The Rel/NF-κB Family Directly Activates Expression of the Apoptosis Inhibitor Bcl-xL (original) (raw)
Abstract
The transcription factors of the Rel/NF-κB family are key regulators of immune and inflammatory responses and contribute to lymphocyte proliferation, survival, and oncogenesis. The absolute correlation between the antiapoptotic and oncogenic activities of the Rel/NF-κB oncoprotein v-Rel emphasizes the importance of characterizing the death antagonists under NF-κB control. Our recent finding that the prosurvival Bcl-2 homolog Bfl-1 (also called A1) is a direct transcriptional target of NF-κB raised the issue of whether NF-κB is a specific or global regulator of death antagonists in the Bcl-2 family. Here, we demonstrate that NF-κB differentially regulates the expression of particular Bcl-2-related death inhibitors and that it directly activates the expression of Bcl-xL. While Bcl-xL was significantly upregulated by c-Rel and RelA, Bcl-2 was not. Importantly, stimuli that activate endogenous NF-κB factors also upregulated bcl-x gene expression and this effect was antagonized by an inhibitor of NF-κB activity. The expression of bcl-x suppressed apoptosis in the presence or absence of NF-κB activity. Functional analysis of the bcl-x promoter demonstrated that it is directly controlled by c-Rel. These results establish that NF-κB directly regulates the expression of distinct prosurvival factors in the Bcl-2 family, such as Bcl-xL and Bfl-1/A1. These findings raise the possibility that some of these factors may contribute to oncogenesis associated with aberrant Rel/NF-κB activity.
Apoptosis is an inducible suicide program that occurs at all stages of multicellular life. It is required for normal development, immune system function, tissue remodeling, and prevention of inappropriate cellular proliferation. Dysregulated cell death is associated with various pathological conditions, including neurodegenerative disorders, autoimmune diseases, and cancer (reviewed in reference 17). Experimental evidence suggests a proactive role for the Rel/NF-κB family of transcription factors in the inhibition of programmed cell death, as shown by the following observations. (i) Homozygous inactivation of RelA in mice led to extensive apoptosis in the liver (6). (ii) Inactivation of endogenous Rel/NF-κB factors by superrepressor forms of the inhibitory protein IκBα sensitized cells to stimulus-induced apoptosis (32, 54, 57, 62). (iii) Lymphoid cells transformed by the Rel/NF-κB oncoprotein v-Rel required continuous expression of v-Rel for survival. v-Rel inactivation by a temperature-sensitive mutation or through tetracycline-regulated control resulted in the rapid onset of apoptosis (59, 68). (iv) Transcriptionally competent Rel/NF-κB factors (v-Rel, c-Rel, and RelA) blocked apoptosis induced by tumor necrosis factor alpha (TNF-α), whereas those defective for transactivation did not (5, 13, 32, 67).
Consistent with the notion that NF-κB regulates the expression of genes that antagonize cell death, its protective activity is dependent on RNA and protein synthesis (reviewed in reference 55). Candidate target genes recently identified include those encoding the caspase inhibitors c-IAP1, c-IAP2, and X-IAP, the TNF receptor-associated factors TRAF1 and TRAF2, and the zinc finger protein A20 and the immediate-early response gene IEX-1L (15, 30, 50, 58, 63, 66). Thus, in addition to regulating the expression of genes important for immune and inflammatory responses, Rel/NF-κB also controls the transcription of genes that confer resistance to death-inducing signals.
The Bcl-2 family of cell death regulators is critical for determining cell fate in the apoptotic pathway. Bcl-2 and its mammalian homologs Bcl-xL, Bfl-1 (also called A1), and Mcl-1 block cell death, while Bax, Bcl-xS, Nbk (also called Bik), Bak, and Bad promote apoptosis (reviewed in reference 1). Each of these factors influences the cleavage-mediated activation of caspases, which act as the ultimate downstream effectors of the suicide program. While little is known about the signaling pathways that control the expression of Bcl-2-related factors and of the transcription factors involved in their regulation, exogenous expression of prosurvival Bcl-2-related proteins was shown to block apoptosis in lymphoid cells under conditions in which NF-κB activity was inhibited (62). This raised the possibility that some of these factors may lie downstream of NF-κB in the survival cascade. The recent demonstration that the prosurvival Bcl-2 homolog Bfl-1 is transcriptionally controlled by Rel/NF-κB is consistent with these results (23, 31, 56, 69). It is therefore important to determine whether NF-κB is a general or specific regulator of death antagonists in the Bcl-2 family.
Here, we show that NF-κB can differentially regulate the expression of prosurvival Bcl-2 family proteins. Whereas c-Rel and RelA had no effect on Bcl-2 expression in HT1080 and HtTA cells, they strongly upregulated the expression of Bcl-xL. bcl-x promoter activation was dependent on an NF-κB DNA site, and its protective activity was correlated with that of c-Rel. These results indicate that NF-κB activates distinct prosurvival Bcl-2 family proteins and suggest a role for these factors in the inhibition of cell death by Rel/NF-κB.
MATERIALS AND METHODS
Plasmids.
The human c-rel gene (hc-rel, a gift from N. Rice, ABL-NCI, Frederick, Md.) was stably expressed in a Tet-OFF system, under the control of a minimal cytomegalovirus (CMV) promoter and seven tetracycline operator sites from plasmid pUHD10-3-hygro (13) (pUHD10-3-hygro-hc-rel). In transient transfection assays, hc-rel was expressed from the CMV promoter of pJDCMV19SV (pCMV-hc-rel). Vectors expressing the death antagonists Bcl-2 and Bcl-xL from the CMV promoter of pcDNA3.0 (Invitrogen) were a gift from C. Labrie (CHUL, Québec, Canada). pCMV-β-gal expressed the β-galactosidase gene (β-gal) from a CMV promoter (69).
Cell culture and endogenous NF-κB activation.
Human HT1080 fibrosarcoma cells, HeLa cervical carcinoma cells, Jurkat T lymphocytic leukemia cells, CEM T cells, and 293 embryonal kidney cells were obtained from the American Type Culture Collection. Jurkat T cell lines expressing wild-type IκBα (IκBα-wt) or a constitutive IκBα inhibitor (IκBαΔN) were a gift from D. W. Ballard (Vanderbilt University, Nashville, Tenn.) (15). The cell line RC-K8, derived from a diffused large cell lymphoma, was a gift from N. Zeleznik-Le (University of Chicago, Chicago, Ill.) (33).
Endogenous NF-κB activity was induced upon treatment with TNF-α (1,000 U per ml; Sigma) for 2 h (CEM) or with phorbol 12-myristate 13-acetate (PMA) (50 ng per ml) plus ionomycin (concentration, 1 μM in 0.05% dimethylsulfoxide) for 2 h (Jurkat).
HeLa HtTA-derived cell clones conditionally expressing p65 (also known as RelA) or p50 (also known as NF-κB1) under tetracycline-regulated control were previously described (67, 69). Human HT1080-tTA fibrosarcoma cells stably expressing the tTA (tetracycline-regulated transactivator) protein were generated by cotransfecting HT1080 cells with pUHD15-1 (21) and pSV2Neo, using SuperFect (Qiagen). Neomycin-resistant HT1080-tTA cell clones were selected with G418 (200-μg potency units) in the presence of tetracycline-HCl (2 μg per ml) and screened for luciferase gene activation following transient transfection of the pUHD13-3 luciferase expression plasmid (21). The HT1080-hc-rel cell line that conditionally expressed hc-rel under tetracycline control was obtained by transfecting HT1080-tTA cells with pUHD10-3-hygro-hc-rel. Cell clones were selected in the presence of hygromycin B (400 μg per ml; Calbiochem) and screened for inducible expression of hc-Rel by immunoblotting. HT1080-hc-rel cells were maintained in minimum essential medium supplemented with 10% fetal bovine serum, 2 mM glutamine, Earle's balanced salt solution containing sodium bicarbonate (1.5 g per liter), 1× nonessential amino acids, 0.1 mM sodium pyruvate, and antibiotics (100 U of penicillin per ml and 100 μg of streptomycin per ml). Cells were maintained at 37°C in an atmosphere of 5% CO2. All cell clones were maintained in the presence of tetracycline (2 μg per ml) and refed every other day.
Immunoblotting.
Cells were induced to express the c-Rel, RelA, or p50 protein upon removal of tetracycline from the cell culture medium. Extracts were prepared in lysis buffer (50 mM Tris HCl [pH 7.5], 150 mM sodium chloride, 1% sodium deoxycholate, 1% Triton X-100, 10 μg of leupeptin per ml, 10 μg of pepstatin per ml, 20 μg of aprotinin per ml, 10 mM sodium pyrophosphate, 50 mM sodium fluoride, and 0.5 mM sodium orthovanadate) (42) and quantitated by the method of Bradford (10). Proteins (20 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Immunoblotting was performed by enhanced chemiluminescence (ECL; Amersham). Processing of procaspase 3 and procaspase 8 in HT1080-hc-rel cells was induced by treatment with TNF-α (5 ng per ml) plus cycloheximide (CHX; 5 μg per ml) before or after a 48-h induction of c-Rel expression. The hc-Rel protein was detected with rabbit polyclonal antibody SC-272 (Santa Cruz Biotechnology). Antibodies to caspase 3, Bcl-xL/S, and Bcl-2 were purchased from PharMingen. The anti-caspase 8 antibody was from Zymed Laboratories. An antiactin antibody (Sigma) was used as a control.
Northern blot analysis.
Total RNA (20 μg) extracted with RNAzol B (TEL-TEST) was fractionated in 1% agarose-formaldehyde gels and transferred onto Hybond-NX membranes (Amersham). Membranes were baked for 10 min at 80°C under vacuum and UV cross-linked with a Stratalinker (Stratagene). Probes were generated by random priming with Klenow DNA polymerase in the presence of [α-32P] dCTP and [α-32P] dGTP (19). A 28S oligonucleotide probe (5′-AAGGATCAGAGTAGTGGTATTTCACC-3′) was labeled with [γ-32P]ATP and T4 polynucleotide kinase. Membranes were hybridized in ExpressHyb (Clontech) according to the manufacturer's recommended procedure, or in 5× SSC (0.75 M NaCl plus 75 mM Na citrate [pH 7.0]), 5× Denhardt's solution, 0.5% SDS, and sheared salmon sperm DNA (100 μg per ml) at 65°C overnight. Membranes were washed twice in 2× SSC–0.1% SDS and twice in 1× SSC–0.1% SDS at 65°C, followed by autoradiography.
Apoptosis assays.
Cell resistance to TNF-α- or anti-Fas antibody-induced apoptosis in transient assays was examined as described previously (60). HT1080 cells (2 × 105) were cotransfected with pCMV-β-gal (0.5 μg) together with pCMV vectors expressing Bcl-xL or hc-Rel (1.5 μg), using SuperFect reagent (Qiagen). An empty pCMV vector was used as a control. After 30 h, the cells were treated with CHX either alone (30 μg per ml) or together with TNF-α (10 ng per ml; Sigma) or with anti-Fas antibodies (1 μg per ml; Calbiochem) for 14 h. After fixation and staining with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), cells were counted from a minimum of 10 fields chosen at random. Quantitation of cell survival represents the ratio of the number of cells expressing β-gal in wells treated with TNF-α or anti-Fas plus CHX over that in wells treated with CHX alone. In assays of cell death performed in the absence of cycloheximide, HeLa cells (3 × 106) were coelectroporated using a Bio-Rad Gene Pulser (220 V, 960 μF) with pCMV-β-gal (3 μg), an empty CMV vector, or pCMV-IκBαM (12 μg) to constitutively repress NF-κB, alone or together with pCMV-bcl-xl (6 μg). Cells were then distributed equally into two 35-mm wells and treated 24 h later with TNF-α (10 ng per ml) for 16 h. Cells were counted from a minimum of 10 fields chosen at random.
Cloning of the human bcl-x promoter region and transient CAT assays.
The human bcl-x promoter region was isolated by nested PCR amplification using a Genome Walker PromoterFinder kit (Clontech) and cloned in a promoterless vector expressing a chloramphenicol acetyltransferase (CAT) reporter gene (pCAT-basic; Promega). bcl-x promoter activity was analyzed by transient transfection of HT1080 cells with _bcl-x_-CAT reporter plasmids (0.8 μg) in the presence of a CMV-hc-rel expression vector (1.2 μg) or an empty pCMV vector as a control. Assays were performed with 20 μg of protein for 1.5 h. The relative CAT activity represents the average of three independent experiments. The bcl-x promoter region (from positions −298 to +22) cloned in pAlter-1 was subjected to site-directed mutagenesis to inactivate the NF-κB motif (mutated bases are underlined) at position −232 (TTTACTGCCC; −298/+22 mκB; Altered Sites Mutagenesis System [Promega]). Mutation of the NF-κB site was confirmed by sequencing.
DNA-binding assays.
The binding of Rel/NF-κB factors to the κB DNA site found in the bcl-x promoter was assayed in human 293 cells transiently transfected with CMV expression vectors for p50, p65, or c-Rel. Nuclear extracts (3 μg) were incubated with a double-stranded 32P-labeled NF-κB oligonucleotide probe derived from the bcl-x promoter region (5′-AGTGGGGGCGGGGGGGACTGCCCCCTCTCCTT-3′) or a control interleukin 6 (IL-6)-κB oligonucleotide probe (4 × 104 cpm) (64) in 12.5 mM HEPES (pH 7.9), 12% glycerol, 5 mM MgCl2, 60 mM KCl, 0.2 mM EDTA, 1 mM dithiothreitol, bovine serum albumin (1 μg per μl), poly(dI-dC) (2 μg) and analyzed on 5% native polyacrylamide gels as described previously (64). Where indicated, nuclear extracts were prepared from HT1080-hc-rel cells induced to express c-Rel for 0, 24, 48, 72, or 96 h following the removal of tetracycline. Extracts (3 μg) were analyzed for binding to the bcl-x κB DNA oligonucleotide probe in gel retardation assays, as described above.
RESULTS
c-Rel and RelA promote the expression of Bcl-xL in HT1080 and HtTA cells but have no effect on Bcl-2.
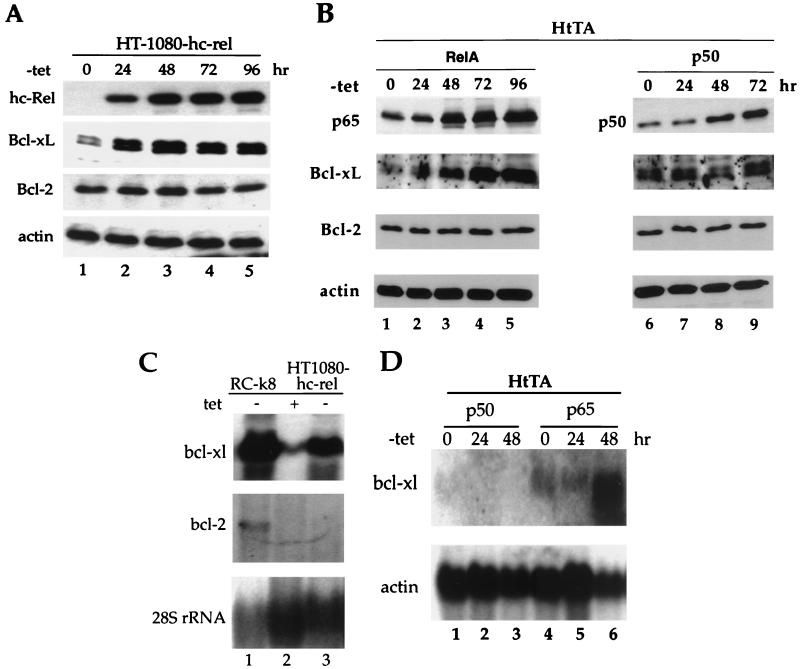
To investigate whether NF-κB is a specific or global regulator of death antagonists in the Bcl-2 family, we examined the effect of c-Rel on the steady-state levels of endogenous Bcl-2 and Bcl-xL proteins in human HT1080-hc-rel cells that conditionally expressed the human c-rel gene under tetracycline-regulated control. Similar to the HeLa-derived HtTA-CCR43 cell line that we previously characterized (67), HT1080-hc-rel cells showed significant protection from TNF-α- or anti-Fas antibody-induced apoptosis following induction of c-Rel expression upon withdrawal of tetracycline (data not shown). Interestingly, expression of c-Rel in these cells was accompanied by a four- to sixfold increase in Bcl-xL protein levels (Fig. 1A, lanes 1 to 5). The 29-kDa protein doublet that was detected with anti-Bcl-x antibodies is typical of Bcl-xL (28). In contrast to the strong induction of Bcl-xL, the steady-state levels of Bcl-2 remained unchanged following c-Rel expression (Fig. 1A, lanes 1 to 5).
FIG. 1.
Rel/NF-κB factors lead to a specific increase in Bcl-xL expression. (A) Induction of c-Rel expression correlates with increased levels of Bcl-xL protein but not Bcl-2. Cell extracts (30 μg) from HT1080-hc-rel cells maintained in the presence (lane 1) or absence (lanes 2 to 5) of tetracycline were resolved by SDS-PAGE and analyzed by immunoblotting with antibodies specific for c-Rel (hc-Rel), Bcl-xL/S, Bcl-2, or actin. Proteins were visualized by ECL. (B) Expression of p65 but not p50 correlates with increased levels of Bcl-xL protein but not Bcl-2. Cell extracts (30 μg) from HtTA-RelA (lanes 1 to 5) or HtTA-p50 cells (lanes 6 to 9) maintained in the presence (lanes 1 and 6) or absence (lanes 2 to 5 and 7 to 9) of tetracycline were resolved by SDS-PAGE and analyzed by immunoblotting with antibodies specific for c-Rel, Bcl-xL, Bcl-2, or actin. Proteins were visualized by ECL. (C) bcl-x gene expression is induced by ectopic expression of c-Rel. Expression of bcl-x transcripts in human RC-K8 cells (lane 1) or HT1080-hc-rel cells (lanes 2 and 3) maintained in the presence (lane 2) or absence (lanes 1 and 3) of tetracycline for 72 h. The blot was successively hybridized to bcl-x, bcl-2, and 28S rRNA probes. (D) The p65 subunit of NF-κB but not the p50 subunit upregulates bcl-xl transcripts. bcl-x gene expression in HtTA-p50 (lanes 1 to 3) or HtTA-RelA cells (lanes 4 to 6) maintained in the presence (lanes 1 and 4) or absence (lanes 2, 3, 5, and 6) of tetracycline for 24 (lanes 2 and 5) or 48 h (lanes 3 and 6). The blot was hybridized to bcl-x and actin probes. tet, tetracycline.
Bcl-xL protein levels were also significantly enhanced in cells expressing the c-Rel-related protein p65 (Fig. 1B, compare lanes 1 to 5). As with c-Rel-expressing cells, the accumulation of Bcl-xL paralleled that of p65. In contrast to these findings, cells expressing the p50 subunit that is devoid of transcriptional activity on most κB site-containing promoters failed to show any increase in Bcl-xL protein levels (Fig. 1B, lanes 6 to 9). Together, these results suggested a correlation between the expression of transcriptionally competent Rel/NF-κB proteins and the specific accumulation of the prosurvival factor Bcl-xL.
The increased accumulation of Bcl-xL protein correlated with an increase in the steady-state levels of bcl-xl transcripts in c-Rel-expressing cells. Northern blots showed a strong enhancement in bcl-x gene expression in HT1080-hc-rel cells induced to express c-Rel (14-fold increase) (Fig. 1C, compare lanes 2 and 3). On the contrary, bcl-2 mRNAs were virtually undetectable in HT1080-hc-rel cells and remained unaffected by the expression of c-Rel (Fig. 1C, lanes 2 and 3). Similarly, bcl-xl was found to be highly expressed in human RC-K8 cells in comparison to the low levels of bcl-2 transcripts (Fig. 1C, lane 1). These cells are derived from a human diffused large cell lymphoma associated with overexpression of a rearranged c-rel gene (33).
Consistent with these results, bcl-x gene expression was also strongly induced by p65. Northern blot analysis showed a ninefold increase in bcl-x transcript levels 48 h after the removal of tetracycline from the HeLa-derived HtTA-RelA cell line to induce expression of p65 (Fig. 1D, compare lanes 4, 5, and 6). As anticipated, p50 failed to promote bcl-x gene expression (Fig. 1D, lanes 1 to 3). These data indicated a selective effect of transcriptionally competent Rel/NF-κB subunits on bcl-xl expression but not on bcl-2 expression.
Stimuli that activate NF-κB also upregulate bcl-x gene expression.
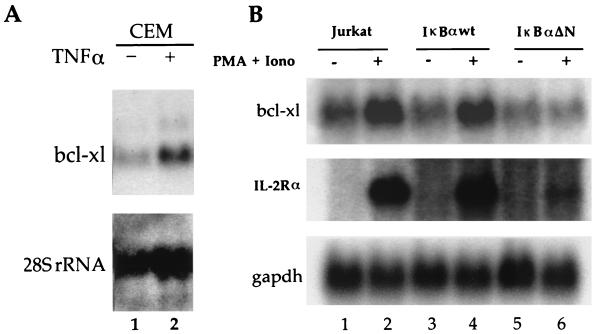
To further investigate the functional regulation of Bcl-xL, we verified the effects of endogenous NF-κB activation on bcl-x gene expression. The basal levels of bcl-x transcripts were significantly increased upon stimulation of human CEM T cells with TNF-α (Fig. 2A, lanes 1 and 2). The participation of NF-κB in bcl-x gene expression was further substantiated by the analysis of human Jurkat T cells stimulated with PMA plus ionomycin to activate endogenous NF-κB factors. Basal levels of bcl-x transcripts were increased threefold following PMA-plus-ionomycin treatment of both parental Jurkat T cells and Jurkat T cells expressing IκBα-wt in comparison to untreated cells (Fig. 2B, lanes 1 to 4). This enhanced expression correlated with the induction of mRNAs for IL-2 receptor alpha (IL2Rα), a known target gene for NF-κB. Importantly, the constitutive repression of NF-κB in Jurkat-IκBαΔN cells by a mutant of IκBα that is resistant to proteolytic degradation (15) markedly decreased the response of bcl-x (Fig. 2B, compare lanes 5 and 6). Together, these data indicated that the activation of NF-κB enhances bcl-x gene expression.
FIG. 2.
Expression of bcl-x is dependent on endogenous Rel/NF-κB activity. (A) bcl-x gene expression is induced by endogenous NF-κB activation. Human CEM T cells were left untreated (lane 1) or were treated with TNF-α for 2 h (lane 2). Total RNA (20 μg) was hybridized with 32P-labeled probes for bcl-x or 28S rRNA. (B) Induction of bcl-x gene expression is blocked by IκBαΔN, a dominant inhibitor of NF-κB. Parental Jurkat T cells (lanes 1 and 2) and Jurkat cells expressing IκBα-wt (lanes 3 and 4) or IκBαΔN (lanes 5 and 6) were treated with PMA plus ionomycin (Iono) for 2 h to induce endogenous NF-κB activity (lanes 2, 4, and 6) or with dimethyl sulfoxide alone as a control (lanes 1, 3, and 5). Total RNA (20 μg) was hybridized to 32P-labeled probes for bcl-x or the gene encoding IL2Rα or glyceraldehyde-3-phosphate dehydrogenase (gapdh).
The protective activity of Bcl-xL is correlated with that of c-Rel and can substitute for NF-κB to block TNF-α-induced cell death.
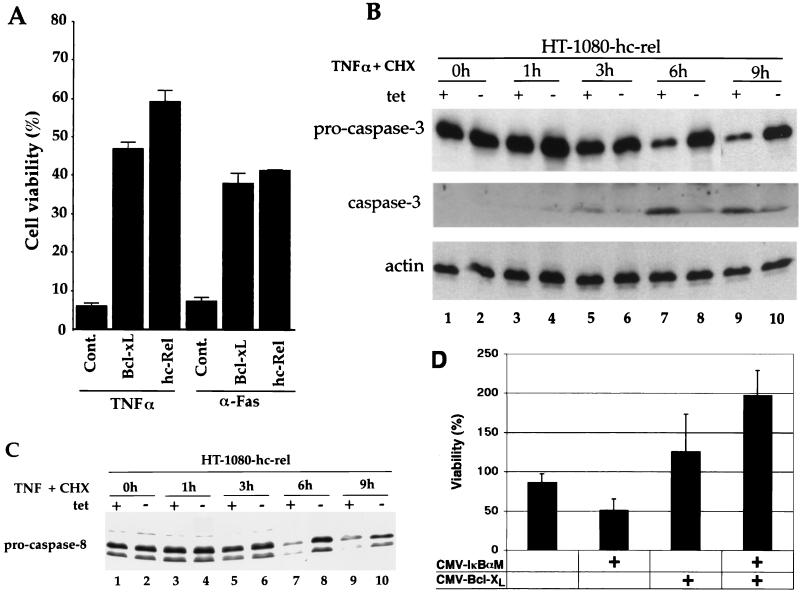
We investigated the protective activities of Bcl-xL and c-Rel in transient transfection assays. HT1080 cells were cotransfected with a CMV-β-gal reporter plasmid together with pCMV vectors expressing either Bcl-xL or c-Rel or an empty vector as a control. Cells were treated with CHX either alone or together with TNF-α or anti-Fas antibodies. Cell survival represents the ratio of the number of cells expressing β-gal in wells treated with TNF-α plus CHX or anti-Fas plus CHX to that in wells treated with CHX alone. The cotransfection of pCMV-hc-rel with CMV-β-gal significantly suppressed cell killing induced either by TNF-α or by Fas receptor cross-linking with an anti-Fas antibody (Fig. 3A). c-Rel increased cell survival 10-fold following addition of TNF-α and 5.5-fold following treatment with anti-Fas antibodies. Consistent with the protective activity of c-Rel in this assay, bcl-xl also efficiently repressed apoptosis induced by either stimulus (eightfold for TNF-α and fivefold for anti-Fas [Fig. 3A]). The correlation between the protective activities of c-Rel and Bcl-xL is consistent with the possibility of a functional relationship between these factors.
FIG. 3.
The protective activity of Bcl-xL is correlated with that of c-Rel. (A) Both Bcl-xL and c-Rel suppress apoptosis induced by TNF-α or Fas receptor cross-linking. HT1080 cells were cotransfected with pCMV-β-gal together with an empty pCMV vector control (Cont.), pCMV-bcl-xl, or pCMV-hc-rel. The cells were treated with CHX alone or together with TNF-α or anti-Fas antibodies for 14 h and stained with X-Gal to quantitate cell survival. Cell viability represents the ratio of the number of cells expressing β-gal in wells treated with TNF-α or anti-Fas antibodies together with CHX to that in wells treated with CHX alone. Cells were counted from a minimum of 10 fields chosen at random. The average survival from three independent experiments is shown. Error bars show standard deviations. (B) Similarly to Bcl-xL, c-Rel prevents the processing of procaspase 3. HT1080-hc-rel cells were maintained in the presence (lanes 1, 3, 5, 7, and 9) or absence (lanes 2, 4, 6, 8, and 10) of tetracycline for 48 h. Cells were left untreated (lanes 1 and 2) or treated with TNF-α plus CHX for 1, 3, 6, or 9 h (lanes 3 to 10). Cell extracts (30 μg) were resolved by SDS-PAGE and analyzed by immunoblotting with antibodies specific for caspase 3 or actin. The 17-kDa cleavage product of procaspase 3 processing was detected only after long exposure times and is shown in the blot of the middle row. (C) c-Rel suppresses the processing of procaspase 8. HT1080-hc-rel cells were maintained in the presence (lanes 1, 3, 5, 7, and 9) or absence (lanes 2, 4, 6, 8, and 10) of tetracycline for 48 h. Cells were left untreated (lanes 1 and 2) or treated with TNF-α plus CHX for 1, 3, 6 or 9 h (lanes 3 to 10). Cell extracts (30 μg) were resolved by SDS-PAGE and analyzed by immunoblotting with an antibody specific for caspase 8. The 55-kDa doublet is characteristic of procaspase 8. (D) Bcl-xL can suppress TNF-α-induced cell death under conditions in which NF-κB activity is suppressed. HeLa cells were cotransfected with pCMV-β-gal and an empty pCMV vector or pCMV-IκBαM, alone or together with pCMV-bcl-xl. The cells were treated with TNF-α alone for 16 h and stained with X-Gal. Cell survival represents the ratio of the number of cells expressing β-gal in wells treated with TNF-α to that in wells left untreated. The average survival from three experiments is shown. Error bars show standard deviations. tet, tetracycline.
Prior work indicated that Bcl-xL efficiently blocked the cleavage-mediated activation of procaspase 3 (CPP32), a critical downstream effector caspase in the apoptotic pathway (14, 18, 46). Likewise, the induction of c-Rel expression in HT1080-hc-rel cells blocked cleavage-mediated activation of procaspase 3 in response to TNF-α-plus-CHX treatment (Fig. 3B). HT1080-hc-rel cells maintained in the presence of tetracycline to block c-Rel expression underwent significant processing of procaspase 3 within 6 h of TNF-α-plus-CHX treatment, as shown by the disappearance of the procaspase 3 band (Fig. 3B, compare lanes 7 and 9 to lane 1). The 17-kDa cleavage product of procaspase 3 processing was detected only after long exposure times and is shown in the middle panel. In contrast, the caspase 3 proenzyme was significantly more resistant to cleavage in cells induced to express c-Rel following the removal of tetracycline (Fig. 3B, lanes 8 and 10).
Interestingly, the induction of c-Rel expression also suppressed the cleavage-mediated activation of FADD-like IL-1β convertase enzyme (FLICE; also known as procaspase 8), the apical caspase in the death receptor caspase cascade (Fig. 3C, compare lanes 8 and 10 to lanes 7 and 9). While Bcl-xL was reported to function downstream of FLICE (8), others showed that it can prevent the oligomerization of FADD and the recruitment of FLICE coincident with the inhibition of apoptosis (38). Although more studies are needed to establish the extent to which Bcl-xL contributed to this activity, the conditional expression of c-Rel in HT1080-hc-rel cells conferred a protective activity similar to that provided by Bcl-xL, consistent with the upregulation of bcl-xl transcripts by Rel/NF-κB factors.
Importantly, Bcl-xL also suppressed TNF-α-induced killing in cells in which endogenous NF-κB activity was suppressed by a serine-to-alanine mutant of IκBα that is resistant to signal-induced degradation (IκBαM) (54). While the expression of IκBαM sensitized HeLa cells to killing induced by TNF-α in the absence of CHX, the cotransfection of Bcl-xL rescued the cells from apoptosis (Fig. 3D). Together, the results support a role for Bcl-xL as one of several death antagonists that function in the NF-κB pathway for cell survival.
c-Rel directly regulates the bcl-x promoter.
To clarify the nature of the response of bcl-xl to Rel/NF-κB, we isolated and characterized the regulatory sequences that lie upstream of the bcl-x cDNA. Nested PCR amplification of an adapter-ligated human genomic library generated a product of 435 bp with a 3′ end derived from the 5′ end of the bcl-xl cDNA and extending 5′ into adjacent genomic sequences (Genome Walker PromoterFinder kit; Clontech). The sequence was identical to that previously reported (DDBJ, EMBL, and GenBank accession no. D30746). A putative NF-κB DNA binding site was identified at position −232 relative to the first nucleotide in the human bcl-xl cDNA sequence (DDBJ, EMBL, and GenBank accession no. L20121; GGGACTGCCC; position −77 relative to the transcription start site) and is conserved in the mouse bcl-x promoter region (22).
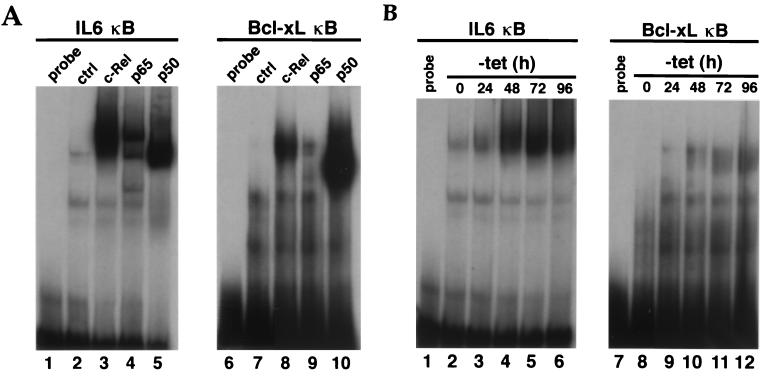
Gel retardation assays confirmed the binding of c-Rel, p65, and p50 to an oligonucleotide probe containing this site (Fig. 4A, lanes 8 to 10). A consensus IL-6 κB oligonucleotide probe was used as a control for Rel/NF-κB protein expression (Fig. 4A, lanes 3 to 5). A time course gel shift analysis was then used to monitor the binding of Rel factors to this site in induced HT1080-hc-rel cells and to see its relationship to endogenous Bcl-xL expression (Fig. 4B). In this assay, Rel factor binding to the bcl-x κB DNA site was correlated with the time course for the induction of Bcl-xL in these cells (Fig. 1A).
FIG. 4.
Rel/NF-κB factors bind to a κB DNA site derived from the human bcl-x promoter region. (A) Binding of Rel/NF-κB factors to the bcl-x κB DNA site. Nuclear extracts from human 293 cells transiently transfected with CMV expression vectors for c-Rel (lanes 3 and 8), p65 (lanes 4 and 9), or p50 (lanes 5 and 10) were incubated with double-stranded 32P-labeled oligonucleotides containing the bcl-x κB DNA site at position −232 (lanes 6 to 10) or an IL-6–κB DNA-binding motif as a control (ctrl) for NF-κB protein expression (lanes 1 to 5). Mock transfected cells (lanes 2 and 7) and probes alone (lanes 1 and 6) were used as controls. DNA-protein complexes were resolved from unbound probes in a 5% native polyacrylamide gel. (B) Binding of induced Rel factors to the bcl-x κB DNA site is correlated with induction of endogenous Bcl-xL expression. Nuclear extracts from HT1080-hc-rel cells induced to express c-Rel for 0, 24, 48, 72, or 96 h upon removal of tetracycline (tet) were incubated with double-stranded 32P-labeled oligonucleotides containing the bcl-x κB DNA site at position −232 (lanes 8 to 12) or a control IL-6–κB DNA-binding motif (lanes 2 to 6). The probes alone (lanes 1 and 7) were used as controls. DNA-protein complexes were resolved from unbound probes in a 5% native polyacrylamide gel.
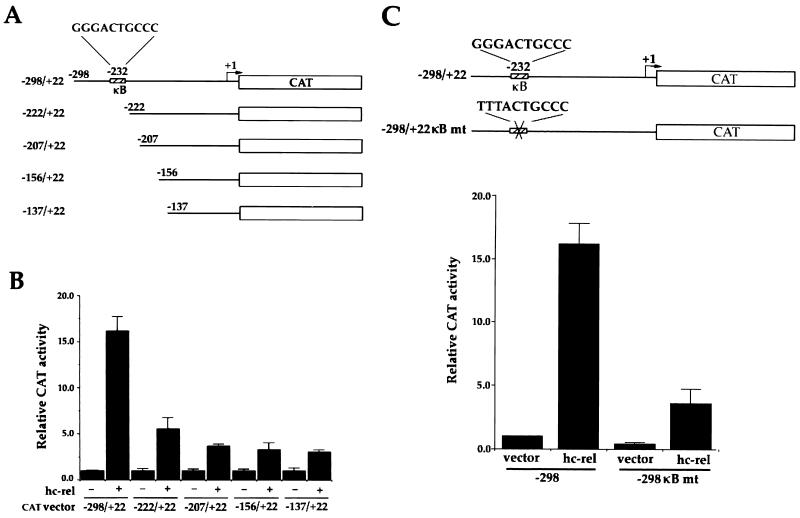
After directional cloning into a promoterless CAT reporter plasmid (Fig. 5A), bcl-x promoter activity was assayed by transient transfection of HT1080 cells in the presence or absence of CMV-hc-rel. The −298/+22 _bcl-x_-CAT reporter construct showed little activity on its own, but the cotransfection of CMV-hc-rel increased the construct's expression 16-fold (Fig. 5B). Progressive deletions removing 5′ sequences from the bcl-x promoter region rapidly interfered with its response to c-Rel. Importantly, a mutant promoter with a deletion endpoint mapping only 10 nucleotides downstream of the NF-κB motif showed a 65% reduction in activity in comparison to the wild-type promoter construct (−222/+22 _bcl-x_-CAT) (Fig. 5B). These results are consistent with our data showing increased expression of Bcl-xL in response to Rel/NF-κB factors. Site-directed mutagenesis was used to inactivate the NF-κB site at position −232 to confirm its role in the Rel-mediated induction of the bcl-x promoter (TTTACTGCCC; −298/+22 mκB). While this mutation slightly decreased the basal activity of the bcl-x promoter, it greatly reduced its responsiveness to c-Rel (Fig. 5C). The response of the −298/+22 mκB _bcl-x_-CAT mutant promoter construct to c-Rel was decreased down to 3.5-fold, in comparison to the 16-fold induction observed with the wild-type −298/+22 _bcl-x_-CAT reporter plasmid. Together, these results demonstrate a direct role for Rel/NF-κB in the regulation of bcl-xl expression.
FIG. 5.
The human bcl-x promoter contains a κB DNA site responsible for its Rel-dependent induction. (A) Schematic representation of bcl-x CAT reporter gene constructs. CAT reporter plasmids driven by various regions of the human bcl-x promoter are shown. The NF-κB site at position −232 relative to the first nucleotide in the bcl-xl cDNA is indicated. (B) c-Rel-dependent transactivation of the bcl-x promoter. HT1080 cells were cotransfected with _bcl-x_-CAT reporter plasmids together with pCMV-c-rel or an empty pCMV vector as a control. The average CAT activity from three independent experiments is shown. (C) NF-κB site-dependent activation of the bcl-x promoter. HT1080 cells were cotransfected with _bcl-x_-CAT reporter plasmid (−298/+22) or the κB site mutant (mt) plasmid (−298/+22 mκB) together with pCMV-hc-rel, or an empty pCMV vector as a control. The average CAT activity from three independent experiments is shown. The arrow indicates the transcription start site for bcl-x. Error bars show standard deviations.
DISCUSSION
The strong correlation that exists between the antiapoptotic and oncogenic activities of Rel/NF-κB factors has elicited a fervent search for the target genes that participate in the NF-κB survival pathway. Recent work from members of our group and others demonstrated that NF-κB directly regulates expression of the prosurvival Bcl-2 family protein Bfl-1 and its mouse homolog A1 (23, 31, 56, 69). The positioning of one member of the important Bcl-2 family of death antagonists in the NF-κB survival pathway raised the question of whether NF-κB also controls other death inhibitors from the Bcl-2 family. Here, we demonstrate that Bcl-xL is also a transcriptional target of NF-κB. We show that ectopically expressed and endogenous Rel/NF-κB factors specifically upregulated the expression of bcl-xl. This effect was dependent on NF-κB activity, as a mutant form of IκBα that can physiologically inhibit NF-κB antagonized bcl-x gene induction. Functionally, both c-Rel and Bcl-xL exhibited similar antiapoptotic activities toward cell death induced by TNF-α or by Fas receptor cross-linking. This agrees with studies indicating that expression of either Bcl-xL or Rel/NF-κB factors antagonizes apoptosis in response to various death-inducing stimuli (2, 8, 14, 32, 43, 49, 54, 57, 62, 67). The mapping of a functional κB DNA site in the human bcl-x promoter region confirmed its Rel-dependent response. Together, these data demonstrate the direct participation of NF-κB in the transcriptional control of the prosurvival factor Bcl-xL.
Our finding that NF-κB is an important inducer of bcl-xl is consistent with recent reports showing the NF-κB-dependent upregulation of bcl-xl upon CD40-mediated activation of B lymphocytes, in primary neurons stimulated with TNF, and in mouse T cells expressing human T-cell leukemia virus type 1 (HTLV-1) Tax (31, 51, 52). However, the regulation of bcl-xl expression appears to be quite complex and the contribution of NF-κB to this process may depend on cell type, differentiation stage and/or activating stimuli. For instance, while the studies described above point to a proactive role for NF-κB in inducing bcl-xl, other analyses looking for death antagonists under NF-κB control failed to reveal alterations in bcl-xl transcript levels (23, 58, 63). Similarly, no change in bcl-xl expression was observed in IL-3-dependent mouse B cells expressing HTLV-1 Tax (34). Understanding of the relationship between NF-κB and Bcl-xL is also complicated by data implicating NF-κB as a negative regulator of Bcl-xL in double-positive thymocytes (24) and by reports pointing to the ability of Bcl-xL to interfere with NF-κB activation upstream of IκBα degradation (3). Additional work is thus needed to sort out the functional and regulatory relationships between NF-κB and Bcl-xL and the conditions under which they take place.
Our findings do not exclude the possibility that NF-κB may function in conjunction with other transcription factors to regulate bcl-x gene expression. Similarly to NF-κB, transcription factors Stat1, Stat5, and Ets2 were also recently implicated in the regulation of bcl-xl expression (20, 45, 47) and multiple binding sites for other transcription factors were identified in the bcl-x promoter (22). The regulation of bcl-xl induction by multiple transcription factors may thus explain why significant levels of bcl-xl transcripts have been observed in the absence of NF-κB activation. In this respect, it is noteworthy that the response of bcl-x to endogenous NF-κB activation differed somewhat from the response that our group and others previously described for its homolog bfl-1/a1 (31, 69). Whereas cells that lack nuclear NF-κB activity failed to show any expression of bfl-1/a1, a basal level of endogenous bcl-x gene expression was observed in several cell lines, which was further induced upon NF-κB activation. These data are consistent with a model in which NF-κB may act in concert with other transcription factors to control the expression of bcl-xl depending on cell type and/or activating stimuli. Nevertheless, the results described herein indicate that NF-κB is one of several important inducers of bcl-xl.
As a result of alternative splicing, bcl-x encodes two death regulators that exhibit opposing activities (7). Bcl-xL exerts a protective effect, whereas the shorter form, Bcl-xS, is proapoptotic. The 29-kDa protein doublet detected in our immunoblots with anti-Bcl-x antibodies is typical of Bcl-xL (Fig. 1A) (28). No change was observed at the position for its proapoptotic isoform, Bcl-xS. Our results therefore highlight the participation of NF-κB in the control of bcl-x gene activation to block cell death through upregulation of the prosurvival Bcl-xL factor. In a recent study, Tsukahara et al. also observed NF-κB-mediated induction of bcl-xl but not bcl-xs in CTLL-2 cells expressing Tax (52). It will be interesting to investigate the mechanism underlying the selective induction of the bcl-x promoter by NF-κB that leads to production of the antiapoptotic long form rather than the proapoptotic short form.
The ability of c-Rel to transcriptionally regulate bcl-x and its homolog bfl-1/a1 is in contrast with its failure to induce bcl-2 gene expression in our cells. While NF-κB has been implicated in the induction of bcl-2 in certain cells (51) but not others (52), the absence of a bcl-2 response in our system implies that NF-κB is able to selectively regulate particular prosurvival genes in the Bcl-2 family in defined cellular environments. This differential regulation is consistent with the fact that Bcl-xL and Bcl-2 can display different patterns of expression in various hematopoietic cells and tissues. For instance, bcl-2 is intensely expressed in naive and memory B cells but is mostly absent from germinal center B cells. In contrast, bcl-xl is weakly expressed in naive and memory B cells but is abundant in germinal center B cells where c-rel is constitutively expressed (12, 28, 35). Thus, despite the sequence and functional homology between Bcl-xL and Bcl-2, their different modes of regulation enable them to exhibit complementary protective activities in hematolymphoid tissues by allowing them to participate in distinct survival programs in different cells and at different stages of differentiation (reviewed in references 41 and 65).
In experiments not shown here, we observed that transcripts for the Bcl-2-related protein Mcl-1 were also upregulated by c-Rel and NF-κB activation and that mcl-1 mRNAs were abundantly expressed in immune tissue where NF-κB is active (data not shown). However, we have so far been unable to demonstrate that NF-κB activity is required for mcl-1 gene expression. While NF-κB activation in Jurkat T cells treated with PMA plus ionomycin efficiently promoted mcl-1 gene expression, a similar degree of induction was observed in Jurkat-IκBαΔN cells in which endogenous NF-κB activity was constitutively repressed (data not shown). This is in contrast to the results that members of our group obtained with bcl-xl and bfl-1/a1 (Fig. 2B) (69). Further work is therefore needed to characterize the mcl-1 promoter region and to assess the role of NF-κB in its regulation. Taken together, our findings indicate that NF-κB can specifically regulate particular cell death inhibitors in the Bcl-2 family.
Dysregulated Rel/NF-κB activity has been implicated in several hematopoietic and solid tumors (reviewed in references 34 and 40). For example, c-rel is amplified in human extranodal diffuse large cell lymphomas and constitutive NF-κB/Rel activity was observed in Hodgkin's lymphoma and in breast, colon, and prostate carcinoma (4, 9, 11, 16, 25, 29, 39, 48, 61; F. Payvandi, W. X. Zong, C. Gélinas, P. Amenta, and A. B. Rabson, unpublished data). Although the mechanisms involved remain to be clarified, the ability of NF-κB to promote cell survival is likely to contribute to malignancy. Interestingly, bcl-xl is overexpressed in lymphoid and hematopoietic tumors as well as in a high proportion of colorectal, prostate, and primary breast carcinomas (26, 27, 37, 44, 53). While Bcl-xL is unlikely to be the only antiapoptotic factor responsible for the protective activity of NF-κB, these observations suggest that the dysregulated expression of Bcl-xL, Bfl-1, and other prosurvival factors under NF-κB control may be important for the oncogenic process. Future challenges will be to elucidate the pathways responsible for the differential regulation of death antagonists controlled by NF-κB and to establish their respective contribution to tumors associated with aberrant Rel/NF-κB activity.
ACKNOWLEDGMENTS
We are very grateful to D. W. Ballard (HHMI, Vanderbilt University) for Jurkat-IκBα-wt and Jurkat-IκBαΔN cells, C. Labrie (CHUL) for bcl-2, bcl-xl, and mcl-1 cDNAs, D. Perez and E. White (HHMI, CABM, Piscataway, N.J.) for anti-Bcl-2 antibodies, N. Rice (ABL-NCI) for hc-rel cDNA, H. Bujard (Zentrum fur Molekulare Biologie der Universitat Heidelberg, Heidelberg, Germany) for the gift of pUHD vectors, and to N. Zeleznik-Le (University of Chicago) for the RC-K8 cell line. We thank P. Lizzul, A. Rabson, B. Rayet, E. White, and W.-X. Zong for helpful comments on the manuscript.
This work was supported by a grant from the National Institutes of Health (CA54999) and by the New Jersey Commission on Science and Technology to C.G. C.C. was a postdoctoral fellow of the New Jersey Commission on Cancer Research and The Foundation of UMDNJ. L.C.E. was partially supported by NIH Biotechnology predoctoral training grant GM08339 and by NIH predoctoral training grant in Biochemistry and Molecular Biology GM08360.
REFERENCES
- 1.Adams J M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong R C, Aja T, Xiang J, Gaur S, Krebs J F, Hoang K, Bai X, Korsmeyer S J, Karanewsky D S, Fritz L C, Tomaselli K J. Fas-induced activation of the cell death-related protease CPP32 is inhibited by Bcl-2 and by ICE family protease inhibitors. J Biol Chem. 1996;271:16850–16855. doi: 10.1074/jbc.271.28.16850. [DOI] [PubMed] [Google Scholar]
- 3.Badrichani A Z, Stroka D M, Bilbao G, Curiel D T, Bach F H, Ferran C. Bcl-2 and Bcl-xL serve an anti-inflammatory function in endothelial cells through inhibition of NF-κB. J Clin Investig. 1999;103:543–553. doi: 10.1172/JCI2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bargou R, Emmerich F, Krappmann D, Bommert K, Mapara M, Arnold W, Royer H, Grinstein E, Greiner A, Scheidereit C, Dorken B. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. J Clin Investig. 1997;100:2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beg A A, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 6.Beg A A, Sha W C, Bronson R T, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 7.Boise L, Gonzalez-Garcia M, Postema C, Ding L, Lindsten T, Turka L, Mao X, Nunez G, Thompson C. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 8.Boise L H, Thompson C B. Bcl-x(L) can inhibit apoptosis in cells that have undergone Fas-induced protease activation. Proc Natl Acad Sci USA. 1997;94:3759–3764. doi: 10.1073/pnas.94.8.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bours V, Dejardin E, Goujon-Letawe F, Merville M-P, Castronovo V. The NF-κB transcription factor and cancer: high expression of NF-κB- and IκB-related proteins in tumor cell lines. Biochem Pharmacol. 1994;47:145–149. doi: 10.1016/0006-2952(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 10.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 11.Cabannes E, Khan G, Aillet F, Jarrett R, Hay R. Mutations in the IκBα gene in Hodgkin's disease suggest a tumour suppressor role for Ikappa B alpha. Oncogene. 1999;18:3063–3070. doi: 10.1038/sj.onc.1202893. [DOI] [PubMed] [Google Scholar]
- 12.Carrasco D, Weih F, Bravo R. Developmental expression of the mouse c-rel proto-oncogene in hematopoietic organs. Development. 1994;120:2991–3004. doi: 10.1242/dev.120.10.2991. [DOI] [PubMed] [Google Scholar]
- 13.Chen C, Agnès F, Gélinas C. Mapping of a serine-rich domain essential for the transcriptional, antiapoptotic, and transforming activities of the v-Rel oncoprotein. Mol Cell Biol. 1999;19:307–316. doi: 10.1128/mcb.19.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chinnaiyan A, Orth K, O'Rourke K, Duan H, Poirier G, Dixit V. Molecular ordering of the cell death pathway. Bcl-2 and Bcl-xL function upstream of the CED-3-like apoptotic proteases. J Biol Chem. 1996;271:4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- 15.Chu Z-L, McKinsey T A, Liu L, Gentry J J, Malim M H, Ballard D W. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-κB control. Proc Natl Acad Sci USA. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dejardin E, Bonizzi G, Bellahcene A, Castronovo V, Merville M-P, Bours V. Highly expressed p100/p52 (NFκB2) sequesters other NFκB-related proteins in the cytoplasm of human breast cancer cells. Oncogene. 1995;11:1835–1841. [PubMed] [Google Scholar]
- 17.Dragovich T, Rudin C M, Thompson C B. Signal transduction pathways that regulate cell survival and cell death. Oncogene. 1998;17:3207–3213. doi: 10.1038/sj.onc.1202587. [DOI] [PubMed] [Google Scholar]
- 18.Erhardt P, Cooper G M. Activation of the CPP32 apoptotic protease by distinct signaling pathways with differential sensitivity to Bcl-xL. J Biol Chem. 1996;271:17601–17604. doi: 10.1074/jbc.271.30.17601. [DOI] [PubMed] [Google Scholar]
- 19.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 20.Fujio Y, Kunisada K, Hirota H, Yamauchi-Takihara K, Kishimoto T. Signals through gp130 upregulate bcl-x gene expression via STAT1-binding cis-element in cardiac myocytes. J Clin Investig. 1997;99:2898–2905. doi: 10.1172/JCI119484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grillot D A M, González-Garcia M, Ekhterae D, Duan L, Inohara N, Ohta S, Seldin M F, Nunez G. Genomic organization, promoter region analysis, and chromosome localization of the mouse bcl-x gene. J Immunol. 1997;158:4750–4757. [PubMed] [Google Scholar]
- 23.Grumont R J, Rourke I J, Gerondakis S. Rel-dependent induction of A1 transcription is required to protect B cells from antigen receptor ligation-induced apoptosis. Genes Dev. 1999;13:400–411. doi: 10.1101/gad.13.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hettmann T, DiDonato J, Karin M, Leiden J M. An essential role for nuclear factor kappaB in promoting double positive thymocyte apoptosis. J Exp Med. 1999;189:145–158. doi: 10.1084/jem.189.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houldsworth J, Mathew S, Rao P H, Dyomina K, Louie D C, Parsa N, Offit K, Chaganti R S K. REL proto-oncogene is frequently amplified in extranodal diffuse large cell lymphoma. Blood. 1996;87:25–29. [PubMed] [Google Scholar]
- 26.Krajewska M, Krajewski S, Epstein J, Shabaik A, Sauvageot J, Song K, Kitada S, Reed J. Immunohistochemical analysis of bcl-2, bax, bcl-x, and mcl-1 expression in prostate cancers. Am J Pathol. 1996;148:1567–1576. [PMC free article] [PubMed] [Google Scholar]
- 27.Krajewska M, Moss S, Krajewski S, Song K, Holt P, Reed J. Elevated expression of Bcl-x and reduced Bak in primary colorectal adenocarcinomas. Cancer Res. 1996;56:2422–2427. [PubMed] [Google Scholar]
- 28.Krajewski S, Krajewska M, Shabaik A, Wang H G, Irie S, Fong L, Reed J C. Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Res. 1994;54:5501–5507. [PubMed] [Google Scholar]
- 29.Krappmann D, Emmerich F, Kordes U, Scharschmidt E, Dorken B, Scheidereit C. Molecular mechanisms of constitutive NF-kappaB/Rel activation in Hodgkin/Reed-Sternberg cells. Oncogene. 1999;18:943–953. doi: 10.1038/sj.onc.1202351. [DOI] [PubMed] [Google Scholar]
- 30.Krikos A, Laherty C D, Dixit V M. Transcriptional activation of the tumor necrosis factor α-inducible zinc finger protein, A20, is mediated by κB elements. J Biol Chem. 1992;267:17971–17976. [PubMed] [Google Scholar]
- 31.Lee H H, Dadgostar H, Cheng Q, Shu J, Cheng G. NF-κB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc Natl Acad Sci USA. 1999;96:9136–9141. doi: 10.1073/pnas.96.16.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 33.Lu D, Thompson J D, Gorski G K, Rice N R, Mayer M G, Yunis J J. Alterations at the rel locus in human lymphoma. Oncogene. 1991;6:1235–1241. [PubMed] [Google Scholar]
- 34.Luque I, Gélinas C. Rel/NF-κB and IκB factors in oncogenesis. Semin Cancer Biol. 1997;8:103–111. doi: 10.1006/scbi.1997.0061. [DOI] [PubMed] [Google Scholar]
- 35.Miyazaki T, Liu Z J, Taniguchi T. Selective cooperation of HTLV-I-encoded p40tax-1 with cellular oncoproteins in the induction of hematopoietic cell proliferation. Oncogene. 1996;12:2403–2408. [PubMed] [Google Scholar]
- 36.Ohta K, Iwai K, Kasahara Y, Taniguchi N, Krajewski S, Reed J C, Miyawaki T. Immunoblot analysis of cellular expression of Bcl-2 family proteins, Bcl-2, Bax, Bcl-X and Mcl-1, in human peripheral blood and lymphoid tissues. Int Immunol. 1995;7:1817–1825. doi: 10.1093/intimm/7.11.1817. [DOI] [PubMed] [Google Scholar]
- 37.Pallis M, Zhu Y M, Russell N H. Bcl-x(L) is heterogenously expressed by acute myeloblastic leukaemia cells and is associated with autonomous growth in vitro and with P-glycoprotein expression. Leukemia. 1997;11:945–949. doi: 10.1038/sj.leu.2400705. [DOI] [PubMed] [Google Scholar]
- 38.Perez D, White E. E1B 19K inhibits Fas-mediated apoptosis through FADD-dependent sequestration of FLICE. J Cell Biol. 1998;141:1255–1266. doi: 10.1083/jcb.141.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao P H, Houldsworth J, Dyomina K, Parsa N Z, Cigudosa J C, Louie D C, Popplewell L, Offit K, Jhanwar S C, Chaganti R S K. Chromosomal and gene amplification in diffuse large B-cell lymphoma. Blood. 1998;92:234–240. [PubMed] [Google Scholar]
- 40.Rayet B, Gélinas C. Aberrant rel/nfκb genes and activity in human cancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 41.Reed J C. Mechanisms of Bcl-2 family protein function and dysfunction in health and disease. Behring Inst Mitt. 1996;97:72–100. [PubMed] [Google Scholar]
- 42.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider T J, Grillot D, Foote L C, Nunez G E, Rothstein T L. Bcl-x protects primary B cells against Fas-mediated apoptosis. J Immunol. 1997;159:4834–4839. [PubMed] [Google Scholar]
- 44.Schott A F, Apel I J, Nunez G, Clarke M F. Bcl-XL protects cancer cells from p53-mediated apoptosis. Oncogene. 1995;11:1389–1394. [PubMed] [Google Scholar]
- 45.Sevilla L, Aperlo C, Dulic V, Chambard J C, Boutonnet C, Pasquier O, Pognonec P, Boulukos K E. The Ets2 transcription factor inhibits apoptosis induced by colony-stimulating factor 1 deprivation of macrophages through a Bcl-xL-dependent mechanism. Mol Cell Biol. 1999;19:2624–2634. doi: 10.1128/mcb.19.4.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimizu S, Eguchi Y, Kamiike W, Matsuda H, Tsujimoto Y. Bcl-2 expression prevents activation of the ICE protease cascade. Oncogene. 1996;12:2251–2257. [PubMed] [Google Scholar]
- 47.Socolovsky M, Fallon A E J, Wang S, Brugnara C, Lodish H F. Fetal anemia and apoptosis of red cell progenitors in Stat5a-/-5b-/- mice: a direct role for Stat5 in Bcl-XL induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 48.Sovak M, Bellas R, Kim D, Zanieski G, Rogers A, Traish A, Sonenshein G. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Investig. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srinivasan A, Li F, Wong A, Kodandapani L, Smidt R, Jr, Krebs J F, Fritz L C, Wu J C, Tomaselli K J. Bcl-xL functions downstream of caspase-8 to inhibit Fas- and tumor necrosis factor receptor 1-induced apoptosis of MCF7 breast carcinoma cells. J Biol Chem. 1998;273:4523–4529. doi: 10.1074/jbc.273.8.4523. [DOI] [PubMed] [Google Scholar]
- 50.Stehlik C, de Martin R, Kumabashiri I, Schmid J A, Binder B R, Lipp J. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med. 1998;188:211–216. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamatani M, Che Y H, Matsuzaki H, Ogawa S, Okado H, Miyake S-I, Mizuno T, Tohyama M. Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NFκB activation in primary hippocampal neurons. J Biol Chem. 1999;274:8531–8538. doi: 10.1074/jbc.274.13.8531. [DOI] [PubMed] [Google Scholar]
- 52.Tsukahara T, Kannagi M, Ohashi T, Kato H, Arai M, Nunez G, Iwanaga Y, Yamamoto N, Ohtani K, Nakamura M, Fujii M. Induction of Bcl-xL expression by human T-cell leukemia virus type I Tax through NF-κB in apoptosis-resistant T-cell transfectants with Tax. J Virol. 1999;73:7981–7987. doi: 10.1128/jvi.73.10.7981-7987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tu Y, Renner S, Xu F, Fleishman A, Taylor J, Weisz J, Vescio R, Rettig M, Berenson J, Krajewski S, Reed J C, Lichtenstein A. Bcl-x expression in multiple myeloma: possible indicator of chemoresistance. Cancer Res. 1998;58:256–262. [PubMed] [Google Scholar]
- 54.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 55.Van Antwerp D J, Martin S J, Verma I M, Green D R. Inhibition of TNF-induced apoptosis by NF-κB. Trends Cell Biol. 1998;8:107–111. doi: 10.1016/s0962-8924(97)01215-4. [DOI] [PubMed] [Google Scholar]
- 56.Wang C-Y, Guttridge D C, Mayo M W, Baldwin A S., Jr NF-κB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol. 1999;19:5923–5929. doi: 10.1128/mcb.19.9.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang C-Y, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 58.Wang C-Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S., Jr NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 59.White D W, Roy A, Gilmore T D. The v-Rel oncoprotein blocks apoptosis and proteolysis of IκBα in transformed chicken spleen cells. Oncogene. 1995;10:857–868. [PubMed] [Google Scholar]
- 60.White E, Sabbatini P, Debbas M, Wold W S M, Kusher D I, Gooding L R. The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor α. Mol Cell Biol. 1992;12:2570–2580. doi: 10.1128/mcb.12.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wood K, Roff M, Hay R. Defective IκBα in Hodgkin cell lines with constitutively active NF-κB. Oncogene. 1998;16:2131–2139. doi: 10.1038/sj.onc.1201735. [DOI] [PubMed] [Google Scholar]
- 62.Wu M, Lee H, Bellas R E, Schauer S L, Arsura M, Katz D, FitzGerald M J, Rothstein T L, Sherr D H, Sonenshein G E. Inhibition of NF-κB/Rel induces apoptosis of murine B cells. EMBO J. 1996;15:4682–4690. [PMC free article] [PubMed] [Google Scholar]
- 63.Wu M X, Ao Z, Prasad K V S, Wu R, Schlossman S F. IEX-1L, an apoptosis inhibitor involved in NF-κB-mediated cell survival. Science. 1998;281:998–1001. doi: 10.1126/science.281.5379.998. [DOI] [PubMed] [Google Scholar]
- 64.Xu X, Gélinas C. A mutant Rel-homology domain promotes transcription by p50/NF-κB1. Oncogene. 1997;14:1521–1530. doi: 10.1038/sj.onc.1200985. [DOI] [PubMed] [Google Scholar]
- 65.Yang E, Korsmeyer S J. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- 66.You M, Ku P-T, Hrdlicková R, Bose H R., Jr ch-IAP1, a member of the inhibitor-of-apoptosis protein family, is a mediator of the antiapoptotic activity of the v-Rel oncoprotein. Mol Cell Biol. 1997;17:7328–7341. doi: 10.1128/mcb.17.12.7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zong W-X, Bash J, Gélinas C. Rel blocks both anti-Fas and TNFα-induced apoptosis and an intact Rel transactivation domain is essential for this effect. Cell Death Differ. 1998;5:963–972. doi: 10.1038/sj.cdd.4400441. [DOI] [PubMed] [Google Scholar]
- 68.Zong W-X, Farrell M, Bash J, Gélinas C. v-Rel prevents apoptosis in transformed lymphoid cells and blocks TNFα-induced cell death. Oncogene. 1997;15:971–980. doi: 10.1038/sj.onc.1201266. [DOI] [PubMed] [Google Scholar]
- 69.Zong W X, Edelstein L C, Chen C, Bash J, Gélinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-κB that blocks TNFα-induced apoptosis. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]