Glomerular endothelial cells and podocytes can express CD80 in patients with minimal change disease during relapse (original) (raw)
. Author manuscript; available in PMC: 2021 Oct 20.
Published in final edited form as: Pediatr Nephrol. 2020 May 12;35(10):1887–1896. doi: 10.1007/s00467-020-04541-3
Abstract
Background.
Urinary CD80 has emerged as potential biomarker in idiopathic nephrotic syndrome (INS). However, its cellular source remains controversial. The aim of the study was to assess whether CD80 is truly expressed by glomerular cells in INS patients during relapse and in the LPS mouse model of podocyte injury.
Methods.
The presence of CD80 in glomeruli was evaluated by combining immunohistochemistry, immunogold labeling and in situ hybridization techniques.
Results.
CD80 was present along the surface of glomerular endothelial cells (GEC) and rarely in podocytes in six of nine minimal change disease (MCD) patients in relapse, two of eleven patients with focal segmental glomerulosclerosis in relapse and absent in controls. In mice, CD80 was upregulated at mRNA and protein level in GEC and podocytes, in a similar pattern to that seen in MCD patients.
Conclusion.
Glomerular endothelial cells and podocytes can express CD80 in patients with MCD during relapse. A better understanding of the role of CD80 in glomerular cells may provide further insights into the mechanisms of proteinuria in INS.
Keywords: CD80, biomarker, nephrotic syndrome, glomerular endothelial cell, podocyte
Introduction
Idiopathic nephrotic syndrome (INS) refers to a heterogeneous group of glomerular disorders, including minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS), characterized by severe proteinuria and podocyte foot processes effacement [1]. While MCD is usually associated with favorable steroid response and long-term outcome, FSGS often leads to progressive chronic kidney disease (CKD) and/or end-stage renal disease (ESRD) [2]. Nevertheless, the clinical and histological distinction between these entities can be challenging in early stages. Currently, there are no validated biomarkers to help with diagnosis, predict outcomes or to individualize treatment.
The pathogenesis of these disorders remains elusive [2]. Immune dysregulation is thought to contribute to podocyte injury and proteinuria [3]. CD80 (also known as B7.1), a co-stimulatory molecule expressed by antigen presenting cells (APC), plays a key role in initiating and modulating the immune response by activating (when bound to CD28) or inactivating (when bound to CTLA-4) T cells. In vivo and in vitro models suggest that nontraditionally APCs, such as podocytes, express CD80 under certain circumstances and that CD80 may play a role in proteinuria [4–7], actin reorganization [4, 8–9] and podocyte migration [10]. CD80, thought to be shed into urine by “stimulated” podocytes, has also emerged as potential biomarker in INS [5, 11–18]. When measured in urine using ELISA, CD80 levels are consistently higher in patients with steroid sensitive nephrotic syndrome (SSNS) and/or MCD patients during relapse compared to those in remission or with FSGS [11–17]. Elevated urinary CD80 seems also associated to good long-term outcome and to favorable response to anti-CD80 therapy [18–20]. Despite the encouraging clinical data, the cellular source of urinary CD80 remains unclear. Using immunohistochemistry (IHC), some groups reported CD80 expression on podocytes as distinctive marker for MCD [12] or FSGS [10] patients whereas others could not replicate any of these observations [21–23]. Given the potential role of CD80 as non-invasive biomarker, the identification of its cellular source may provide further insights into the pathogenesis of INS.
In this study, we combined IHC, immunogold labeling, in situ hybridization and cell-specific RNA sequencing analysis, and identified glomerular endothelial cells (GEC) and podocytes as potential sources of urinary CD80 in a subset of MCD patients. Using the LPS model of podocyte injury, we also confirmed that CD80 is expressed in glomerular endothelial cells and podocytes. The role of glomerular CD80 warrants further investigation.
Methods
Human subjects
All patients were considered in relapse at time of the biopsy since they all had nephrotic range proteinuria and edema. Only two patients had a serum albumin equal or greater than 3.5 g/dl. The study was approved by the local Institutional Review Board (Vall D’Hebron Hospital, Barcelona, Spain) and informed consent was obtained from each participant. Healthy human kidney and tonsil tissue were obtained from The Tissue Core of the University of Michigan Comprehensive Cancer Center. This study was performed in accordance with the Declaration of Helsinki.
Reagents and antibodies
The following primary antibodies were used CD80 (AF740, R&D) at 1:100 dilution; CD80 (MAB140, clone 37711, R&D) at 1:20; caveolin 1 (D46G3, #3267, Cell Signaling) at 1:800, nephrin (provided by Verma R), ICAM-1 (AF796, R&D) at 1:1000, synaptopodin (10R-2373, Fitzgerald) at 1:20. Lipopolysaccharide from Escherichia Coli O111:B4 was obtained from Sigma-Aldrich.
Transient LPS-induced podocyte injury
Eight to twelve week-old B6 background mice were injected intraperitoneally with LPS (10 μg/g body weight in 200 μl PBS) or an equal volume of sterile PBS. Spot urine samples were collected prior to and 24 hours following intraperitoneal injection of LPS. Albuminuria was measured as per the manufacturer’s protocol using ELISA Albumin Kit (Bethyl Laboratories) and normalized to urine creatinine. All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Michigan.
Immunohistochemistry staining
Mouse kidney and spleen samples were fixed in 10% formalin and paraffin embedded. Tissues were sectioned at 3-μm, deparaffinised and rehydrated according to standard techniques. Antigen retrieval was performed with Tris-EDTA buffer at 96° C for 30 minutes and permeabilized with 0.1% Triton X-100 for 10 minutes. Slides were blocked with 1% Bovine Serum Albumin (BSA) for 1 hour followed by overnight incubation with the primary antibody. This was followed by three PBS washes and incubation with the Alexa Fluor secondary antibodies at 1:400 for 2 hours at room temperature. Paraffin-embedded human kidney and tonsil samples were processed in a similar fashion. Validation of anti-CD80 antibodies (human and mouse) is shown in Supplementary Figure 1. Silver immunostaining was performed according to standard techniques. Images were captured using a Leica Microscope.
Immunogold electron microscopy
Samples were embedded in London Resin (LR) and post embedding protocols were followed according to manufactures protocols (Electron Microscopy Sciences, Hatfield, PA). Briefly, specimens were mounted on 200 mesh Nickle grids, blocked using a combination of normal 5% goat (host) serum, 0.1% cold water fish-skin gelatin, and 1% BSA (Electron Microscopy Sciences, Hatfield, PA). Later incubated overnight with primary antibody (1:100 dilution) in 0.1%–0.2% Aurion BSA-c incubation Buffer (Electron Microscopy Sciences). After quick rinses, specimens were re-incubated with nanogold conjugated goat anti-rabbit (ultra-small, Electron Microscopy Sciences) diluted (1:50 dilution) in incubation buffer for 2–3 hours at room temperature processed for silver enhancement per manufacturer’s instructions (Electron Microscopy Sciences). Specimens were later contrast enhanced with Uranyl acetate and lead citrate solution based on standard protocols and analyzed using a JEM-1400Plus Transmission Electron Microscope at the Microscopy and Image Analysis Core Facility, University of Michigan, Ann Arbor, USA.
Transmission electron microscopy
Preparation of mouse kidneys for transmission electron microscopy was performed using standard methods. Kidneys were sectioned in small pieces and fixed with 2% paraformaldehyde and 2.5% glutaraldehyde. Tissues were processed at the Microscopy and Image Analysis Laboratory at the University of Michigan.
In situ hybridization
In situ RNA hybridization was performed using multiplex RNAscope technology (Advanced Cell Diagnostics, Hayward, CA). The following probes were used: CD80 (Cat No. 416741), podocin (Cat No. 507041), PPIB (positive control, Cat No. 313911) and ICAM-1 (Cat No. 320269). Fresh frozen samples were sectioned at 14 μm and processed according to the manufacturer’s protocol for the RNAscope Multiplex Fluorescent Assay, with RNAscope probes hybridized for 2 hours at 40°C. The fluorescent signal was visualized and captured using a Leica microscope, where each mRNA molecule hybridized to a probe appears as a separate small fluorescent dot.
Statistical Analysis
Statistical analysis performed using GraphPad Prism 7.0. Mann-Whitney tests were applied to evaluate differences in age, proteinuria, serum albumin and creatinine and eGFR between MCD and FSGS patient groups. Mouse albumin to creatinine ratio levels were analyzed using Mann-Whitney test and presented as mean ± SEM. P<0.05 was considered to be statistically significant.
Results
CD80 is expressed by glomerular endothelial cells and podocytes in a subset of patients with MCD
Nine and eleven patients with biopsy-proven MCD and FSGS, respectively, were included in this study. Clinical characteristics are displayed in Table 1. Patients in the MCD group were younger and had a significantly higher estimated glomerular filtration rate (eGFR) than those in the FSGS group (p 0.006 and 0.001 respectively). All but two patients from the FSGS group had an eGFR greater than 60 ml/min/1.73 m2. No statistical differences were observed in proteinuria or serum albumin levels among groups.
Table 1.
Demographics and laboratory data from patients with idiopathic nephrotic syndrome
| Patients with MCD during relapse | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Gender | Age (years) † | Proteinuria (g/day)†† | Serum albumin (g/dl)ʅ | Serum creatinine (mg/dl)ʅʅ | eGFR (ml/min/1.73m2)ɫ | DM /HTN | Steroid resistant | Medications |
| 1 | F | 28 | 8.2 | 2.1 | 0.7 | 105.9 | No/No | No | No |
| 2 | F | 45 | 15 | 2.4 | 0.8 | 82.4 | No/No | No | No |
| 3 | M | 43 | 7.9 | 2.3 | 1 | 86.7 | No/No | No | No |
| 4 | M | 42 | 6.7 | 2.7 | 0.8 | 112.7 | No/No | No | No |
| 5 | F | 21 | 9.2 | 1.9 | 0.8 | 96.2 | No/No | No | No |
| 6 | M | 19 | 16 | 1.8 | 0.9 | 115.5 | No/No | No | No |
| 7 | M | 35 | 16 | 2.3 | 0.7 | 136.4 | No/No | No | No |
| 8 | M | 32 | 7.5 | 2.6 | 0.9 | 103.9 | No/No | Yes | No |
| 9 | M | 26 | 8.4 | 2.4 | 0.7 | 144.9 | No/No | Yes | No |
| mean±SD | 32.3±9.6 | 10.5±3.9 | 2.2±0.2 | 0.8±0.1 | 109.4±20.9 | ||||
| Patients with FSGS during relapse | |||||||||
| 1 | M | 61 | 6.3 | 2.2 | 1.2 | 65.4 | No/Yes | Yes | ARB |
| 2 | M | 61 | 13 | 2.9 | 1.9 | 38.5 | No/Yes | Yes | ARB+ amlodipine |
| 3 | M | 39 | 4 | 3.1 | 1.2 | 71.6 | No/no | Yes | No |
| 4 | M | 56 | 7.1 | 2 | 0.9 | 92.8 | No/No | Yes | No |
| 5 | M | 60 | 8.1 | 1.9 | 1.2 | 65.6 | No/No | Yes | ARB+ furosemide + amlodipine |
| 6 | M | 57 | 6.9 | 3.6 | 1.1 | 73.3 | No/No | No | No |
| 7 | F | 49 | 4 | 2.6 | 0.8 | 81 | No/No | No | No |
| 8 | M | 62 | 4.3 | 3.5 | 1.3 | 59.5 | Yes/Yes | Yes | Insulin+ACEI + furosemide |
| 9 | M | 35 | 11 | 2.8 | 1.3 | 66.8 | No/No | No | No |
| 10 | M | 43 | 3.8 | 3.4 | 1.1 | 77.7 | No/No | No | No |
| 11 | F | 22 | 18 | 1.5 | 0.6 | 132.9 | No/No | No | No |
| mean±SD | 49±5 | 7.8±4.4 | 2.6±0.7 | 1.1 ±0.3 | 75±23.5 |
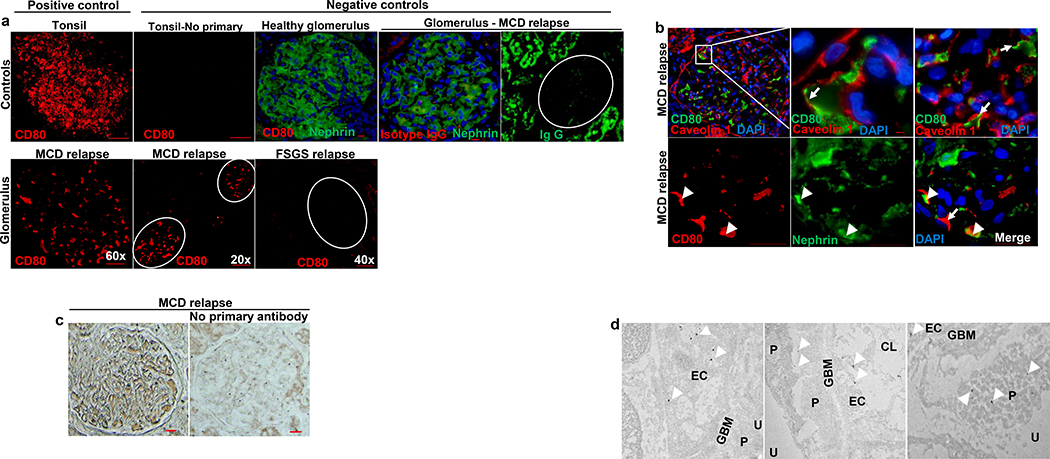
CD80 immunostaining was performed in paraffin-embedded kidney specimens (Figure 1), where CD80 was present in glomeruli from six of nine patients with MCD and two of eleven FSGS patients (Figure 1A). In nephrotic states, hypogammaglobinemia is usually observed due to loss of immunoglobulins (including IgG) in the urine. To exclude a possible cross-reactivity between CD80 and IgG, we performed IgG staining. This was mostly limited to tubules (Figure 1A), likely reflecting increased tubular uptake or non-specific binding in nephrotic states.
Figure 1. CD80 is overexpressed by glomerular cells in a subset of patients with idiopathic nephrotic syndrome.
(A) Immunostaining with a mouse monoclonal anti human CD80 (red) was performed on paraffin embedded sections from patients with MCD and FSGS. Positive and negative controls are shown (upper panel). Upregulation of glomerular CD80 was noted in most of MCD but few FSGS patients (lower panel). (B) CD80 (green, upper panel) colocalized with caveolin 1 in an endothelial specific pattern (arrows). To less extent, CD80 (red, lower panel) colocalized with nephrin (arrowheads). (C) Specificity of immunostaining was confirmed by silver staining. No signal detected in kidney tissue sample from same MCD patient in relapse when primary antibody was omitted. (D) Electron microscopy (EM) from kidney tissue from patient with MCD is shown. CD80 colocalized with glomerular endothelial cells and rarely podocytes. Original EM images captured at 6000x (far right) and 30000x. Podocyte (P), capillary lumen (CL), endothelial cell (EC), urinary space (U), glomerular basement membrane (GBM). Scale bars: 100 μm.
Co-labeling studies showed CD80 lining the capillary lumen along with caveolin-1 (used as endothelial marker) and rarely co-localizing with nephrin (podocyte marker), suggesting that CD80 is likely expressed by the glomerular endothelial cells (GECs) and/or inflammatory cells attached to GECs, and by podocytes (Figure 1B). These findings were validated using silver staining and immunogold electron microscopy (Figure 1C and 1D respectively).
CD80 is expressed by glomerular cells in LPS-treated mice
Because puromycin and LPS cause reversible podocyte injury in mice, these models are commonly used to study steroid sensitive INS and/or MCD. Since LPS recapitulates infection, a common trigger of INS [24], we used LPS to induce podocyte injury in mice. As expected, LPS induced albuminuria and foot processed effacement 24 hours after intraperitoneal administration (Figure 2A and 2B respectively). Following LPS, CD80 was expressed in mouse glomeruli and around the periphery of glomeruli. The latter likely reflecting inflammatory cells or parietal epithelial cells (Figure 3A). CD80 colocalized with ICAM1 (used as marker of endothelial activation) and synaptopodin (podocyte marker) (Figure 3B). By in situ hybridization, we confirmed that CD80 mRNA and ICAM1 mRNA were upregulated in glomeruli 24 hours following LPS (Figure 3C). Compared to controls, we also found that CD80 and ICAM-1 mRNA molecules were increased as early as 6 hours after LPS (Figure 3D). Since ICAM1 can be expressed by activated endothelial cells and inflammatory cells, we analyzed recent published data from endothelial-specific translating ribosome affinity purification to determine whether endothelial kidney cells are a potential source of CD80 [25]. Indeed, CD80 was upregulated in kidney endothelial cells isolated in vivo as soon as 4 hours following LPS (6.1-fold as compared to control, adjusted p-value <0.01, 3 mice per group). This coincided with a significant upregulation of another co-stimulatory molecule, CD40, in isolated kidney endothelial cells after LPS (3.5-fold, adjusted p-value 0.02) demonstrating that the kidney endothelial cells can be potential sources of co-stimulatory molecules under pathologic conditions [25].
Figure 2. LPS-induced albuminuria and podocyte foot process effacement.
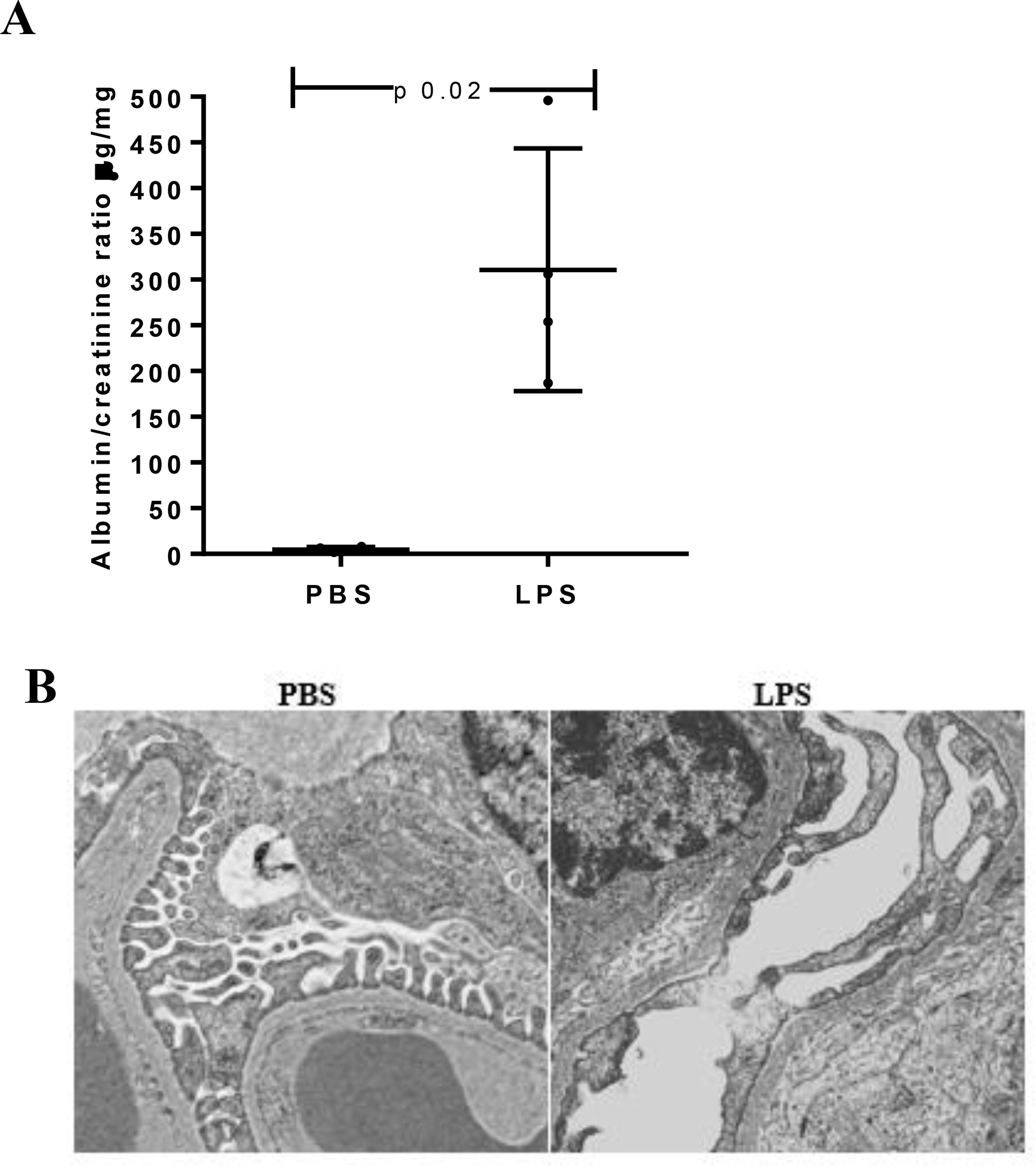
(A) Albuminuria was significantly increased in LPS-injected mice compared to PBS-mice (n 4 mice per group). (B) Transmission electron microscopy showed foot processes effacement 24 h following LPS whereas preserved podocyte structure was noted in control mice. Original magnification 20000x.
Figure 3. CD80 is upregulated in glomeruli from LPS-treated mice.
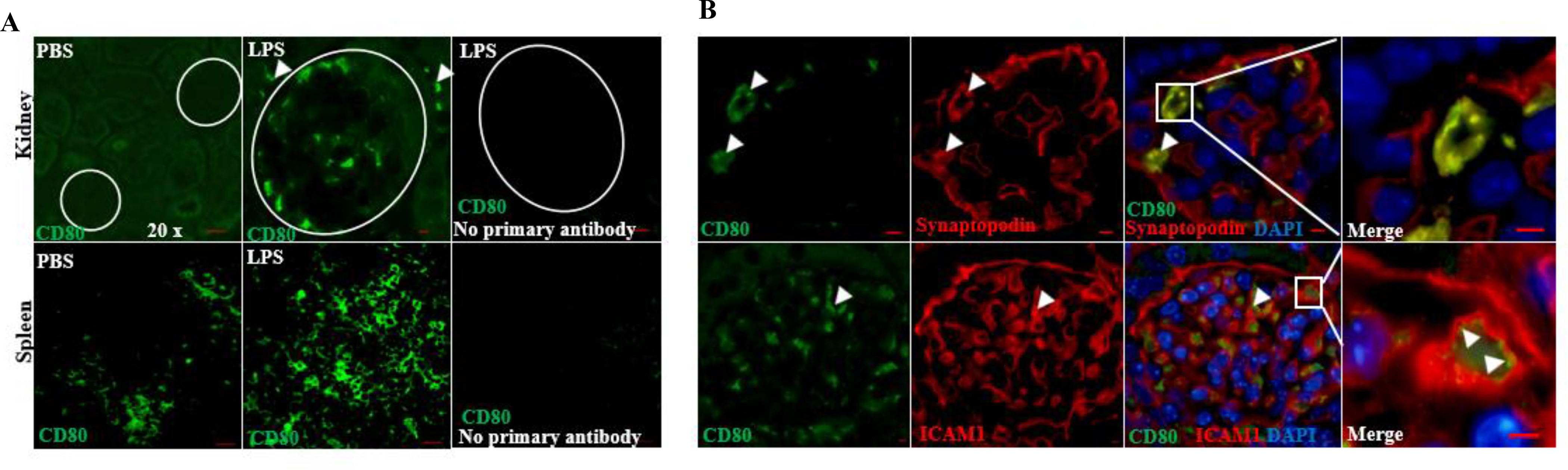
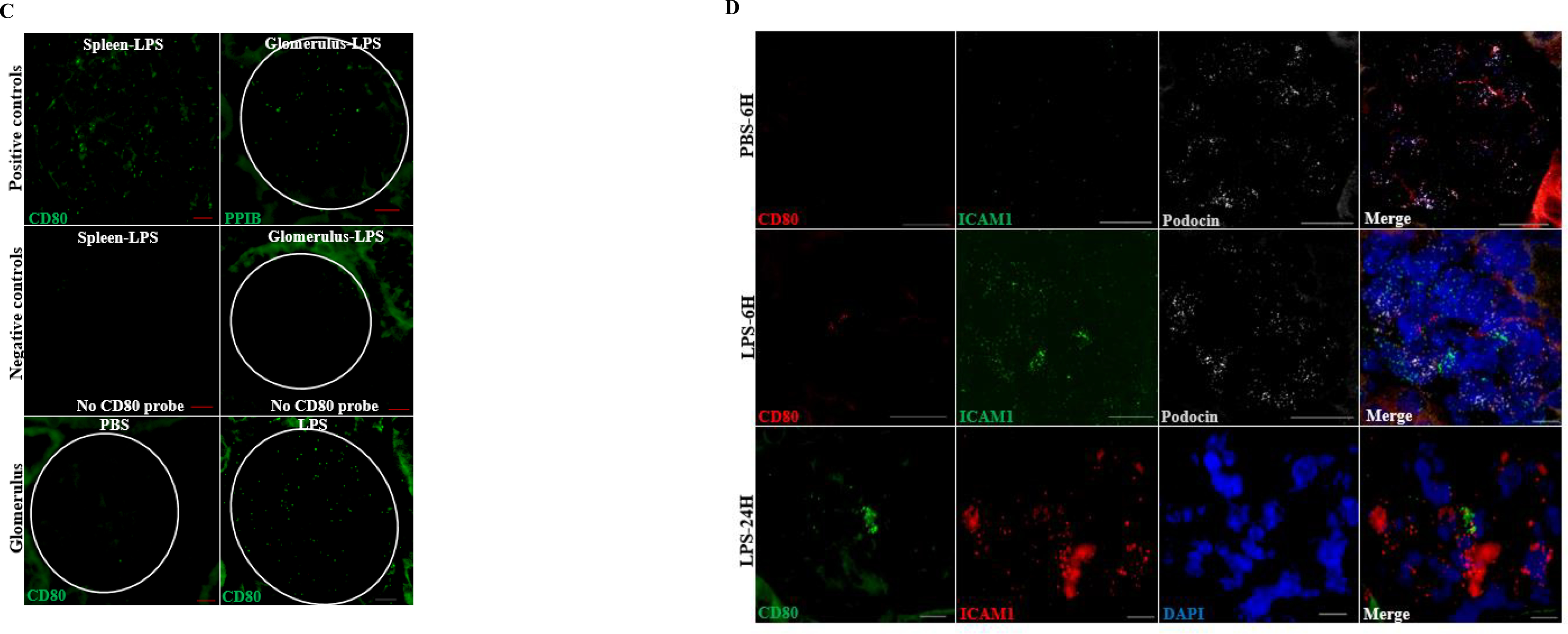
(A) Immunostaining was performed on paraffin embedded sections from mice treated with PBS (controls) and LPS. Positive and negative controls are shown. CD80 was upregulated in glomeruli following LPS. Scattered infiltrating inflammatory and/or parietal cells were also observed after LPS (upper panel, arrowheads). (B) By immunostaining, CD80 (green) colocalized to some extent with synaptopodin (podocyte marker, red) and lined the capillary lumen co-localizing with ICAM1 (red), used as marker of endothelial cell activation. (C) Using in situ hybridization, we confirmed the presence of CD80 RNA molecules in spleen (positive control) and glomeruli from LPS mice (24 hours). PPIB (housekeeping gene coding for peptidylprolyl isomerase B) probe was also used as positive control. Omission of CD80 probes on spleen and kidney were used as negative controls. (D) CD80 and ICAM1 mRNA were upregulated in LPS-treated mice, compared to controls, as early as 6 hours following LPS. Scale bars: 100 μm.
Discussion
The nature of podocyte injury in INS remains poorly understood. Given the heterogeneity of these disorders and the lack of targeted therapies, there is an urge to discover non-invasive biomarkers to optimize our ability to establish a more precise diagnosis, prognosis and to tailor therapies according to a certain molecular signature. Urinary CD80 has emerged as a promising non-invasive biomarker in INS [11–18]. However, the cellular source of urinary CD80 remains a matter of debate (Table 2). In this study, we combined different techniques to rigorously test whether CD80 is expressed by glomerular cells in INS patients and LPS-injected mice.
Table 2.
CD80 expression in kidney tissue from patients with idiopathic nephrotic syndrome
| Study | N | Results | Methods | Comments |
|---|---|---|---|---|
| Yu10† | 4 Primary FSGS, active5 Primary MCD, relapse5 Recurrent FSGS, active | 3/4, podocyte CD80+3/5, podocyte CD80+5/5, podocyte CD80+ | *Immunohistochemistry of frozen fresh tissue*Anti-human polyclonal CD80 goat antibody (R&D systems) | *No tubular CD80 observed*Adults and children†Including data from supplementary appendix |
| Garin12 | 7 Primary MCD, relapse1 Primary MCD, remission2 Primary FSGS, active | 7/7, podocyte CD80+0/1, glomerular CD80+0/2, glomerular CD80+ | *Immunohistochemistry of frozen fresh tissue*Anti-human polyclonal CD80 goat antibody (R&D systems) | *No tubular CD80 observed*Children |
| Larsen21 | 19 Primary MCD, relapse28 Primary FSGS, active | Podocyte CD80 − in all patients. | *Immunoperoxidase staining on paraffin-embedded tissue using a mouse monoclonal CD80 antibody (R&D systems)*Immunofluorescence on fresh tissue (when available) using a polyclonal CD80 goat antibody (R&D systems) | *Adults* Focal interstitial inflammatory cells CD80 + observed |
| Novelli22 | 11 Primary MCD,5 relapse13 Primary FSGS, all active | Podocyte CD80− in all patients. | *Immunofluorescence on fresh tissue (when available) using a polyclonal CD80 goat antibody (R&D systems)*Immunoperoxidase staining on paraffin-embedded tissue using a mouse monoclonal CD80 antibody (R&D systems) | *Adults*CD80+ in interstitial inflammatory cells and injured epithelial cells |
| Lee23 | 28 Primary MCD, relapse | 0/28 podocyte CD80+21/28 tubular CD80+ | *Immunohistochemistry on paraffin-embedded tissue using a mouse monoclonal CD80 antibody (R&D systems) | *Adults |
The first major finding in this study was to identify CD80 on the surface of glomerular endothelial cells as well as podocytes in a subset of MCD patients. These observations are supported by appropriate positive and negative controls. The discrepancy regarding data on glomerular CD80 across the spectrum of INS likely reflects the heterogeneity of the disease, which may account for the variability of urinary CD80 among INS patients. While several groups have shown that urinary CD80 levels are significantly higher in SSNS and/or MCD patients in relapse compared to remission and/or FSGS [11–18], Minamikawa et. al concluded that urinary CD80 was an unreliable biomarker to differentiate MCD and FSGS [26]. However, this conclusion might be premature since this study only included 4 FSGS patients. Interestingly, these same authors found that 1/3 of MCD patients in relapse had a very high urinary CD80 levels compared to the rest of MCD patients in relapse or remission. So, one may speculate that urinary CD80 may be a biomarker that could potentially identify subsets of patients with glomerular diseases associated with CD80 expression, especially as we were not able to find CD80 in the tubular compartments in these patients. If the glomerulus is indeed the source of urinary CD80, one might anticipate that CD80 will be absent in glomeruli from patients with low urinary CD80 levels. The use of different detection techniques and/or timing of biopsy (early versus prolonged relapse) might have also contributed to the discrepancy in CD80 immunohistochemistry. Lee et. al found tubular, but no glomerular, CD80 expression in 28 adults with MCD during relapse [23]. Most of those patients had significant tubular-interstitial or glomerular lesions indicative of a more advanced kidney damage. Novelli et al did not find glomerular CD80 expression in 15 MCD patients. However, only five of them had nephrotic range proteinuria at the time of biopsy [22]. Larsen et al. studied paraffin and frozen samples in 19 MCD patients in relapse using two different primary antibodies [21]. They could not identify presence of CD80 on podocytes in any patient. As previously mentioned, the study of different populations might yield different results.
The second major finding was to demonstrate that CD80 is significantly upregulated at protein and mRNA level in GEC and podocytes following LPS. Several groups have previously reported the in vivo up-regulation of glomerular CD80 by RT-PCR, immunostaining and western blotting in this model [4, 5, 27]. However, other groups could not demonstrate CD80 expression, by immunostaining, in mouse glomeruli following LPS [22, 28]. Novelli found that CD80 expression in LPS-injected mice was limited to CD11c-positive antigen presenting cells [22]. In that study, a higher LPS dose was used compared to other groups. Baye showed that high dose of IP LPS (300 and 400 ug/mouse) led to mice death within 24 h [28]. When a lower, more standardly used dose of LPS (200 ug/mouse) was used, no glomerular CD80 was detected. The caveat is that those mice only developed mild proteinuria. Consistent with our findings, Chen et al found podocyte CD80 following LPS by immunostaining and in situ hybridization [29].
Whether CD80 plays a role in proteinuria either in INS and/or the LPS model or merely represent a marker of cell activation is unclear. On one hand, CD80 induces cytoskeletal rearrangement and podocyte migration by binding Neph1 and β1 integrins in cultured podocytes [10, 30]. So it is tentative to postulate that CD80 overexpression in GEC and/or podocytes may alter the permeability of the glomerular filtration barrier. So far, experimental models only provide indirect evidence of CD80 as mediator of proteinuria. Reiser et al showed that CD80 knockout mice are protected from LPS albuminuria, suggesting a link between CD80 and albuminuria [4]. Whereas mice used in this study were globally deficient in CD80, a chimeric approach suggested that CD80 is indeed required on non-hematopoietic cells for mice to develop albuminuria following LPS [6]. In MCD patients with elevated urinary CD80, we observed a rapid decline in proteinuria and dramatic decrease in number of relapses in the long-term, suggesting that CD80 may contribute to proteinuria in some patients with INS [19, 20].
Lastly, our results suggest that GECs are activated in a subset of MCD patients and in LPS mice. Endothelial cells are lining the vascular tree, thereby being the first cells in the kidney to come in contact with circulating microbial products. Interestingly, upper respiratory infections are the most common known trigger of relapses [31]. It is plausible that circulating microbial particles target GEC, thereby initiating a local immune response and crosstalk with other glomerular cells. Thus, GEC activation may represent the first hit to the glomerulus in a subset of patients. In support of that, several markers of endothelial dysfunction are elevated in children and adults with MCD during relapse [32–34]. A second hit to the glomerulus may be required to induce and perpetuate more significant podocyte foot processes effacement and proteinuria. This could come in the form of an exaggerated local immune response (mediated by podocyte CD80 overexpression or impaired CTLA4 expression [35]) and/or impaired mechanisms of podocyte repair (such as nephrin phosphorylation or impaired nephrin recycling). Interestingly, patients with MCD in relapse have less regulatory T cell (CTLA4 +) in glomeruli [36], which may contribute to an imbalanced CD80/CTLA4 ratio locally, and a reduction in phosphorylated nephrin compared to patients during remission [37].
This study has limitations. Our study population was limited to adults, so it remains unclear whether the CD80 pattern of expression is similar in children. In addition, urine samples were not available so we could not investigate the correlation between glomerular and urinary CD80. However, the primary strength of the study is that we utilized a wide variety of methods that were more extensive than previous studies and that included immunostaining, immunogold electron microscopy and in situ hybridization, all with multiple controls, that provides authentication of our findings.
In summary, we identified glomerular endothelial cells and podocytes as potential cellular sources of CD80 in INS and LPS mice. In light of these findings, clinical studies to determine the correlation between CD80 and markers of endothelial activation, as well as experimental studies including CD80 overexpression in GECs and/or podocytes are warranted. A better understanding of the role of CD80 in glomerular cells may provide further insights into the mechanisms of proteinuria in INS.
Supplementary Material
Supp Figure
Supplementary Figure 1. In vitro validation of CD80 antibodies. (A) Mouse monoclonal anti-human CD80 antibodies and (B) goat polyclonal anti-mouse CD80 antibody bound to CD80 using an overexpression system where a construct expressing flag-tagged CD80 was transfected in COS7 cells. Scale bars: 100 μm.
Figure 4. Proposed model for glomerular endothelial and podocyte activation in subsets of INS patients and LPS model of podocyte injury.
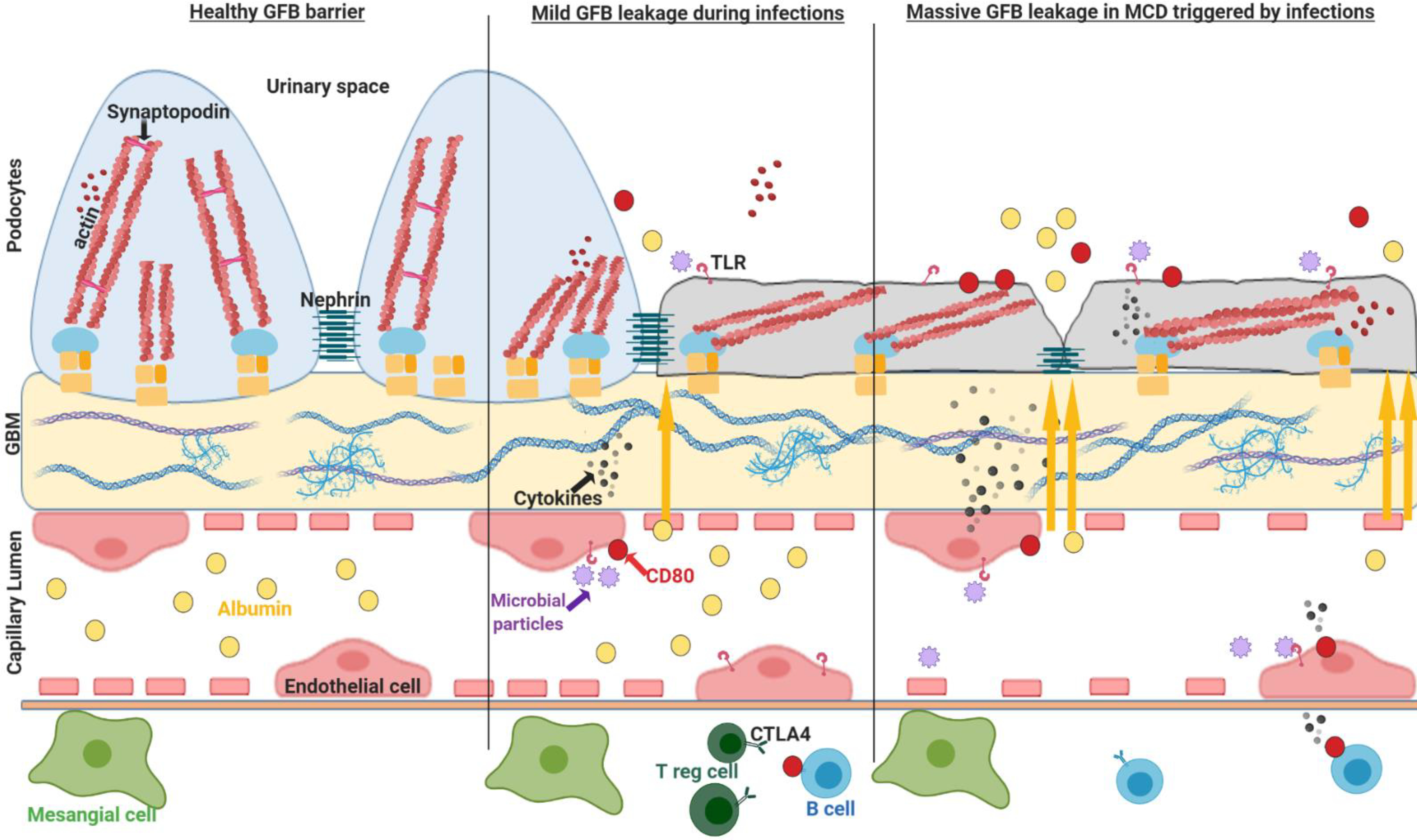
Glomerular endothelial cells, lining the vasculature tree, express toll-like receptors acting as a sentinel organ for circulating microbial particles. During infections (toll–like receptors ligands for mice), GECs are activated, as supported by the expression of co-stimulatory molecules CD80 and CD40, releasing a myriad of cytokines that may cross talk with extracellular matrix and podocytes. This probably contribute to podocyte activation, CD80 upregulation and shedding into urine along with transient albuminuria. In INS, one may speculate that a dysregulated glomerular crosstalk may result in a local hyper-immune response (excessive local cytokine production, Th17 cells, B cells/IL-4, CD80, etc) that along with the presence of defective mechanisms of podocyte repair (nephrin phosphorylation/trafficking, deficient T reg cells/CTLA-4, etc) could possibly aggravate and/or perpetuate proteinuria in these patients. GFB glomerular filtration barrier; GBM glomerular basement membrane, GECs glomerular endothelial cells; INS idiopathic nephrotic syndrome. Cartoon created with BioRender.com.
Acknowledgment
We thank Dr Eduardo H Garin for his suggestions.
Support This study was supported by NIH K12 HD028820-27 to GCF.
Footnotes
Disclosure Authors declare no conflict of interest.
References
- 1.Barisoni L, Schnaper HW, Kopp JB (2007) A proposed taxonomy for the podocytopathies: a reassessment of the primary nephrotic diseases. CJASN 2(3):529–42. [DOI] [PubMed] [Google Scholar]
- 2.Vivarelli M, Massella L, Ruggiero B, Emma F (2017) Minimal Change Disease. CJASN 12(2):332–45. doi: 10.2215/CJN.05000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colucci M, Corpetti G, Emma F, Vivarelli M (2018) Immunology of idiopathic nephrotic syndrome. Pediatr Nephrol 33(4):573–84. doi: 10.1007/s00467-017-3677-5. [DOI] [PubMed] [Google Scholar]
- 4.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P (2004) Induction of B7–1 in podocytes is associated with nephrotic syndrome. J Clin Invest 113(10):1390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishimoto T, Shimada M, Gabriela G, Kosugi T, Sato W, Lee PY, Lanaspa MA, Rivard C, Maruyama S, Garin EH, Johnson RJ (2013) Toll-like receptor 3 ligand, polyIC, induces proteinuria and glomerular CD80, and increases urinary CD80 in mice. Nephrol Dial Transplant 28(6):1439–46. doi: 10.1093/ndt/gfs543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain N, Khullar B, Oswal N, Banoth B, Joshi P, Ravindran B, Panda S, Basak S, George A, Rath S, Bal V, Sopory S (2016) TLR-mediated albuminuria needs TNFalpha-mediated cooperativity between TLRs present in hematopoietic tissues and CD80 present on non-hematopoietic tissues in mice. Dis Model Mech 9(6):707–17. doi: 10.1242/dmm.023440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivard CJ, Tanabe T, Lanaspa MA, Watanabe H, Nomura S, Andres-Hernando A, Garth K, Sekijima M, Ishimoto T, Ariyoshi Y, Garcia GE, Shah J, Lennan B, Tasaki M, Pomposelli T, Shimizu A, Sachs DH, Johnson RJ, Yamada K (2018) Upregulation of CD80 on glomerular podocytes plays an important role in development of proteinuria following pig-to-baboon xeno-renal transplantation - an experimental study. Transpl Int 31(10):1164–77. doi: 10.1111/tri.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimada M, Ishimoto T, Lee PY, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Wymer DT, Yamabe H, Mathieson PW, Saleem MA, Garin EH, Johnson RJ (2012) Toll-like receptor 3 ligands induce CD80 expression in human podocytes via an NF-kappaB-dependent pathway. Nephrol Dial Transplant 27(1):81–9. doi: 10.1093/ndt/gfr271. [DOI] [PubMed] [Google Scholar]
- 9.Gurkan S, Cabinian A, Lopez V, Bhaumik M, Chang JM, Rabson AB, Mundel P (2013) Inhibition of type I interferon signalling prevents TLR ligand-mediated proteinuria. J Pathol 231(2):248–56. [DOI] [PubMed] [Google Scholar]
- 10.Yu CC, Fornoni A, Weins A, Hakroush S, Maiguel D, Sageshima J, Chen L, Ciancio G, Faridi MH, Behr D, Campbell KN, Chang JM, Chen HC, Oh J, Faul C, Arnaout MA, Fiorina P, Gupta V, Greka A, Burke GW 3rd, Mundel P (2013) Abatacept in B7–1-positive proteinuric kidney disease. N ENgl J Med 369(25):2416–23. doi: 10.1056/NEJMoa1304572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garin EH, Diaz LN, Mu W, Wasserfall C, Araya C, Segal M, Johnson RJ (2009) Urinary CD80 excretion increases in idiopathic minimal-change disease. J Am Soc Nephrol 20(2):260–6. doi: 10.1681/ASN.2007080836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garin EH, Mu W, Arthur JM, Rivard CJ, Araya CE, Shimada M, Johnson RJ (2010) Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int 78(3):296–302. doi: 10.1038/ki.2010.143. [DOI] [PubMed] [Google Scholar]
- 13.Cara-Fuentes G, Wei C, Segarra A, Ishimoto T, Rivard C, Johnson RJ, Reiser J, Garin EH (2014) CD80 and suPAR in patients with minimal change disease and focal segmental glomerulosclerosis: diagnostic and pathogenic significance. Pediatr Nephrol 29(8):1363–71. doi: 10.1007/s00467-013-2679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cara-Fuentes G, Wasserfall CH, Wang H, Johnson RJ, Garin EH (2014) Minimal change disease: a dysregulation of the podocyte CD80-CTLA-4 axis? Pediatr Nephrol 29(12):2333–40. doi: 10.1007/s00467-014-2874-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ling C, Liu X, Shen Y, Chen Z, Fan J, Jiang Y, Meng Q (2015) Urinary CD80 levels as a diagnostic biomarker of minimal change disease. Pediatr Nephrol 30(2):309–16. doi: 10.1007/s00467-014-2915-3. [DOI] [PubMed] [Google Scholar]
- 16.Mishra OP, Kumar R, Narayan G, Srivastava P, Abhinay A, Prasad R, Singh A, Batra VV (2017) Toll-like receptor 3 (TLR-3), TLR-4 and CD80 expression in peripheral blood mononuclear cells and urinary CD80 levels in children with idiopathic nephrotic syndrome. Pediatr Nephrol 32(8):1355–61. doi: 10.1007/s00467-017-3613-8. [DOI] [PubMed] [Google Scholar]
- 17.Bhatia D, Sinha A, Hari P, Sopory S, Saini S, Puraswani M, Saini H, Mitra DK, Bagga A (2018) Rituximab modulates T- and B-lymphocyte subsets and urinary CD80 excretion in patients with steroid-dependent nephrotic syndrome. Pediatr Res 84(4):520–6. doi: 10.1038/s41390-018-0088-7. [DOI] [PubMed] [Google Scholar]
- 18.Ling C, Liu X, Shen Y, Chen Z, Fan J, Jiang Y, Meng Q (2018) Urinary CD80 excretion is a predictor of good outcome in children with primary nephrotic syndrome. Pediatr Nephrol 33(7):1183–7. doi: 10.1007/s00467-018-3885-7. [DOI] [PubMed] [Google Scholar]
- 19.Garin EH, Reiser J, Cara-Fuentes G, Wei C, Matar D, Wang H, Alachkar N, Johnson RJ (2015) Case series: CTLA4-IgG1 therapy in minimal change disease and focal segmental glomerulosclerosis. Pediatr Nephrol 30(3):469–77. doi: 10.1007/s00467-014-2957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isom R, Shoor S, Higgins J, Cara-Fuentes G, Johnson RJ (2019) Abatacept in Steroid-Dependent Minimal Change Disease and CD80-uria. Kidney Int Rep 4(9):1349–53. doi: 10.1016/j.ekir.2019.05.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen CP, Messias NC, Walker PD (2014) B7–1 immunostaining in proteinuric kidney disease. Am J Kidney Dis 64(6):1001–3. doi: 10.1053/j.ajkd.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Novelli R, Gagliardini E, Ruggiero B, Benigni A, Remuzzi G (2016) Any value of podocyte B7–1 as a biomarker in human MCD and FSGS? Am J Physiol Renal Physiol 310(5):F335–41. doi: 10.1152/ajprenal.00510.2015. [DOI] [PubMed] [Google Scholar]
- 23.Lee SW, Baek SH, Paik JH, Kim S, Na KY, Chae DW, Chin HJ (2017) Tubular B7–1 expression parallels proteinuria levels, but not clinical outcomes in adult minimal change disease patients. Sci Rep 7:41859. doi: 10.1038/srep41859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alwadhi RK, Mathew JL, Rath B (2004) Clinical profile of children with nephrotic syndrome not on glucorticoid therapy, but presenting with infection. J Pediatr Child Health 40(1–2):28–32. [DOI] [PubMed] [Google Scholar]
- 25.Cleuren ACA, van der Ent MA, Jiang H, Hunker KL, Yee A, Siemieniak DR, Molema G, Aird WC, Ganesh SK, Ginsburg D (2019) The in vivo endothelial cell translatome is highly heterogeneous across vascular beds. Proc Natl Acad Sci U S A 19;116(47):23618–23624. doi: 10.1073/pnas.1912409116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minamikawa S, Nozu K, Maeta S, Yamamura T, Nakanishi K, Fujimura J, Horinouchi T, Nagano C, Sakakibara N, Nagase H, Shima H, Noda K, Ninchoji T, Kaito H, Iijima K (2018) The utility of urinary CD80 as a diagnostic marker in patients with renal diseases. Sci Rep 8(1):17322. doi: 10.1038/s41598-018-35798-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P, Zhou S, Ge Y, Lu M, Liu Z, Gong R (2017) Valproate hampers podocyte acquisition of immune phenotypes via intercepting the GSK3beta facilitated NFkB activation. Oncotarget 8(51):88332–44. doi: 10.18632/oncotarget.19917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baye E, Gallazzini M, Delville M, Legendre C, Terzi F, Canaud G (2016) The costimulatory receptor B7–1 is not induced in injured podocytes. Kidney Int 90(5):1037–44. doi: 10.1016/j.kint.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W, Wu Y, Zhang G, Wang M, Yang H, Li Q (2018) gammadeltaT Cells Exacerbate Podocyte Injury via the CD28/B7–1-Phosphor-SRC Kinase Pathway. Biomed Res Int 5647120. doi: 10.1155/2018/5647120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khullar B, Balyan R, Oswal N, Jain N, Sharma A, Abdin MZ, Bagga A, Bhatnagar S, Wadhwa N, Natchu UCM, George A, Rath S,2, Bal V, Sopory S (2018) Interaction of CD80 with Neph1: a potential mechanism of podocyte injury. Clin Exp Nephrol 22(3):508–16. doi: 10.1007/s10157-017-1489-3. [DOI] [PubMed] [Google Scholar]
- 31.MacDonald NE, Wolfish N, McLaine P, Phipps P, Rossier E (1986) Role of respiratory viruses in exacerbations of primary nephrotic syndrome. J Pediatr 108(3):378–82. [DOI] [PubMed] [Google Scholar]
- 32.Tkaczyk M, Czupryniak A, Owczarek D, Lukamowicz J, Nowicki M (2008) Markers of endothelial dysfunction in children with idiopathic nephrotic syndrome. Am J Nephrol 28(2):197–202. [DOI] [PubMed] [Google Scholar]
- 33.Sharma B, Saha A, Dubey NK, Kapoor K, Anubhuti, Batra VV, Upadhayay AD (2014) Endothelial dysfuntion in children with idiopathic nephrotic syndrome. Atherosclerosis 233(2):704–6. doi: 10.1016/j.atherosclerosis.2014.01.055. [DOI] [PubMed] [Google Scholar]
- 34.Chaves MMS, Mendes MS, Schwermann MP, Queiroz R, Coelho RF, Salmito FTS, Meneses GC, Martins AMC, Moreira ACOM, Liborio AB (2018) Angiopoietin-2: A Potential Mediator of the Glycocalyx Injury in Adult Nephrotic Patients. J Clin Med 7(11). doi: 10.3390/jcm7110401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohl K, Eberhardt C, Spink C, Zahn K, Wagner N, Eggermann T, Kemper MJ, Querfeld U, Hoppe B, Harendza S, Tenbrock K (2014) CTLA4 polymorphisms in minimal change nephrotic syndrome in children: a case-control study. AM J Kidney Dis 63(6):1074–5. doi: 10.1053/j.ajkd.2014.01.427. [DOI] [PubMed] [Google Scholar]
- 36.Benz K, Buttner M, Dittrich K, Campean V, Dotsch J, Amann K (2010) Characterisation of renal immune cell infiltrates in children with nephrotic syndrome. Pediatri Nephrol 25(7):1291–8. doi: 10.1007/s00467-010-1507-0. [DOI] [PubMed] [Google Scholar]
- 37.Uchida K, Suzuki K, Iwamoto M, Kawachi H, Ohno M, Horita S, Nitta K (2008) Decreased tyrosine phosphorylation of nephrin in rat and human nephrosis. Kidney Int 73(8):926–32. doi: 10.1038/ki.2008.19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp Figure
Supplementary Figure 1. In vitro validation of CD80 antibodies. (A) Mouse monoclonal anti-human CD80 antibodies and (B) goat polyclonal anti-mouse CD80 antibody bound to CD80 using an overexpression system where a construct expressing flag-tagged CD80 was transfected in COS7 cells. Scale bars: 100 μm.