A Biomarker-Based Score for Risk of Hospitalization for Heart Failure in Patients With Diabetes (original) (raw)
Abstract
OBJECTIVE
Heart failure (HF) is an impactful complication of type 2 diabetes mellitus (T2DM). We aimed to develop and validate a risk score for hospitalization for HF (HHF) incorporating biomarkers and clinical factor(s) in patients with T2DM.
RESEARCH DESIGN AND METHODS
We derived a risk score for HHF using clinical data, high-sensitivity troponin T (hsTnT), and N-terminal prohormone of B-type natriuretic peptide (NT-proBNP) from 6,106 placebo-treated patients with T2DM in SAVOR-TIMI 53 (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53). Candidate variables were assessed using Cox regression. The strongest indicators of HHF risk were included in the score using integer weights. The score was externally validated in 7,251 placebo-treated patients in DECLARE-TIMI 58 (Dapagliflozin Effect on CardiovascuLAR Events–Thrombolysis in Myocardial Infarction 58). The effect of dapagliflozin on HHF was assessed by risk category in DECLARE-TIMI 58.
RESULTS
The strongest indicators of HHF risk were NT-proBNP, prior HF, and hsTnT (each P < 0.001). A risk score using these three variables identified a gradient of HHF risk (_P_-trend <0.001) in the derivation and validation cohorts, with C-indices of 0.87 (95% CI, 0.84–0.89) and 0.84 (0.81–0.86), respectively. Whereas there was no significant effect of dapagliflozin versus placebo on HHF in the low-risk group (hazard ratio [HR] 0.98 [95% CI 0.50–1.92]), dapagliflozin significantly reduced HHF in the intermediate-, high-, and very-high-risk groups (HR 0.64 [0.43–0.95], 0.63 [0.43–0.94], and 0.72 [0.54–0.96], respectively). Correspondingly, absolute risk reductions (95% CI) increased across these latter 3 groups: 1.0% (0.0–1.9), 3.0% (0.7–5.3), and 4.4% (−0.2 to 8.9) (_P_-trend <0.001).
CONCLUSIONS
We developed and validated a risk score for HHF in T2DM that incorporated NT-proBNP, prior HF, and hsTnT. The risk score identifies patients at higher risk of HHF who derive greater absolute benefit from dapagliflozin.
Introduction
More than 400 million people have type 2 diabetes mellitus (T2DM) globally, and the burden of diabetes-related cardiovascular complications is increasing (1). In addition to its marked adverse impact on patient quality of life and health care utilization, heart failure (HF) is an especially prognostically important complication of T2DM because it is associated with a 4- to 10-fold higher mortality risk compared with those without HF (2,3). Despite the significance of this complication, assessing individual risk for HF in patients with T2DM poses a challenge given the clinical heterogeneity of T2DM and complex pathobiological processes connecting T2DM and HF (4). At the same time, the emergence of sodium–glucose cotransporter 2 (SGLT2) inhibitors, a class of glucose-lowering therapies that robustly reduces the risk of incident and recurrent HF events in patients with T2DM (5–9), has highlighted the potential value of improved HF risk assessment in this population in order to personalize prevention and treatment approaches.
The Thrombolysis in Myocardial Infarction (TIMI) Risk Score for Heart Failure in Diabetes (TRS-HFDM) is a simple clinical risk score that predicts the risk of hospitalization for heart failure (HHF) in patients with T2DM (10). This practical tool includes five clinical variables that are routinely assessed in clinical practice—prior HF, coronary artery disease, atrial fibrillation, estimated glomerular filtration rate (eGFR), and urine albumin-to-creatinine ratio (UACR) (10).
Beyond clinical risk profiling, multiple studies have suggested that widely available circulating biomarkers of cardiovascular disease, including natriuretic peptides and high-sensitivity cardiac troponin, may forecast the risk of incident and recurrent HF events in patients with T2DM (11–13). We therefore aimed to improve HF risk assessment beyond TRS-HFDM by developing and validating a novel risk score incorporating these prognostically important biomarkers. In addition, we sought to evaluate whether this score could identify high-risk patients with T2DM, including patients without a history of HF, who have the greatest reduction in HHF risk with an SGLT2 inhibitor.
Research Design and Methods
Study Populations
The SAVOR-TIMI 53 (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53) and DECLARE-TIMI 58 (Dapagliflozin Effect on CardiovascuLAR Events–Thrombolysis in Myocardial Infarction 58) trials were multinational, randomized, placebo-controlled trials enrolling patients with T2DM and a history of established atherosclerotic cardiovascular disease or multiple risk factors for atherosclerotic cardiovascular disease. SAVOR-TIMI 53 evaluated the cardiovascular safety and efficacy of the dipeptidyl peptidase-4 inhibitor saxagliptin (5 mg daily) in 16,492 patients monitored for a median of 2.1 years; baseline blood samples for biomarker assessment were collected in 12,310 patients (74.6%) (14). The DECLARE-TIMI 58 trial evaluated the cardiovascular safety and efficacy of dapagliflozin (10 mg daily) in 17,160 patients monitored for a median of 4.2 years; 14,565 patients (84.9%) elected to participate in the nested prospective biomarker substudy (8). In both studies, prior history of HF was captured in the case record form based on the history obtained by the investigator and assessment of the medical record.
For the present analyses, 6,106 patients with available baseline biomarker data from the placebo arm of SAVOR-TIMI 53 served as the derivation cohort, and 7,251 patients with available baseline biomarker data from the placebo arm of DECLARE-TIMI 58 served as the validation cohort. The ethics committees at participating centers approved the protocols for each trial. Written informed consent was obtained from all patients. We encourage parties interested in collaboration and data sharing to contact the corresponding author directly for further discussion.
Circulating Protein Biomarkers
Baseline blood samples were collected on the day of randomization. Samples were collected in EDTA anticoagulant tubes, and isolated plasma was stored at −20°C or colder until shipped on dry ice to the central laboratory, where plasma was stored at −70°C or colder until thawed for analysis at the TIMI Clinical Trials Laboratory (Boston, MA). High-sensitivity troponin T (hsTnT) and N-terminal prohormone of B-type natriuretic peptide (NT-proBNP) concentrations were measured with immunoassays on the cobas e 601 (Roche Diagnostics). The limit of quantitation of the hsTnT assay is 6 ng/L, and the 99th percentile upper reference limit is 14 ng/L (15). The limit of quantitation of the NT-proBNP assay is 50 pg/mL, and 125 pg/mL and 450 pg/mL have both been used as thresholds for risk stratification in patients with T2DM (13,16).
Clinical Outcomes
The primary outcome for this analysis was HHF, which was prospectively collected and centrally adjudicated by the TIMI Clinical Events Committee using established, virtually identical definitions in both the SAVOR-TIMI 53 and DECLARE-TIMI 58 trials (8,14). Specifically, HHF required 1) hospital admission for at least 12 h (SAVOR-TIMI 53) or 24 h (DECLARE-TIMI 58), 2) objective clinical manifestations of HF (e.g., physical examination findings, radiological evidence of pulmonary congestion, elevated natriuretic peptide levels), and 3) intensification of HF therapies (e.g., intravenous diuretic or inotropic agents).
Statistical Methods
Log-transformed biomarker concentrations were entered into a multivariable Cox regression model for HHF along with the five clinical risk indicators included in TRS-HFDM. UACR was also log-transformed, and all continuous variables were included in the regression model as linear terms. Of note, 20 additional candidate risk indicators were considered in the development of TRS-HFDM (age, sex, race, BMI, duration of T2DM, glycated hemoglobin [HbA1c], baseline insulin use, history of diabetic retinopathy, history of diabetic nephropathy, previous myocardial infarction, established peripheral artery disease, previous ischemic stroke, previous percutaneous coronary intervention, previous coronary artery bypass grafting, dyslipidemia, hypertension, current smoking, heart rate, systolic blood pressure, and diastolic blood pressure); however, these variables did not significantly improve discrimination of the clinical risk model beyond the five clinical variables that were included (10).
The strength of the association between each of the seven variables and risk of HHF in the saturated multivariable risk model was assessed based on partial Wald χ2 values. To develop an optimized parsimonious risk model, the model was narrowed to the strongest risk indicators. To estimate the relative prognostic information provided by the more parsimonious three-variable biomarker-based model compared with the comprehensive seven-variable model (i.e., TRS-HFDM plus the circulating biomarkers), we approximated the comprehensive model using an ordinary least-squares model in which the estimated linear predictor from the comprehensive model was the outcome variable and the three variables from the parsimonious model were the covariates. For ease of clinical application, each biomarker was modeled using categorical cut points framed by established clinical thresholds (hsTnT: <6 ng/L, 6 to <10 ng/L, 10 to <14 ng/L, and ≥14 ng/L; NT-proBNP: <50 pg/mL, 50 to <125 pg/mL, 125 to <450 pg/mL, and ≥450 pg/mL), and each covariate was assigned an integer weight proportional to the magnitude of the regression coefficient in the final multivariable model. Risk categories were defined based on the distribution of HHF event rates across integer risk scores in the derivation cohort: low risk (0–3 points), intermediate risk (4–6 points), high risk (7–8 points), and very high risk (9–11 points).
The risk score model was internally validated using 1,000 bootstrap samples and was externally validated in 7,251 patients from the placebo arm of DECLARE-TIMI 58. Discrimination was assessed using the Harrell c-index. We performed a sensitivity analysis restricted to patients without prior HF to specifically assess the ability of the risk score to discriminate incident HHF risk. Calibration was assessed in the external validation cohort by calculating the Greenwood-Nam-D’Agostino statistic and by graphically comparing observed versus predicted HHF event rates. To compare the predictive performance of the biomarker-based risk score to the clinical variable-only risk score (TRS-HFDM) in the external validation cohort, we compared bootstrapped 95% CI for the respective c-indices.
To compare absolute differences in the treatment effect of dapagliflozin versus placebo according to baseline HHF risk in the full DECLARE-TIMI 58 biomarker cohort (N = 14,548), we calculated the absolute risk reduction (ARR) by subtracting the Kaplan-Meier event rates for HHF at 4 years in patients treated with dapagliflozin from the Kaplan-Meier event rates for HHF at 4 years in patients treated with placebo across each risk category. To assess the trend of ARR in HHF with dapagliflozin by baseline HHF risk, we used an inverse-variance weighted least squares model, regressing ARR on risk score category. As an exploratory analysis, we also assessed the relative and absolute risk difference in HHF at 2 years with saxagliptin versus placebo by modeled risk category using the same approach in the full SAVOR-TIMI 53 biomarker cohort (N = 12,176).
Statistical analyses were performed in SAS 9.4 (SAS Institute, Cary, NC) and R 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria) software. All P values are two-sided unless otherwise specified.
Results
Biomarker Distributions and Baseline Characteristics
The median baseline hsTnT and NT-proBNP values in the 6,106 patients in the derivation cohort were 11.9 (25th–75th percentiles, 8.1–18.5) ng/L and 140 (25th–75th percentiles, 63–329) pg/mL, respectively. The median baseline hsTnT and NT-proBNP values in the 7,251 patients in the validation cohort were 10.2 (25th–75th percentiles, 6.9–15.4) ng/L and 75 (25th–75th percentiles, 36–168) pg/mL, respectively. A history of HF was documented in 13% of the patients in the derivation cohort compared with 10% in the validation cohort. Baseline characteristics of the derivation cohort stratified by prespecified biomarker categories are shown in Table 1, and those for the validation cohort are shown in Supplementary Table 1. Patients in the highest categories of hsTnT and NT-proBNP were older and had a greater burden of established cardiovascular and kidney disease at the time of randomization. There were 168 HHF events in the derivation cohort and 251 HHF events in the validation cohort. Of these, 57% were incident HHF events in patients without a history of HF.
Table 1.
Baseline characteristics by established biomarker categories in the derivation cohort
| hsTnT (ng/L) | NT-proBNP (pg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <6 (n = 633) | 6 to <10 (n = 1,700) | 10 to <14 (n = 1,343) | ≥14 (n = 2,430) | P value | <50 (n = 1,170) | 50 to <125 (n = 1,665) | 125 to <450 (n = 2,121) | ≥450 (n = 1,150) | P value | |
| Demographics | ||||||||||
| Age, years | 61 (55–65) | 63 (59–69) | 65 (60–71) | 67 (62–74) | <0.001 | 61 (56–66) | 64 (59–69) | 67 (61–72) | 69 (63–74) | <0.001 |
| Female sex | 389 (61.5) | 746 (43.9) | 378 (28.1) | 511 (21.0) | <0.001 | 289 (24.7) | 581 (34.9) | 769 (36.3) | 385 (33.5) | <0.001 |
| White race | 447 (70.6) | 1,350 (79.4) | 1,101 (82.0) | 1,997 (82.2) | <0.001 | 899 (76.8) | 1,296 (77.8) | 1,742 (82.1) | 958 (83.3) | <0.001 |
| BMI, kg/m2 | 30.3 (27.2–34.5) | 30.8 (27.7–34.7) | 30.7 (27.6–34.8) | 30.8 (27.7–35.0) | 0.218 | 31.1 (28.0–35.3) | 30.8 (27.7–34.5) | 30.5 (27.4–34.9) | 30.4 (27.3–34.5) | 0.004 |
| Diabetes history | ||||||||||
| Duration of T2DM, years | 7.2 (3.6–12.5) | 8.8 (4.4–14.8) | 10.2 (5.2–15.9) | 12.6 (6.8–20.2) | <0.001 | 8.0 (4.1–13.3) | 10.0 (5.3–15.7) | 10.7 (5.4–18.3) | 12.8 (6.8–20.3) | <0.001 |
| HbA1c, % | 7.5 (6.8–8.5) | 7.5 (6.9–8.6) | 7.6 (6.9–8.7) | 7.7 (7.0–8.9) | <0.001 | 7.7 (6.9–8.8) | 7.7 (6.9–8.8) | 7.5 (6.9–8.5) | 7.7 (6.9–8.7) | 0.029 |
| Baseline insulin use | 177 (28.0) | 578 (34.0) | 517 (38.5) | 1,233 (50.7) | <0.001 | 403 (34.4) | 662 (39.8) | 885 (41.7) | 555 (48.3) | <0.001 |
| Diabetic retinopathy | 29 (4.6) | 149 (8.8) | 154 (11.5) | 412 (17.0) | <0.001 | 99 (8.5) | 182 (10.9) | 278 (13.1) | 185 (16.1) | <0.001 |
| Diabetic nephropathy | 39 (6.2) | 173 (10.2) | 182 (13.6) | 647 (26.6) | <0.001 | 123 (10.5) | 223 (13.4) | 369 (17.4) | 326 (28.3) | <0.001 |
| eGFR, mL/min/1.73 m2 | 91 (80–98) | 84 (71–94) | 75 (62–89) | 64 (47–80) | <0.001 | 88 (75–96) | 81 (68–93) | 72 (57–86) | 58 (43–76) | <0.001 |
| UACR, mg/g | 8 (4–19) | 10 (5–30) | 14 (6–51) | 35 (10–178) | <0.001 | 9 (5–29) | 11 (5–38) | 18 (7–70) | 51 (14–279) | <0.001 |
| Other comorbidities | ||||||||||
| Coronary artery disease | 280 (44.2) | 943 (55.5) | 879 (65.5) | 1,728 (71.1) | <0.001 | 525 (44.9) | 896 (53.8) | 1,505 (71.0) | 904 (78.6) | <0.001 |
| Prior myocardial infarction | 177 (28.0) | 575 (33.8) | 550 (41.0) | 1,068 (44.0) | <0.001 | 314 (26.8) | 550 (33.0) | 899 (42.4) | 607 (52.8) | <0.001 |
| Peripheral artery disease | 95 (15.0) | 168 (9.9) | 152 (11.3) | 371 (15.3) | <0.001 | 143 (12.2) | 223 (13.4) | 261 (12.3) | 159 (13.8) | 0.497 |
| Ischemic stroke | 68 (10.7) | 194 (11.4) | 144 (10.7) | 310 (12.8) | 0.207 | 128 (10.9) | 201 (12.1) | 234 (11.0) | 153 (13.3) | 0.200 |
| Prior heart failure | 34 (5.4) | 144 (8.5) | 153 (11.4) | 473 (19.5) | <0.001 | 57 (4.9) | 126 (7.6) | 273 (12.9) | 348 (30.3) | <0.001 |
| Atrial fibrillation | 13 (2.1) | 79 (4.6) | 92 (6.9) | 286 (11.8) | <0.001 | 10 (0.9) | 54 (3.2) | 142 (6.7) | 264 (23.0) | <0.001 |
| Percutaneous coronary intervention | 115 (18.2) | 390 (22.9) | 313 (23.3) | 630 (25.9) | <0.001 | 252 (21.5) | 362 (21.7) | 556 (26.2) | 278 (24.2) | 0.003 |
| Coronary artery bypass grafting | 67 (10.6) | 313 (18.4) | 342 (25.5) | 763 (31.4) | <0.001 | 97 (8.3) | 300 (18.0) | 641 (30.2) | 447 (38.9) | <0.001 |
| Dyslipidemia | 423 (66.8) | 1,177 (69.3) | 968 (72.1) | 1,757 (72.3) | 0.014 | 816 (69.8) | 1,171 (70.4) | 1,491 (70.3) | 847 (73.7) | 0.139 |
| Hypertension | 471 (74.4) | 1,384 (81.4) | 1,137 (84.7) | 2,042 (84.1) | <0.001 | 919 (78.6) | 1,361 (81.7) | 1,785 (84.2) | 969 (84.3) | <0.001 |
| Current smoker | 85 (13.4) | 260 (15.3) | 186 (13.8) | 309 (12.7) | 0.129 | 211 (18.0) | 242 (14.5) | 265 (12.5) | 122 (10.6) | <0.001 |
| Vital signs | ||||||||||
| Heart rate, bpm | 72 (64–78) | 70 (63–78) | 69 (62–76) | 70 (62–78) | <0.001 | 72 (66–80) | 70 (63–78) | 68 (62–76) | 70 (63–78) | <0.001 |
| Systolic blood pressure, mmHg | 135 (124–145) | 137 (126–146) | 138 (125–147) | 138 (125–149) | 0.007 | 135 (124–144) | 138 (125–147) | 138 (127–149) | 137 (123–148) | <0.001 |
| Diastolic blood pressure, mmHg | 80 (74–86) | 80 (73–86) | 80 (71–85) | 79 (70–85) | <0.001 | 81 (74–87) | 80 (73–86) | 80 (70–85) | 78 (70–85) | <0.001 |
Development of a Biomarker-Based Risk Score for HHF
In a multivariable risk model incorporating NT-proBNP, hsTnT, and the five components of TRS-HFDM, the strongest indicators of HHF risk were NT-proBNP, prior HF, and hsTnT (each P < 0.001) (Supplementary Fig. 1). These three variables, which accounted for 95.1% of the prognostic information provided by the full seven-variable model, were selected for inclusion in the final model. For ease of clinical application, cut points for NT-proBNP and hsTnT were used to develop the final risk score. Given the direct monotonic relationships between baseline biomarker concentration and risk of HHF (Supplementary Fig. 2), multiple biomarker cut points were used to capture these risk gradients rather than a single cut point. The specific levels were selected a priori framed by established clinical thresholds. Finally, integer weights were assigned to each variable in proportion to the magnitude of the regression coefficients in the multivariable model. The resulting integer score, named the TIMI Biomarker Score for Heart Failure in Diabetes, has a maximum value of 11 points (Table 2). Risk categories were defined based on the distribution of HHF event rates across integer risk scores in the derivation cohort.
Table 2.
TIMI Biomarker Score for Heart Failure in Diabetes in the derivation cohort
| Variable | β Coefficient | Points |
|---|---|---|
| Prior HF | 1.078 | 2 |
| hsTnT | ||
| <6 ng/L | Reference | 0 |
| 6 to <10 ng/L | 0.441 | 1 |
| 10 to <14 ng/L | 1.241 | 2 |
| ≥14 ng/L | 2.155 | 3 |
| NT-proBNP | ||
| <50 ng/L | Reference | 0 |
| 50 to <125 ng/L | 1.460 | 2 |
| 125 to <450 ng/L | 2.598 | 4 |
| ≥450 ng/L | 3.704 | 6 |
| Maximum possible score = 11 points |
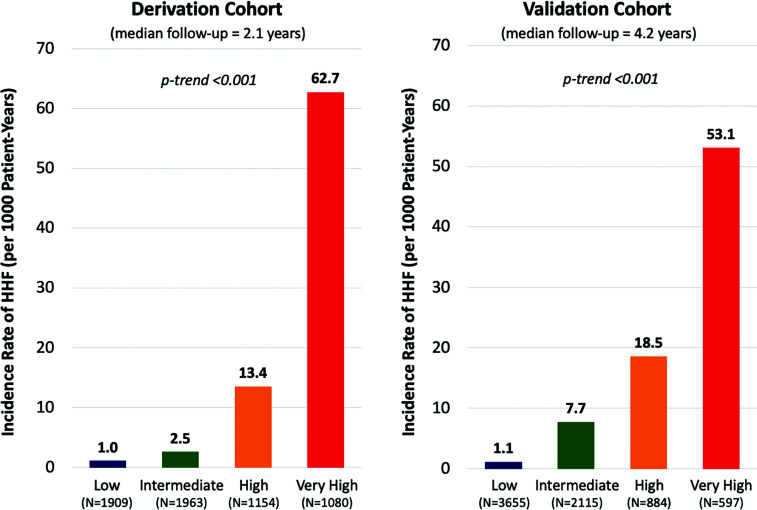
The biomarker-based integer risk score identified a significant gradient of HHF risk (Fig. 1) and had a c-index of 0.87 (95% CI 0.84–0.89) for the prediction of HHF in the derivation cohort. The internal bootstrap validation suggested minimal overfitting of the model (optimism-corrected c-index 0.87). In the external validation cohort, the score also had excellent discrimination (c-index 0.84 [95% CI 0.81–0.86]), identifying a similar gradient of HHF risk across risk categories (Fig. 1). Furthermore, these risk assessments identified stable, approximately linear HHF risk out to at least a 4-year time horizon in the external validation cohort (Fig. 2). Finally, the biomarker-based integer risk score had excellent discrimination for the composite of cardiovascular death and HHF, the coprimary end point in DECLARE-TIMI 58 (Supplementary Fig. 3).
Figure 1.
Incidence rates of HHF by risk category in the derivation and validation cohorts. Risk was categorized as low risk (0–3 points), intermediate risk (4–6 points), high risk (7–8 points), and very high risk (9–11 points). Incidence rates of HHF are shown for each risk category. The biomarker-based risk score identified a gradient of HHF risk with comparable incidence rates in each cohort.
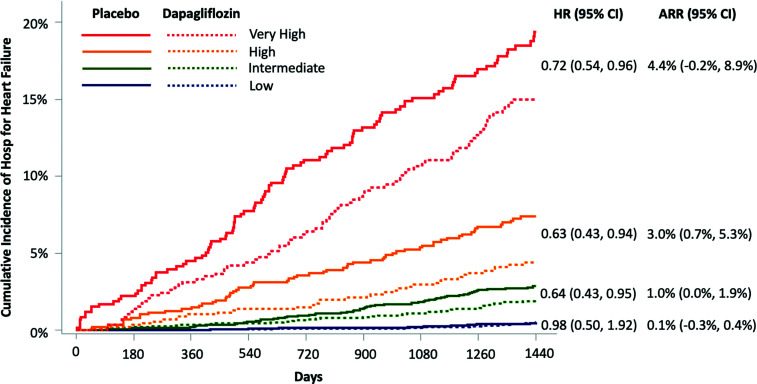
Figure 2.
Treatment effect of dapagliflozin by risk category predicted by the biomarker-based risk score. ARR was calculated by subtracting the Kaplan-Meier event rates for HHF at 4 years in patients treated with dapagliflozin from the Kaplan-Meier event rates for HHF at 4 years in patients treated with placebo across each risk score category. There was a significant gradient of increasing ARR with increasing risk category.
In a sensitivity analysis restricted to patients without prior HF (in whom the maximum score was therefore only 9 points), there was a consistent gradient of HHF risk (Supplementary Fig. 4), and the c-indices for the biomarker-based risk score were 0.84 (95% CI 0.81–0.88) in the derivation cohort and 0.80 (95% CI 0.77–0.83) in the external validation cohort.
The score had nearly perfect calibration in the external validation cohort, with a Greenwood-Nam-D’Agostino statistic of P = 0.93 (nonsignificant P values indicate adequate calibration) (Supplementary Fig. 5). Finally, when the biomarker-based risk score was compared with the clinical variable-only risk score (TRS-HFDM) in the external validation cohort, the biomarker-based risk score had superior discrimination (c-index 0.84 [95% CI 0.813–0.859] vs. 0.78 [95% CI 0.751–0.811]).
TIMI Biomarker Score for Heart Failure in Diabetes and Clinical Benefit of Dapagliflozin
When applied to the full biomarker cohort from the DECLARE-TIMI 58 trial, the TIMI Biomarker Score for Heart Failure in Diabetes identified significant gradients of HHF risk among dapagliflozin-treated patients (log-rank _P_-trend <0.001). Patients in the low-risk group had very low HHF event rates irrespective of treatment group assignment (Kaplan-Meier estimates at 4 years ∼0.5%). Moreover, dapagliflozin did not significantly reduce HHF risk in this low-risk group over the time horizon of the trial (hazard ratio [HR] for dapagliflozin vs. placebo 0.98 [95% CI 0.50–1.92]). By contrast, patients in the intermediate-, high-, and very-high-risk categories all derived a significant treatment benefit from dapagliflozin, with HRs of 0.64 (95% CI 0.43–0.95), 0.63 (0.43–0.94), and 0.72 (0.54–0.96), respectively. Although these relative risk reductions were similar, the absolute treatment benefit from dapagliflozin was greater in those at higher baseline risk, with ARRs of 1.0% (95% CI 0.0–1.9), 3.0% (0.7–5.3), and 4.4% (−0.2 to 8.9) at 4 years among patients in the intermediate-, high-, and very-high-risk categories, respectively (_P_-trend for ARR <0.001) (Fig. 2). In a sensitivity analysis restricted to patients without prior HF, there was a consistent gradient of absolute treatment benefit from dapagliflozin (Supplementary Fig. 6).
TIMI Biomarker Score for Heart Failure in Diabetes and Safety of Saxagliptin
When applied to the full biomarker cohort from the SAVOR-TIMI 53 trial, the TIMI Biomarker Score for Heart Failure in Diabetes also identified a significant gradient of HHF risk among saxagliptin-treated patients (log-rank _P_-trend <0.001). Patients in the low-risk group had a very low HHF event rate at 2 years, and saxagliptin treatment was not associated with an increased risk of HHF in this group (HR for saxagliptin vs. placebo 0.99 [95% CI 0.25–3.95]). Among patients in the intermediate-, high-, and very-high-risk categories, there was a higher risk of HHF in patients treated with saxagliptin vs. placebo (HR 1.26 [95% CI 1.03–1.55]), with a gradient of absolute risk increase according to risk category (_P_-trend for absolute risk difference = 0.003) (Supplementary Fig. 7).
Conclusions
In this study, we developed and validated a novel biomarker-based risk score for predicting HHF in two large clinical trial cohorts of patients with T2DM at high atherosclerotic cardiovascular risk. The score leverages the prognostic importance of two widely available clinical biomarkers—hsTnT and NT-proBNP—and the single clinical variable of prior HF. The score performs very well when restricted to patients without a history of HF (effectively leveraging the prognostic performance of the biomarkers alone), demonstrating its value for forecasting incident HF events. This biomarker-based risk score is well-calibrated between cohorts and further refines risk estimates when compared with a clinical variable-only risk score. Furthermore, it identifies higher-risk individuals with and without prior HF who derive greater treatment benefit from SGLT2 inhibitors with respect to absolute reduction in risk of HHF over a 4-year time horizon. Results from these analyses highlight a role for comprehensive cardiovascular risk profiling in patients with T2DM and provide a potentially helpful decision support tool to clinicians who care for patients with T2DM.
Biomarkers for HF Risk Assessment
The natriuretic peptides, including BNP and its prohormone fragment NT-proBNP, are the gold standard biomarkers for the diagnosis and assessment of HF (17). Most widely embraced for their role in HF diagnosis (18,19), the natriuretic peptides also predict incident and recurrent HF events in patients with acute and chronic HF syndromes, acute coronary syndromes, stable atherothrombotic disease, and even those without clinically overt cardiovascular disease (20–23).
Despite the primacy of the natriuretic peptides for HF prognostication, elevated concentrations of cardiac troponin, the canonical biomarker of myocardial injury, are also associated with increased HF risk in patients with acute and chronic HF syndromes as well as pre-HF conditions such as stable atherothrombotic disease (24,25). On the basis of the available data, natriuretic peptides and cardiac troponin have class I indications for risk assessment in current HF guidelines (26). Moreover, the widespread use and availability of natriuretic peptide and cardiac troponin assays make their application to clinical practice particularly attractive.
As recognized by the American College of Cardiology/American Heart Association HF classification system and the recent Universal Definition and Classification of Heart Failure consensus statement, T2DM is an “at risk for HF” disease state (“stage A”) with a continuum of disease progression toward clinical HF (27,28). The mechanisms by which dysglycemia contributes to HF pathogenesis include progressive microangiopathy and endothelial dysfunction, which in turn lead to cardiomyocyte stress and cardiomyocyte injury (1). Circulating biomarkers reflecting these subclinical myocardial structural changes thus provide the opportunity to detect underlying disease progression better than do clinical variables alone. The fact that NT-proBNP and hsTnT emerged as stronger indicators of incident and recurrent HHF risk than most other clinical variables in this analysis highlights this point.
Potential Application of the TIMI Biomarker Score for Heart Failure in Diabetes in Clinical Practice
Strategies for mitigating cardiovascular disease risk in patients with T2DM have traditionally focused on reducing atherothrombotic complications. By contrast, the significance of HF complicating T2DM has been largely underappreciated, and until recently, HHF has not been included as a component of the primary end point of clinical trials enrolling patients with T2DM (29,30). Furthermore, in contrast to atherothrombotic risk, HF risk in patients with T2DM persists even when other cardiovascular risk factors (e.g., LDL-C and hypertension) are within therapeutic target ranges (31). As such, identifying and targeting HF risk is critical to improving cardiovascular outcomes in the global pandemic of T2DM.
The TIMI Biomarker Score for Heart Failure in Diabetes offers a high-performing new tool for comprehensive cardiovascular risk assessment of patients with T2DM. Expanding on the framework created by TRS-HFDM, this biomarker-based risk score may allow clinicians to more effectively counsel patients about their HF risk and identify particularly strong candidates with and without prior HF for early initiation of SGLT2 inhibitors. It may also be used to identify low-risk patients in whom saxagliptin may be used safely. While TRS-HFDM has the advantage of using patient characteristics that can be readily obtained from the medical record of a typical patient with T2DM (and thus does not require any additional testing), the biomarker-based risk score offers superior discrimination and better assessment of SGLT2 inhibitor-related HHF risk reduction. In the era of precision medicine, these improvements lend support to the value of biologically based risk tools and the use of circulating biomarkers in the management of cardiovascular disease more broadly.
Limitations
Several limitations of this analysis deserve mention. First, the biomarker-based risk score was derived and validated in two clinical trial cohorts with selected populations of patients with T2DM, which may affect the generalizability of the results. Reassuringly, despite the differences in cardiovascular and kidney disease burden between the derivation and external validation cohorts, the score was well calibrated and had excellent discrimination in each, suggesting that the score performs well in diverse populations of patients with T2DM. Nevertheless, additional validation efforts in unselected community-based cohorts would be valuable.
Second, patients with severe kidney disease (i.e., eGFR <30 mL/min/1.73 m2) were excluded from both trials and were therefore not represented in the derivation or validation cohorts. Since the concentrations of both hsTnT and NT-proBNP are affected by impaired renal clearance, additional validation studies would be needed to assess the performance of the biomarker-based risk score in patients with eGFR <30 mL/min/1.73 m2.
Third, this derivation of the biomarker-based risk score uses cut points for the high-sensitivity cardiac troponin assay and natriuretic peptide assay used in this study, which cannot automatically be applied to other assays. Future studies are needed to define analogous cut points for BNP and assays for high-sensitivity troponin I (hsTnI).
Fourth, since prior history of HF was not a qualifying condition for the SAVOR-TIMI 53 or DECLARE-TIMI 58 trials, this variable relied on investigator report.
Fifth, the derivation cohort database did not include echocardiographic data, such as left ventricular ejection fraction, so we did not include left ventricular ejection fraction as a candidate risk indicator and did not separately model risk of HHF with HF with reduced ejection fraction versus with preserved ejection fraction.
Finally, SGLT2 inhibitors have pleiotropic metabolic and cardiorenal effects favoring their use in broad populations, including lowering HbA1c and systolic blood pressure, promoting weight loss, and slowing the progression of kidney disease. It should therefore be emphasized that the biomarker-based risk score predicts the benefit of SGLT2 inhibitors with respect to HHF risk reduction and is not designed to predict other potential benefits.
In conclusion, circulating biomarkers improve HF risk assessment in patients with T2DM, providing insight into cardiovascular disease progression and severity. We developed and validated a biomarker-based score for predicting HHF risk in patients with T2DM that incorporates NT-proBNP, hsTnT, and prior HF. This simple biomarker-based risk tool provides superior discrimination to a clinical variable-only risk tool and identifies higher-risk patients who derive a greater absolute treatment benefit from SGLT2 inhibitors.
Article Information
Funding. D.D.B., S.D.W., B.M.S., M.S.S., and D.A.M., were supported for the present analysis by the American Heart Association Cardiometabolic Health & Type 2 Diabetes Mellitus Strategically Focused Research Network (20SFRN35120087). The SAVOR-TIMI 53 and DECLARE-TIMI 58 trials were supported by institutional research grants to Brigham and Women’s Hospital from AstraZeneca. Biomarker testing was supported by grants from Roche Diagnostics (reagent only). D.D.B. is supported by Harvard Catalyst KL2/CMeRIT (National Institutes of Health, National Center for Advancing Translational Sciences grant UL 1TR002541). T.A.Z. reports research grants from the German Research Foundation, and Austrian Science Funds.
Duality of Interest. D.D.B. has received research grant support to his institution from AstraZeneca and Pfizer, and consulting fees from AstraZeneca. S.D.W. reports grants from Amgen, Arena, AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Eisai, Eli Lilly, Janssen, Merck, and Sanofi, and consulting fees from Arena, AstraZeneca, Aegerion, Allergan, AngelMed, Boehringer-Ingelheim, Boston Clinical Research Institute, Bristol-Myers Squibb, Daiichi Sankyo, Eisai, Eli Lilly, Icon Clinical, Janssen, Lexicon, Merck, Servier, St. Jude Medical, and Xoma. His spouse, Dr. Caroline Fox, is an employee of Merck. B.M.S. reports research grants via Brigham and Women's Hospital from AstraZeneca, Eisai, Novartis, and Merck, consulting fees from AstraZeneca, Biogen Idec, Boehringer Ingelheim, Covance, Dr. Reddy's Laboratory, Eisai, Elsevier PracticeUpdate Cardiology, GlaxoSmithKline, Lexicon, Merck, Novo Nordisk, Sanofi, and St. Jude's Medical, and equity in Health [at] Scale. T.A.Z. reports honoraria from AstraZeneca and Boehringer Ingelheim. E.L.G. reports grants from AstraZeneca during the conduct of the study. P.Ja. has received research support from Abbott Laboratories, Amgen Inc., AstraZeneca, Daiichi Sankyo, Inc., Eisai, Inc., GlaxoSmith Kline, Merck & Co., Inc., Regeneron Pharmaceuticals, Inc., Roche Diagnostics Corp., Siemens Healthineers, Takeda Global Research and Development Center, and Waters Technologies Corp., and consulting fees from Roche Diagnostics. O.M. sits on advisory boards for Novo Nordisk, Eli Lilly, Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim, Novartis, AstraZeneca, and BOL Pharma, has received research grant support through Hadassah Hebrew University Hospital from Novo Nordisk and AstraZeneca, and has received speaker’s fees from AstraZeneca, Novo Nordisk, Eli Lilly, Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim, and Jansen. A.C. reports grants and personal fees from AstraZeneca and Novo Nordisk and personal fees from Abbott, Eli Lilly, Sanofi, Boehringer Ingelheim, Merck Sharp & Dohme, Medial Early-Sign, and GlucoMe. D.L.B. discloses the following relationships: advisory board for Cardax, CellProthera, Cereno Scientific, Elsevier PracticeUpdate Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, and Regado Biosciences; board of directors for Boston VA Research Institute, Society of Cardiovascular Patient Care, and TobeSoft; inaugural chair, American Heart Association Quality Oversight Committee; data monitoring committees for Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the CENTERA THV System in Intermediate Risk Patients Who Have Symptomatic, Severe, Calcific, Aortic Stenosis [ExCEED] trial, funded by Edwards), Contego Medical (Chair, Protection Against Emboli During Carotid Artery Stenting Using the Neuroguard IEP System [PERFORMANCE 2]), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the Edoxaban Compared to Standard Care After Heart Valve Replacement Using a Catheter in Patients With Atrial Fibrillation [ENVISAGE] trial, funded by Daiichi Sankyo), Novartis, and Population Health Research Institute; honoraria from Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, Evaluation of Dual Therapy With Dabigatran vs. Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting [RE-DUAL PCI] clinical trial steering committee funded by Boehringer Ingelheim, Study to Investigate CSL112 in Subjects With Acute Coronary Syndrome [AEGIS-II] executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the A Trial Comparing Cardiovascular Safety of Degarelix Versus Leuprolide in Patients With Advanced Prostate Cancer and Cardiovascular Disease [PRONOUNCE] trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor, Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (Continuing Medical Education steering committees), MJH Life Sciences, Population Health Research Institute (for the Cardiovascular OutcoMes for People Using Anticoagulation StrategieS [COMPASS] operations committee, publications committee, steering committee, and U.S. national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), and WebMD (Continuing Medical Education steering committees); and other for Clinical Cardiology (Deputy Editor), National Cardiovascular Data Registry-Acute Coronary Treatment and Intervention Outcomes Network [NCDR-ACTION] Registry Steering Committee (Chair), Veterans Health Administration Clinical Assessment, Reporting and Tracking System for Cath Labs [VA CART] Research and Publications Committee (Chair); research funding from Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Lexicon, Eli Lilly, Medtronic, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, The Medicines Company, and 89Bio; royalties from Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); site co-investigator for Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Philips, and Svelte; trustee for American College of Cardiology; and unfunded research for FlowCo, Merck, and Takeda. L.A.L. reports speaker’s bureau/honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi, and Servier, consulting Fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi, and Servier, and participation in clinical trials funded by AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Janssen, Novo Nordisk, and Sanofi. D.K.M. has received personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Janssen, Lexicon, Merck, Merck Sharp & Dohme, Novo Nordisk, Sanofi, Eisai, Esperion, GlaxoSmithKline, Eli Lilly, Pfizer, Metavant, Applied Therapeutics, Afimmune, and CSL Behring. J.P.H.W. reports grants, consultancy fees (paid to his institution), and personal fees for lectures and trial steering committee participation from AstraZeneca, grants, consultancy fees (paid to his institution), and personal fees for lectures from Novo Nordisk, consultancy fees (paid to his institution) and personal fees for lectures from Boehringer Ingelheim, Janssen, Napp, Mundipharma, Eli Lilly, Takeda, and Sanofi, and consultancy fees (paid to his institution) from Wilmington Healthcare. P.Jo. and A.M.L. are employees and shareholders of AstraZeneca. I.R. reports personal fees from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Concenter BioPharma and Silkim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, Orgenesis, Pfizer, Sanofi, SmartZyme Innovation, Panaxia, FuturRx, Insuline Medical, Medial EarlySign, CameraEyes, Exscopia, Dermal Biomics, Johnson & Johnson, Novartis, Teva, GlucoMe, and DarioHealth. E.B. reports grants to his institution from AstraZeneca, Daiichi Sankyo, Merck, and Novartis, and personal fees for consultancies with Amgen, Cardurion, MyoKardia, Novo Nordisk, and Verve. M.S.S. reports research grant support through Brigham and Women’s Hospital from Amgen, Anthos Therapeutics, AstraZeneca, Bayer, Daiichi Sankyo, Eisai, Intarcia, Medicines Company, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, and Takeda, and consulting for Althera, Amgen, Anthos Therapeutics, AstraZeneca, Bristol-Myers Squibb, CVS Caremark, DalCor, Dr. Reddy’s Laboratories, Dyrnamix, Esperion, IFM Therapeutics, Intarcia, Janssen Research and Development, Medicines Company, MedImmune, Merck, and Novartis. D.A.M. reports grants to the TIMI Study Group from Abbott Laboratories, Amgen Anthos Therapeutics, AstraZeneca, BRAHMS, Eisai, GlaxoSmithKline, Medicines Co., Merck, Novartis, Pfizer, Roche Diagnostics, Quark, Siemens, and Takeda, and consultant fees from InCardia, Merck & Co, Novartis, and Roche Diagnostics. D.D.B., S.D.W., B.M.S., E.L.G., E.B., M.S.S., and D.A.M. are members of the TIMI Study Group, which has received institutional research grant support through Brigham and Women’s Hospital from Abbott, Amgen, Anthos Therapeutics, ARCA Biopharma, AstraZeneca, Bayer, Daiichi-Sankyo, Eisai, Intarcia, Ionis Pharmaceuticals, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Regeneron Pharmaceuticals, Roche, Siemens Healthcare Diagnostics, The Medicines Company, and Zora Biosciences. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. D.D.B. drafted the manuscript. D.D.B., S.D.W., M.S.S., and D.A.M. developed the study concept and design and interpreted the data. S.D.W., B.M.S., T.A.Z., E.L.G., P.Ja., O.M., A.C., D.L.B., L.A.L., D.K.M., J.P.H.W., P.Jo., A.M.L., I.R., E.B., M.S.S., and D.A.M. critically revised the manuscript. E.L.G. performed the statistical analyses. D.D.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the American Heart Association Virtual Scientific Sessions, 13–17 November 2020.
Footnotes
References
- 1.Braunwald E. Diabetes, heart failure, and renal dysfunction: the vicious circles. Prog Cardiovasc Dis 2019;62:298–302 [DOI] [PubMed] [Google Scholar]
- 2.Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC Jr. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 2004;27:699–703 [DOI] [PubMed] [Google Scholar]
- 3.Carr AA, Kowey PR, Devereux RB, et al. Hospitalizations for new heart failure among subjects with diabetes mellitus in the RENAAL and LIFE studies. Am J Cardiol 2005;96:1530–1536 [DOI] [PubMed] [Google Scholar]
- 4.Stefan N, Fritsche A, Schick F, Häring HU. Phenotypes of prediabetes and stratification of cardiometabolic risk. Lancet Diabetes Endocrinol 2016;4:789–798 [DOI] [PubMed] [Google Scholar]
- 5.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393: 31–39 [DOI] [PubMed] [Google Scholar]
- 6.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 7.Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377:644–657 [DOI] [PubMed] [Google Scholar]
- 8.Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 9.Cannon CP, Pratley R, Dagogo-Jack S, et al.; VERTIS CV Investigators . Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020;383:1425–1435 [DOI] [PubMed] [Google Scholar]
- 10.Berg DD, Wiviott SD, Scirica BM, et al. Heart failure risk stratification and efficacy of sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes mellitus. Circulation 2019;140: 1569–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zelniker TA, Morrow DA, Mosenzon O, et al. Relationship between baseline cardiac biomarkers and cardiovascular death or hospitalization for heart failure with and without SGLT2 inhibitor therapy in DECLARE-TIMI 58. Eur J Heart Fail 2021;23:1026–1036 [DOI] [PubMed] [Google Scholar]
- 12.Scirica BM, Braunwald E, Raz I, et al.; SAVOR-TIMI 53 Steering Committee and Investigators . Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial [published correction appears in Circulation 2015;132:e198]. Circulation 2014;130:1579–1588 [DOI] [PubMed] [Google Scholar]
- 13.Scirica BM, Bhatt DL, Braunwald E, et al. Prognostic implications of biomarker assessments in patients with type 2 diabetes at high cardio vascular risk: a secondary analysis of a randomized clinical trial. JAMA Cardiol 2016;1:989–998 [DOI] [PubMed] [Google Scholar]
- 14.Scirica BM, Bhatt DL, Braunwald E, et al.; SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326 [DOI] [PubMed] [Google Scholar]
- 15.Jaffe AS, Morrow DA, Scirica BM. High-sensitivity troponin in the triage of acute decompensated heart failure. JACC Heart Fail 2016;4:600–602 [DOI] [PubMed] [Google Scholar]
- 16.Huelsmann M, Neuhold S, Strunk G, et al. NT-proBNP has a high negative predictive value to rule-out short-term cardiovascular events in patients with diabetes mellitus. Eur Heart J 2008;29:2259–2264 [DOI] [PubMed] [Google Scholar]
- 17.Braunwald E. Heart failure. JACC Heart Fail 2013;1:1–20 [DOI] [PubMed] [Google Scholar]
- 18.McDonagh TA, Robb SD, Murdoch DR, et al. Biochemical detection of left-ventricular systolic dysfunction. Lancet 1998;351:9–13 [DOI] [PubMed] [Google Scholar]
- 19.Maisel AS, Krishnaswamy P, Nowak RM, et al.; Breathing Not Properly Multinational Study Investigators . Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002;347:161–167 [DOI] [PubMed] [Google Scholar]
- 20.de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med 2001;345:1014–1021 [DOI] [PubMed] [Google Scholar]
- 21.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004;350:655–663 [DOI] [PubMed] [Google Scholar]
- 22.Omland T, Sabatine MS, Jablonski KA, et al.; PEACE Investigators . Prognostic value of B-type natriuretic peptides in patients with stable coronary artery disease: the PEACE Trial. J Am Coll Cardiol 2007;50:205–214 [DOI] [PubMed] [Google Scholar]
- 23.Berger R, Huelsman M, Strecker K, et al. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation 2002;105:2392–2397 [DOI] [PubMed] [Google Scholar]
- 24.Aimo A, Januzzi JL Jr, Vergaro G, et al. Prognostic value of high-sensitivity troponin T in chronic heart failure: an individual patient data meta-analysis. Circulation 2018;137:286–297 [DOI] [PubMed] [Google Scholar]
- 25.Omland T, de Lemos JA, Sabatine MS, et al.; Prevention of Events with Angiotensin Converting Enzyme Inhibition (PEACE) Trial Investigators . A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med 2009;361: 2538–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136:e137–e161 [DOI] [PubMed] [Google Scholar]
- 27.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:1810–1852 [DOI] [PubMed] [Google Scholar]
- 28.Bozkurt B, Coats AJ, Tsutsui H, et al. Universal Definition and Classification of Heart Failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail 2021;27:P387–P413 [Google Scholar]
- 29.McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol 2014;2:843–851 [DOI] [PubMed] [Google Scholar]
- 30.Standl E, Schnell O, McGuire DK. Heart failure considerations of antihyperglycemic medications for type 2 diabetes. Circ Res 2016;118:1830–1843 [DOI] [PubMed] [Google Scholar]
- 31.Rawshani A, Rawshani A, Franzén S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2018;379:633–644 [DOI] [PubMed] [Google Scholar]