Expression of Interferon Consensus Sequence Binding Protein (ICSBP) Is Downregulated in Bcr-Abl-Induced Murine Chronic Myelogenous Leukemia-Like Disease, and Forced Coexpression of ICSBP Inhibits Bcr-Abl-Induced Myeloproliferative Disorder (original) (raw)
Abstract
Chronic myelogenous leukemia (CML) is a clonal myeloproliferative disorder resulting from the neoplastic transformation of a hematopoietic stem cell. The majority of cases of CML are associated with the (9;22) chromosome translocation that generates the bcr-abl chimeric gene. Alpha interferon (IFN-α) treatment induces hematological remission and prolongs life in 75% of CML patients in the chronic phase. It has been shown that mice deficient in interferon consensus sequence binding protein (ICSBP), a member of the interferon regulatory factor family, manifest a CML-like syndrome. We have shown that expression of Bcr-Abl in bone marrow (BM) cells from 5-fluorouracil (5-FU)-treated mice by retroviral transduction efficiently induces a myeloproliferative disease in mice resembling human CML. To directly test whether icsbp can function as a tumor suppressor gene, we examined the effect of ICSBP on Bcr-Abl-induced CML-like disease using this murine model for CML. We found that expression of the ICSBP protein was significantly decreased in Bcr-Abl-induced CML-like disease. Forced coexpression of ICSBP inhibited the Bcr-Abl-induced colony formation of BM cells from 5-FU-treated mice in vitro and Bcr-Abl-induced CML-like disease in vivo. Interestingly, coexpression of ICSBP and Bcr-Abl induced a transient B-lymphoproliferative disorder in the murine model of Bcr-Abl-induced CML-like disease. Overexpression of ICSBP consistently promotes rather than inhibits Bcr-Abl-induced B lymphoproliferation in a murine model where BM cells from non-5-FU-treated donors were used, indicating that ICSBP has a specific antitumor activity toward myeloid neoplasms. We also found that overexpression of ICSBP negatively regulated normal hematopoiesis. These data provide direct evidence that ICSBP can act as a tumor suppressor that regulates normal and neoplastic proliferation of hematopoietic cells.
Chronic myelogenous leukemia (CML), which accounts for 15 to 20% of all human leukemias, is a myeloproliferative disorder resulting from the neoplastic transformation of hematopoietic stem cells (reviewed in reference 21). More than 90% of cases of CML are associated with the Philadelphia chromosome, a product of t(9;22) chromosome translocation which generates the bcr-abl chimeric gene (reviewed in reference 24). The disease usually has a biphasic course. The initial, chronic phase is characterized by increased proliferation and maturation of myeloid cells, with granulocytes predominating. Progression of the disease, after 3 to 5 years, to the terminal blast crisis stage is characterized by accelerated accumulation of immature myeloid or lymphoid cells. Apart from curative allogeneic or syngeneic bone marrow transplantation, combined approaches with high doses of alpha interferon (IFN-α) and chemotherapy are the most effective treatments. Such treatments can achieve clinical, hematological, and even cytogenetic remissions (reviewed in reference 40) and thus prolong life in a majority (>75%) of CML patients treated in the chronic phase. However the treatment rarely cures CML. Elucidating the molecular mechanisms of IFN-α treatment and identifying the specific mediators of IFN-α in treating CML is therefore critical for developing improved therapies for CML.
IFNs are a family of multifunctional cytokines that play important roles in the induction of antiviral activities, inhibition of cell growth, induction of cell differentiation, and immunomodulation (reviewed in reference 31). IFN-α also restores normal adhesion of CML progenitors to bone marrow stroma (2). IFNs function by inducing a group of transcriptional factors called IFN regulatory factors (IRFs) (reviewed in reference 13). IRFs regulate the expression of IFN-stimulated genes by binding to specific DNA sequences, i.e., IFN-stimulated response element or gamma activation sequence, in promoters of the genes regulated by IFNs. The IRF protein family includes IRF-1, IRF-2, IRF-3, IRF-4/ICSAT/Pip, IRF-5, IRF-6, and IRF-7, ISGF-3γ (interferon-stimulated gene factor 3γ), and ICSBP (interferon consensus sequence binding protein) (reviewed in reference 27). The members of the IRF family have significant homology in the first 115 amino acids, which comprise the DNA binding domain, and contain a divergent C-terminal region that serves as the regulatory domain.
ICSBP, a ∼50-kDa protein, is a member of the IRF family and is expressed predominantly in hematopoietic cells (6, 25, 32). Its expression can be strongly induced by IFN-γ (6, 32, 33, 43). IFN-α also can induce icsbp gene expression in vivo (37). ICSBP can selectively suppress the expression of some IFN- responsive genes, such as the major histocompatibility complex type 1 gene, and activate others, such as the interleukin-12 (IL-12) gene, depending on the context of the promoters (11, 26, 36, 42, 43). It has been shown that ICSBP interacts with IRF-1, IRF-2, and a hematopoietic cell-specific Ets protein, PU.1. These interactions include the formation of a complex with PU.1 on Ets/IRF composite elements, and cooperation with PU.1 and IRF-1 to increase gp91(phox) expression (3, 7, 8). In vitro studies have demonstrated that direct binding of ICSBP to DNA is prevented by tyrosine phosphorylation, but phosphorylated ICSBP can bind DNA through the association with tyrosine-phosphorylated IRF-1 and IRF-2 (39).
ICSBP plays an important role in regulating immune responses and hematopoiesis. ICSBP-deficient mice exhibit enhanced susceptibility to viral and intracellular parasite infections, possibly due to impaired IL-12 production (11, 16, 36, 44). Interestingly, ICSBP-deficient mice manifest a CML-like syndrome, suggesting that icsbp may function as a tumor suppressor gene (16). Consistent with this idea, it was found by a reverse transcription-PCR assay that in CML patients the number of icsbp transcripts was decreased and that this reduction of icsbp transcripts could be reversed by IFN-α treatment (37). However, there has not been a direct demonstration that ICSBP can suppress tumor growth. Since ICSBP deficiency can affect the expression of other proteins, such as IRF-2 (16), it is not known whether the development of CML-like disease in ICSBP-deficient mice is directly due to the lack of ICSBP protein.
In this study we examined the role of ICSBP on Bcr-Abl-induced CML-like disease by using a recently developed efficient mouse model for Bcr-Abl-induced CML (28, 45). We found that the expression of ICSBP protein was significantly decreased in Bcr-Abl-induced CML-like disease and that forced coexpression of ICSBP inhibited the Bcr-Abl-induced colony formation of bone marrow (BM) cells from 5-fluorouracil (5-FU)-treated mice (5-FU BM cells) in vitro and the CML-like disease in vivo. We also found that overexpression of ICSBP negatively regulated the growth of normal hematopoietic cells. Our data provide direct evidence that ICSBP can act as a tumor suppressor that regulates normal and neoplastic proliferation of hematopoietic cells.
MATERIALS AND METHODS
DNA constructs.
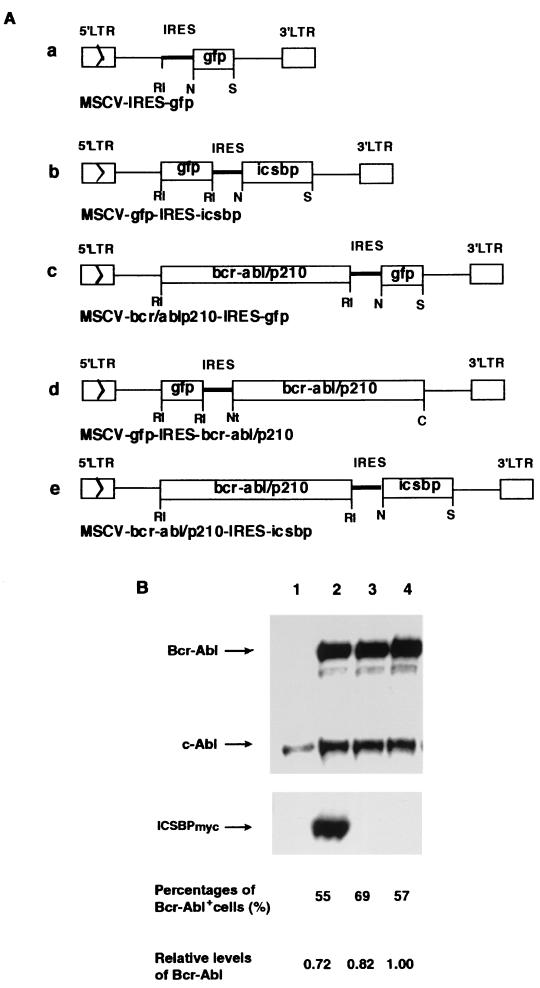
Construction of MSCV-IRES-gfp (see Fig. 2A, panel a) and MSCV-_bcr-abl/p210_-IRES-gfp (see Fig. 2A, panel c) was described previously (45). For construction of MSCV-gfp-IRES-icsbp and MSCV-_bcr-abl/p210_-IRES-icsbp, the human icsbp gene was amplified by PCR with a human icsbp cDNA (6) as template with a 5′ primer containing the _Nco_I site (5′-CAT GCC ATG GCA TGT GAC CGG AAT GGT GGT GGG C-3′) and a 3′ primer containing the _Sal_I site, _Not_I site, and 23 nucleotides from the 3′ end of coding sequences of ICSBP (5′-ACG CGT CGA CTT AGG CGG CCG CGA GGG TGA TCT GTT GGT TTT CTC-3′). The ∼1.4-kb _Nco_I-_Sal_I icsbp fragment was then cloned into pCITE (Novagen, Madison, Wis.) between the _Nco_I and _Sal_I sites. A DNA adapter containing the myc tag coding sequences (5′-GAA CAA AAG CTT ATT TCT GAA GAA GAC TTG TAA-3′) with _Not_I and _Sal_I cohesive ends was inserted in frame into pCITE/icsbp between the _Not_I and _Sal_I sites. To avoid possible mutations introduced by PCR, we swapped the _Stu_I-_Tth_IIII fragment (∼0.8 kb within icsbp) in pCITE/icsbp with the original icsbp DNA. The rest of the icsbp DNA amplified by PCR was examined by DNA sequencing, and no mutation was found. The 2.1-kb _Eco_RI-_Sal_I IRES-icsbp fragment was then cloned into the pMSCV vector (14) between the _Eco_RI and _Sal_I sites. Finally, MSCV-_gfp_-IRES-icsbp and MSCV-_bcr-abl/p210_-IRES-icsbp (see Fig. 2A, panels b and e) were generated by cloning the gfp or bcr-abl gene into the _Eco_RI site of pMSCV-IRES-icsbp. For MSCV-_gfp_-IRES-bcr-abl/p210 (construct d), the bcr-abl cDNA was cloned into pMSCV-IRES vector through a _Not_I site introduced downstream of the internal ribosome entry site (IRES) (Baum and R. Ren, unpublished data). The following nucleotide sequences from the IRES and the _Not_I site were fused into the 5′ end of the bcr-abl coding region: ATG GCC ACA ACC ATG GCG GCC GCC (the _Not_I site is underlined), which encodes the amino acid sequence MATTMAAA. The gfp gene was then cloned into the _Eco_RI site of pMSCV-IRES-bcr-abl.
FIG. 2.
(A) Retroviral constructs used to transduce the bcr-abl/p210+gfp, gfp+bcr-abl/p210, bcr-abl/p210+icsbp, gfp, and gfp+icsbp genes. LTR, long terminal repeat; MSCV, murine stem cell virus vector; RI, _Eco_RI; N, _Nco_I; S, _Sal_I; C, _Cla_I; Nt, _Not_I. (B) Ectopic expression of Bcr-Abl and ICSBPmyc in NIH 3T3 cells as examined by immunoblotting with the anti-Abl monoclonal antibody Ab-3 (top panel) and a polyclonal anti-ICSBP antibody (bottom panel). Percentages of Bcr-Abl-positive NIH 3T3 cells detected by intracellular immunostaining are indicated. The amount of Bcr-Abl protein detected by immunoblotting was quantified as relative integrated optical densities by Gel Doc 1000 with Molecular Analyst 2.1.1 software. Lanes: 1, NIH 3T3 cells; 2, _bcr-abl+icsbp_-infected NIH 3T3 cells; 3: _gfp+bcr-abl_-infected NIH 3T3 cells; 4, _bcr-abl+gfp_-infected NIH 3T3 cells.
Cell culture and retrovirus preparation.
Bosc23 cells (29) were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml (GIBCO BRL, Grand Island, N.Y.). NIH 3T3 fibroblast cells were grown in DMEM containing 10% calf serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml.
Retroviruses were produced by transfecting Bosc23 cells with retroviral vectors and tested on NIH 3T3 cells essentially as described previously (12). Two days after transfection, the culture supernatant containing the retroviruses was collected and used to infect BM and NIH 3T3 cells. For titer determination of retroviruses carrying the gfp gene, 105 NIH 3T3 cells were plated in 60-mm plates and infected the next day with 0.1 to 0.5 ml of viral supernatant for 4 h in a total volume made up to 2 ml with medium plus 8 μg of Polybrene (Sigma, St. Louis, Mo.) per ml. Two days after infection, NIH 3T3 cells were tested for expression of green fluorescent protein (GFP) by flow cytometry, and the relative virus titer was measured as the percentage of GFP+ NIH 3T3 cells. The amount of viral supernatant used to infect NIH 3T3 cells was directly proportional to the percentage of GFP+ cells in a range up to 50% GFP+. The intracellular immunostaining method (described below) was used to quantify cells infected with retroviruses without the gfp gene.
BM transduction, transplantation, and colony assays.
BM transduction and transplantation with 5-FU BM cells were performed as previously described (45). For non-5-FU BM transduction and transplantation, normal BM cells from male donor BALB/cByJ mice (The Jackson Laboratory, Bar Harbor, Maine) were infected for 2 days in DMEM–15% FCS–5% WEHI-conditioned medium–33% viral supernatant–2 μg of Polybrene per ml–2 mM l-glutamine (Gibco BRL)–100 μg of streptomycin per ml–100 U of penicillin per ml–0.25 μg of amphotericin B (Gibco BRL) per ml–7 ng of recombinant murine IL-3 (R&D Systems, Inc., Minneapolis, Minn.) per ml–12 ng of recombinant human IL-6 (R&D Systems) per ml–56 ng of recombinant murine stem cell factor (R&D Systems) per ml–10 ng of recombinant murine IL-7 (R&D Systems), per ml as described previously (22). After 1 day of infection, the cells were collected and infected for one more day in a freshly made retrovirus cocktail as above. Then infected BM cells were washed and resuspended in phosphate-buffered saline (Gibco BRL), and 106 BM cells were injected into the tail vein of each of the lethally irradiated (2 × 450 rads, 4 h between each dose) female recipient BALB/cByJ mice.
Statistical analysis of survival curve data was performed with Survival Tools for StatView 5 (Abacus Concepts, Inc., Berkeley, Calif.) using the Kaplan-Meier survival analysis and Mantel-Cox (log-rank) test functions.
In vitro soft agar colony assays were performed as described previously (35), with modifications. From the pool of infected cells used for BM transplantation (BMT), 105 infected 5-FU BM cells were plated per 35-mm well in DMEM–20% FCS, 100 μg of streptomycin per ml–100 U of penicillin per ml–0.25 μg of amphotericin B per ml–50 μM 2-mercaptoethanol (Sigma)–0.3% Bacto Agar, on top of a layer of medium with 0.6% Bacto Agar. Colonies were counted and compared on days 7 and 9.
Southern blot analysis.
Mouse splenocytes were treated with red blood cell lysis solution ACK (0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM disodium EDTA [pH 7.3]). Genomic DNAs were isolated from these cells and NIH 3T3 cells with the QIAamp blood kit (Qiagen, Santa Clara, Calif.). About 10 μg of the DNA was digested with _Xba_I (two _Xba_I sites are located in the 5′ and 3′ long terminal repeats LTR of the MSCV vector), separated on a 0.7% agarose gel, transferred to a Hybond-N+ membrane (Amersham, Arlington Heights, Ill.), and hybridized with a probe of a 32P-labeled 1.2-kb _Sgr_I-_Bgl_II fragment from the 3′ end of human c-abl cDNA. The radioactive DNA on the washed membrane were detected by PhosphoImager analysis.
Immunoblotting.
NIH 3T3 cells (107) infected with various retroviruses were washed once in ice-cold phosphate-buffered saline and lysed in 1 ml of lysis buffer (50 mmol of HEPES [pH 7.4] per liter, 150 mmol of NaCl per liter, 10% glycerol, 1% Triton X-100, 1 mmol of EGTA per liter, 1.5 mmol of MgCl2 per liter, 10 mmol of NaF per liter, 1 mmol of sodium orthovanadate per liter, 1× Complete proteinase inhibitor cocktail [Boehringer, Mannheim, Germany]). The total protein concentration was determined with the Coomassie protein assay reagent (Pierce, Rockford, Ill.). Peripheral blood cells were treated with ACK, suspended in PBS (100 μl of PBS per 1 × 106 white blood cells (WBCs) for detecting overexpressed Bcr-Abl and ICSBPmyc, and 30 μl of PBS per 2 × 106 WBCs for detecting the endogenous ICSBP), mixed with an equal volume of 2× Laemmli sample buffer, boiled at 100°C for 10 min, and immediately cooled on ice for 2 min. Immunoblotting was performed as previously described (34). The relative amount of proteins detected by immunoblotting were quantified as integrated optical densities with Gel Doc 1000 (Bio-Rad Laboratories, Hercules, Calif.) and Molecular Analyst 2.1.1 software.
Intracellular immunostaining.
Cells were fixed and permeabilized with Cytofix/Cytoperm (Pharmingen, San Diego, Calif.) or with 80% ethanol at −20°C for 30 min. The anti-Abl monoclonal antibody Ab-3 (Oncogene Research Products, Cambridge, Mass.) was used as the primary antibody, and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G or allophycocyanin (APC)-conjugated goat anti-mouse immunoglobulin G (Molecular Probes, Eugene, Oreg.) was used as the secondary antibody. Stained cells were analyzed on a FACSCalibur (Beckton Dickinson, San Jose, Calif.). We found that intracellular immunofluorescent staining analysis of overexpression of Bcr-Abl protein gave a similar result to flow cytometric analysis of GFP expression in Bcr-Abl+GFP-containing hematopoietic cells and NIH 3T3 cells (see Fig. 6) (data not shown).
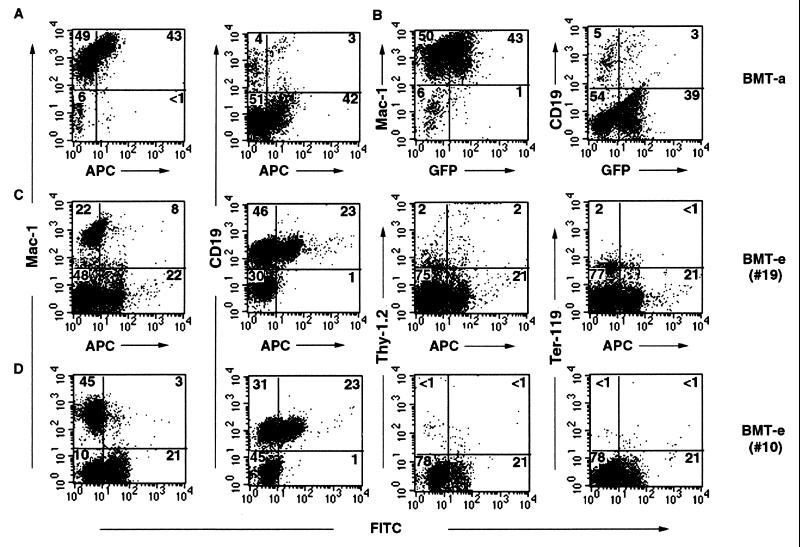
FIG. 6.
Immunophenotyping of peripheral blood cells of Bcr-Abl+GFP-BMT mice and Bcr-Abl+ICSBP-BMT mice. Peripheral blood WBCs from a Bcr-Abl+GFP-BMT mouse (A and B) and two Bcr-Abl+ICSBP-BMT mice (C and D) were first stained by PE-conjugated Mac-1, Thy-1.2, CD19, or Ter-119 antibodies. These cells were then intracellularly stained with anti-Abl monoclonal antibody, Ab-3, and APC- or FITC-conjugated goat anti-mouse antibody. The same batch of WBCs from the Bcr-Abl+GFP-BMT mouse, which are stained by PE-conjugated Mac-1 or CD19 and anti-Abl antibody/APC-conjugated goat anti-mouse antibody, and express GFP, is shown in either a PE versus APC (A) or a PE versus GFP (B) dot plot. The WBCs from the two Bcr-Abl+ICSBP-BMT mice stained with the anti-Abl primary antibody and either APC- or FITC-conjugated secondary antibody is shown in either a PE versus APC (C) or a PE versus FITC (D) dot-plot.
Flow cytometry.
Standard protocols for antibody staining of cell surface proteins were followed (4). Peripheral blood or spleen cells were treated with ACK, resuspended in staining buffer (Hanks balanced salt solution, 5% fetal bovine serum, 0.1% sodium azide), and blocked with anti-mouse CD16/CD32 (2.4G2; Pharmingen). The cells were then stained with phycoerytherin (PE)-conjugated Mac-1 (M1/70), Thy-1.2 (53-2.1), CD19 (1D3), or Ter-119. Flow cytometry measurements were made on a FACSCalibur machine, and data were analyzed with CellQuest software (Becton Dickinson). GFP+ cells or Mac-1+/GFP+ and Mac-1+/GFP− cells were sorted on a FACSorter (Beckton Dickinson).
RESULTS
Decrease of ICSBP expression in Bcr-Abl-induced CML-like disease.
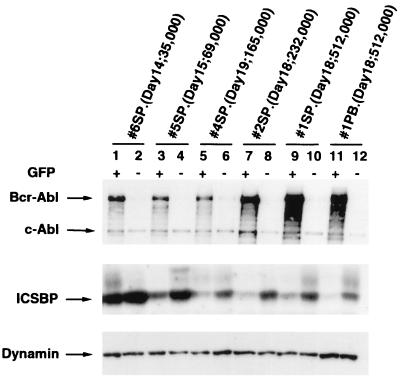
We have previously shown that Bcr-Abl induced a fatal myeloproliferative disease in mice in about 3 weeks and that both _bcr-abl_-infected and noninfected myeloid cells, distinguished by GFP expression, can be expanded in mice with Bcr-Abl-induced CML-like disease (45). To examine if the expression of ICSBP is changed in Bcr-Abl-induced CML-like disease, we sorted the Mac-1+/GFP+ and Mac-1+/GFP− cells from the mice with Bcr-Abl-induced CML-like disease on different days after BMT. Expression of Bcr-Abl and the endogenous ICSBP in these sorted cells was examined by immunoblotting with anti-Abl and anti-ICSBP antibodies (Fig. 1). The expression of dynamin (Fig. 1) and the expression of actin (which gave a similar result [data not shown]), as detected with anti-dynamin and anti-actin monoclonal antibodies, respectively, were used as loading controls. As expected, only GFP+ cells expressed Bcr-Abl (Fig. 1). It is noted that the expression level of Bcr-Abl increased as disease progressed (Fig. 1, compare results for mice with low WBC counts to those for mice with high WBC counts). This phenomenon may reflect an outgrowth of tumor cells with high levels of Bcr-Abl expression.
FIG. 1.
Decrease of expression of the endogenous ICSBP in Bcr-Abl+GFP-BMT mice. Expression of Bcr-Abl/p210 and the endogenous ICSBP in the Mac-1+/GFP+ and Mac-1+/GFP− cells isolated from Bcr-Abl+GFP-BMT mice (mice 1, 2, 3, 4, 5, and 6) at days 14, 15, 18, and 19 after BMT was examined by immunoblotting with the anti-Abl monoclonal antibody Ab-3 and a polyclonal anti-ICSBP antibody. Positions of Bcr-Abl/p210, c-Abl, and ICSBP are indicated by arrows. Expression of dynamin in the same samples was used as an internal loading control. The total WBC count of each Bcr-Abl+GFP-BMT mouse is shown in parentheses, along with the time after BMT when the mouse was examined. SP, splenocytes; PB, WBCs from peripheral blood.
The endogenous ICSBP was detected in both GFP+ and GFP− myeloid cells (Fig. 1). However, in each Bcr-Abl+GFP-BMT mouse, expression of ICSBP was significantly lower (by two- to ninefold) in the GFP+ myeloid cells than in the GFP− myeloid cells. A greater reduction of ICSBP expression in the GFP+ myeloid cells was seen in mice with more advanced disease. For example, the expression of ICSBP in the GFP+ myeloid cells isolated from the spleens of Bcr-Abl-BMT mice 1 (with a WBC count of 512,000/mm3) and 2 (with a WBC count of 232,000/mm3) on day 18 after BMT (Fig. 1, lanes 9 and 7, respectively) was decreased by four- and eightfold, respectively, compared to that of Bcr-Abl+GFP-BMT mouse 6 (with a WBC count of 35,000/mm3) on day 14 after BMT (lane 1). A modest decrease (less than twofold) of ICSBP expression also was observed in the number of GFP− myeloid cells in mice with more advanced myeloproliferative disease (Fig. 1). This inverse relationship of the level of ICSBP protein and expansion of Bcr-Abl-expressing myeloid cells indicates that ICSBP expression is downregulated in Bcr-Abl-stimulated hyperproliferative myeloid cells.
Coexpression of ICSBP inhibits Bcr-Abl stimulated colony formation of 5-FU BM cells.
To examine if forced coexpression of ICSBP inhibits Bcr-Abl-induced CML-like disease, we constructed retroviruses as represented in Fig. 2A. We first examined the influence of coexpressing ICSBP versus GFP on the expression of Bcr-Abl. Western blot analysis using an anti-Abl monoclonal antibody, Ab3, demonstrated that the expression level of Bcr-Abl in NIH 3T3 cells infected with retrovirus e (NIH 3T3-e, containing _bcr-abl_-IRES-icsbp) was similar to that in NIH 3T3-d (containing _gfp_-IRES-bcr-abl) but slightly lower than that in NIH 3T3-c (containing _bcr-abl_-IRES-gfp) (Fig. 2B, lanes 2, 3, and 4). The effect of coexpressing ICSBP on expression of Bcr-Abl was not specific since the expression of a human placenta alkaline phosphatase also was decreased in NIH 3T3 cells infected with a retrovirus carrying _plap_-IRES-icsbp (data not shown). As shown below, MSCV-_gfp_-IRES-bcr-abl still induced a CML-like disease in mice similar to that induced by MSCV-_bcr-abl_-IRES-gfp (see Fig. 4). The Western blot analysis also shows that ICSBP was expressed in NIH 3T3-e only, as expected (Fig. 2B, lane 2, bottom panel).
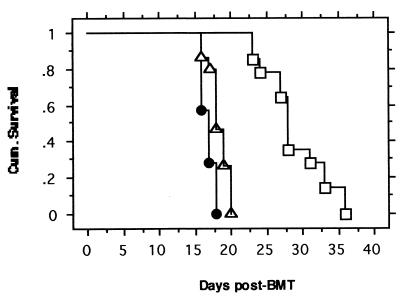
FIG. 4.
Survival of mice receiving transplantation of 5-FU BM cells infected with retroviruses containing various genes bcr-abl+gfp (●), bcr-abl+icsbp (□), and gfp+bcr-abl (▵). Curves were generated by Kaplan-Meier survival analysis. The number of mice used was as follows; 7 for Bcr-Abl+GFP-BMT, 14 for Bcr-Abl+ICSBP-BMT, and 15 for GFP+Bcr-Abl-BMT. A Mantel-Cox (log rank) test of survival between Bcr-Abl+GFP-BMT mice and GFP+Bcr-Abl-BMT mice yielded P = 0.0059, while the test of survival between Bcr-Abl+ICSBP-BMT mice and both Bcr-Abl+GFP-BMT mice and GFP+Bcr-Abl-BMT mice yielded P < 0.0001.
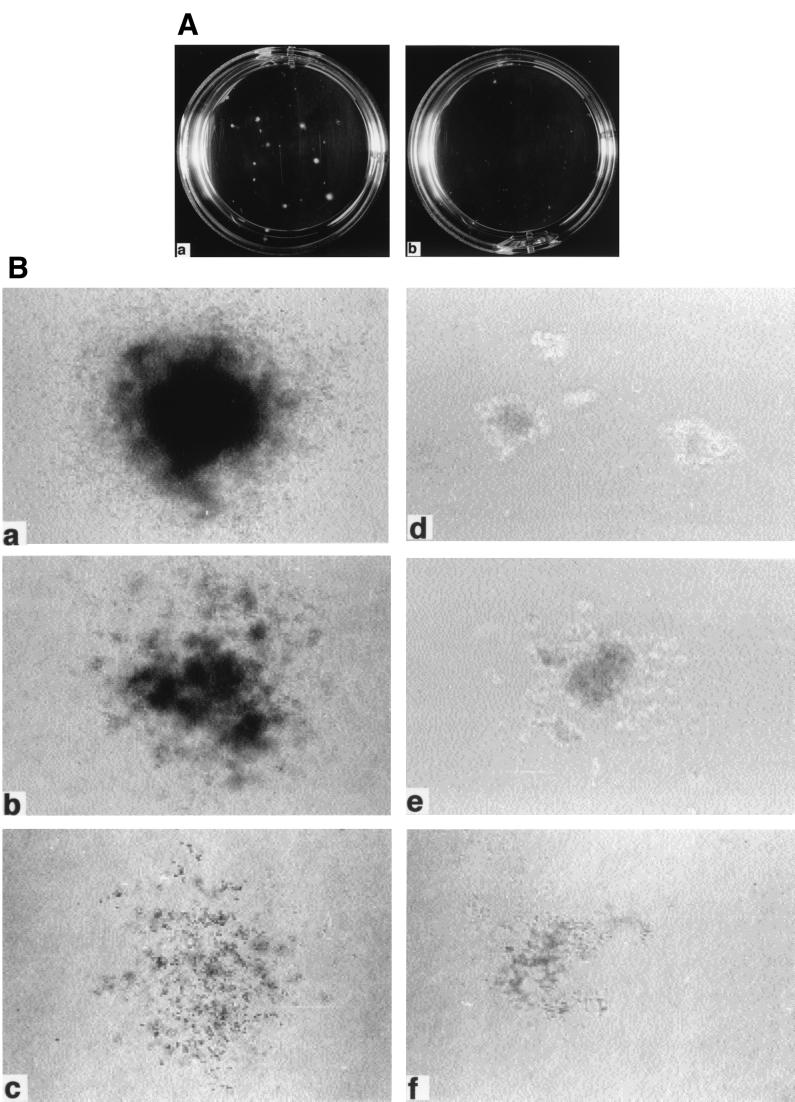
We then compared the abilities of the titer-matched retrovirus-e and retrovirus-d to stimulate colony formation of 5-FU BM cells. The 5-FU BM cells were infected twice in 2 days with the retroviruses by incubating the cells in retrovirus-containing medium in the presence of stem cell factor, IL-3, and IL-6, as described previously (45), and then plated into semisolid cultures in the absence of added cytokines, as described previously (20). While no colonies formed in the control uninfected and _gfp_-infected cultures in a 2-week observation period (data not shown), colonies of various sizes were observed in _bcr-abl_-containing retroviruses-infected cultures within 1 week. Interestingly, both the number and size of the colonies formed in the _bcr-abl_-IRES-_icsbp_-infected cultures (Fig. 3A panel b and Fig. 3B panels d to f) were significantly reduced compared to those in the _gfp_-IRES-_bcr-abl_-infected cultures (Fig. 3A panel a and Fig. 3B panels a, b, and c). The number of colonies formed in _gfp_-IRES-_bcr-abl_-infected cultures and _bcr-abl_-IRES-_icsbp_-infected cultures in the experiment shown in Fig. 3 were 32 ± 7 (mean ± standard deviation) and 11 ± 4, respectively (data collected from nine 35-mm wells for each virus). Microscopic examination showed that _gfp_-IRES-bcr-abl colonies and _bcr-abl_-IRES-icsbp colonies contained similar types of myeloid cells, including granulocytes, monocytes, and their precursors (data not shown), suggesting that proliferation rather than differentiation of Bcr-Abl-expressing bone marrow cells is inhibited by ICSBP.
FIG. 3.
Growth of soft agar colonies from infected 5-FU BM cells. (A) Soft agar cultures of 5-FU BM cells infected with MSCV-_gfp_-IRES-bcr-abl/p210 (a) or MSCV-_bcr-abl/p210_-IRES-icsbp (b) in 35-mm plates on day 8 postplating are shown. Large colonies can be seen in the _gfp+bcr-abl_-infected culture. (B) Microscopic view of the colonies. Three major types of colonies are shown in both _gfp+bcr-abl_-infected colonies (magnifications: ×40 [a], ×40 [b], and ×40 [c]) and _bcr-abl+icsbp_-infected colonies (magnifications: ×100 [d], ×100 [e], and ×40 [f]).
Coexpression of ICSBP with Bcr-Abl prolongs the life of the mice with Bcr-Abl-induced disease.
To examine the effect of ICSBP on Bcr-Abl-induced CML in mice, we transplanted the _bcr-abl_-IRES-_gfp_-, _gfp_-IRES-_bcr-abl_-, or _bcr-abl_-IRES-_icsbp_-infected 5-FU BM cells into lethally irradiated syngeneic recipient mice, as described previously (45). Both Bcr-Abl+GFP-BMT and GFP+Bcr-Abl-BMT mice developed a lethal myeloproliferative disease in approximately 3 weeks, while GFP-BMT and GFP+ICSBP-BMT mice showed no signs of disease in more than 3 months of observation (Fig. 4 and data not shown). The GFP+Bcr-Abl-BMT mice survived slightly and somewhat significantly (P = 0.0059) longer than did the Bcr-Abl+GFP-BMT mice, suggesting that the expression level of Bcr-Abl affects the rate of disease progression. Interestingly, mice transplanted with 5-FU BM cells infected with the retrovirus containing both bcr-abl and icsbp survived much longer than did both Bcr-Abl+GFP-BMT mice and GFP+Bcr-Abl-BMT mice (Fig. 4). The differences between the survival periods of the Bcr-Abl+ICSBP-BMT mice and both the Bcr-Abl+GFP-BMT and GFP+Bcr-Abl-BMT mice are highly significant (P < 0.0001). These results indicate that forced coexpression of ICSBP can prolong the life of mice with Bcr-Abl-induced disease.
Coexpression of ICSBP with Bcr-Abl inhibits the Bcr-Abl-induced myeloproliferative disorder but induces a transient B-lymphoproliferative disorder.
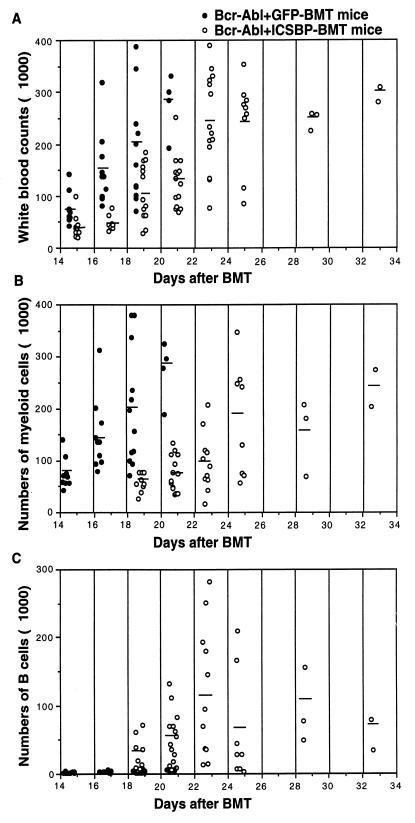
We further compared the diseases developed in Bcr-Abl+GFP-BMT mice, GFP+Bcr-Abl-BMT mice, and Bcr-Abl+ICSBP-BMT mice. Since Bcr-Abl+GFP-BMT mice and GFP+Bcr-Abl-BMT mice have an identical disease phenotype besides a slight difference in disease latency, here we describe only the comparison of disease phenotypes of Bcr-Abl+GFP-BMT mice and Bcr-Abl+ICSBP-BMT mice unless otherwise stated. The peripheral WBC count of both Bcr-Abl+GFP-BMT mice and Bcr-Abl+ICSBP-BMT mice was progressively elevated (Fig. 5A). The elevation of WBCs in the majority of Bcr-Abl+ICSBP-BMT mice was slightly delayed, and the average total number of WBCs of Bcr-Abl+ICSBP-BMT mice was generally lower than that of Bcr-Abl+GFP-BMT mice (Fig. 5A). Dramatic differences were found when the types of elevated blood cells in these two groups were analyzed. In diseased Bcr-Abl+GFP-BMT mice, over 90% of the peripheral WBCs were myeloid cells (Mac-1+) (Fig. 6A and B), with granulocyte predominance (data not shown). In these mice, about half of the Mac-1+ cells were GFP positive, a typical phenomenon of the expansion of both _bcr-abl_-infected and bystander myeloid cells in the Bcr-Abl+GFP-BMT mice (45). No significant B-lymphoid cell (CD-19+) expansion was observed in Bcr-Abl+GFP-BMT mice.
FIG. 5.
Total and differential WBC counts of Bcr-Abl+GFP-BMT mice and Bcr-Abl+ICSBP-BMT mice during disease development. Total WBCs (A), myeloid cells (B), and B-lymphoid cells (C) in the peripheral blood of Bcr-Abl+GFP-BMT mice and Bcr-Abl+ICSBP-BMT mice were counted every other day since 2 weeks after BMT. The differential WBCs were counted on peripheral blood smears under the microscope. The quality of the counts were confirmed by immunophenotyping samples of blood with flow cytometry.
In contrast, the percentage of myeloid cells in Bcr-Abl+ICSBP-BMT mice was significantly reduced (compare Fig. 6C and D with Fig. 6A and B). A dramatic delay and reduced increase of the total number of myeloid cells in Bcr-Abl+ICSBP-BMT mice were observed (Fig. 5B). Correlating with this delayed myeloproliferative disorder, a proliferative disorder of the B-lymphoid lineage was observed in Bcr-Abl+ICSBP-BMT mice (Fig. 5C and 6C and D). Similar to Bcr-Abl+GFP-BMT mice (data not shown), few Thy-1+ cells (including T lymphocytes and early hematopoietic progenitors) and Ter119+ cells (erythrocytes) were detected in Bcr-Abl+ICSBP-BMT mice (Fig. 6C and D). The level of B-lymphoid cell expansion varied among Bcr-Abl+ICSBP-BMT mice. For example, in one of the experiments (Table 1, experiment 2), at their highest level, 5 out of 14 Bcr-Abl+ICSBP-BMT mice accumulated over 80% B lymphoid cells, 6 mice accumulated 30 to 80%, and 3 mice accumulated 10 to 30%. The average B-lymphoid cell expansion peaked around 3 to 4 weeks after BMT and then declined.
TABLE 1.
Summary of disease phenotypes in Bcr-Abl+GFP-BMT and Bcr-Abl+ICSBP-BMT mice
| Mouse | No. of mice with myeloproliferative disease/total no. (%) | No. of mice with myelo- and lymphoproliferative disease/total no. (%) | ||
|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 1 | Expt 2 | |
| Bcr-Abl+GFP | 11/11 (100%) | 7/7 (100%) | 0/11 (0%) | 0/7 (0%) |
| GFP+Bcr-Abl | NAa | 15/15 (100%) | NA | 0/15 (0%) |
| Bcr-Abl+ICSBP | 3/11 (27.3%) | 0/14 (0%) | 8/11 (72.7%) | 14/14 (100%) |
Since no GFP marker was available in _bcr-abl_-IRES-_icsbp_-infected cells, intracellular immunofluorescent staining of the overexpressed Bcr-Abl was used to identify the _bcr-abl_-IRES-_icsbp_-infected hematopoietic cells from Bcr-Abl+ICSBP-BMT mice. The intracellular immunofluorescent staining was shown to be able to detect Bcr-Abl-expressing hematopoietic cells from Bcr-Abl+GFP-BMT mice, since the proportion of Bcr-Abl-positive and -negative cells determined by the intracellular immunofluorescent staining method was the same as that of GFP-positive and -negative cells (Fig. 6A and B). Using this method, we found that both Bcr-Abl-positive and Bcr-Abl-negative B-lymphoid cells were present in the peripheral blood of Bcr-Abl+ICSBP-BMT mice. Surprisingly, the majority of Mac-1+ cells in Bcr-Abl+ICSBP-BMT mice were Bcr-Abl negative (Fig. 6C and D). These results indicate that as a result of forced coexpression of ICSBP, Bcr-Abl-induced myeloproliferative disorder is suppressed while a B-lymphoproliferative disorder appears. The results also suggest that the Bcr-Abl-expressing cells may stimulate the expansion of bystander B lymphoid cells and myeloid cells, probably by overproducing hematopoietic growth factors. Overproduction of IL-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF) by the _bcr-abl_-infected hematopoietic cells in mice with Bcr-Abl-induced CML-like disease has been found previously (45).
However, coexpression of ICSBP did not permanently suppress the Bcr-Abl-induced myeloproliferative disorder. A myeloproliferative disorder characterized by both Bcr-Abl-expressing and non-Bcr-Abl-expressing myeloid cells (data not shown) gradually became predominant as the number of B-lymphoid cells decreased (Fig. 5B and C). The majority of Bcr-Abl+ICSBP-BMT mice eventually died of the myeloproliferative disease. In general, the percentages of B-lymphoid cell expansion inversely correlated with the aggressiveness of the myeloid cell expansion and disease progression. The numbers of Bcr-Abl+GFP-BMT mice, GFP+Bcr-Abl-BMT mice, and Bcr-Abl+ICSBP-BMT mice that suffered from myeloproliferative disorder (>90% myeloid cells with granulocyte predominance and <5% B-lymphoid cells) or mixed myeloproliferative and lymphoproliferative disorder (high WBC count with >10% B-lymphoid cells) in two separate experiments are summarized in Table 1.
Differences between the disease in Bcr-Abl+ICSBP-BMT mice and in Bcr-Abl+GFP-BMT mice also were revealed pathologically. Hepatosplenomegaly and pulmonary hemorrhages were common pathological findings in Bcr-Abl+GFP-BMT mice, as previously described (45). The three Bcr-Abl+ICSBP-BMT mice that had lower B-lymphoid cell expansion and died earlier had similar lesions to the Bcr-Abl+GFP-BMT mice. For the Bcr-Abl+ICSBP-BMT mice that died later, pulmonary hemorrhages were usually not obvious (data not shown). Hepatosplenomegaly in Bcr-Abl+ICSBP-BMT mice was also not as severe as that in Bcr-Abl+GFP-BMT mice.
In summary, the disease in the majority of Bcr-Abl+ICSBP-BMT mice has two phases. The first phase is characterized by an increased proliferation of infected B-lymphoid cells (Fig. 6C and D). The numbers of bystander myeloid cells also are expanded in this phase (Fig. 6C and D). A delayed myeloproliferative disorder, containing both infected and noninfected myeloid cells, becomes predominant in the second phase (Fig. 5 and data not shown). In this late phase, B lymphoproliferation decreases or disappears. Most Bcr-Abl+ICSBP-BMT mice die of the myeloproliferative disease.
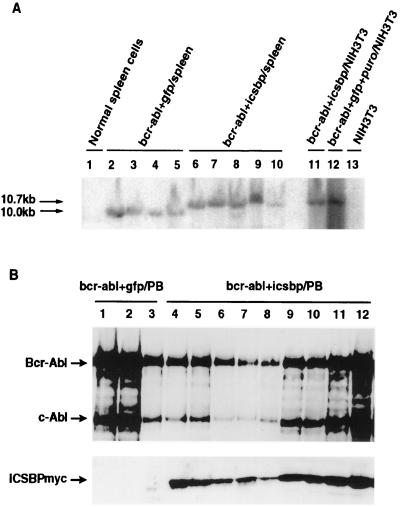
To demonstrate that tumor cells contain an intact MSCV-_bcr-abl_-IRES-gfp or MSCV-_bcr-abl_-IRES-icsbp provirus, genomic DNA isolated from peripheral WBCs was digested with _Xba_I and subjected to Southern blot analysis with an abl probe (Fig. 7A). All tumor cells from Bcr-Abl+GFP-BMT mice contained a single 10-kb band, while all tumor cells from Bcr-Abl+ICSBP-BMT mice contained a single 10.7-kb band, corresponding to the intact MSCV-_bcr-abl_-IRES-gfp and MSCV-_bcr-abl_-IRES-icsbp proviruses, respectively, as expected. Expression of the ICSBP and/or Bcr-Abl proteins in peripheral WBCs of the diseased Bcr-Abl+ICSBP-BMT and Bcr-Abl+GFP-BMT mice was detected by immunoblotting with anti-ICSBP and anti-Abl antibodies (Fig. 7B). The expression levels of Bcr-Abl in tumor cells from diseased Bcr-Abl+ICSBP-BMT and Bcr-Abl+GFP-BMT mice are comparable. The results shown above demonstrate that forced coexpression of ICSBP specifically inhibits the development of Bcr-Abl-induced myeloproliferative disease in mice, although it cannot completely block development of the disease.
FIG. 7.
(A) Analysis of MSCV-_bcr-abl/p210_-IRES-gfp and MSCV-_bcr-abl/p210_-IRES-icsbp provirus in diseased mice. Genomic DNAs isolated from splenocytes of Bcr-Abl+GFP-BMT and Bcr-Abl+ICSBP-BMT mice as well as _bcr-abl/p210_-IRES-_icsbp_- and _bcr-abl/p210_-IRES-_gfp_-SV40-_puro_-infected NIH 3T3 cells were digested with _Xba_I and subjected to Southern blot analysis with a 32P-labeled 1.2-kb _Sgr_I-_Bgl_II fragment from the 3′ end of the human c-abl cDNA as a probe. Only one band was detected in each sample. A ∼10-kb band was found in genomic DNA from Bcr-Abl+GFP-BMT mice and a ∼10.7-kb band was found in genomic DNA from Bcr-Abl+ICSBP-BMT mice and NIH 3T3 cells infected by MSCV-_bcr-abl/p210_-IRES-icsbp and MSCV-_bcr-abl/p210_-IRES-_gfp_-SV40-puro retroviruses. (B) Expression of Bcr-Abl and ICSBP in peripheral blood WBCs of the diseased Bcr-Abl+GFP-BMT mice (lanes 1 to 3) and Bcr-Abl+ICSBP-BMT mice (lanes 4 to 12). Overexpression of ICSBPmyc was detected only in Bcr-Abl+ICSBP-BMT mice (lanes 4 to 12). Expression of the endogenous ICSBP was not detected under the conditions used for this experiment in most samples.
The myeloproliferative disorder in both Bcr-Abl+GFP-BMT and Bcr-Abl+ICSBP-BMT mice can be transplanted to secondary recipient mice.
It has been shown that Bcr-Abl-induced CML-like disease can be transplanted to secondary recipient mice (45). To examine whether ICSBP affects the long-term repopulating ability of Bcr-Abl-induced myeloproliferative disorder, we transferred BM cells from two Bcr-Abl+GFP-BMT mice on day 20 after BMT and six Bcr-Abl+ICSBP-BMT mice, four of them on day 23 and two on day 38 after BMT to sets of three to five sublethally irradiated recipient mice. The secondary recipients of cells from one of the two primary Bcr-Abl+GFP-BMT mice developed a myeloproliferative disease and died 6 weeks after transplantation (data not shown). Secondary recipients of cells from two out of six primary Bcr-Abl+ICSBP-BMT mice developed CML-like disease. One of the two groups of the secondary recipient mice (a total of three, transferred on day 38 after BMT) died between 6 and 8 weeks. Three of four secondary recipient mice in the other group (transferred on day 23 after BMT, when the primary Bcr-Abl-ICSBP-BMT mouse had a mixed myeloproliferative and lymphoproliferative disorder) died between 57 and 75 days after transplantation. The fourth secondary recipient mouse in this group developed a myeloproliferative disorder initially but went into remission later and lived without obvious disease for 4 months of observation (data not shown). These results indicate that ICSBP also inhibits but cannot completely block the long-term repopulating myeloproliferative disease induced by Bcr-Abl.
Overexpression of ICSBP does not inhibit Bcr-Abl-induced B lymphoproliferation.
The observation that coexpression of ICSBP with Bcr-Abl induced a transient B-lymphoproliferative disorder while it inhibited the Bcr-Abl-induced myeloproliferative disease raised questions about the function of ICSBP in B lymphoproliferation. Bcr-Abl can induce both myeloproliferative disease and lymphoid leukemia in mice, depending on the cells into which bcr-abl is targeted (5, 9, 15, 17–19, 22, 28, 41, 45). In the murine bone marrow (from 5-FU-treated donors) transduction-transplantation model used above, the bcr-abl oncogene is targeted into multipotential hematopoietic stem/progenitor cells. Although _bcr-abl_-positive B-lymphoid cells are produced in Bcr-Abl+GFP-BMT mice, the wild-type Bcr-Abl induces only myeloproliferative disease. One possible reason why the myeloid lineage but not lymphoid lineage was massively expanded in Bcr-Abl+GFP-BMT mice is that the expansion of myeloid cells may suppress or compete with the expansion of lymphoid cells. Therefore, a possible reason why Bcr-Abl+ICSBP induces the proliferative disorder of B-lymphoid cells is that a strong inhibition of Bcr-Abl-induced myeloproliferation by coexpression of ICSBP temporarily releases B-lymphoid cell proliferation from the suppression and/or competition by myeloid cells. Alternatively, ICSBP may cooperate with Bcr-Abl to promote the proliferation of B-lymphoid cells.
To directly address the effect of ICSBP on Bcr-Abl-induced B-lymphoproliferation, we transplanted _bcr-abl_-IRES-_gfp_- or _bcr-abl_-IRES-_icsbp_-infected BM cells from non-5-FU-treated donors into lethally irradiated syngeneic recipient mice as described previously (22). Different from mice receiving _bcr-abl_-transduced 5-FU BM cells, mice receiving _bcr-abl_-transduced non-5-FU BM cells developed several distinct hematopoietic neoplasms. (i) Of 10 Bcr-Abl+GFP-BMT mice, 3 developed a myeloproliferative disease with the same characteristics as mice receiving _bcr-abl_-transduced 5-FU BM cells, including markedly elevated WBC with granulocyte predominance, hepatosplenomegaly, and pulmonary hemorrhage. These mice died within 3 weeks after BMT. (ii) One of the others developed a mixed myeloproliferative disorder and B-lymphoblastic leukemia and died within 4 weeks after BMT. (iii) One other Bcr-Abl+GFP-BMT mouse developed monocyte/macrophage tumors. (iv) Three Bcr-Abl+GFP-BMT mice developed B-lymphoid malignancy. One of the three manifest B lymphoblastic leukemia/lymphoma characterized by a modestly high WBC count, modest splenomegaly, lymphadenopathy with infiltration of B lymphoblasts, meningeal tumor as described for v-Abl-induced lymphosarcoma (1), and a bloody pleural effusion containing large number of GFP-positive B lymphoblasts. The other two mice with B lymphoblastic malignancy exhibited large lymphomas or hindlimb paralysis, possibly due to infiltration of lymphoblasts into the central nervous system. The B lymphoblasts in all these mice were shown to be in the early B-cell developmental stage previously defined as fraction B and/or C (B220+/CD43+/HSA+/BP-1+/−/sIgM−). The other two Bcr-Abl+GFP-BMT mice died before a diagnosis could be made.
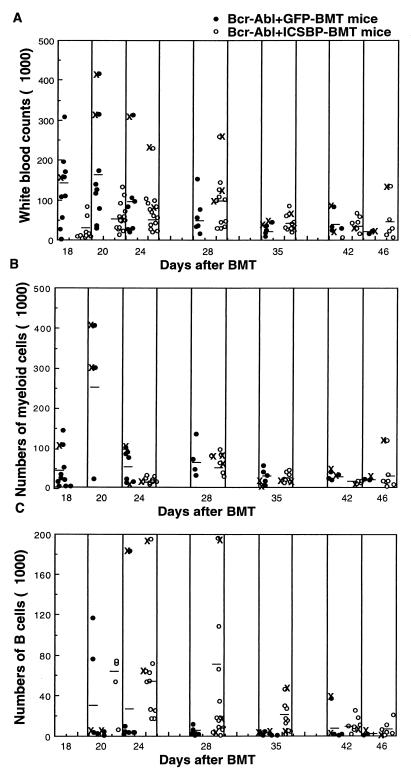
Of the 14 mice receiving _bcr-abl+icsbp_-transduced non-5-FU BM cells, only 2 developed myeloproliferative disease with lower WBC counts (Fig. 8) and an extended latency (more than 4 weeks). The majority of the Bcr-Abl+ICSBP-BMT mice did develop pre-B leukemia or lymphosarcoma as described above. Figure 8 shows a comparison of the WBC count, the number of myeloid cells (Mac-1 positive), and the number of B-lymphoid cells (CD-19 positive) in peripheral blood from Bcr-Abl+GFP-BMT mice and Bcr-Abl+ICSBP-BMT mice. In Bcr-Abl+ICSBP-BMT mice, myeloid cells were not expanded to as high a level as in a few Bcr-Abl+GFP-BMT mice while B lymphoid cells were generally expanded more than that in Bcr-Abl+GFP-BMT mice. These results indicate that overexpression of ICSBP inhibits Bcr-Abl-induced myeloproliferative disease but mostly promotes rather than inhibits the Bcr-Abl-induced B lymphoproliferation (Fig. 8).
FIG. 8.
Total and differential WBC counts of mice receiving MSCV-_bcr-abl/p210_-IRES-_gfp_- and MSCV-_bcr-abl/p210_-IRES-_icsbp_-transduced non-5-FU BM cells during disease development. Total WBCs (A), myeloid cells (B), and B-lymphoid cells (C) in the peripheral blood of Bcr-Abl+GFP-BMT mice (solid circles) and Bcr-Abl+ICSBP-BMT mice (open circles) were counted since 18 days after BMT. The differential WBCs were calculated by multiplying the total WBC counts by the percentages of Mac-1+ and CD19+ cells determined by flow cytometric analysis. Results for mice that died or were sacrificed due to moribund condition on the day of analysis or before the next analysis are labeled ×.
Overexpression of ICSBP inhibits reconstitution of hematopoietic cells in irradiated recipient mice.
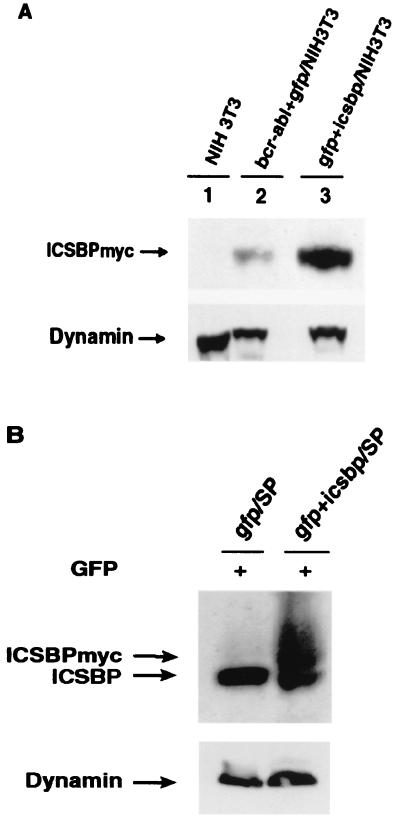
We have shown that overexpression of ICSBP significantly inhibits Bcr-Abl-induced myeloproliferative disorder but enhances the proliferation of Bcr-Abl-expressing B-lymphoid cells. We wondered whether ICSBP functions in a similar way in normal hematopoietic cells. To address this question, we compared the effects of GFP and ICSBP on reconstitution of hematopoietic cells in irradiated recipient mice. We noted that the expression level of ICSBP in _gfp_-IRES-_icsbp_-infected NIH 3T3 cells was about four times as high as that in the _bcr-abl_-IRES-_icsbp_-infected NIH 3T3 cells (Fig. 9A). The lower expression of ICSBP protein in _bcr-abl_-IRES-icsbp infected NIH 3T3 cells is possibly due to a stronger interference of ICSBP translation by bcr-abl sequences. In the bone marrow reconstitution experiment, titer-matched retroviruses carrying gfp or _gfp_-IRES-icsbp genes were used to infect 5-FU BM cells for 2 days and the infected BM cells were then transplanted into lethally irradiated mice. To demonstrate expression of the introduced ICSBP in vivo, we sorted GFP+ cells from the spleens of GFP-BMT mice and GFP+ICSBP-BMT mice at 9 weeks after BMT. Expression of the exogenous ICSBPmyc and the endogenous ICSBP in these sorted cells was examined by immunoblotting with an anti-ICSBP polyclonal antibody. As shown in Fig. 9B, GFP+ cells isolated from GFP+ICSBP-BMT mice do express ICSBPmyc.
FIG. 9.
(A) Expression of ICSBPmyc in _bcr-abl+icsbp_-infected NIH 3T3 cells (lane 2) and _gfp+icsbp_-infected NIH 3T3 cells (lane 3) as examined by immunoblotting with the polyclonal anti-ICSBP antibody. NIH 3T3 cells (lane 1) were used as a negative control. Expression of dynamin was used as an internal loading control. (B) Expression of ICSBPmyc and/or the endogenous ICSBP in the sorted GFP+ splenocytes from GFP-BMT mouse (lane 1) and GFP+ICSBP-BMT mouse (lane 2) at 9 weeks after BMT.
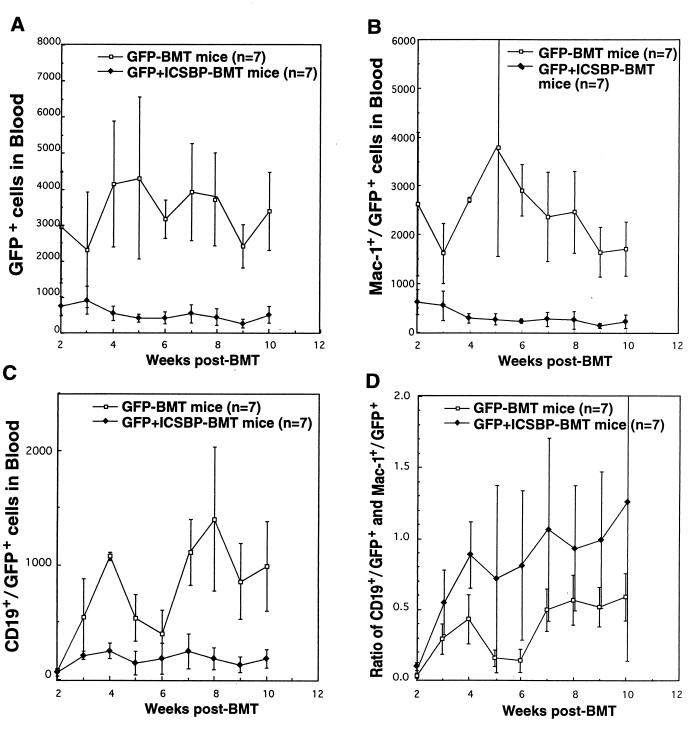
To reveal the effect of overexpression of ICSBP on bone marrow reconstitution, we examined the number of WBCs and GFP+ WBCs of various lineages in peripheral blood from GFP-BMT mice and GFP+ICSBP-BMT mice weekly from 2 to 10 weeks after BMT by flow cytometric analysis (Fig. 10). The total number of WBCs and the percentages of myeloid and B-lymphoid cells were comparable between the two groups of mice (data not shown). However, the number of total GFP+ WBCs in the peripheral blood of GFP+ICSBP-BMT mice was significantly smaller than that in GFP-BMT mice at all time points (Fig. 10A), indicating that overexpression of ICSBP inhibits reconstitution of the targeted BM cells. The levels of GFP+ myeloid cells were significantly lower in GFP+ICSBP-BMT mice beginning 2 weeks after BMT (Fig. 10B). The numbers of GFP+ B-lymphoid cells in GFP-BMT mice were similar to those of GFP+ICSBP-BMT mice at 2 weeks after BMT (Fig. 10C). However, after 3 weeks, GFP+ B-lymphoid cell levels rose rapidly in GFP-BMT mice while the level of GFP+ B-lymphoid cells stayed low in GFP+ICSBP-BMT mice. Although overexpression of ICSBP inhibited the growth of both myeloid and B-lymphoid cells, it appears that ICSBP inhibited the growth of myeloid cells more than it did the growth of B-lymphoid cells, since the ratio of B lymphoid cells to myeloid cells in GFP+ICSBP-BMT mice was higher than that in GFP-BMT mice (Fig. 10D). These results demonstrate that overexpression of ICSBP inhibits proliferation of normal hematopoietic cells.
FIG. 10.
Effect of ICSBP on the reconstitution of hematopoietic cells in irradiated recipient mice. (A) Total number of GFP+ cells in GFP+ICSBP-BMT mice versus GFP-BMT mice. (B) The number of GFP+ myeloid cells in GFP+ICSBP-BMT mice versus GFP-BMT mice. (C) GFP+ B-lymphoid cells in GFP+ICSBP-BMT mice versus GFP-BMT mice. (D) The ratio of GFP+ B lymphoid cells and myeloid cells in GFP+ICSBP-BMT mice versus GFP-BMT mice. The number of mice (n) used in the experiment for each retrovirus construct is indicated.
DISCUSSION
This study shows that expression of the ICSBP protein was downregulated in Bcr-Abl-induced murine CML-like myeloproliferative disease (Fig. 1) and that forced coexpression of ICSBP inhibited Bcr-Abl-stimulated colony formation of 5-FU BM cells in vitro (Fig. 3) and Bcr-Abl-induced CML-like myeloproliferative disease in vivo (Fig. 4 to 6). These findings provide direct evidence that ICSBP can act as a tumor suppressor for Bcr-Abl-induced CML-like disease. Interestingly, the inhibitory effect of ICSBP on Bcr-Abl-stimulated cell growth was specific to the myeloid lineage. In mice receiving _bcr-abl+icsbp_-transduced 5-FU BM cells, coexpression of ICSBP and Bcr-Abl induced a transient B-lymphoproliferative disorder (Fig. 5 and 6). Using the non-5-FU BM cell transduction/transplantation model, we showed that ICSBP did not inhibit, and actually promoted, the development of Bcr-Abl-induced B-lymphoid neoplasms (Fig. 8). These results indicate that ICSBP has a specific antitumor activity toward myeloid neoplasms and that the inhibitory effect of ICSBP on Bcr-Abl-induced myeloproliferative disorders is not due to a nonspecific cytostatic or cytotoxic effect of overexpression of this transcription factor.
One possible explanation of the differential antiproliferative effect of ICSBP on Bcr-Abl-stimulated growth of myeloid and lymphoid cells is that ICSBP may inhibit Bcr-Abl activated signaling pathway(s) required for inducing myeloproliferative but not lymphoproliferative disease. Indeed, we have found that certain mutations in Bcr-Abl inhibits its potential to induce myeloid but not lymphoid neoplasms (12). Alternatively, the differential antiproliferative activity of ICSBP in myeloid versus lymphoid cells may reflect the normal function of ICSBP. Consistent with this idea, ICSBP is expressed constitutively throughout B-cell development and in both resting and activated cells under physiological conditions (25), suggesting that ICSBP at least does not inhibit the proliferation of B-lymphoid cells. In addition, in ICSBP-deficient mice, proliferation of myeloid cells is markedly enhanced while proliferation of lymphoid cells is moderately increased (16).
As opposed to its effect on Bcr-Abl-stimulated cell growth, overexpression of ICSBP markedly inhibited reconstitution of both myeloid and, to a lesser extent, lymphoid cells in recipient mice (Fig. 10). This result is consistent with the finding that proliferation of multiple hematopoietic lineages is enhanced in ICSBP-deficient mice (16). The effect of ICSBP on normal hematopoiesis may be due to a negative regulation of the proliferation of early hematopoietic progenitors by ICSBP (16). Together, our results and published data suggest that the effect of ICSBP on cell proliferation is cellular context dependent. ICSBP may exert an antiproliferation activity in early hematopoietic progenitor cells and myeloid cells, while it may promote proliferation of B-lymphoid cells. It may exert its growth-promoting function in B-lymphoid cells by regulating the expression of proteins in cell growth signaling pathways or by regulating cytokine production.
We have previously shown that both _bcr-abl_-infected and noninfected myeloid cells can be expanded in mice with Bcr-Abl-induced myeloproliferative disorder and that the _bcr-abl_-infected cells express excess IL-3 and GM-CSF (45). In this study, the expansion of noninfected myeloid cells was found in Bcr-Abl+ICSBP-BMT mice during both B-lymphoproliferative disorder and myeloproliferative disorder stages (Fig. 6). This result indicates that both Bcr-Abl-expressing myeloid cells and lymphoid cells can produce excess cytokines that stimulate hematopoiesis and that overexpression of ICSBP does not inhibit this.
Although overexpression of ICSBP prolonged the lives of mice with Bcr-Abl-induced disease, it did not permanently suppress the Bcr-Abl-induced CML-like disease. This effect of ICSBP on the Bcr-Abl-induced CML-like disease resembles the effect of IFN-α on human CML, where IFN-α effectively inhibits the development of CML and prolongs the lives of CML patients but does not cure the disease. This suggests that IFN-α and ICSBP may effectively inhibit the proliferation of CML progenitors but do not effectively eliminate these cells.
The mechanism by which ICSBP exerts its antiproliferative activity in hematopoietic cells is not known. One possible mechanism is that ICSBP functions by regulating the activity of its interacting proteins. It was shown that ICSBP can form a complex with PU.1 (7). Since ICSBP does not contain a transcriptional activation domain, expression of excess ICSBP may inhibit PU.1 function, which is required for the development of myeloid and lymphoid lineages (reviewed in reference 10). It also might enhance the function of IRF-1, a tumor suppressor gene (reviewed in reference 13). Another way in which ICSBP may inhibit hematopoiesis is to directly regulate the expression of growth control genes. One of the target genes of ICSBP is IL-12, which plays an important role in IFN-γ induction (11, 16, 36, 44). However, IL-12-deficient mice have no obvious developmental abnormalities (23).
It has been shown that IFN-α inhibits the expression of stimulatory cytokines (e.g. GM-CSF, G-CSF, IL-1, and IL-11) and induces the expression of negative regulators (e.g., IL-1RA and MIP-1α) in the hematopoietic environment. It also restores adhesion of CML progenitors to bone marrow stroma and enhances Fas receptor expression on CML progenitor cells (2, 30, 38). ICSBP may be involved in mediating some of these functions of IFN-α. It may also suppress tumor by exerting its function in regulating immune responses. However, since a transient proliferative disorder of B-lymphoid cells is not associated with the IFN-α treatment of human CML, other IRFs may also play a role in mediating the antitumor activity of IFN-α. In any event, the experimental systems we presented here will help us to study the mechanism by which ICSBP regulates the normal and neoplastic proliferation of hematopoietic cells. Our studies also suggest that up-regulating ICSBP expression may have therapeutic values for treating CML.
ACKNOWLEDGMENTS
We thank K. Ozato for kindly providing the human icsbp cDNA, and we thank X. Zhang and B. Wang for technical assistance.
This work was supported by National Cancer Institute grant CA68008 (to R.R.). R.R. is a recipient of Leukemia Society of America Scholar Award.
REFERENCES
- 1.Abelson H T, Rabstein L S. Lymphosarcoma: virus-induced thymic-independent disease in mice. Cancer Res. 1970;30:2213–2222. [PubMed] [Google Scholar]
- 2.Bhatia R, Wayner E A, McGlave P B, Verfaillie C M. Interferon-α restores normal adhesion of chronic myelogenous leukemia hematopoietic progenitors to bone marrow stroma by correcting impaired β1 integrin receptor function. J Clin Investig. 1994;94:384–391. doi: 10.1172/JCI117333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bovolenta C, Driggers P H, Marks M S, Medin J A, Politis A D, Vogel S N, Levy D E, Sakaguchi K, Appella E, Coligan J E, et al. Molecular interactions between interferon consensus sequence binding protein and members of the interferon regulatory factor family. Proc Natl Acad Sci USA. 1994;91:5046–5050. doi: 10.1073/pnas.91.11.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1996. [Google Scholar]
- 5.Daley G Q, Van Etten R A, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210 bcr/abl gene of the philadelphia chromosome. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 6.Driggers P H, Ennist D L, Gleason S L, Mak W H, Marks M S, Levi B Z, Flanagan J R, Appella E, Ozato K. An interferon gamma-regulated protein that binds the interferon- inducible enhancer element of major histocompatibility complex class I genes. Proc Natl Acad Sci USA. 1990;87:3743–3747. doi: 10.1073/pnas.87.10.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenbeis C F, Singh H, Storb U. Pip, a novel IRF family member, is a lymphoid-specific, PU.1-dependent transcriptional activator. Genes Dev. 1995;9:1377–1387. doi: 10.1101/gad.9.11.1377. [DOI] [PubMed] [Google Scholar]
- 8.Eklund E A, Jalava A, Kakar R. PU.1, interferon regulatory factor 1, and interferon consensus sequence- binding protein cooperate to increase gp91(phox) expression. J Biol Chem. 1998;273:13957–13965. doi: 10.1074/jbc.273.22.13957. [DOI] [PubMed] [Google Scholar]
- 9.Elefanty A G, Hariharan I K, Cory S. bcr-abl, the hallmark of chronic myeloid leukemia in man, induces multiple hematopoietic neoplasms in mice. EMBO J. 1990;9:1069–1078. doi: 10.1002/j.1460-2075.1990.tb08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher R C, Scott E W. Role of PU.1 in hematopoiesis. Stem Cells. 1998;16:25–37. doi: 10.1002/stem.160025. [DOI] [PubMed] [Google Scholar]
- 11.Giese N A, Gabriele L, Doherty T M, Klinman D M, Tadesse-Heath L, Contursi C, Epstein S L, Morse H C., III Interferon (IFN) consensus sequence-binding protein, a transcription factor of the IFN regulatory factor family, regulates immune responses in vivo through control of interleukin 12 expression. J Exp Med. 1997;186:1535–1546. doi: 10.1084/jem.186.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross A W, Zhang X, Ren R. Bcr-Abl with an SH3 deletion retains the ability to induce a myeloproliferative disease in mice, yet c-Abl activated by an SH3 deletion induces only lymphoid malignancy. Mol Cell Biol. 1999;19:6918–6928. doi: 10.1128/mcb.19.10.6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada H, Taniguchi T, Tanaka N. The role of interferon regulatory factors in the interferon system and cell growth control. Biochimie. 1998;80:641–650. doi: 10.1016/s0300-9084(99)80017-0. [DOI] [PubMed] [Google Scholar]
- 14.Hawley R G, Lieu F H L, Fong A Z C, Hawley T S. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1995;1:136–138. [PubMed] [Google Scholar]
- 15.Heisterkamp N, Jenster G, ten Hoeve J, Zovich D, Pattengale P K, Groffen J. Acute leukemia in bcr/abl transgenic mice. Nature. 1990;344:251–253. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- 16.Holtschke T, Lohler J, Kanno Y, Fehr T, Giese N, Rosenbauer F, Lou J, Knobeloch K P, Gabriele L, Waring J F, Bachmann M F, Zinkernagel R M, Morse III H C, Ozato K, Horak I. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell. 1996;87:307–317. doi: 10.1016/s0092-8674(00)81348-3. [DOI] [PubMed] [Google Scholar]
- 17.Honda H, Fujii T, Takatoku M, Mano H, Witte O N, Yazaki Y, Hirai H. Expression of p210bcr-abl by metallothionein promoter induced T-cell leukemia in transgenic mice. Blood. 1995;85:2853–2861. [PubMed] [Google Scholar]
- 18.Honda H, Oda H, Suzuki T, Takahashi T, Witte O N, Ozawa K, Ishikawa T, Yazaki Y, Hirai H. Development of acute lymphoblastic leukemia and myeloproliferative disorder in transgenic mice expressing p210bcr/abl: a novel transgenic model for human Ph1-positive leukemias. Blood. 1998;91:2067–2075. [PubMed] [Google Scholar]
- 19.Kelliher M A, McLaughlin J, Witte O N, Rosenberg N. Induction of a chronic myelogenous leukemia-like syndrome in mice with v-abl and BCR/ABL. Proc Natl Acad Sci USA. 1990;87:6649–6653. doi: 10.1073/pnas.87.17.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelliher M A, Weckstein D J, Knott A G, Wortis H H, Rosenberg N. ABL oncogenes directly stimulate two distinct target cells in bone marrow from 5-fluorouracil-treated mice. Oncogene. 1993;8:1249–1256. [PubMed] [Google Scholar]
- 21.Kurzrock R, Gutterman J, Talpaz M. The molecular genetics of Philadelphia chromosome-positive leukemias. N Engl J Med. 1988;319:990–998. doi: 10.1056/NEJM198810133191506. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Ilaria R L, Million R P, Daley G Q, Van Etten R A. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J Exp Med. 1999;189:1399–1412. doi: 10.1084/jem.189.9.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magram J, Sfarra J, Connaughton S, Faherty D, Warrier R, Carvajal D, Wu C Y, Stewart C, Sarmiento U, Gately M K. IL-12-deficient mice are defective but not devoid of type 1 cytokine responses. Ann N Y Acad Sci. 1996;795:60–70. doi: 10.1111/j.1749-6632.1996.tb52655.x. [DOI] [PubMed] [Google Scholar]
- 24.Melo J V. The diversity of Bcr-Abl fusion proteins and their relationship to leukemia phenotype. Blood. 1996;88:2375–2384. [PubMed] [Google Scholar]
- 25.Nelson N, Kanno Y, Hong C, Contursi C, Fujita T, Fowlkes B J, O'Connell E, Hu-Li J, Paul W E, Jankovic D, Sher A F, Coligan J E, Thornton A, Appella E, Yang Y, Ozato K. Expression of IFN regulatory factor family proteins in lymphocytes. Induction of Stat-1 and IFN consensus sequence binding protein expression by T cell activation. J Immunol. 1996;156:3711–3720. [PubMed] [Google Scholar]
- 26.Nelson N, Marks M S, Driggers P H, Ozato K. Interferon consensus sequence-binding protein, a member of the interferon regulatory factor family, suppresses interferon-induced gene transcription. Mol Cell Biol. 1993;13:588–599. doi: 10.1128/mcb.13.1.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen H, Hiscott J, Pitha P M. The growing family of interferon regulatory factors. Cytokine Growth Factor Rev. 1998;8:293–312. doi: 10.1016/s1359-6101(97)00019-1. [DOI] [PubMed] [Google Scholar]
- 28.Pear W S, Miller J P, Xu L, Pui J C, Soffer B, Quackenbush R C, Pendergast A M, Bronson R, Aster J C, Scott M L, Baltimore D. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 1998;92:3780–3792. [PubMed] [Google Scholar]
- 29.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peschel C, Aulitzky W E, Huber C. Influence of interferon-alpha on cytokine expression by the bone marrow microenvironment—impact on treatment of myeloproliferative disorders. Leuk Lymphoma. 1996;22(Suppl. 1):129–134. doi: 10.3109/10428199609074370. [DOI] [PubMed] [Google Scholar]
- 31.Pfeffer L M, Dinarello C A, Herberman R B, William B R G, Borden E C, Bordens R, Walter M R, Nagabhushan T L, Trotta P P, Pestka S. Biological properties of recombinant alpha-interferon: 40th anniversary of the discovery of interferons. Cancer Res. 1998;58:2489–2499. [PubMed] [Google Scholar]
- 32.Politis A D, Ozato K, Coligan J E, Vogel S N. Regulation of IFN-gamma-induced nuclear expression of IFN consensus sequence binding protein in murine peritoneal macrophages. J Immunol. 1994;152:2270–2278. [PubMed] [Google Scholar]
- 33.Politis A D, Sivo J, Driggers P H, Ozato K, Vogel S N. Modulation of interferon consensus sequence binding protein mRNA in murine peritoneal macrophages. Induction by IFN-gamma and down-regulation by IFN-alpha, dexamethasone, and protein kinase inhibitors. J Immunol. 1992;148:801–807. [PubMed] [Google Scholar]
- 34.Ren R, Ye Z, Baltimore D. Abl protein-tyrosine kinase selects the Crk adapter as a substrate using SH3-binding sites. Genes Dev. 1994;8:783–795. doi: 10.1101/gad.8.7.783. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg N, Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976;143:1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scharton-Kersten T, Contursi C, Masumi A, Sher A, Ozato K. Interferon consensus sequence binding protein-deficient mice display impaired resistance to intracellular infection due to a primary defect in interleukin 12 p40 induction. J Exp Med. 1997;186:1523–1534. doi: 10.1084/jem.186.9.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt M, Nagel S, Proba J, Thiede C, Ritter M, Waring J F, Rosenbauer F, Huhn D, Wittig B, Horak I, Neubauer A. Lack of interferon consensus sequence binding protein (ICSBP) transcripts in human myeloid leukemias. Blood. 1998;91:22–29. [PubMed] [Google Scholar]
- 38.Selleri C, Sato T, Del Vecchio L, Luciano L, Barrett A J, Rotoli B, Young N S, Maciejewski J P. Involvement of Fas-mediated apoptosis in the inhibitory effects of interferon-alpha in chronic myelogenous leukemia. Blood. 1997;89:957–964. [PubMed] [Google Scholar]
- 39.Sharf R, Meraro D, Azriel A, Thornton A M, Ozato K, Petricoin E F, Larner A C, Schaper F, Hauser H, Levi B Z. Phosphorylation events modulate the ability of interferon consensus sequence binding protein to interact with interferon regulatory factors and to bind DNA. J Biol Chem. 1997;272:9785–9792. doi: 10.1074/jbc.272.15.9785. [DOI] [PubMed] [Google Scholar]
- 40.Talpaz M. Use of interferons in the treatment of chronic myeloid leukemia. Semin Oncol. 1994;21(Suppl. 14):3–7. [PubMed] [Google Scholar]
- 41.Voncken J W, Kaartinen V, Pattengale P K, Germeraad W T, Groffen J, Heisterkamp N. BCR/ABL P210 and P190 cause distinct leukemia in transgenic mice. Blood. 1995;86:4603–4611. [PubMed] [Google Scholar]
- 42.Weisz A, Kirchhoff S, Levi B Z. IFN consensus sequence binding protein (ICSBP) is a conditional repressor of IFN inducible promoters. Int Immunol. 1994;6:1125–1131. doi: 10.1093/intimm/6.8.1125. [DOI] [PubMed] [Google Scholar]
- 43.Weisz A, Marx P, Sharf R, Appella E, Driggers P H, Ozato K, Levi B Z. Human interferon consensus sequence binding protein is a negative regulator of enhancer elements common to interferon-inducible genes. J Biol Chem. 1992;267:25589–25596. [PubMed] [Google Scholar]
- 44.Wu C Y, Maeda H, Contursi C, Ozato K, Seder R A. Differential requirement of IFN consensus sequence binding protein for the production of IL-12 and induction of Th1-type cells in response to IFN-gamma. J Immunol. 1999;162:807–812. [PubMed] [Google Scholar]
- 45.Zhang X, Ren R. Bcr-Abl efficiently induces a myeloproliferative disease and production of excess interleukin-3 and granulocyte-macrophage colony-stimulating factor in mice: a novel model for chronic myelogenous leukemia. Blood. 1998;92:3829–3940. [PubMed] [Google Scholar]