The Transmembrane Mutation G380R in Fibroblast Growth Factor Receptor 3 Uncouples Ligand-Mediated Receptor Activation from Down-Regulation (original) (raw)
Abstract
A point mutation, Gly380Arg, in the transmembrane domain of fibroblast growth factor receptor 3 (FGFR3) leads to achondroplasia, the most common form of genetic dwarfism in humans. This substitution was suggested to enhance mutant receptor dimerization, leading to constitutive, ligand-independent activation. We found that dimerization and activation of the G380R mutant receptor are predominantly ligand dependent. However, using both transient and stable transfections, we found significant overexpression only of the mutant receptor protein. Metabolic pulse-chase experiments, cell surface labeling, and kinetics of uptake of radiolabeled ligand demonstrated a selective delay in the down-regulation of the mutant receptor. Moreover, this receptor was now resistant to ligand-mediated internalization, even at saturating ligand concentrations. Finally, transgenic mice expressing the human G380R mutant receptor under the mouse receptor transcriptional control demonstrated a markedly expanded area of FGFR3 immunoreactivity within their epiphyseal growth plates, compatible with an in vivo defect in receptor down-regulation. We propose that the achondroplasia mutation G380R uncouples ligand-mediated receptor activation from down-regulation at a site where the levels and kinetics of FGFR3 signals are crucial for chondrocyte maturation and bone formation.
Fibroblast growth factor (FGF) receptors (FGFR) constitute a family of four genes that encode multiple receptor isoforms, all of which have intrinsic tyrosine kinase activity (8, 12). Upon binding of a ligand, receptor dimerization is induced, leading to auto- and transphosphorylation followed by receptor internalization and down-regulation. These lead to the controlled activation of specific signal transduction pathways and the expression of FGF target genes, critically required during embryogenesis, tissue repair, and angiogenesis (1).
Multiple mutations in FGFR 1, 2, and 3 (FGFR1, FGFR2, and FGFR3, respectively) give rise to a variety of inherited skeletal malformations (40). Mutations in FGFR3 are responsible for disorders predominantly of the long bones, including achondroplasia, the most common form of human genetic dwarfism (27, 29). Over 97% of cases of achondroplasia result from either a G-to-A transition or a G-to-C transversion, changing the codon for Gly380 (GGG) to Arg (AGG or CGG) in the transmembrane domain of FGFR3. An Asn540Lys mutation in the proximal tyrosine kinase domain of FGFR3 is found in the milder disorder of hypochondroplasia (2), while substitution to a cysteine of residue 248, 249, 370, or 371 in the extracellular domain or a Lys650Glu mutation in the kinase activation loop gives rise to the most severe and neonatal lethal thanatophoric dysplasia (TD) types, I and II, respectively (28, 33). All of these skeletal malformations represent autosomal dominant disorders characterized by disproportionately short limbs and relative macrocephaly (23).
The cellular basis underlying the clinical features of achondroplasia is a defect in chondrocyte function during endochondral bone formation, the primary mechanism by which long bones elongate (22). This formation involves a linear process of chondrocyte proliferation and maturation in a very strictly time- and space-controlled manner (10). The cartilage progenitor cells which arise at the top of the epiphyseal growth plate proceed to form columns of proliferating chondrocytes that later differentiate to produce the calcified matrix. This matrix is subsequently remodeled by invading osteoblasts to form the typical bone structure underlying the cartilaginous growth plate (3). A disturbance in any of the stages in this tightly coordinated process is quickly reflected by an alteration in the normal pattern of the growth plate, resulting in inappropriate bone growth and short stature. The remarkably similar phenotype shared by achondroplasia-affected individuals, irrespective of their genetic background, suggests a unique mechanism responsible for this inhibition of endochondral bone formation.
FGFR3 is highly expressed during embryonic development in the precartilaginous condensing mesenchyme and in bony and cartilaginous structures of the developing vertebrae (20). Later in development, during endochondral ossification, it is concentrated in the perichondrium, the resting cartilage, and the maturation and upper hypertrophic zones of the growth plate, where it may play a key role in terminal chondrocyte differentiation. Mice deficient in FGFR3 show remarkable skeletal overgrowth with wider growth plates, suggesting that FGFR3 exerts a negative effect on bone growth (5, 7). Moreover, transgenic mice overexpressing FGF2 driven by the collagen type II promoter show shortening of their long bones and macrocephaly (4).
Little is known about the molecular mechanisms underlying the various chondrodysplasia syndromes. The current dogma is that constitutive, ligand-independent activation of mutant receptors and their downstream signaling is the mechanism shared by all of these disorders (15, 18, 38). This notion is supported by the finding that many of these disorders result from substitutions to unpaired cysteine residues in the extracellular domain of FGFR, leading to covalently disulfide-linked receptor dimers, as in TD type I, or from direct activating mutations in the kinase domain of these receptors, as in hypochondroplasia and TD type II; both types of mutations can lead to ligand-independent constitutive phosphorylation (25, 28, 36). We found a specific defect in ligand-mediated receptor down-modulation of G380R which may lead to inappropriate levels and kinetics of receptor activation at a site where both are crucial for chondrocyte maturation and bone formation.
MATERIALS AND METHODS
Cell lines.
Nontransformed rat chondrocytes derived from fetal calvaria (RCJ 3.1C5.18), a generous gift from J. Aubin, and human embryonal kidney cells expressing large T antigen (293T) were cultured in Dulbecco modified Eagle medium (DMEM) containing 10% fetal calf serum.
Expression of human wt and G380R mutant FGFR3.
Wild-type (wt) or mutant receptor cDNA in expression vector pcDNA3 (Invitrogen) was transfected into RCJ or 293T cells by the calcium phosphate method. For stable expression, clones were selected in G418 (GibcoBRL, Gaithersberg, Md.) (0.5 mg/ml) and screened for FGFR3 expression by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and Western blotting. Retroviruses were produced in 293T cells cotransfected with vector pLXSN containing either wt or mutant FGFR3 and a Psi helper phage. Virus-containing medium, collected every 8 h, was filtered and kept at −80°C until further use. Infections were performed by incubating RCJ cells with virus-enriched medium and 8 μg of Polybrene per ml for 6 h followed by selection in G418 (0.5 mg/ml). Positive pools were screened for FGFR3 expression by SDS-PAGE and Western blotting.
Immunoprecipitation.
Cells were lysed in lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1 mM MgCl2, 0.1 mM ZnCl2, 0.5% Nonidet P-40, 1 mg of aprotonin per ml, 1 mg of leupeptin per ml, 2 mM phenylmethylsulfonyl fluoride) and clarified by centrifugation at 12,000 × g for 15 min. The lysates were immunoprecipitated for 16 h at 4°C with anti-FGFR3 C terminus antibody (Santa Cruz, Santa Cruz, Calif.) and analyzed by SDS–6% PAGE and Western blotting. Protein bands were visualized with horseradish peroxidase and an ECL kit (Amersham) according to the manufacturer's instructions.
Covalent cross-linking.
Cells were washed twice with binding buffer (DMEM, 25 mM HEPES, 1% bovine serum albumin) and incubated at 4°C without or with FGF9 (50 ng/ml). After 2 h, chemical cross-linking was performed with 1 mM bis-(sulfosuccinimidyl)-suberate for 30 min at room temperature. Cells were washed twice with binding buffer and lysed, and the lysates were precipitated with protein A-immobilized FGFR3 antibodies. The immunoprecipitates were transferred to a nitrocellulose membrane and blotted with anti-FGFR3 antibodies (generated in rabbits with a purified recombinant kinase domain of mouse FGFR3).
Analysis of pMAPK.
Cells were grown in six-well plates. After starvation for 16 h with serum-free DMEM, the cells were incubated for 9 min either without or with 50 ng of FGF9 per ml and lysed. Equal amounts of each lysate were separated by SDS–7.5% PAGE, and the presence of phosphorylated MAPK (pMAPK) was determined by Western immunoblotting with antibodies against phosphorylated mitogen-activated protein kinase (MAPK).
Fos-luciferase assay.
RCJ cells were cultured in 60-mm plates at 37°C and transfected in duplicate with 0.5 μg of β-galactosidase cDNA with or without 2 μg of a c-fos promoter–luciferase cDNA. After 24 h, the cells were serum starved for 16 h, treated for 9 min with FGF9, lysed, and assayed for luciferase activity. Luciferase activity was measured by use of a Turner luminometer with luciferase reaction buffer (100 mM Tris acetate, 10 mM magnesium acetate, 1 mM EDTA, 0.07 mM luciferin, 2 mM ATP). To account for transfection efficiencies, luciferase values from each transfection were normalized for the corresponding β-galactosidase activities.
Metabolic pulse-chase labeling experiments.
RCJ cells were cultured in methionine-depleted medium for 3 h, after which 35S-methionine (150 μCi/ml) was added for 30 min. Cells were washed extensively with DMEM and incubated at 37°C for various times. Cells were extracted, and lysates were precipitated with immobilized anti-FGFR3 antibodies. Proteins were separated by SDS–6% PAGE and visualized by autoradiography.
Cell surface biotinylation.
RCJ cells were washed twice in ice-cold phosphate-buffered saline and incubated at 4°C with 0.5 mg of water-soluble biotin-X-NHS (Pierce) per ml in borate buffer (10 mM boric acid [pH 8], 150 mM NaCl). After 45 min, the coupling of biotin was blocked by an extensive wash with 15 mM glycine in phosphate-buffered saline. Cells were incubated at 37°C for various times. To evaluate cell surface receptors, the cells were extracted and their lysates were precipitated with immobilized streptavidin (Pierce). The precipitated proteins were separated by SDS–6% PAGE, transferred to a nitrocellulose membrane, and probed with anti-FGFR3 antibodies.
Radiolabeled ligand internalization assay.
FGF2 was labeled with 125I-Na (1 mCi) by the chloramine-T method (16) and separated from free iodine on a heparin-Sepharose column. Cells cultured in 12-well plates were washed with binding buffer (DMEM, 100 mM HEPES [pH 7.5], 1% bovine serum albumin) and incubated in the presence of radiolabeled 125I-FGF2 (5 ng/ml) without or with a 200-fold excess of cold ligand for 2 h at 4°C. To allow for ligand internalization, cells were transferred to 37°C for various times, at the end of which the cells were placed on ice and washed twice with low-affinity buffer (25 mM HEPES [pH 7.5], 1.6 M NaCl). The cellular distribution of the radiolabeled ligand was determined by use of a low-pH extraction buffer (25 mM HEPES [pH 4], 1.6 M NaCl) to remove cell surface receptor-associated ligand; the remaining radioactivity, solubilized in 100 mM NaOH, represented the internalized ligand. The extent of nonspecific binding was determined in the presence of a 100-fold excess of unlabeled FGF2 and was subtracted from all data points.
Immunohistochemistry.
Isolated bones were fixed in 4% paraformaldehyde (pH 7.4) containing cetylpyridinium chloride (0.5%), decalcified in EDTA, dehydrated with an ethanol gradient, and embedded in paraffin. Sections (5 μm) were cut, stained with anti-FGFR3 antibodies (Santa Cruz), and visualized with fluorescein-conjugated anti-rabbit immunoglobulin G (Vectra Laboratory). Slides were counterstained with methyl green (Vector) and mounted with Lemonvitrex (Carlo Erba). Negative controls were obtained by substituting normal rabbit serum for the specific antibodies.
RESULTS
Expression of wt and G380R mutant FGFR3 in chondrocytes.
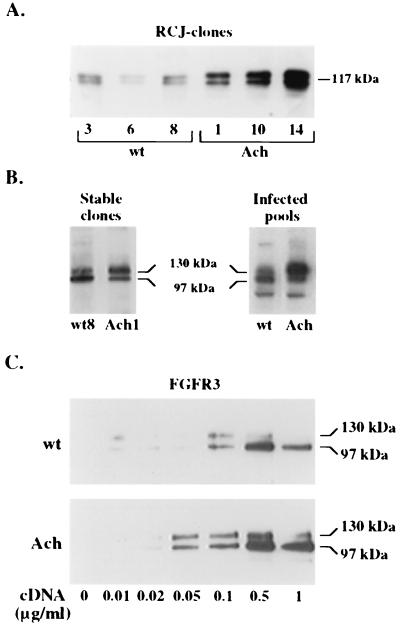
Since the cells most affected in achondroplasia are chondrocytes, we chose to study FGFR3 functions in the nontransformed RCJ 3.1C5.18 cell line derived from multipotential mesenchymal rat stem cells and expressing chondrocyte-specific differentiation markers (9, 17). Human wt FGFR3- or G380R mutant FGFR3-encoding plasmids were introduced into RCJ cells by stable transfection with full-length human FGFR3 (hFGFR3) cDNA driven by a cytomegalovirus promoter. Six independent clones expressing the transduced genes, as evident by SDS-PAGE and immunoblot analysis, were selected. All clones transduced with the G380R mutant receptor were found to express higher receptor protein levels than those expressing the wt receptor (Fig. 1A). Two stable clones which expressed comparable levels of the two receptors (Fig. 1B) were chosen for further studies.
FIG. 1.
Expression of wild-type and G380R mutant hFGFR3 in RCJ and 293T cells. (A and B) Lysates of RCJ cells expressing the wt receptor or the G380R mutant receptor were precipitated and probed with anti-FGFR3 antibodies. Three different clones expressing wt or mutant (Ach) FGFR3 (A) or stable clones expressing equal amounts of wt or mutant FGFR3 and RCJ pools infected with wt FGFR3- or mutant FGFR3-encoding retroviruses (B) are shown. (C) 293T cells transfected with the indicated concentrations of either receptor cDNA were lysed, precipitated, and analyzed by SDS-PAGE and Western blotting with anti-FGFR3 antibodies.
Differential overexpression of G380R mutant FGFR3.
Repeated transfections with different cell lines have always yielded, on average, more clones expressing the mutant receptor than the wt receptor as well as higher protein levels for the G380R mutant gene than for the wt gene under identical conditions. This finding suggested a posttranscriptional mechanism as the potential cause for the selective accumulation of the mutant receptor. To rule out the possibility that the apparent selective overexpression of the mutant receptor was due to clonal variation, the receptors were subcloned into a retroviral expression vector, and parental RCJ cells were infected with the retrovirus encoding either wt or mutant FGFR3. Pools of infected cells expressing either receptor were obtained and analyzed (Fig. 1B). FGFR3 migrates on SDS-PAGE as two discrete bands; the upper band is the mature, membrane-associated glycoprotein (130 kDa), while the lower band is the immature, nonprocessed form (97 kDa) (13). A comparison of immunoprecipitates from wt FGFR3- and G380R mutant FGFR3-expressing cells revealed a clear difference in the ratio of the 97-kDa form to the 130-kDa form. While in wt receptor-expressing cells the protein was present mainly as the 97-kDa form, in cells expressing the mutant receptor, more of the protein accumulated as the 130-kDa, mature form (Fig. 1B). The phenomenon of higher total expression levels for the mutant receptor was not unique to chondrocytes, as it was similarly observed when equal amounts of wt receptor- and mutant receptor-encoding cDNAs were transiently transfected into 293T human embryonal kidney cells (Fig. 1C). At such high expression levels, even the mutant receptor accumulated more as the 97-kDa form, most likely due to saturation of the posttranslational and trafficking machinery. Nevertheless, the expression of wt FGFR3 could be observed only at DNA concentrations of at least 0.1 μg/ml, while that of the mutant receptor could be easily detected at half that amount.
Ligand-mediated dimerization and activation of G380R mutant FGFR3.
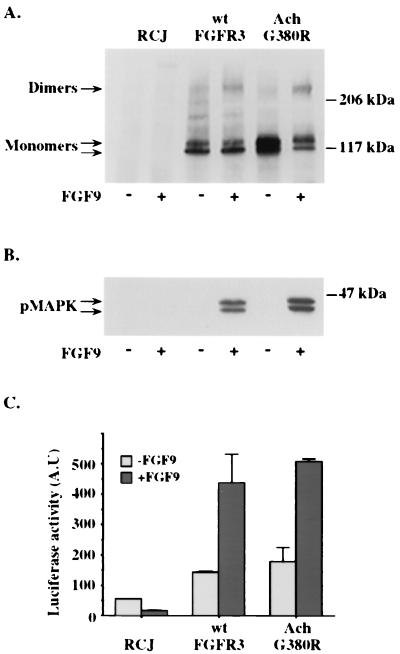
Since receptor overexpression may by itself lead to spontaneous dimerization of receptor tyrosine kinases, we investigated the ability of wt and mutant FGFR3 expressed in RCJ cells to form ligand-dependent and -independent dimers. Chemical cross-linking followed by Western immunoblot analysis failed to detect dimers of either wt or mutant FGFR3 expressed at moderate levels (Fig. 2A) in the absence of FGF9, a preferred ligand for FGFR3 (10). At very high expression levels, both receptors underwent spontaneous, ligand-independent dimerization and autophosphorylation (data not shown), which were more pronounced for the G380R mutant receptor due to its abnormal accumulation at the plasma membrane. Significant levels of receptor dimers were, however, formed only upon ligand binding to both wt and mutant receptors. Activation of downstream signaling pathways, such as MAPK (Fig. 2B) or that of the immediate-early gene c-fos, were also strictly ligand dependent for both receptor types (Fig. 2C). A more detailed analysis, including the activation of FRS2, JNK, phospholipase C-γ (PLC-γ), and Stat1 and using several different cell lines, revealed identical results (P. David, R. Ben-Levi, and A. Yayon, unpublished data). These results suggest that at least at moderate expression levels, the G380R mutant receptor, like its wt counterpart, does not form spontaneous ligand-independent dimers.
FIG. 2.
Receptor dimerization, MAPK activation, and c-fos induction by wt and G380R mutant FGFR3. (A) Stable clones of RCJ cells expressing either the wt receptor or the G380R mutant (Ach) receptor were incubated for 2 h at 4°C in the absence (−) or presence (+) of 50 ng of FGF9 per ml. After chemical cross-linking, cells were lysed, immunoprecipitated with anti-FGFR3 C terminus antibodies, and probed on an immunoblot with a polyclonal antibody to the kinase domain of FGFR3. (B) Cells incubated with (+) or without (−) FGF9 for 9 min at 37°C were lysed, and their lysates were separated by SDS-PAGE and immunoblotted with an anti-pMAPK antibody. (C) Cells transfected with the c-fos luciferase vector were incubated with or without FGF9, lysed, and analyzed for luciferase activity. The data shown represent the means ± standard deviations of duplicate transfections normalized for β-galactosidase activity. A.U, arbitrary units.
G380R mutant FGFR3 is specifically defective in internalization and accumulates at the cell surface.
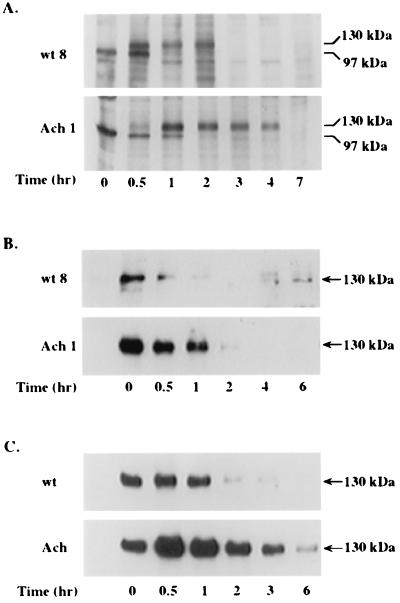
In order to obtain more insight into the mechanisms responsible for the apparent difference in processing of wt FGFR3 and mutant FGFR3, de novo receptor synthesis and translocation to and from the plasma membrane were followed by a short pulse of metabolic labeling of RCJ cells expressing either of the two receptors (Fig. 3A). The immature form (97 kDa) of both receptors could be detected within 30 min after labeling with 35S-methionine. It took another 30 min for processing and for the 130-kDa form to appear. There did not seem to be a significant difference in posttranslational processing and trafficking to the membrane between the two receptors (Fig. 3A). However, a clear difference in the rates of internalization and degradation between the two receptors could be observed. While the wt receptor remained intact for up to 2 h before it was internalized and degraded, the mutant receptor was not internalized during this time period and could be traced even after a 4-h chase (Fig. 3A).
FIG. 3.
Metabolic labeling and surface biotinylation of wt and G380R mutant FGFR3 expressed in RCJ cells. (A) Stable clones of RCJ cells expressing wt or G380R mutant (Ach) receptors were pulse-labeled with 35S-methionine for 30 min. At the indicated times, cell lysates were immunoprecipitated with anti-FGFR3 antibodies and resolved by SDS–6% PAGE and autoradiography. (B and C) Stable clones (B) or infected pools (C) of RCJ cells were cell surface biotinylated for 45 min. At the indicated times, cell lysates were precipitated with immobilized avidin, analyzed by SDS–6% PAGE, blotted onto a nitrocellulose membrane, and probed with anti-FGFR3 antibodies. Results represent three independent experiments repeated with different RCJ clones.
In another independent technique used to monitor the fate of the plasma membrane resident receptor, total cell surface proteins were labeled for 45 min with membrane-impermeable, water-soluble biotin-X-NHS. Stable RCJ clones (Fig. 3B) and infected RCJ pools (Fig. 3C) expressing either the wt or the mutant receptor were biotinylated, and their cell lysates were precipitated with immobilized avidin, separated by SDS-PAGE, and immunoblotted with antibodies to FGFR3 (Fig. 3B and C). As expected, only the mature, 130-kDa, membrane-associated protein band of both receptors could be detected by that method. Similar to the results obtained by metabolic labeling (Fig. 3A), the biotin-labeled wt receptor disappeared from the cell surface within 2 h after labeling, while the G380R mutant receptor remained stable on the cell surface for more than 3 h (Fig. 3B and C). Some variation in the time scale for internalization but not in the selective delay in mutant receptor down-regulation was observed between different experiments, most likely due to the variation in receptor expression levels. Taken together, these results suggest that the selective accumulation of the mutant receptor may be a direct consequence of its slower rate of internalization and/or degradation.
Ligand-mediated receptor internalization is selectively abrogated in G380R mutant FGFR3.
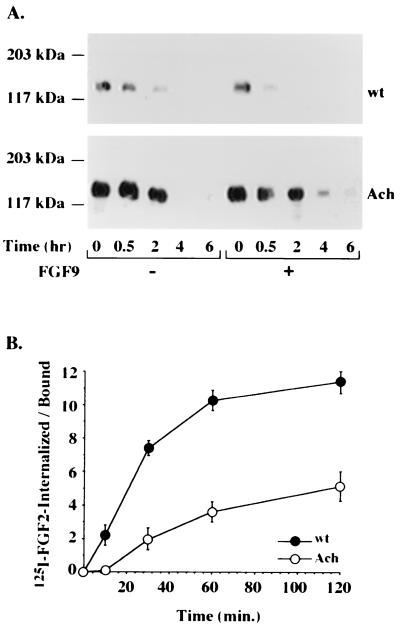
Cell surface receptors, including receptor tyrosine kinases, are internalized upon ligand binding (30), leading to receptor down-regulation and signal transduction attenuation. We therefore examined the internalization and degradation rates of biotin-labeled receptors in response to a ligand (Fig. 4A). A major difference in the rates of internalization of wt FGFR3 and mutant FGFR3 was revealed. The addition of a ligand had a dramatic effect, as expected, on the wt receptor half-life, as in less than 30 min, most of the labeled receptor was internalized and targeted for degradation. Strikingly, the rate of internalization of the mutant receptor was not affected by the addition of a ligand in several different clones (data not shown), even at saturating ligand concentrations (Fig. 4A). Using a kinase-deficient version of the wt or mutant receptor, we found no effect of the ligand on the half-life of either receptor (data not shown); this result suggests that FGFR internalization may be strictly regulated by interactions with the intracellular domain, which in turn may depend on its kinase activity.
FIG. 4.
Ligand-mediated receptor internalization. (A) RCJ cells expressing wt or mutant (Ach) receptors were cell surface biotinylated for 45 min and incubated with (+) or without (−) FGF9 (50 ng/ml) for the indicated times. The cell lysates were precipitated with immobilized avidin, analyzed by SDS–6% PAGE, and probed with anti-FGFR3 antibodies. (B) Cells were incubated for 2 h at 4°C with 125I-FGF2 and then transferred to 37°C for the indicated times. At the end of each incubation, low-affinity heparan sulfate-bound ligand was removed with a high-salt buffer, high-affinity receptor-bound ligand was dissociated under low-pH conditions, and the retained intracellular radioactivity was determined by solubilizing the cells in 100 mM NaOH. The results shown are the averages ± standard deviations of triplicate measurements, calculated as the ratio between the internalized ligand and the receptor-bound ligand. Nonspecific binding was subtracted from all data points.
In a more quantitative approach, the effect of FGF ligands on receptor internalization was examined by monitoring the level of the accumulated radiolabeled ligand with time. We found marked differences in the kinetics of radiolabeled FGF2 accumulation between the cells (Fig. 4B). Cells expressing the mutant receptor bound significantly higher levels of the ligand than wt receptor-expressing cells, reflecting the higher levels of expression of the mutant receptor but, nevertheless, internalized the labeled ligand at a very slow rate. Cells expressing wt FGFR3, despite having a smaller number of binding sites for FGF2, internalized most of this ligand in less than 30 min, while the internalization of the radiolabeled ligand by G380R mutant FGFR3-expressing cells was far from being completed even after 120 min (Fig. 4B).
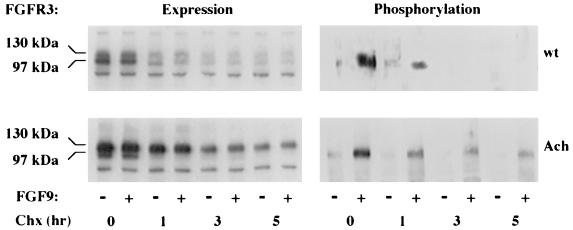
To investigate the effect of receptor stabilization on receptor activation, RCJ cells expressing wt or G380R mutant FGFR3 were treated with cycloheximide, which blocks the production of newly synthesized receptors. Monitoring of FGFR3 levels at different times revealed prolonged expression of the mutant receptor compared to the wt receptor (Fig. 5), in agreement with previous results (Fig. 3). This finding was, however, accompanied by a sustained capacity of the mutant receptor to undergo ligand-dependent phosphorylation, suggesting that as long as the receptor is on the cell surface, it is capable of ligand binding and transphosphorylation. The mutant receptor, therefore, not only accumulates at the surface but also is capable of signaling over a longer period of time than its normal counterpart.
FIG. 5.
Expression and phosphorylation of wt and G380R mutant FGFR3. RCJ cells expressing either wt or mutant (Ach) receptors were treated for the indicated times with 10 μg of cyclohexamide (Chx) per ml at 37°C. Then, the cells were incubated for 10 min with 50 ng of FGF9 per ml and lysed. Each lysate (1 mg of protein) was immunoprecipitated with an antiphosphotyrosine antibody. Samples were separated by SDS–6% PAGE, transferred to nitrocellulose, and blotted with antibodies to FGFR3.
Expanded expression of FGFR3 in growth plates of transgenic mice carrying the human mutant receptor.
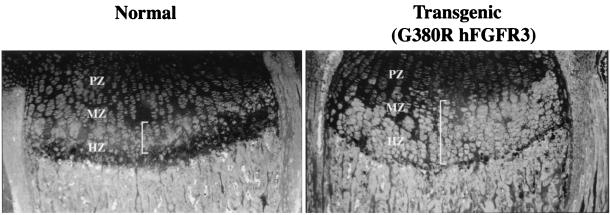
To address the relevance of such a specific delay in receptor internalization to the localization of FGFR3 in the growth plate, we analyzed epiphyseal growth plates of transgenic mice which were engineered to express G380R mutant hFGFR3 under mouse FGFR3 transcriptional control and which show a phenotype remarkably similar to that of afflicted humans (28a). Immunostaining with anti-FGFR3 antibodies revealed that while the localization of this receptor in the growth plates of normal mice was restricted to cells in the upper hypertrophic zone (Fig. 6, left), transgenic littermates expressed the receptor in a significantly wider area of the growth plates comprising several layers of cells, including the lower hypertrophic zone (Fig. 6, right). No such difference was observed at the RNA level, as detected by in situ hybridization (data not shown). This result suggests that the mutant receptor may not be down-regulated in vivo at the time and differentiation stage at which the normal receptor is down-regulated.
FIG. 6.
Immunohistochemical analysis of epiphyseal growth plates from normal and transgenic G380R mutant hFGFR3-expressing mice. Immunostaining for FGFR3 was performed on sections of proximal tibia growth plates from 8-day-old normal and transgenic littermates with anti-FGFR3 and fluorescein-conjugated antibodies. Cells expressing FGFR3 are indicated by white brackets. The different zones of the growth plate (PZ, proliferating zone; MZ, maturation zone; MZ, hypertrophic zone) are noted.
DISCUSSION
Substitution of a glycine with an arginine in the transmembrane domain of FGFR3, as in achondroplasia, results primarily in the stabilization and accumulation of the mutant receptor at the cell surface. Transgenic mice expressing G380R mutant hFGFR3 driven by the mouse FGFR3-specific promoter and enhancer demonstrate a significantly extended area of FGFR3 expression similar to that observed in sections from human TD type I cases (6) and compatible with the detected prolonged expression of the mutant receptor in genetically engineered chondrocytes. The direct consequence of this specific defect in internalization is uncontrolled, prolonged, ligand-dependent activation of this receptor, which may contribute to receptor overactivation (15, 18, 25, 36, 38) and the observed inhibition of chondrocyte terminal differentiation typical of achondroplasia. This suggestion is well in line with the current concept that multiple mutations in FGFR responsible for human skeletal dysplasias are all gain-of-function mutations. Whether the observed selective overexpression of the FGFR3 protein in TD type I (6) and possibly of other mutants of FGFR3 results from a similar defect in receptor down-regulation has yet to be determined.
Ligand-induced dimerization is a key event in the transmembrane signaling of receptor tyrosine kinases. A Val-Glu point mutation in the transmembrane domain of the Neu/ErbB-2 tyrosine kinase leads to constitutive receptor phosphorylation due to ligand-independent dimerization and activation (39). The G380R mutation in FGFR3, being in a position analogous to that of the neu mutation, was implied to function in a similar manner to constitutively activate this receptor (38). Constitutive activation of the mutant receptor was supported by experiments with chimeric proteins of FGFR3 having the kinase domain of either FGFR1 (18) or the neu oncogene (37), having the extracellular domain of the platelet-derived growth factor receptor (24), or having a full-length homologous mutant of FGFR2 (15). Our results, like those of Raffioni et al. (24) and Thompson and coworkers (34), suggest that at moderate expression levels, the G380R mutant receptor, like its normal counterpart, requires a ligand for dimerization and activation. We have also found an equal capacity of the mutant receptor to dimerize with either a wild-type or a mutant receptor counterpart upon ligand binding, as evidenced by coexpression and immunoprecipitation experiments (data not shown). This finding may argue against mutant homodimerization via hydrogen bonding as the sole mechanism driving constitutive receptor activation in human achondroplasia. The discrepancy between the reported, albeit partial, ligand-independent activation and our above-mentioned results may be due to the use of different cellular systems, which may express variable levels of these receptors or their endogenous ligands (1) acting in an autocrine manner to stimulate the overexpressed receptor. Indeed, from our experience, it is not uncommon to detect significant basal phosphorylation levels of the wild-type receptor as well. One cannot rule out, however, the possibility that both constitutive activation and selective overexpression of the mutant receptor may act in concert to locally enhance FGFR3 signals.
Tyrosine kinase receptors undergo ligand-mediated internalization following their dimerization and activation (31). Although specific data concerning FGFR3 internalization are not available, the mechanisms responsible for the internalization of other tyrosine kinase receptors are relevant to FGFR3, as evidenced by the increased rate of internalization of this receptor in response to a ligand and the lack of this effect in a kinase-deficient receptor. Our studies of internalization rates by several independent methods, such as pulse-chase metabolic labeling, surface biotinylation, and quantitative measurements of receptor-mediated internalization of a radiolabeled ligand, clearly establish a primary defect in the down-regulation of the G380R mutant receptor. The pattern of expression of the mutant receptor is distinct from that of the wt receptor in both total receptor levels and the ratio between the immature, unglycosylated form and the mature, membrane form of the receptor. The selective accumulation of the mutant receptor in the mature form is of particular importance, as it is this form which is capable of binding and responding to extracellular ligands by dimerization and downstream signal transduction. Such an accumulation of a fully functional receptor may also support the prolonged and persistent signals induced by a locally expressed ligand. It is expected that in the heterozygous achondroplasia state, a block in the internalization of the mutant receptor may eventually lead, through the process of ligand-mediated receptor heterodimerization (35, 39), to the excess accumulation and activation of wt FGFR as well. Such a transdominant positive effect of the mutant receptor can further contribute to the overall excessive and prolonged activation of FGFR-specific pathways.
The molecular basis for the selective accumulation of the mutant receptor is not clear. It could be related to differential, posttranslational processing of the receptor. Introducing a positively charged residue within the interior of the hydrophobic membrane may also present an ionic barrier for membrane endocytosis or cause a change in receptor protein conformation crucial for the interaction of the receptor with intracellular components responsible for internalization. Alternatively, the lack of down-regulation may be due to a specific block in the transmittance of an internalization signal by the mutant receptor. FGFR1, with a mutation in Y766 autophosphorylation and in the PLC-γ binding site, is defective in internalization and degradation (32) and leads to a hypomorphic gain-of-function allele resulting in vertebral abnormalities in mice targeted for this mutation (26). The disrupted down-regulation signal in achondroplasia may be different from that of MAPK, JNK, and Stat1 as well as from PLC-γ-mediated signals, which seem to be properly activated by the mutant receptor. The fact that this phenomenon is also observed for 293T human embryonal kidney cells, L8 myoblasts, and CHO epithelial cells suggests that the internalization defect of the mutant receptor is an intrinsic characteristic of the mutant protein and is not cell or tissue specific, as could be implied from the selective defect in bone formation.
Signals from FGFR3 seem to exert a selective negative effect on bone growth, as evidenced from the skeletal overgrowth of FGFR3-null mice (5, 7) and the severe skeletal growth retardation of mice overexpressing FGF2 (4). Moreover, FGFR3 mutations selectively inhibit endochondral ossification and long-bone formation without detectable abnormalities in other tissues, where FGFR3 is highly expressed (20, 41). The cellular context in which FGFR function seems to be of great importance, and their tissue-specific expression as well as the expression of their ligands is highly coordinated throughout development. Several FGF ligands were found to be expressed by growth plate chondrocytes (11, 14, 20, 21), and receptor activation in the growth plate can be readily explained by either autocrine or paracrine mechanisms. Ligand-mediated receptor overactivation is therefore an attractive mechanism to support an exclusive effect of mutated FGFR3 on long-bone formation due to the specific combination of ligands, receptors, and coreceptors, such as heparan sulfate proteoglycans, in this tissue and at different stages along the pathway of differentiation of epiphyseal chondrocytes.
The process of longitudinal bone growth involves coordination and precise balance among chondrocyte proliferation, differentiation, cartilage matrix production, and mineralization within the developing growth plate. These cellular activities are under the influence of a variety of hormonal and local factors, such as FGF ligands (19), whose relative concentrations, sites, and sequence of appearance vary critically during development. It is this tightly coordinated process which may make the growth plate a most sensitive organ to a variety of insults, such as in achondroplasia.
ACKNOWLEDGMENTS
We are grateful to Yuri Sheinin for assistance with the immunolocalization studies, Peer David for most helpful discussions, Magda David for excellent technical assistance, and Moshe Oren for the Fos-luciferase construct.
This work was supported in part by the Israel Academy of Sciences and Humanities and by Prochon Biotech Ltd.
REFERENCES
- 1.Basilico C, Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- 2.Bellus G A, McIntosh I, Szabo J, Aylsworth A, Kaitila I, Francomano C A. Hypochondroplasia: molecular analysis of the fibroblast growth factor receptor 3 gene. Ann N Y Acad Sci. 1996;785:182–187. doi: 10.1111/j.1749-6632.1996.tb56257.x. [DOI] [PubMed] [Google Scholar]
- 3.Cancedda R, Descalzi Cancedda F, Castagnola P. Chondrocyte differentiation. Int Rev Cytol. 1995;159:265–358. doi: 10.1016/s0074-7696(08)62109-9. [DOI] [PubMed] [Google Scholar]
- 4.Coffin J D, Florkiewicz R Z, Neumann J, Mort-Hopkins T, Dorn II G W, Lightfoot P, German R, Howles P N, Kier A, O'Toole B A, et al. Abnormal bone growth and selective translational regulation in basic fibroblast growth factor (FGF-2) transgenic mice. Mol Biol Cell. 1995;6:1861–1873. doi: 10.1091/mbc.6.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colvin J S, Bohne B A, Harding G W, McEwen D G, Ornitz D M. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–397. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- 6.Delezoide A L, Lasselin-Benoist C, Legeai-Mallet L, Brice P, Senee V, Yayon A, Munnich A, Vekemans M, Bonaventure J. Abnormal FGFR 3 expression in cartilage of thanatophoric dysplasia fetuses. Hum Mol Genet. 1997;6:1899–1906. doi: 10.1093/hmg/6.11.1899. [DOI] [PubMed] [Google Scholar]
- 7.Deng C, Wynshaw B A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 8.Givol D, Yayon A. Complexity of FGF receptors: genetic basis for structural diversity and functional specificity. FASEB J. 1992;6:3362–3369. [PubMed] [Google Scholar]
- 9.Grigoriadis A E, Heersche J N, Aubin J E. Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: effect of dexamethasone. J Cell Biol. 1988;106:2139–2151. doi: 10.1083/jcb.106.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horton W A, Machado M A, Ellard J, Campbell D, Putnam E A, Aulthouse A L, Sun X, Sandell L J. An experimental model of human chondrocyte differentiation. Prog Clin Biol Res. 1993;3838:533–540. [PubMed] [Google Scholar]
- 11.Jingushi S, Scully S P, Joyce M E, Sugioka Y, Bolander M E. Transforming growth factor-beta 1 and fibroblast growth factors in rat growth plate. J Orthop Res. 1995;13:761–768. doi: 10.1002/jor.1100130516. [DOI] [PubMed] [Google Scholar]
- 12.Johnson D E, Williams L T. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- 13.Keegan K, Meyer S, Hayman M J. Structural and biosynthetic characterization of the fibroblast growth factor receptor 3 (FGFR-3) protein. Oncogene. 1991;6:2229–2236. [PubMed] [Google Scholar]
- 14.Leach R J, Sokol C, McMurtry J P. Immunolocalization of basic fibroblast growth factor in porcine epiphyseal growth plate. Domest Anim Endocrinol. 1997;14:129–132. doi: 10.1016/s0739-7240(96)00120-8. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Mangasarian K, Mansukhani A, Basilico C. Activation of FGF receptors by mutations in the transmembrane domain. Oncogene. 1997;14:1397–1406. doi: 10.1038/sj.onc.1200983. [DOI] [PubMed] [Google Scholar]
- 16.McConahey P J, Dixon F J. Radioiodination of proteins by the use of the chloramine-T method. Methods Enzymol. 1980;70:210–213. doi: 10.1016/s0076-6879(80)70050-2. [DOI] [PubMed] [Google Scholar]
- 17.McDougall S, Fu Y H, Lowe G N, Williams A, Polendo R, Benya P D, Iida-Klein A, Fang M A, Hahn T J. Surface adhesion-mediated regulation of chondrocyte-specific gene expression in the nontransformed RCJ 3.1C5.18 rat chondrocyte cell line. J Bone Miner Res. 1996;11:1130–1138. doi: 10.1002/jbmr.5650110812. [DOI] [PubMed] [Google Scholar]
- 18.Naski M C, Wang Q, Xu J, Ornitz D M. Graded activation of fibroblast growth factor receptor 3 by mutations causing achondroplasia and thanatophoric dysplasia. Nat Genet. 1996;13:233–237. doi: 10.1038/ng0696-233. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson A, Ohlsson C, Isaksson O G, Lindahl A, Isgaard J. Hormonal regulation of longitudinal bone growth. Eur J Clin Nutr. 1994;48(Suppl. 1):S150–158. doi: 10.1007/BF02558817. [DOI] [PubMed] [Google Scholar]
- 20.Peters K, Ornitz D, Werner S, Williams L. Unique expression pattern of the FGF receptor 3 gene during mouse organogenesis. Dev Biol. 1993;155:423–430. doi: 10.1006/dbio.1993.1040. [DOI] [PubMed] [Google Scholar]
- 21.Peters K G, Werner S, Chen G, Williams L T. Two FGF receptor genes are differentially expressed in epithelial and mesenchymal tissues during limb formation and organogenesis in the mouse. Development. 1992;114:233–243. doi: 10.1242/dev.114.1.233. [DOI] [PubMed] [Google Scholar]
- 22.Ponseti I V. Skeletal growth in achondroplasia. J Bone Jt Surg Am Vol. 1970;52:701–716. [PubMed] [Google Scholar]
- 23.Ponseti I V. Bone formation in achondroplasia. Basic Life Sci. 1988;48:109–122. doi: 10.1007/978-1-4684-8712-1_15. [DOI] [PubMed] [Google Scholar]
- 24.Raffioni S, Zhu Y Z, Bradshaw R A, Thompson L M. Effect of transmembrane and kinase domain mutations on fibroblast growth factor receptor 3 chimera signaling in PC12 cells. A model for the control of receptor tyrosine kinase activation. J Biol Chem. 1998;273:35250–35259. doi: 10.1074/jbc.273.52.35250. [DOI] [PubMed] [Google Scholar]
- 25.Robertson S C, Meyer A N, Hart K C, Galvin B D, Webster M K, Donoghue D J. Activating mutations in the extracellular domain of the fibroblast growth factor receptor 2 function by disruption of the disulfide bond in the third immunoglobulin-like domain. Proc Natl Acad Sci USA. 1998;95:4567–4572. doi: 10.1073/pnas.95.8.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossant J, Ciruna B, Partanen J. FGF signaling in mouse gastrulation and anteroposterior patterning. Cold Spring Harbor Symp Quant Biol. 1997;62:127–133. [PubMed] [Google Scholar]
- 27.Rousseau F, Bonaventure J, Legeai M L, Pelet A, Rozet J M, Maroteaux P, Le M M, Munnich A. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature. 1994;371:252–254. doi: 10.1038/371252a0. [DOI] [PubMed] [Google Scholar]
- 28.Rousseau F, el Ghouzzi V, Delezoide A L, Legeai-Mallet L, Le Merrer M, Munnich A, Bonaventure J. Missense FGFR3 mutations create cysteine residues in thanatophoric dwarfism type I (TD1) Hum Mol Genet. 1996;5:509–512. doi: 10.1093/hmg/5.4.509. [DOI] [PubMed] [Google Scholar]
- 28a.Segev, O., I. Chumakov, Z. Nevo, D. Givol, L. Madar-Shapiro, Y. Sheinin, M. Weinreb, and A. Yayon. Restrained chondrocyte proliferation and maturation with abnormal growth plate vascularization and ossification in human FGFR-3G380R transgenic mice. Hum. Mol. Genet., in press. [DOI] [PubMed]
- 29.Shiang R, Thompson L M, Zhu Y Z, Church D M, Fielder T J, Bocian M, Winokur S T, Wasmuth J J. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78:335–342. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 30.Sorkin A, Helin K, Waters C M, Carpenter G, Beguinot L. Multiple autophosphorylation sites of the epidermal growth factor receptor are essential for receptor kinase activity and internalization. Contrasting significance of tyrosine 992 in the native and truncated receptors. J Biol Chem. 1992;267:8672–8678. [PubMed] [Google Scholar]
- 31.Sorkin A, Waters C, Overholser K A, Carpenter G. Multiple autophosphorylation site mutations of the epidermal growth factor receptor. Analysis of kinase activity and endocytosis. J Biol Chem. 1991;266:8355–8362. [PubMed] [Google Scholar]
- 32.Sorokin A, Mohammadi M, Huang J, Schlessinger J. Internalization of fibroblast growth factor receptor is inhibited by a point mutation at tyrosine 766. J Biol Chem. 1994;269:17056–17061. [PubMed] [Google Scholar]
- 33.Tavormina P L, Shiang R, Thompson L M, Zhu Y Z, Wilkin D J, Lachman R S, Wilcox W R, Rimoin D L, Cohn D H, Wasmuth J J. Thanatophoric dysplasia (types I and II) caused by distinct mutations in fibroblast growth factor receptor 3. Nat Genet. 1995;9:321–328. doi: 10.1038/ng0395-321. [DOI] [PubMed] [Google Scholar]
- 34.Thompson L M, Raffioni S, Wasmuth J J, Bradshaw R A. Chimeras of the native form or achondroplasia mutant (G375C) of human fibroblast growth factor receptor 3 induce ligand-dependent differentiation of PC12 cells. Mol Cell Biol. 1997;17:4169–4177. doi: 10.1128/mcb.17.7.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 36.Webster M K, D'Avis P Y, Robertson S C, Donoghue D J. Profound ligand-independent kinase activation of fibroblast growth factor receptor 3 by the activation loop mutation responsible for a lethal skeletal dysplasia, thanatophoric dysplasia type II. Mol Cell Biol. 1996;16:4081–4087. doi: 10.1128/mcb.16.8.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webster M K, Donoghue D J. Constitutive activation of fibroblast growth factor receptor 3 by the transmembrane domain point mutation found in achondroplasia. EMBO J. 1996;15:520–527. [PMC free article] [PubMed] [Google Scholar]
- 38.Webster M K, Donoghue D J. FGFR activation in skeletal disorders: too much of a good thing. Trends Genet. 1997;13:178–182. doi: 10.1016/s0168-9525(97)01131-1. [DOI] [PubMed] [Google Scholar]
- 39.Weiner D B, Liu J, Cohen J A, Williams W V, Greene M I. A point mutation in the neu oncogene mimics ligand induction of receptor aggregation. Nature. 1989;339:230–231. doi: 10.1038/339230a0. [DOI] [PubMed] [Google Scholar]
- 40.Wilkie A O, Morriss K G, Jones E Y, Heath J K. Functions of fibroblast growth factors and their receptors. Curr Biol. 1995;5:500–507. doi: 10.1016/s0960-9822(95)00102-3. [DOI] [PubMed] [Google Scholar]
- 41.Wuechner C, Nordqvist A C, Winterpacht A, Zabel B, Schalling M. Developmental expression of splicing variants of fibroblast growth factor receptor 3 (FGFR3) in mouse. Int J Dev Biol. 1996;40:1185–1188. [PubMed] [Google Scholar]