rhodamine-123 fluorescence. Mitochondrial inhibitors, rotenone and antimycin A, reduced TPA-induced cell death in PKCδ-overexpressing keratinocytes. These results indicate that PKCδ can initiate a death pathway in keratinocytes that involves direct interaction with mitochondria and alterations of mitochondrial function.
Epidermal differentiation, like that of other stratified squamous epithelia, requires a coordinated program of sequential gene expression, migratory controls, and temporal regulation of a death pathway to achieve the proper balance of functional but nonviable mature squames and viable cells to replenish them. Studies on cultured keratinocytes from murine and human skin have revealed components of the regulatory pathways responsible for this complex maturation process (16, 50). While the control of gene expression and cell migration has attracted much research interest, the regulation of the death pathway has not been as fully explored. In fact, it is still uncertain whether maturation-induced cell death is an active or a passive process that is simply the result of production of toxic structural components and the formation of cornified envelopes. For example, inappropriate expression of keratin 10 in proliferating keratinocytes leads to growth arrest and cell loss (25, 36). Even the nature of the death pathway is in dispute, having neither complete characteristics of apoptosis nor necrosis, suggesting that it may be a unique program customized for the important functions that a nonviable epidermal keratinocyte must perform without the intervention of phagocytosis (37).
For the most part, correlations have directed interpretations of keratinocyte-programmed cell death pathways. The pro-apoptotic protein Bax is increased in differentiating keratinocytes, while bcl-2 and procaspase 3 are found in proliferating cells (31, 44). Furthermore, keratinocytes isolated from transgenic mice overexpressing bcl-2 in the epidermis have a prolonged in vitro lifespan (40). These studies have been interpreted to support an apoptotic mechanism for terminal cell death in keratinocytes. The observation that the phorbol ester tumor promoter 12-_O_-tetradecanoylphorbol-13-acetate (TPA) enhances terminal cell death in cultured keratinocytes and epidermis in vivo (51) has implicated protein kinase C (PKC) as a mediator of the terminal phase of keratinocyte maturation. In vitro, PKC inhibitors effectively block keratinocyte terminal differentiation induced by calcium or TPA (13). However, keratinocytes express five isoforms of PKC (α, δ, ɛ, η, and ζ), and it has been unclear if all or only specific isoforms are involved in terminal cell death based on activation or inhibition studies. Recently, PKCδ and -η have been identified as inducers of keratinocyte transglutaminase and protein cross-linking in studies utilizing adenoviral vectors to overexpress these isoforms in cultured human keratinocytes (34). These findings were consistent with previous studies indicating that PKCδ translocated and was activated during calcium-induced keratinocyte differentiation and that PKCη increased in amount in late stages of epidermal maturation in vitro and in vivo (8, 27). Furthermore, recent studies have strongly implied that the activation of PKCδ was required for keratinocyte apoptosis induced by UV light (9). PKCδ is a ubiquitously expressed isoform of PKC that regulates pathways in a cell-type-specific manner (21, 26, 29). Under certain conditions, PKCδ can stimulate or inhibit proliferation, promote secretion, suppress tumor formation or in vitro transformation, and stimulate differentiation. In several model systems, PKCδ contributes to apoptosis induced by DNA damage and death receptors. PKCδ has several unique characteristics that distinguish it from other isoforms. It is protected from downregulation by bryostatin 1 in a specific dose response, and this is regulated by its catalytic domain (30). It is also subject to phosphorylation on tyrosine residues by a variety of stimuli, particularly after activation of cells by growth factor receptors (7, 21). Tyrosine phosphorylation may result in activation or inactivation, and the direction of change in catalytic activity may be substrate dependent (6, 28, 45).
We have focused on PKCδ as a keratinocyte death inducer from data obtained in studies of neoplastic keratinocytes, where escape from programmed cell death is essential for tumor development. In mouse keratinocytes transformed by a ras oncogene, PKCδ is catalytically inactivated by tyrosine phosphorylation (6), and this is associated with resistance to terminal cell death induced by calcium or TPA. Furthermore, inhibition of tyrosine phosphorylation of PKCδ by kinase inhibitors reverses the differentiation block in vitro and causes tumor regression in vivo (6, 46). In addition, PKCδ is specifically downmodulated when human keratinocytes are neoplastically transformed by an oncogenic ras gene (17). Because of the potential to exploit the endogenous keratinocyte death pathway in the treatment or prevention of squamous tumor development, we constructed a replication-deficient adenovirus carrying PKCδ to determine whether this isoform can induce keratinocyte cell death directly in order to define the characteristics of the death program and to elucidate the cellular targets involved.
MATERIALS AND METHODS
Chemicals and antibodies.
Polyclonal antibodies to PKCδ, PKCα, PKCɛ, PKCζ, and monoclonal antibody to FLAG peptide (M2) were from Sigma BioScience (St. Louis, Mo.). TPA was purchased from Alexis Co. (San Diego, Calif.). MitoTracker red was purchased from Molecular Probes, Inc. (Portland, Oreg.). Protein kinase inhibitors GF109203X, Ro-32-0432, PD98059 and H89 were purchased from CalBiochem (La Jolla, Calif.). Antimycin A, rotenone, and other chemicals or reagents were from Sigma Chemical Co. (St. Louis, Mo.).
Cell culture.
Primary mouse keratinocytes from newborn BALB/c mouse epidermis were prepared by a trypsin flotation procedure (11). The isolated primary keratinocytes were plated at a density of 2 × 106 to 5 × 106 cells per 60-mm dish in Eagle minimum essential medium (Ca2+ and Mg2+ free; BioWhittaker, Walkersville, Md.) supplemented with 8% Chelex (Bio-Rad Laboratories, Richmond, Calif.)-treated fetal bovine serum (FBS) (Gemini Bio-Products, Inc., Calabasas, Calif.) and 0.05 mM Ca2+. The cells were maintained at 36°C in a humidified incubator with 7% CO2 for 6 to 9 days. Fresh medium was added daily. Mouse neoplastic keratinocyte cell lines SP-1 and 308 were maintained in the same medium as the primary mouse keratinocytes (47). 293 and HeLa cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FBS. SQCC-Y1, a human squamous carcinoma-derived cell line, was kindly provided by J. Rheinwald of Harvard University (Boston, Mass.), and HPV-18 infected immortalized human keratinocytes were a gift from R. Schlegel (Georgetown University, Washington, D.C.). Both cell lines were grown in DMEM supplemented with 10% FBS.
Generation of adenovirus carrying PKCδ.
Two complementary single stranded DNAs, GGTACCCTCGAGTATACGCGTGACTACAAGGACGACGATGACAAGTAGAATTCGGGCC and CGAATTCTACTTGTCATCGTCGTCCTTGTAGTCACGCGTATACTCGAGGGTACCGC were synthesized and annealed at 50°C. This double-stranded DNA, containing the FLAG sequence followed by a stop codon, several restriction sites, and protruding ends, was cloned into the _Sac_II and _Apa_I sites of pGEM vector (Promega, Madison, Wis.). A mouse PKCδ cDNA fragment (_Xho_I-_Mlu_I) lacking the stop codon was digested from the MTH vector (32) and cloned 5′ to the FLAG sequence in the modified pGEM vector (pGEM-FPKCδ). The sequence was verified by automated DNA sequencing by using a DNA sequencing kit (Perkin-Elmer, Branchburg, N.J.). The FLAG-epitope-tagged PKCδ cDNA was then excised from the pGEM-FPKCδ with _Xho_I-_Eco_RI and ligated into the plasmid vector, pCA4 (Microbix Biosystems, Inc., Toronto, Ontario, Canada), which contains partial adenovirus type 5 sequence deleted in the E1 region with insertion of the cytomegalovirus (CMV) promoter and the simian virus 40 polyadenylation signal into the E1 region. The plasmid pCA4 carrying PKCδ-FLAG was cotransfected with pJM17 (Microbix Biosystems, Inc.) into 293 cells. pJM17 is noninfectious in single transfection of 293 cells. Adenoviral plaques were isolated after 10 to 14 days and reamplified in 293 cells. The expression of PKCδ-FLAG fusion protein was examined by Western blot with both anti-PKCδ and anti-FLAG antibodies. The resulting positive virus was referred to as AdFPKCδ. An adenovirus carrying β-galactosidase (Adβgal) under the control of CMV promoter was used as a viral control vector (22).
Construction of plasmid encoding PKCδ-GFP fusion protein.
A plasmid containing the green fluorescent protein (GFP) cDNA (pEGFP-N1) was purchased from Clontech (Palo Alto, Calif.). pEGFP-N1 and pCA4 carrying PKCδ-FLAG were digested with _Xma_I and _Mlu_I, respectively. The resulting DNA protruding ends were filled in with Klenow fragment (Promega). Both linearized plasmids were then digested with _Xho_I. The PKCδ insert from pCA4 and the vector of pEGFP-N1 were purified by 1% agarose gel and ligated. To construct plasmid expressing the kinase inactive mutant of PKCδ-GFP fusion protein, pPKCδK376R-GFP, a mouse PKCδK376R cDNA fragment (_Xho_I-_Mlu_I) lacking the stop codon was digested from the MTH vector (5, 32) and ligated with the modified pEGFP containing an added _Mlu_I site. The sequences of pPKCδ-GFP and pPKCδK376R-GFP were verified with automated sequencing as described above.
Infection of adenovirus.
The infection of adenovirus was carried out in serum-free medium containing 2.5 μg of Polybrene (Sigma) per ml at 50 PFU/cell for primary mouse keratinocytes and 100 PFU/cell for cell lines, respectively, for 30 min at room temperature. Fresh serum-containing medium was added thereafter. The transducing efficiency of the adenovirus under these condition is over 90%.
Transfection.
pGFP, pPKCδ-GFP, and pPKCδK376R-GFP were transfected into SP-1 cells by using Lipofectamine Plus reagent (Life Technologies, Inc., Gaithersburg, Md.) according to the procedure recommended by the manufacturer. In brief, 4 μg of DNA, 12 μl of lipid, and 12 μl of Plus reagent were used to transfect SP-1 cells growing in 60-mm tissue culture dishes at 50% confluence in 2 ml of medium without serum. Fresh medium with 8% FBS was added 3 h later. The transfection efficiency is ca. 50%.
Subcellular fractionation.
To isolate the particulate and cytosol fractions, cells were collected in buffers containing 25 mM Tris (pH 7.4), 150 mM NaCl, 2 mM MgCl2, 1 mM EGTA, and 1 mM EDTA. Protease inhibitors (10 μg of leupeptin per ml, 10 μg of aprotinin per ml, and 1 mM phenylmethylsulfonyl fluoride) and phosphatase inhibitors (10 μM NaVO4 and 1 mM NaF) were added prior to lysis. The cell lysates were then sonicated and centrifuged at 100,000 × g for 1 h at 4°C. Aliquots of the cytosolic fraction (supernatant) and the particulate fraction (pellet) were subjected to electrophoresis and immunoblotting. To isolate the mitochondrial enriched membrane fractions, cells were collected in phosphate-buffered saline (PBS) and then pelleted by centrifugation. The cell pellets were resuspended in a buffer containing 25 mM Tris (pH 7.4), 250 mM sucrose, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, and 1 mM dithiothreitol with protease inhibitors as described above and homogenized 10 times with a Dounce homogenizer. Unlysed cells and nuclei were removed by centrifugation at 750 × g for 10 min. The supernatant was centrifuged at 10,000 × g for 30 min, and the resulting pellet was washed once with the same buffer and represents the mitochondrial enriched fraction. The supernatant was further spun at 100,000 × g for 1 h, and the supernatant from this final centrifugation represents the cytosol fraction. The centrifugation was carried out at 4°C (49). The protein concentration in each sample was determined by the Bradford method (Bio-Rad, Richmond, Calif.).
Cell viability assay.
Cell viability was measured with a cell proliferation assay kit (The CellTiter 96; Promega) according to the manufacturer’s protocol. In brief, the cells grown on 24-well tissue culture plates were infected with adenovirus for 2 days and treated with TPA in 0.5 ml of medium. After various times, 75 μl of dye solution was added to the cells without medium change, and 0.5 ml of solubilization-stop solution was added 4 h later. Then, 200 μl of the final mixture was transferred to 96-well plates, and the absorbency at 590 nm was examined by a plate reader. Results are presented relative to 100% of cell death as determined by killing cells with three cycles of freeze-thawing. The assay is based on the cellular conversion of a tetrazolium salt (MTT) into a blue formazan product that is detected by a plate reader at 590 nm.
PKC activity.
The PKC activity was assayed by using the Protein Kinase C Assay System (Gibco-BRL) according to the manufacturer’s procedure. In brief, both control and viral-infected cells were lysed in the extraction buffer (20 mM Tris, pH 7.5; 0.5 mM EDTA; 0.5 mM EGTA; 0.5% Triton X-100; 25 μg each of aprotinin and leupeptin per ml). Kinase activities from cell lysate (40 μg) and immunoprecipitation complex were examined by using acetylated myelin basic protein (Ac-MBP) as the substrate, and the nonspecific activity was determined by including peptides containing the pseudosubstrate region of PKC. The assay mixture contains 20 mM Tris (pH 7.5), 20 mM MgCl2, 1 mM CaCl2, 20 μM ATP, and 50 μM Ac-MBP, with 0.1 μCi of [γ-32P]ATP per assay. For the Ca2+-independent kinase activity, 5 mM EGTA was included in the assay mixture. Data represent triplicate determinations.
TUNEL assay.
Apoptotic cells were detected by TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assay by using the ApopTag Direct In Situ Apoptosis Detection Kit with Fluorescein (Oncor, Gaithersburg, Md.). Attached keratinocytes were harvested by trypsinization, combined with unattached cells, fixed in 5% paraformaldehyde, and resuspended in PBS. Next, 105 cells were spread on glass slides by use of a Cytospin (Cytospin 2; Shandon, Pittsburgh, Pa.). Detection of apoptotic cells and counterstaining with propidium iodide (PI) were performed according to the instructions provided by the manufacturer. The slides were viewed and photographed by using a fluorescent Leica microscope.
DNA degradation and caspase 3 activity measurements.
Total DNA was isolated from primary keratinocytes by lysing cells in a buffer containing 5 mM Tris (pH 8.0), 10 mM EDTA, and 0.5% Triton X-100. The lysates were treated with 100 μg of RNase A per ml for 60 min at 37°C and then 200 μg of proteinase K per ml for 50 min at 50°C. DNA was extracted with equal volumes of phenol-chloroform-isoamyl alcohol twice and precipitated with 1/4 volume of NH4 acetate (3 N) and 2 volumes of 100% EtOH. The pellets were washed with 70% ethanol once and resuspended in 50 μl of TE. Then, 10 μl of DNA was used for electrophoresis on a 1.8% agarose gel. The gel was stained with ethidium bromide and photographed under UV light. Caspase 3 activity was determined by using the Caspase-3 Assay Kit from Biomol (Plymouth Meeting, Pa.) according to the instructions provided by the manufacturer. Adenovirus-infected cells were treated with TPA, and cell lysates were collected after 12 h for assay.
Confocal microscopy.
SP-1 cells were transfected with pGFP, pPKCδ-GFP, and pPKCδK376R-GFP by using Lipofectamine as described earlier. After 24 h, cells were treated with TPA (250 nM) for 30 min and then loaded with MitoTracker red (20 nM) for an additional 30 min in culture medium. A time course of pPKCδK376R-GFP translocation was recorded by use of confocal microscopy. Confocal fluorescent images were collected by using a Bio-Rad MRC 1024 confocal scan head mounted on a Nikon Optiphot microscope with a 488-nm excitation light from an argon-krypton laser. Emission filters of 598/40 and 522/32 were used for collecting red and green fluorescence, respectively, in channels one and two. After sequential excitation of 1.0- or 1.5-μm optical sections (z series), red and green fluorescent images of the same cell were collected, saved, and merged for colocalization of GFP with MitoTracker red by using LaserSharp software (Bio-Rad). A time course of PKCδ-GFP translocation in SP-1 cells was also viewed and photographed by using an inverted fluorescent microscope (Zeiss ICM 405).
Detection of mitochondrial membrane potential.
The mitochondrial membrane potential was determined by using rhodomine-123 (Rh123) as described previously (49). Cells were harvested by trypsinization and resuspended in medium at 106 cells/ml. Cells were incubated with Rh123 (5 μM) for 20 min at room temperature, washed once, resuspended in tissue culture medium, and analyzed by flow cytometry. The uncoupling agent, carbonyl cyanide _m_-chlorophenylhydropazone (CCCP; Sigma), was added at 50 μM with Rh123 as a positive control.
RESULTS
Characterization of adenovirus-mediated expression of PKCδ.
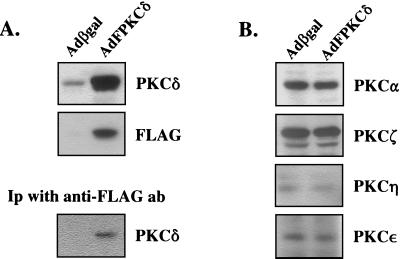
Primary mouse keratinocytes were infected with epitope-tagged PKCδ or β-galactosidase control adenoviruses at 50 PFU/cell, and cell lysates were analyzed by Western blotting after 48 h (Fig. 1A). A 10-fold increase in PKCδ protein was detected in the AdFPKCδ-infected cells by using anti-PKCδ antibodies, and exogenous PKCδ was also abundant on Western blots or by immunoprecipitation when detected by anti-FLAG antibody. The level of the other PKC isoforms was unaffected by infection with either adenovirus (Fig. 1B). More than 90% of keratinocytes were infected under this condition when examined by immunostaining with anti-FLAG antibodies or a β-galactosidase staining assay (not shown).
FIG. 1.
Expression of exogenous and endogenous PKC isozymes in primary mouse keratinocytes. After 3 days in primary culture, mouse keratinocytes were infected with 50 PFU/cell of either AdFPKCδ or Adβgal (vector) for 48 h. (A) Cell lysates from both vector- and AdFPKCδ-infected cells were collected and subjected to either immunoblotting with anti-PKCδ or anti-FLAG antibodies or immunoprecipitation (Ip) with anti-FLAG antibody and immunoblotted with anti-PKCδ antibodies. (B) The expression of endogenous PKC isozymes α, ζ, ɛ, and η was examined by immunoblotting of cell lysates 48 h after infection with either AdFPKCδ or Adβgal.
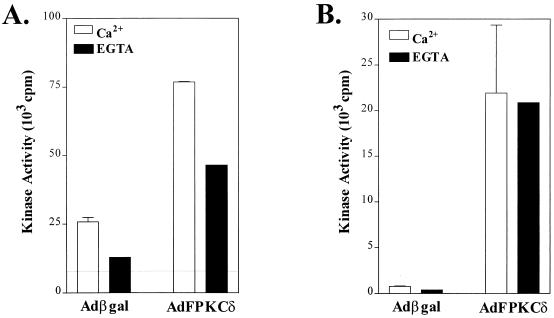
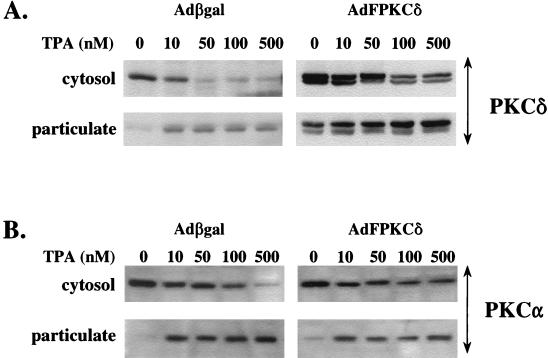
The functional activity of the epitope-tagged PKCδ was examined by PKC activity assay as described in Materials and Methods. Total PKC activity, including the Ca2+-dependent and Ca2+-independent activity, increased three- to fourfold (Fig. 2A) in AdFPKCδ-infected keratinocytes. Since protein levels increased nearly 10-fold according to Western blot, this suggests that some PKCδ is in an inactive state. Most of the PKC activity increase can be accounted for in the Ca2+-independent fraction (i.e., plus EGTA), as would be expected for PKCδ. Exogenous PKCδ was recoverable as a Ca2+-independent PKC activity in anti-FLAG immunoprecipitates from AdFPKCδ but not Adβgal-infected keratinocytes (Fig. 2B). The activation of exogenous and endogenous PKCδ was also tested by measuring translocation from cytosol to the particulate fraction after TPA treatment (Fig. 3A). After infection and expression, exogenous PKCδ was already present in the particulate fraction of untreated cells and frequently appeared as a doublet, suggesting constitutive phosphorylation. TPA caused translocation of both endogenous and exogenous PKCδ from the cytosol to the particulate fraction. In PKCδ-overexpressing cells, there was a preferential increase in the slow mobility band in the particulate fraction, suggesting that phosphorylation occurs preferentially at membranes. A slow mobility band is also formed in the particulate fraction of endogenous PKCδ in TPA-treated Adβgal-infected cells, suggesting that this is a phenomenon associated with activation of this isoform. Endogenous PKCα also translocates in response to TPA (Fig. 3B) in both cell types, indicating it remains active in the presence of excess PKCδ. However, there are slight differences in the dose-response for cytosol depletion among the two infected cell types of unknown significance. Nevertheless, the introduction of PKCδ by adenovirus vector results in a functioning enzyme that remains responsive to phorbol ester activation and translocation signals.
FIG. 2.
PKC activity detected in mouse primary keratinocytes infected with either AdFPKCδ or Adβgal. Primary mouse keratinocytes infected with either AdFPKCδ or Adβgal for 48 h were lysed as described in Materials and Methods. (A) PKC kinase activity from total lysates was assayed in the presence or absence of 5 mM EGTA. (B) PKC activity from immunoprecipitates of anti-FLAG antibody was examined in the presence or absence of 5 mM EGTA. Data presented are the mean ± the standard error of the mean (n = 3). Similar results were obtained in two experiments.
FIG. 3.
TPA-induced translocation of PKCα and -δ from soluble to particulate fractions. SP-1 cells infected with Adβgal or AdFPKCδ for 24 h were treated with various doses of TPA as indicated in the figure for 30 min. Aliquots of cytosol and particulate fractions were analyzed by Western blot with rabbit anti-PKCδ (A) or anti-PKCα (B) antibodies.
Activation of PKCδ by TPA induces cell death.
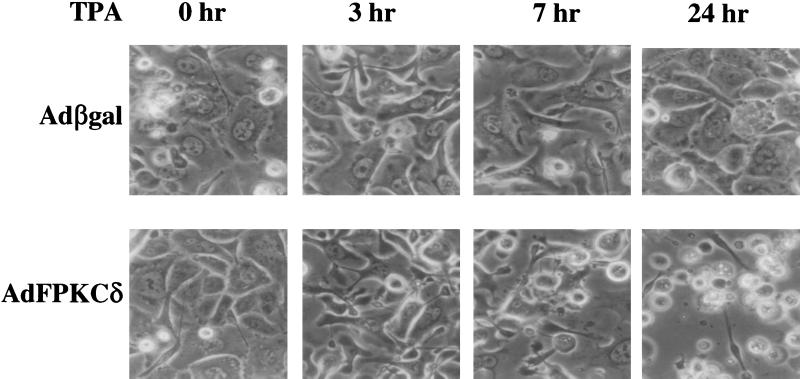
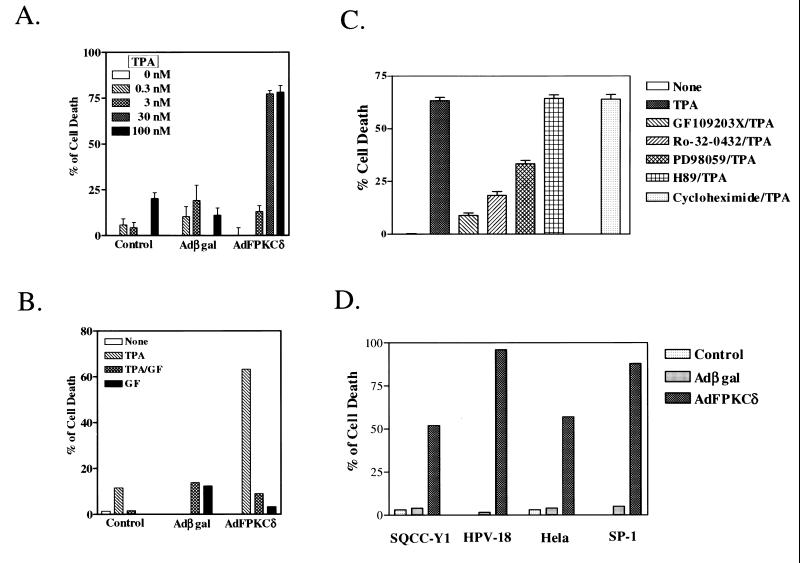
Initially, infection of primary mouse keratinocytes with AdFPKCδ, but not Adβgal, at 100 PFU/cell or higher resulted in cell killing for 3 to 5 days so that it was difficult to explore the pathways involved. Therefore, we reduced adenovirus to 50 PFU/cell and used TPA activation to control biological events. Under these conditions keratinocytes infected with the PKCδ or control adenoviruses were morphologically similar in the absence of activation by TPA (Fig. 4). Within the first few hours after administration of a low dose of TPA (50 nM), cells in both groups became elongated and formed dendritic structures which were reversed in the Adβgal group but became more prominent with time in the AdFPKCδ group. Within 24 h of TPA treatment, a spindle phenotype or cell rounding and detachment was obvious in a majority of AdFPKCδ cells, and by 48 h most of the cells were floating in the medium. Rounded, detached cells were also seen in control cultures, but to a much lower extent. Studies with quantitative endpoints for cell viability (Fig. 5A) indicate that TPA was lethal to AdFPKCδ-infected primary mouse keratinocytes with a 50% effective dose between 3 and 30 nM. Even at 100 nM, TPA did not cause substantial lethality in cultured control keratinocytes (Fig. 5A). At low concentrations of TPA, cell death in PKCδ-overexpressing cells was inhibited by a 10 μM concentration of the PKC selective inhibitor, GF109203X (Fig. 5B). GF109203X itself had no effect on cell growth. To further confirm that the TPA-induced cell death in PKCδ-overexpressing keratinocytes is mediated by PKCδ, we compared the effect of inhibitors for PKA and mitogen-activated protein kinase kinase (MAPKK) to PKC inhibitors (Fig. 5C). As seen before, GF109203X and also Ro-32-0432, both PKC-selective inhibitors, blocked lethality from TPA. In contrast, H89, a PKA inhibitor, had no effect on TPA-induced cell death. Of interest, however, PD98059, a MAPKK inhibitor, partially blocked TPA-induced cell death, suggesting that MAPK may be a downstream target of a PKCδ-activated cell death pathway. Pretreating cells with cycloheximide (5 μg/ml) did not prevent TPA-induced cell death in PKCδ-overexpressing cells (Fig. 5C), suggesting, together with the rapid time course (see below), that PKCδ may directly activate a cell death pathway without a requirement for gene activation or new protein synthesis. To determine whether a differentiation program was induced in PKCδ-overexpressing keratinocytes, Western blots were performed on total cell lysates collected from AdFPKCδ-infected primary keratinocytes treated with TPA after 24 or 48 h. There was no detectable expression of keratins 1 and 10, loricrin, or filaggrin at these time points (not shown).
FIG. 4.
TPA treatment of primary mouse keratinocytes overexpressing PKCδ alters cell morphology. Primary mouse keratinocytes were infected with either AdFPKCδ or Adβgal as described in Materials and Methods. After 24 h (time zero), 50 nM TPA was added to cells, and photographs were taken after 0, 3, 7, and 24 h.
FIG. 5.
TPA induces cell death in PKCδ-overexpressing normal and neoplastic keratinocytes. Primary mouse keratinocytes, infected with either AdFPKCδ or Adβgal for 24 h, were treated with various concentrations of TPA (A) or with a PKC inhibitor, GF109203X (10 μM), with or without 50 nM TPA for 24 h (B). Cell viability was examined with the MTT assay. (C) PKC selective inhibitors Ro-32-0432 (10 μM) and GF109203X (10 μM), PKA inhibitor H89 (10 μM), MAPKK inhibitor PD98059 (10 μM), and the protein synthesis inhibitor cycloheximide (10 μg/ml) were added to primary mouse keratinocytes overexpressing PKCδ together with 50 nM TPA. After 24 h, cell viability was examined with the MTT assay. (D) Neoplastic human and mouse keratinocytes (SQCC-Y1, HPV-18, HeLa, and SP-1) were infected with AdFPKCδ or Adβgal as described in the Materials and Methods for 24 h. TPA (250 nM) was added, and cell viability was determined after 24 h. Values are expressed relative to 100% of cell death as determined by three cycles of freeze-thawing within each experiment. Results (mean ± the SEM) are from one of at least three experiments, each performed in triplicate. Error bars for the 30 nM dose in panel A for control and Adβgal groups are too small to be seen.
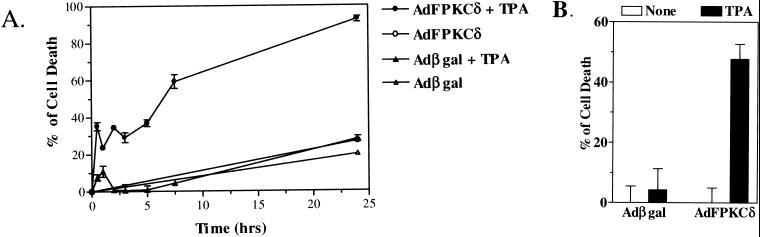
Previous studies have shown that squamous tumor cells are resistant to phorbol ester-induced terminal cell death (24). When AdFPKCδ is introduced into a variety of human or mouse squamous tumor cell lines (Fig. 5D), these cells become responsive to TPA-induced lethality and rapidly detach from the culture dish. Both benign and malignant cells are susceptible, and these changes are blocked by PKC inhibitors (not shown). Time course studies with cell viability assays indicated that PKCδ-mediated lethality occurred rapidly, within 3 to 7 h after the addition of TPA (Fig. 6A). Because TPA-induced cell death was correlated to cell detachment (Fig. 4), we sought to determine whether detachment of keratinocytes preceded or resulted from lethality induced by TPA. Primary mouse keratinocytes, infected with AdFPKCδ or Adβgal, were treated with TPA (100 nM) for 3 h, the attached cells were collected by trypsinization, and 25,000 cells were tested for viability. By 3 h after TPA treatment, there was already a reduction in viability of 50% in PKCδ-overexpressing attached primary keratinocytes, whereas the loss of viability was minimal in vector control cells after TPA at this time (Fig. 6B). Similar results were obtained when cells were treated with phorbol dibutyrate (not shown). This result indicates that lethality precedes detachment in PKCδ-overexpressing keratinocytes.
FIG. 6.
(A) TPA-induced lethality is rapid in PKCδ-overexpressing keratinocytes. Primary mouse keratinocytes, infected with either AdFPKCδ or Adβgal for 24 h, were exposed to TPA (50 nM). Cell viability was assessed at multiple times after TPA treatment by MTT assay. (B) TPA-induced lethality precedes loss of attachment. Primary mouse keratinocytes, infected with either AdFPKCδ or Adβgal for 24 h, were treated with TPA (100 nM) for 3 h. Attached cells were collected by trypsinization, counted, and aliquoted. Then, 25,000 cells were seeded per well in 96-well plates and assayed for cell viability by MTT assay. Data presented are the means ± the standard error of the mean (n = 6). Similar results were obtained from two experiments, and a third experiment was done with phorbol dibutyrate.
PKCδ-mediated cell killing has characteristics of an apoptotic process.
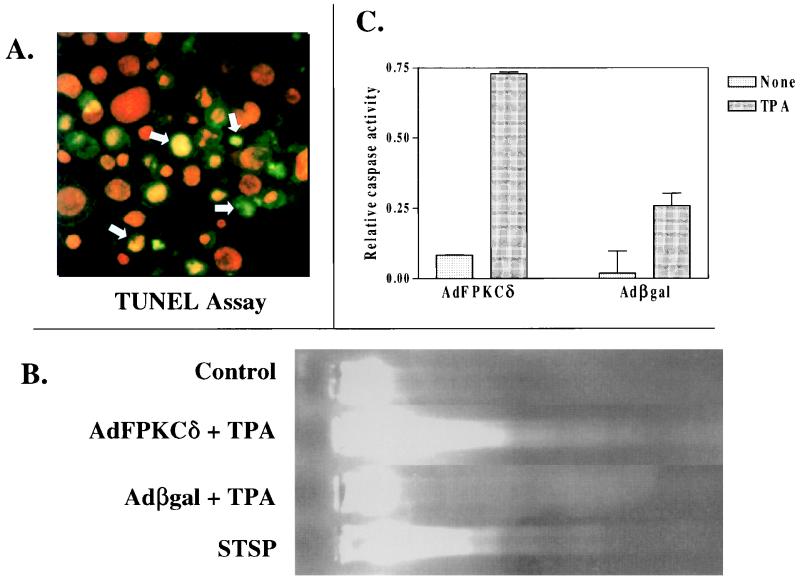
Before exploring the cellular targets for PKCδ-mediated cell killing, we wanted to characterize the nature of the dying cells in order to provide direction to our mechanistic studies. Primary mouse keratinocytes, infected with AdFPKCδ or Adβgal, were treated with TPA and collected after 24 h for TUNEL staining and DNA fragmentation analysis. TUNEL-positive nuclei (Fig. 7A) were detected in TPA-treated cells of both groups, but there were three times more in the PKCδ-overexpressing cells compared to the Adβgal-infected controls (Table 1). Even in the absence of TPA, overexpressing PKCδ increased the number of TUNEL-positive cells substantially. DNA fragmentation was also detected in gel mobility assays of DNA extracted from AdFPKCδ keratinocytes treated for 24 h with TPA, whereas DNA from control keratinocytes treated similarly remained at the origin of the gels (Fig. 7B). However, a well-defined DNA ladder could not be resolved. The activation of caspase plays a central role in the regulation of apoptosis induced by various stimuli (43). Figure 7C indicates that caspase 3 activity is increased in keratinocytes infected with AdFPKCδ relative to keratinocytes infected with Adβgal and, within 12 h of TPA treatment, there is a further 8.6-fold increase above that of the untreated cells. Caspase 3 activity also increases in TPA-treated Adβgal-infected cells, but the activity is only 30% of that in AdFPKCδ-infected cells. Taken together, these findings suggest that TPA induces an apoptosis-like cell death in keratinocytes that is substantially enhanced by overexpression of PKCδ.
FIG. 7.
Apoptotic markers detected in keratinocytes overexpressing PKCδ. (A) Primary mouse keratinocytes infected with AdFPKCδ were treated with TPA (50 nM) for 24 h. Both attached and floating cells were collected and fixed for TUNEL assay. The red fluorescence indicates nuclear counterstaining with PI, and the green fluorescence indicates positive staining for the TUNEL assay. Yellow nuclei (arrows) express both markers. (B) DNA was isolated from AdFPKCδ- or Adβgal-infected primary keratinocytes 24 h after TPA treatment, processed through a 1.8% agarose gel, and stained with ethidium bromide. DNA degradation is detected in PKCδ-overexpressing cells. Parallel cultures were treated with 1 μM STSP as a positive control. (C) Cell lysates from SP-1 cells infected with AdFPKCδ or Adβgal and treated with 250 nM TPA for 12 h were subjected to caspase 3 assay as described in Materials and Methods. The relative activity of caspase 3 was determined as the optical density at 402nm of the reaction mixture. Data presented are mean ± the standard deviation (n = 2). Similar results were obtained from three experiments.
TABLE 1.
Apoptosis induced by TPA in primary keratinocytes infected with AdFPKCδa
| Group | Cells positive for TUNEL assay (%) treated with: | |
|---|---|---|
| DMSO (0.1%) | TPA (30 nM) | |
| Control | 10.3 ± 1.5 | 13.2 ± 2.4 |
| Adβgal | 15.5 ± 5.1 | 13.6 ± 7.8 |
| AdFPKCδ | 27.1 ± 5.9 | 43.8 ± 10.7b |
Overexpressed PKCδ targets mitochondria to induce lethality in keratinocytes.
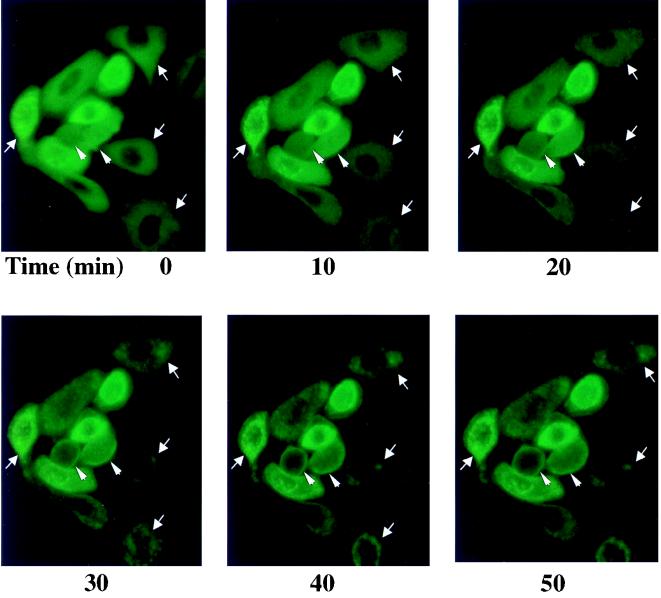
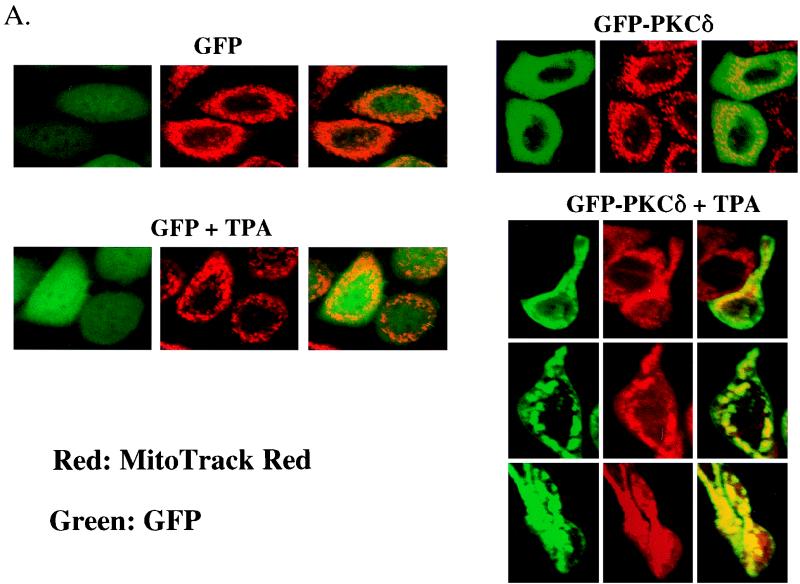
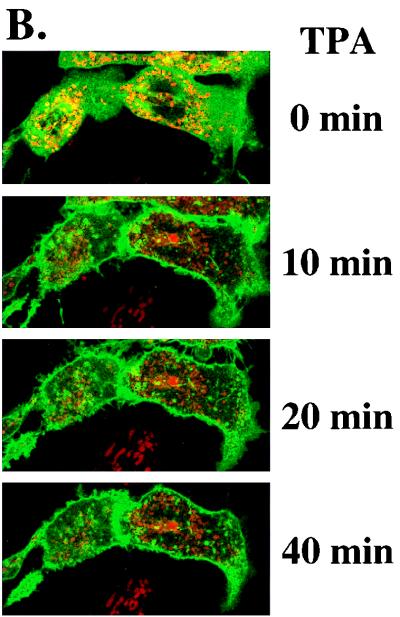
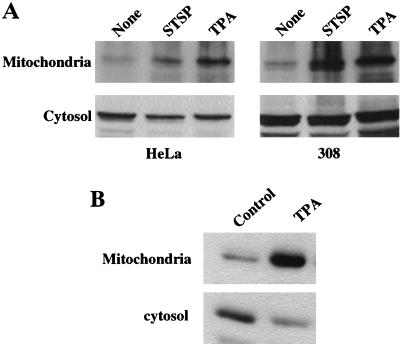
To provide guidance for studies of intracellular targeting of PKCδ in response to TPA activation, we constructed a plasmid expressing a PKCδ-GFP fusion protein, transfected that into SP-1 cells, and treated the cells with TPA. Before TPA treatment, PKCδ-GFP displayed a relatively diffuse cytosolic distribution with perinuclear concentration in some cells (Fig. 8). After TPA treatment, PKCδ-GFP translocated from cytosol to plasma membrane within 10 to 20 min and then concentrated in perinuclear structures consistent with intracellular organelles (Fig. 8). In contrast, the fluorescence pattern did not change in SP-1 cells transfected with pGFP. To determine whether the punctate perinuclear distribution of PKCδ-GFP represents localization to a specific organelle, a fluorescent probe for mitochondria, MitoTracker red, was loaded into SP-1 cells expressing either PKCδ-GFP or GFP. Red and green fluorescence were viewed by a confocal microscope with 598/40 and 522/32 emission filters, respectively. Images of red and green fluorescence of optical sections (1.5 μm) of the same field indicated that PKCδ-GFP colocalized with MitoTracker red after TPA treatment (Fig. 9A). In addition to inducing PKCδ-GFP to colocalize with mitochondria, TPA also caused aggregation of mitochondria and shape changes in SP-1 cells expressing PKCδ-GFP. In contrast, after transfection with the parental GFP vector, the distribution of GFP was diffuse, and GFP localization or cell morphology did change after TPA treatment. Likewise transfection with a kinase-inactive mutant of pPKCδK376R fused to GFP showed the formation of the cytoplasmic granules after TPA treatment that did not colocalize with MitoTracker red (Fig. 9B). However, this mutant did translocate to the plasma membrane after TPA treatment. To confirm the mitochondrial localization of PKCδ, 308 and HeLa cells were infected with AdFPKCδ and treated with TPA, and aliquots of cytosol and mitochondrial enriched fractions were examined by Western blot after 3 h. Translocation of PKCδ to a mitochondrial enriched fraction was detected in both cell lines (Fig. 10A). Of interest, staurosporine (STSP), an agent previously shown to induce terminal cell death in normal and neoplastic keratinocytes through a PKC-dependent mechanism (12), also caused translocation of PKCδ to the mitochondrial fraction. As shown in Fig. 10B, endogenous PKCδ was also detected in the mitochondrial enriched fraction of SP-1 cells, and this was substantially increased after TPA stimulation.
FIG. 8.
Rapid translocation of PKCδ-GFP after TPA treatment. The distribution of green fluorescence of transfected PKCδ-GFP in TPA-treated SP-1 cells was monitored in living cells by use of an inverted fluorescent microscope. SP-1 cells were infected with pPKCδ-GFP and, after 24 h, TPA (500 nM) was added directly to the medium to trigger translocation. Experiments were performed at room temperature. Photographs were taken at the various times indicated. Arrows mark cells where organellular translocation is prominent, and arrowheads mark cells where plasma membrane translocation is prominent.
FIG. 9.
Colocalization of PKCδ-GFP protein with mitochondria. (A) pGFP and pPKCδ-GFP were transfected into SP-1 cells by use of Lipofectamine, and the expression of GFP or GFP-PKCδ protein was viewed in living cells by confocal microscopy. Mitochondria were visualized by staining with MitoTracker red for 30 min. Transfected cells were treated with 250 nM TPA and examined after incubation at 37°C for 1 h. (B) A kinase inactive mutant, pPKCδK376R-GFP fusion protein, was transfected into SP-1 cells, and after a loading with MitoTracker red for 30 min, cells were treated with 250 nM TPA and examined for 40 min by confocal microscopy.
FIG. 10.
TPA induces translocation of PKCδ from cytosolic to mitochondrial enriched fractions. (A) HeLa and 308 cells infected with AdFPKCδ were treated with TPA (250 nM) or STSP (1 μM) for 3 h. Cell lysates were collected, and cytosolic and mitochondrial enriched fractions were isolated as described in Materials and Methods. Western blots with PKCδ antibodies were performed on 20 μg of cytosol protein and 1 μg of mitochondrial enriched protein. (B) Endogenous PKCδ translocates to the mitochondrial enriched fraction in SP-1 cells after TPA treatment. To detect endogenous PKCδ, SP-1 cells were treated with 250 nM TPA for 1 h and subjected to subcellular fractionation. Western blots were performed with PKCδ antibodies on 10 μg of cytosol protein and 10 μg of mitochondrial enriched protein.
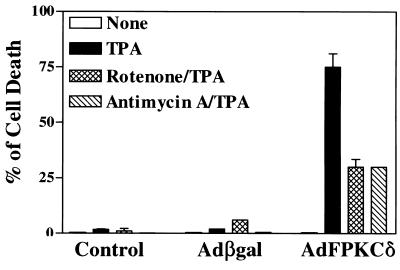
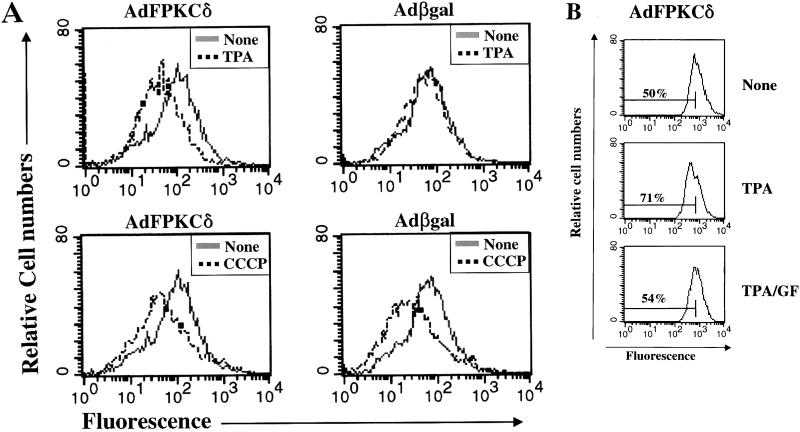
Having identified mitochondria as a potential target for PKCδ-induced lethality, we tested several mitochondrial electron transport inhibitors for activity against TPA-induced cell death in SP-1 cells infected with either AdFPKCδ or Adβgal (Fig. 11). Both antimycin A, an inhibitor of complex III in the respiratory chain, and rotenone, an inhibitor of complex I, substantially decreased TPA-induced cell death at low concentrations. Higher concentrations of these inhibitors could not be tested because they are directly toxic to SP-1 cells. Evidence for a direct effect of PKCδ on mitochondrial membrane potential was obtained by using Rh123 (Fig. 12A). After TPA treatment Rh123 fluorescence was reduced in AdFPKCδ- but not Adβgal-infected SP-1 cells. This change was inhibited by GF109203X (Fig. 12B), indicating it is PKC mediated. In the absence of TPA, Rh123 fluorescence was similar in control and PKCδ overexpressing SP-1 cells and was significantly reduced in SP-1 cells from both groups exposed to an uncoupling agent, CCCP (Fig. 12).
FIG. 11.
Mitochondrial inhibitors, rotenone and antimycin A, inhibit PKCδ mediated cell death. SP-1 cells, infected with either AdFPKCδ or Adβgal for 24 h, were treated with rotenone (0.25 μM) and antimycin A (0.5 μM) with or without 250 nM TPA. After 24 h, surviving cells were measured by assaying the protein content in each sample as described in Materials and Methods. Data presented are the means ± the standard error of the mean (n = 6). Similar results were obtained from three separate experiments.
FIG. 12.
TPA alters mitochondrial membrane potential in PKCδ-overexpressing keratinocytes. (A) SP-1 cells, infected with either AdFPKCδ or Adβgal, were treated with TPA (250 nM) for 3 h and collected by trypsinization. Rh123 (5 μM) was loaded, and the fluorescence intensity was determined by flow cytometry and compared to similarly infected cells not treated with TPA. For a positive control, CCCP, an uncoupling agent, was incubated with cells for 15 min. (B) In a parallel experiment, AdFPKCδ-infected SP-1 cells were treated with PKC inhibitor, GF109203X (1 μM), 15 min prior to the addition of TPA (250 nM). Cells were collected 3 h later, loaded with Rh123, and analyzed for fluorescence intensity. Similar results were obtained from two experiments.
DISCUSSION
Overexpression and activation of PKCδ are lethal to normal and neoplastic keratinocytes.
Earlier observations indicated that inactivation of PKCδ in neoplastic keratinocytes was associated with resistance to cell death that accompanies the terminal phase of keratinocyte differentiation (6). In concert with the specific downregulation of PKCδ detected in human keratinocytes transformed with a ras oncogene (17), these findings implied that PKCδ might have antitumor effects in squamous cell carcinogenesis. This study shows that PKCδ is lethal to normal and neoplastic keratinocytes when overexpressed by an adenoviral vector and activated by TPA. Lethality is rapid, does not require new protein synthesis, and is prevented by selective inhibitors of PKC catalytic activity, suggesting that this kinase targets substrates that are involved in a death pathway. Kuroki and coworkers used PKCδ and -η adenoviral vectors producing high levels of gene expression in human keratinocytes and found that both isoforms caused growth inhibition and transglutaminase I induction (34). However, only the δ isoform caused a spindle cell morphological change similar to what we described for mouse keratinocytes (Fig. 4), suggesting that there is specificity in action among these two isoforms. In that study the longer-term consequences of overexpression on cell viability were not reported, nor was the consequence of activation by phorbol ester examined. Since our study examined both mouse and human keratinocytes and both species were susceptible to cell killing, we must conclude that a lethal response is a general effect of activation of the PKCδ isoform when overexpressed in squamous cell types.
A number of other markers of keratinocyte differentiation previously attributed to PKC activation (8) are not upregulated when PKCδ is overexpressed in mouse or human keratinocytes (this study and Ohba et al. [34]). Thus, PKC signals that regulate particular components of the keratinocyte differentiation and death programs are compartmentalized. Since the PKC pathway is clearly involved in the regulation of differentiation-dependent gene expression in keratinocytes, other isoforms must be responsible for those functions. Although the cellular content of the other PKC isoforms was not altered by overexpressing PKCδ, we cannot exclude alterations in subcellular localization that might affect function of other isoforms, since this was observed in U937 cells overexpressing PKCζ (10). In contrast to our observations, overexpression and activation of PKCδ in mouse myeloid progenitor 32D cells resulted in macrophage differentiation (33), indicating the importance of cell context in evaluating a particular function of a PKC isoform signaling pathway. We were particularly gratified to see that when overexpressed, activated PKCδ could induce lethality in tumor cells of both human and mouse origin and of a benign and malignant phenotype. Even though we were able to detect tyrosine phosphorylation of the exogenous δ in tumor cell extracts (not shown), sufficient active enzyme persists (as shown by in vitro activity assays) to respond to TPA and cause cell death. Currently, we are testing whether in vivo delivery of AdFPKCδ to cutaneous tumors in mouse skin grafts will influence tumor growth and survival.
PKCδ-mediated keratinocyte cell death is an apoptotic process.
The resistance of preneoplastic or neoplastic keratinocytes to cell killing by phorbol esters and UV light, while normal keratinocytes are sensitive, has been proposed to be fundamental to cell selection required for tumor formation in murine and human skin (2, 50). Experimental data now suggest that PKCδ is involved in a pathway leading to cell death of keratinocytes from both phorbol ester and UV light. In human keratinocytes UV light-induced cell death is a PKC-dependent apoptotic process that generates a constitutively active proteolytic product of PKCδ (9). Likewise, TPA activation in PKCδ-overexpressing keratinocytes causes apoptosis-associated changes in keratinocyte morphology, DNA fragmentation and TUNEL-positive staining. As for UV light and other apoptosis inducers, PKCδ activation also increased caspase 3 activity in keratinocytes. In keratinocytes, procaspase 3 is localized to mitochondria and is released and activated by apoptosis inducers such as UV light and STSP (31). PKCδ is proteolytically activated by caspases in several other cell types during apoptosis induction by DNA-damaging agents (14, 15, 18). In those models activation of PKCδ by proteolysis appears to be essential for the apoptotic process. Recent evidence suggests that activated PKCδ phosphorylates DNA-dependent protein kinase, preventing repair of DNA double-strand breaks (1). Such an activity would be consistent with the DNA fragmentation observed in our study, assuming a DNA damage pathway was also stimulated by PKCδ. The efficient killing of HeLa and HPV-18-transformed keratinocytes by overexpressing PKCδ suggests that functional p53 is not required for PKCδ to induce cell death. In contrast, MAPK appears to be involved in PKCδ-mediated cell killing since an inhibitor of this pathway, PD98059, can protect keratinocytes from lethality. In other cell types PKCδ can activate both MEK1 and ERK1, and these pathways have been implicated in apoptosis (48). In collaborative studies, we have also demonstrated that an MAPK cascade is downstream from PKCδ activation in the apoptotic response of LNCaP prostate cancer cells (16a). Thus, this pathway provides a fruitful lead to determine the distal consequences of PKCδ activation.
PKCδ targets mitochondria to induce keratinocyte cell death.
Mitochondria have now been identified as a subcellular target for PKCδ in keratinocytes. Both confocal microscopy of keratinocytes transfected with GFP-PKCδ and subcellular fractionation of keratinocytes infected with AdFPKCδ indicate colocalization with mitochondria. Furthermore, TPA caused rapid translocation of PKCδ from cytosol to the plasma membrane and mitochondria, and affected mitochondria appear to aggregate. Since the kinase-inactive mutant PKCδK376R translocated to the plasma membrane but not to the mitochondria, it appears that kinase activity is required for mitochondrial localization. Studies with SP-1 cells suggest that endogenous PKCδ also translocates to the mitochondria after TPA exposure, but this cannot be confirmed morphologically because antibody staining is not sufficiently powerful to detect endogenous enzyme unambiguously. If translocation of endogenous PKCδ does occur in neoplastic SP-1 cells, further studies will be required to determine whether the resistance of these cells to TPA-induced lethality, in the absence of PKCδ overexpression, is due to the phosphorylation state of the endogenous PKCδ or to some other mechanism of interference.
This is the first indication that PKCδ localizes to the mitochondria. Previous studies in overexpressing NIH 3T3 cells indicated that δ resided in the cytosol and Golgi and translocated to plasma membranes after TPA stimulation (19). In contrast, GFP-tagged PKCδ translocated from cytosol to nuclear and plasma membranes after TPA exposure in transfected CHO cells (35). An earlier study with fluorescence-labeled PKC inhibitors implied that there was mitochondrial localization of an unidentified isoform other than PKCβ1 in TPA-treated rat embryo fibroblasts, and this was associated with mitochondrial shape changes (4). However, PKCδ was not identified as the isoform responsible. In contrast, in HL60 and apoptosis-sensitive human leukemia cells, PKCα was shown to participate in resistance to the apoptotic response to DNA-damaging agents by colocalizing with Bcl-2 in mitochondria and enhancing its antiapoptotic activity by phosphorylation (41). Thus, cell type and isoform type are important variables in localization analysis and the functional response to PKC activation.
Two inhibitors of mitochondrial electron transport, rotenone and antimycin, substantially reduced PKCδ-mediated cell killing at concentrations that are not toxic to keratinocytes. This provides evidence that the mitochondrial target is functionally important and is dependent on changes in the electron transport system. Furthermore, studies with Rh123 indicate that mitochondrial membrane potential decreases within 3 h of PKC activation of AdFPKCδ cells by TPA. These studies suggest that mitochondria are a direct target of PKCδ activity. Since mitochondria are downstream of a number of apoptosis inducers (3, 20), the involvement of PKCδ in several pathways of cell death seems worthy of further study.
While we can only speculate on the identity of the relevant pathways downstream from PKCδ-induced alterations in mitochondrial electron transport, the ceramide-induced apoptotic pathway has certain similarities worth noting. In specific cell types, ceramide induces translocation of PKCδ, stimulates H2O2 production from mitochondria, reduces mitochondrial membrane potential, causes DNA fragmentation, and induces apoptosis that is inhibited by rotenone and antimycin (39). Furthermore, H2O2 is an activator of both PKCδ and MAPKK, and some of its biological effects are inhibited by PKC inhibitors or PD 98059 (42). PKC activation by TPA is known to produce reactive oxygen species and DNA strand breakage in keratinocytes (23, 38). These similarities provide a starting point to design approaches for understanding the mechanism of PKCδ-mediated lethality in normal and neoplastic keratinocytes. Most importantly, they may provide new targets to induce lethality in tumors that arise in squamous epithelia.
ACKNOWLEDGMENTS
We thank Mariana Gerschenson for very helpful discussion and critically reading the manuscript; Toren Finkel for generously providing Adβgal; Susan Garfield and Mark Miller of the core facility in the Division of Basic Sciences, National Cancer Institute, for providing technical support for confocal microscopy and DNA sequencing, respectively; Yajun Zhang for assistance with vector construction and adenovirus production and student interns; and Michael Cheng, Maximillian Soong, and Andrea Ceresa for their helpful technical assistance.
REFERENCES
- 1.Bharti A, Kraeft S K, Gounder M, Pandey P, Jin S, Yuan Z M, Lees-Miller S P, Weichselbaum R, Weaver D, Chen L B, Kufe D, Kharbanda S. Inactivation of DNA-dependent protein kinase by protein kinase Cδ: implications for apoptosis. Mol Cell Biol. 1998;18:6719–6728. doi: 10.1128/mcb.18.11.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brash D E, Ziegler A, Jonason A S, Simon J A, Kunala S, Leffell D J. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc. 1996;1:136–142. [PubMed] [Google Scholar]
- 3.Brenner C, Marzo I, Kroemer G. A revolution in apoptosis: from a nucleocentric to a mitochondriocentric perspective. Exp Gerontol. 1998;33:543–553. doi: 10.1016/s0531-5565(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 4.Chen C S, Poenie M. New fluorescent probes for protein kinase C. Synthesis, characterization, and application. J Biol Chem. 1993;268:15812–15822. [PubMed] [Google Scholar]
- 5.Chen N, Ma Wy, Huang C, Dong Z. Translocation of protein kinase Cɛ and protein kinase Cδ to membrane is required for ultraviolet B-induced activation of mitogen-activated protein kinases and apoptosis. J Biol Chem. 1999;274:15389–15394. doi: 10.1074/jbc.274.22.15389. [DOI] [PubMed] [Google Scholar]
- 6.Denning M F, Dlugosz A A, Howett M K, Yuspa S H. Expression of an oncogenic rasHa gene in murine keratinocytes induces tyrosine phosphorylation and reduced activity of protein kinase C δ. J Biol Chem. 1993;268:26079–26081. [PubMed] [Google Scholar]
- 7.Denning M F, Dlugosz A A, Threadgill D W, Magnuson T, Yuspa S H. Activation of the epidermal growth factor receptor signal transduction pathway stimulates tyrosine phosphorylation of protein kinase C δ. J Biol Chem. 1996;271:5325–5331. doi: 10.1074/jbc.271.10.5325. [DOI] [PubMed] [Google Scholar]
- 8.Denning M F, Dlugosz A A, Williams E K, Szallasi Z, Blumberg P M, Yuspa S H. Specific protein kinase C isozymes mediate the induction of keratinocyte differentiation markers by calcium. Cell Growth Differ. 1995;6:149–157. [PubMed] [Google Scholar]
- 9.Denning M F, Wang Y, Nickoloff B J, Wrone-Smith T. Protein kinase Cδ is activated by caspase-dependent proteolysis during ultraviolet radiation-induced apoptosis of human keratinocytes. J Biol Chem. 1998;273:29995–30002. doi: 10.1074/jbc.273.45.29995. [DOI] [PubMed] [Google Scholar]
- 10.de Vente J, Kiley S, Garris T, Bryant W, Hooker J, Posekany K, Parker P, Cook P, Fletcher D, Ways D K. Phorbol ester treatment of U937 cells with altered protein kinase C content and distribution induces cell death rather than differentiation. Cell Growth Differ. 1995;6:371–382. [PubMed] [Google Scholar]
- 11.Dlugosz A A, Glick A B, Tennenbaum T, Weinberg W C, Yuspa S H. Isolation and utilization of epidermal keratinocytes for oncogene research. In: Vogt P K, Verma I M, editors. Methods in enzymology. New York, N.Y: Academic Press; 1995. pp. 3–20. [DOI] [PubMed] [Google Scholar]
- 12.Dlugosz A A, Yuspa S H. Staurosporine induces protein kinase C agonist effects and maturation of normal and neoplastic mouse keratinocytes in vitro. Cancer Res. 1991;51:4677–4684. [PubMed] [Google Scholar]
- 13.Dlugosz A A, Yuspa S H. Coordinate changes in gene expression which mark the spinous to granular cell transition in epidermis are regulated by protein kinase C. J Cell Biol. 1993;120:217–225. doi: 10.1083/jcb.120.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emoto Y, Kisaki H, Manome Y, Kharbanda S, Kufe D. Activation of protein kinase Cδ in human myeloid leukemia cells treated with 1-β-d-arabinofuranosylcytosine. Blood. 1996;87:1990–1996. [PubMed] [Google Scholar]
- 15.Emoto Y, Manome Y, Meinhardt G, Kisaki H, Kharbanda S, Robertson M, Ghayur T, Wong W W, Kamen R, Weichselbaum R. Proteolytic activation of protein kinase Cδ by an ICE-like protease in apoptotic cells. EMBO J. 1995;14:6148–6156. doi: 10.1002/j.1460-2075.1995.tb00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs E. Epidermal differentiation and keratin gene expression. Princess Takamatsu Symp. 1994;24:290–302. [PubMed] [Google Scholar]
- 16a.Fujii, T., M. L. García-Bermejo, J. L. Bernabó, J. Caamaño, M. Ohba, T. Kuroki, S. H. Yuspa, and M. G. Kazanietz. Unpublished data. [DOI] [PubMed]
- 17.Geiges D, Marks F, Gschwendt M. Loss of protein kinase Cδ from human HaCaT keratinocytes upon ras transfection is mediated by TGF α. Exp Cell Res. 1995;219:299–303. doi: 10.1006/excr.1995.1231. [DOI] [PubMed] [Google Scholar]
- 18.Ghayur T, Hugunin M, Talanian R V, Ratnofsky S, Quinlan C, Emoto Y, Pandey P, Datta R, Huang Y, Kharbanda S, Allen H, Kamen R, Wong W, Kufe D. Proteolytic activation of protein kinase Cδ by an ICE/CED 3-like protease induces characteristics of apoptosis. J Exp Med. 1996;184:2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodnight J A, Mischak H, Kolch W, Mushinski J F. Immunocytochemical localization of eight protein kinase C isozymes overexpressed in NIH 3T3 fibroblasts. Isoform-specific association with microfilaments, Golgi, endoplasmic reticulum, and nuclear and cell membranes. J Biol Chem. 1995;270:9991–10001. doi: 10.1074/jbc.270.17.9991. [DOI] [PubMed] [Google Scholar]
- 20.Green D R, Reed J C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 21.Gschwendt M. Protein kinase Cδ. Eur J Biochem. 1999;259:555–564. doi: 10.1046/j.1432-1327.1999.00120.x. [DOI] [PubMed] [Google Scholar]
- 22.Guzman R J, Hirschowitz E A, Brody S L, Crystal R G, Epstein S E, Finkel T. In vivo suppression of injury-induced vascular smooth muscle cell accumulation using adenovirus-mediated transfer of the herpes simplex virus thymidine kinase gene. Proc Natl Acad Sci USA. 1994;91:10732–10736. doi: 10.1073/pnas.91.22.10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartley J A, Gibson N W, Zwelling L A, Yuspa S H. Association of DNA strand breaks with accelerated terminal differentiation in mouse epidermal cells exposed to tumor promoters. Cancer Res. 1985;45:4864–4870. [PubMed] [Google Scholar]
- 24.Hennings H, Michael D, Lichti U, Yuspa S H. Response of carcinogen-altered mouse epidermal cells to phorbol ester tumor promoters and calcium. J Investig Dermatol. 1987;88:60–65. doi: 10.1111/1523-1747.ep12465014. [DOI] [PubMed] [Google Scholar]
- 25.Kartasova T, Roop D R, Yuspa S H. Relationship between the expression of differentiation-specific keratins 1 and 10 and cell proliferation in epidermal tumors. Mol Carcinog. 1992;6:18–25. doi: 10.1002/mc.2940060105. [DOI] [PubMed] [Google Scholar]
- 26.Kazanietz M G, Blumberg P M. Protein kinase C and signal transduction in normal and neoplastic cells. In: Sirica A E, editor. Cellular and molecular pathogenesis. New York, N.Y: Raven Press; 1996. pp. 389–402. [Google Scholar]
- 27.Koizumi H, Kohno Y, Osada S, Ohno S, Ohkawara A, Kuroki T. Differentiation-associated localization of nPKCθ, a Ca++-independent protein kinase C, in normal human skin and skin diseases. J Investig Dermatol. 1993;101:858–863. doi: 10.1111/1523-1747.ep12371707. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Mischak H, Yu J C, Wang L M, Mushinski J F, Heidaran M A, Pierce J H. Tyrosine phosphorylation of protein kinase C-δ in response to its activation. J Biol Chem. 1994;269:2349–2352. [PubMed] [Google Scholar]
- 29.Liu W S, Heckman C A. The sevenfold way of PKC regulation. Cell Signalling. 1998;10:529–542. doi: 10.1016/s0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzo P S, Bogi K, Acs P, Pettit G R, Blumberg P M. The catalytic domain of protein kinase Cδ conveys protection from down-regulation induced by bryostatin 1. J Biol Chem. 1997;272:33338–33343. doi: 10.1074/jbc.272.52.33338. [DOI] [PubMed] [Google Scholar]
- 31.Mancini M, Nicholson D W, Roy S, Thornberry N A, Peterson E P, Casciola-Rosen L A, Rosen A. The caspase-3 precursor has a cytosolic and mitochondrial distribution: implications for apoptotic signaling. J Cell Biol. 1998;140:1485–1495. doi: 10.1083/jcb.140.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mischak H, Goodnight J, Kolch W, Martiny-Baron G, Schaechtle C, Kazanietz M G, Blumberg P M, Pierce J H, Mushinski J F. Overexpression of protein kinase C-δ and -ɛ in NIH 3T3 cells induces opposite effects of growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993;268:6090–6096. [PubMed] [Google Scholar]
- 33.Mischak H, Pierce J H, Goodnight J, Kazanietz M G, Blumberg P M, Mushinski J F. Phorbol ester-induced myeloid differentiation is mediated by protein kinase C-α and -δ and not by protein kinase C-βII, -ɛ, -zeta and eta. J Biol Chem. 1993;268:20110–20115. [PubMed] [Google Scholar]
- 34.Ohba M, Ishino K, Kashiwagi M, Kawabe S, Chida K, Huh N H, Kuroki T. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the eta and delta isoforms of protein kinase C. Mol Cell Biol. 1998;18:5199–5207. doi: 10.1128/mcb.18.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohmori S, Shirai Y, Sakai N, Fujii M, Konishi H, Kikkawa U, Saito N. Three distinct mechanisms for translocation and activation of the δ subspecies of protein kinase C. Mol Cell Biol. 1998;18:5263–5271. doi: 10.1128/mcb.18.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paramio J M, Casanova M L, Segrelles C, Mittnacht S, Lane E B, Jorcano J L. Modulation of cell proliferation by cytokeratins K10 and K16. Mol Cell Biol. 1999;19:3086–3094. doi: 10.1128/mcb.19.4.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polakowska R R, Piacentini M, Bartlett R, Goldsmith L A, Haake A R. Apoptosis in human skin development: morphogenesis, periderm, and stem cells. Dev Dyn. 1994;199:176–188. doi: 10.1002/aja.1001990303. [DOI] [PubMed] [Google Scholar]
- 38.Przybyszewski J, Box H C, Kulesz-Martin M. Induction of reactive oxygen species without 8-hydroxydeoxyguanosine formation in DNA of initiated mouse keratinocytes treated with 12-O-tetradecanoylphorbol-13-acetate. Carcinogenesis. 1998;19:1467–1474. doi: 10.1093/carcin/19.8.1467. [DOI] [PubMed] [Google Scholar]
- 39.Quillet-Mary A, Jaffrezou J P, Mansat V, Bordier C, Naval J, Laurent G. Implication of mitochondrial hydrogen peroxide generation in ceramide-induced apoptosis. J Biol Chem. 1997;272:21388–21395. doi: 10.1074/jbc.272.34.21388. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Villanueva J, Greenhalgh D, Wang X J, Bundman D, Cho S, Delehedde M, Roop D, McDonnell T J. Human keratin-1.bcl-2 transgenic mice aberrantly express keratin 6, exhibit reduced sensitivity to keratinocyte cell death induction, and are susceptible to skin tumor formation. Oncogene. 1998;16:853–863. doi: 10.1038/sj.onc.1201610. [DOI] [PubMed] [Google Scholar]
- 41.Ruvolo P P, Deng X, Carr B K, May W S. A functional role for mitochondrial protein kinase Cα in Bcl2 phosphorylation and suppression of apoptosis. J Biol Chem. 1998;273:25436–25442. doi: 10.1074/jbc.273.39.25436. [DOI] [PubMed] [Google Scholar]
- 42.Sabri A, Byron K L, Samarel A M, Bell J, Lucchesi P A. Hydrogen peroxide activates mitogen-activated protein kinases and Na+-H+ exchange in neonatal rat cardiac myocytes. Circ Res. 1998;82:1053–1062. doi: 10.1161/01.res.82.10.1053. [DOI] [PubMed] [Google Scholar]
- 43.Salvesen G S, Dixit V M. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 44.Sermadiras S, Dumas M, Joly-Berville R, Bonte F, Meybeck A, Ratinaud M H. Expression of Bcl-2 and Bax in cultured normal human keratinocytes and melanocytes: relationship to differentiation and melanogenesis. Br J Dermatol. 1997;137:883–889. [PubMed] [Google Scholar]
- 45.Smith H, Chang E Y, Szallasi Z, Blumberg P M, Rivera J. Tyrosine phosphorylation of protein kinase C-δ in response to the activation of the high affinity receptor for immunoglobulin E modifies its substrate recognition. Proc Natl Acad Sci USA. 1995;92:9112–9116. doi: 10.1073/pnas.92.20.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strickland J E, Dlugosz A A, Hennings H, Yuspa S H. Inhibition of tumor formation from grafted murine papilloma cells by treatment of grafts with staurosporine, an inducer of squamous differentiation. Carcinogenesis. 1993;14:205–209. doi: 10.1093/carcin/14.2.205. [DOI] [PubMed] [Google Scholar]
- 47.Strickland J E, Greenhalgh D A, Koceva-Chyla A, Hennings H, Restrepo C, Balaschak M, Yuspa S H. Development of murine epidermal cell lines which contain an activated rasHa oncogene and form papillomas in skin grafts on athymic nude mouse hosts. Cancer Res. 1988;48:165–169. [PubMed] [Google Scholar]
- 48.Ueda Y, Hirai Si, Osada Si, Suzuki A, Mizuno K, Ohno S. Protein kinase C δ activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 49.Vander Heiden M G, Chandel N S, Williamson E K, Schumacker P T, Thompson C B. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 50.Yuspa S H. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis. Thirty-third G. H. A. Clowes Memorial Award Lecture. Cancer Res. 1994;54:1178–1189. [PubMed] [Google Scholar]
- 51.Yuspa S H, Ben T, Hennings H, Lichti U. Divergent responses in epidermal basal cells exposed to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1982;42:2344–2349. [PubMed] [Google Scholar]