Genetic Requirements for RAD51- and RAD54-Independent Break-Induced Replication Repair of a Chromosomal Double-Strand Break (original) (raw)
Abstract
Broken chromosomes can be repaired by several homologous recombination mechanisms, including gene conversion and break-induced replication (BIR). In Saccharomyces cerevisiae, an HO endonuclease-induced double-strand break (DSB) is normally repaired by gene conversion. Previously, we have shown that in the absence of RAD52, repair is nearly absent and diploid cells lose the broken chromosome; however, in cells lacking RAD51, gene conversion is absent but cells can repair the DSB by BIR. We now report that gene conversion is also abolished when RAD54, RAD55, and RAD57 are deleted but BIR occurs, as with _rad51_Δ cells. DSB-induced gene conversion is not significantly affected when RAD50, RAD59, TID1 (RDH54), SRS2, or SGS1 is deleted. Various double mutations largely eliminate both gene conversion and BIR, including _rad51Δ rad50Δ, rad51Δ rad59_Δ, and _rad54Δ tid1_Δ. These results demonstrate that there is a _RAD51_- and _RAD54_-independent BIR pathway that requires RAD59, TID1, RAD50, and presumably MRE11 and XRS2. The similar genetic requirements for BIR and telomere maintenance in the absence of telomerase also suggest that these two processes proceed by similar mechanisms.
The repair of broken chromosomes by homologous recombination may occur in several ways (reviewed by Pâques and Haber [46]). If both ends of the broken molecule have homology to sequences on an unbroken chromosome that can serve as a template, repair may proceed by gene conversion. For example, in the G1 stage of the cell cycle, a haploid cell or a diploid with a monosomic chromosome lacks an intact, homologous chromosome that it can use as a template for repair. However, if the centromere-proximal end of the double-strand break (DSB) has homology at or near one end to sequences elsewhere in the genome, a one-ended recombination event may still take place that will repair the broken end of the chromosome by appending a portion of a chromosome arm containing its telomere. A similar situation prevails in cells lacking the telomerase enzyme, which maintains chromosome termini. Here the degrading chromosome ends will only share homology with sequences at a single end of a broken chromosome, and repair again can occur only by a one-ended recombination mechanism. In both of these situations, an alternative mechanism of repair known as break-induced replication (BIR) may be able to restore a telomere to the broken chromosome and thus preserve its integrity.
BIR is a recombination-dependent DNA replication process that has been invoked to explain late DNA replication in phage T4 (36, 43), break-copy-recombination in phage λ (31, 44, 56), and origin-independent DNA replication in Escherichia coli (28, 29). In Saccharomyces cerevisiae, events consistent with BIR following creation of a DSB have been directly demonstrated in several ways. First, when HO endonuclease is used to cleave off the end of one chromosome in a wild-type haploid, repair can occur by BIR, in which sequences centromere proximal to an HO-induced DSB apparently invade a homologous sequence located on another chromosome arm (3). Replication from this site produced a nonreciprocal translocation in which a 30-kb terminal region of a different chromosome arm was found distal to the end produced by HO cleavage. Similar kinds of events have been demonstrated in transformation experiments, where the end of a linearized fragment apparently established a replication fork that could proceed several hundred kilobases to a chromosome end (41).
BIR has also been documented in a diploid experiencing a single HO endonuclease-induced DSB on one chromosome. In wild-type cells, this DSB is efficiently repaired by gene conversion using a homologous chromosome as a template. Gene conversion is abolished by a _rad51_Δ mutation (38), but surprisingly, in the absence of the Rad51p strand exchange protein, repair of the broken chromosome can still occur by a BIR mechanism that causes all markers distal to the site of the DSB to become homozygous (Fig. 1). Although BIR is RAD51 independent, it is RAD52 dependent (38). In the absence of RAD52, the broken chromosome is almost always lost, producing a 2N − 1 viable monosomic diploid.
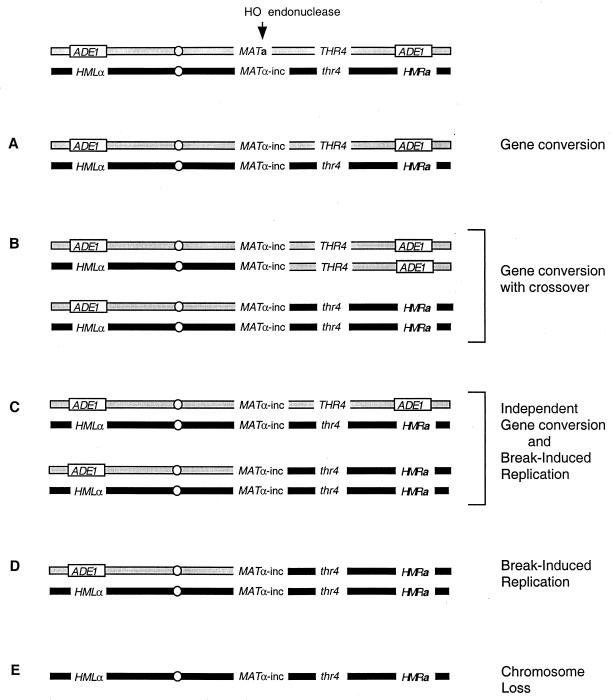
FIG. 1.
Repair of a DSB in a diploid. HO endonuclease cleavage at MATa can be repaired by gene conversion (A) using the uncleavable _MAT_α-inc allele on the homologous chromosome as the donor sequence. Gene conversion can also occur, rarely, using the interchromosomal _HML_α and HMRa loci as donors (38, 40), but these would be cleaved again if HO is continually expressed. If gene conversion occurs in G2 cells, crossing over may generate sectored colonies homozygous for THR4 and for thr4 (B). Colonies that are phenotypically identical to but genotypically different from those shown in panel B can occur if there are two independent DNA repair events, one of which is a gene conversion and the other of which is BIR (C). BIR yields Ade1+ Thr4− cells (D). Failure to repair the DSB leads to chromosome loss and the formation of Ade1+ Thr4− cells (E).
A similar relationship between repair and recombination genes has been found in the survival of strains lacking telomerase. Without telomerase, telomeres slowly degrade until, after many generations, most cells die; however, some survivors appear. Many of these have amplified the subtelomeric Y′ elements to all chromosome ends, whereas others have managed to amplify telomere sequences themselves (37, 62). The appearance of survivors is RAD52 dependent (37). Le et al. (32) found that the appearance of survivors occurred in the absence of RAD51. Their investigation suggested that there were in fact two pathways of telomere maintenance in the absence of telomerase, one of which required RAD51, RAD54, RAD55, and RAD57 and the other of which used RAD50, MRE11, and XRS2. Consequently, there were no survivors in a _rad51_Δ _rad50_Δ double mutant. Recent studies (6, 61, 62) have strongly supported this idea, showing that there are indeed two types of telomerase-independent recombination products and that one of the two types is eliminated in _rad51_Δ and the other in _rad50_Δ strains.
Although it is likely that the repair processes involved in telomere maintenance in the absence of telomerase are based on BIR, it is not possible to examine telomere repair events in detail to determine if they yield nonreciprocal translocations. Hence we have returned to the system where it was first demonstrated that the BIR could occur in the absence of RAD51 to repair a single DSB in the middle of a chromosome (38). We have examined the effects of deleting most of the other members of the RAD52 epistasis group. Although deletions of these genes cause sensitivity to ionizing radiation and to radiomimetic drugs, it is evident that they fall into several distinctive subgroups (reviewed by Pâques and Haber [46]). RAD52 stands alone in being required for essentially all homologous recombination events, although even without RAD52 there remains a crossover-associated pathway of recombination that is accompanied by chromosome loss (16, 38).
Deletion of RAD51 leads to the loss of gene conversions between chromosomal sites, but gene conversions between inverted repeats on plasmids still occur (20, 58). A _rad51_Δ strain is not impaired in another homologous recombinational repair process—single-strand annealing (SSA). Spontaneous heteroallelic recombination in S. cerevisiae is also reduced in _rad52_Δ strains but is only mildly affected by _rad51_Δ (49, 50). Insofar as they have been tested, deletions of RAD54, RAD55, and RAD57 resemble _rad51_Δ. In some assays, _rad55_Δ and _rad57_Δ strains are defective only at low temperatures and this defect can be suppressed by overexpressing RAD51 (17, 35). This observation and biochemical studies have led to the suggestion that Rad55p and Rad57p help load Rad51p onto DNA for recombination (60). For HO-induced MAT gene switching, RAD55 and RAD57 are required at any temperature, possibly because the recombination process is more demanding when the donor sequences are heterochromatic and are probably more difficult to invade (58).
A third set of proteins includes Mre11p, Rad50p, and Xrs2p, which form a complex (64). Loss of any of these three proteins has a similar effect on both homologous and nonhomologous recombination (4, 19, 21, 40). They are actually hyperrecombinational for spontaneous heteroallelic recombination and only mildly defective in the completion of both gene conversion and SSA.
Recently two other genes have been identified that fall into the RAD52 epistasis group, TID1 and RAD59. The Tid1 (or Rdh54) protein is homologous to Rad54p. A _tid1_Δ strain is defective in spontaneous mitotic interhomologue recombination between heteroalleles but appears to be unaffected in intrachromosomal or intersister chromatid recombination (27, 54). In some strains, the effect of deleting TID1 can be seen only when RAD54 has been deleted. The role of Tid1p in DSB-induced events has not been tested.
Finally, Rad59p has been shown to be a homologue of Rad52p (1). Overexpression of RAD52 partially complements the defects of _rad59_Δ, but the converse is not observed. Loss of Rad59p impairs SSA and reduces HO-induced gene conversions on plasmids (2, 22, 48, 57). In spontaneous recombination, _rad59_Δ has no strong phenotype by itself, but a _rad51Δ rad59_Δ double mutant is nearly as severely impaired in spontaneous heteroallelic recombination as _rad52_Δ. This has led to the suggestion that RAD59 functions in a _RAD51_-independent pathway (1). The role of Rad59p in BIR is unknown. In gene conversions induced by DSBs, _rad59_Δ has a relatively minor role when the lengths of homology flanking the DSB are several kilobases; however, as the length of homology decreases, Rad59p plays an increasingly important role, suggesting that it may help stabilize the initial encounter between the DSB end and the donor sequence (57).
In this paper we examine the roles of the RAD52 family of homologous recombination genes in gene conversion and BIR in a diploid with a single HO-induced DSB. We provide evidence that BIR may proceed by a _RAD52_-dependent pathway, independent of RAD51, RAD54, RAD55, and RAD57 but requiring RAD50, RAD59, and TID1.
MATERIALS AND METHODS
Experiments were carried out using two sets of isogenic diploids, closely related to each other. One set of diploids is isogenic to those described by Malkova et al. (38) and is the result of crossing derivatives of haploid strains EI515 and AM133 (Table 1). A second set of diploids was constructed by crossing derivatives of EI515 with those of LS23, which is a segregant of a cross of AM133 with AM9 (MATa ade1-100 his4-519 ura3-52 leu2-3,112).
TABLE 1.
Strains
| Strain | Genotype |
|---|---|
| Haploid strains | |
| EI515 | MATaTHR4 hmlΔ::ADE1 hmrΔ::ADE1 ade1 ura3-52 leu2-3,112 lys5 GAL3::HO |
| AM133 | _MAT_α-inc leu2-3,112 thr4 met13 trp1 ura3-52 ade1 HMLα HMRa |
| LS23 | _MAT_α-inc his4 leu2-3,112 thr4 met13 trp1 ura3-52 ade1 HMLα HMRa |
| Diploid strains | |
| YLS100 | EI515 × LS23 |
| YLS101 | EI515 _rad51_Δ × LS23 _rad51_Δ |
| YLS102 | EI515 _rad54_Δ × LS23 _rad54_Δ |
| YLS103 | EI515 _rad55_Δ × LS23 _rad55_Δ |
| YLS104 | EI515 _rad57_Δ × LS23 _rad57_Δ |
| YLS105 | EI515 _rad1_Δ × LS23 _rad1_Δ |
| YLS106 | EI515 _rad1_Δ _rad51_Δ × LS23 _rad1_Δ _rad51_Δ |
| JZ605 | EI515 _sgs1_Δ × LS23 _sgs1_Δ |
| JZ605 | EI515 _sgs1_Δ _rad51_Δ × LS23 _sgs1_Δ _rad51_Δ |
| MLN120 | EI515 _rad51_Δ _tid1_Δ × AM133 _rad51_Δ _tid1_Δ |
| MLN123 | EI515 _rad51_Δ × AM133 _rad51_Δ |
| MLN126 | EI515 _tid1_Δ × AM133 _tid1_Δ |
| MLN127 | EI515 _rad54_Δ _tid1_Δ × AM133 _rad54_Δ _tid1_Δ |
| MLN128 | MN130 _rad51_Δ _rad59_Δ × AM482 _rad51_Δ _rad59_Δ |
| MLN154 | AM483 _rad51_Δ _rad50_Δ × AM484 _rad51_Δ _rad50_Δ |
Deletions of the RAD genes were introduced into strain EI515, AM133, or LS23 by a one-step gene disruption method (5) using the plasmid or PCR fragment listed below. Details of the deletions of RAD50, RAD54, RAD55, RAD57, RAD59, and TID1 are available on request. Linearized DNA fragments derived from the following plasmids or PCR amplifications were used to disrupt RAD genes: pJH683 (_rad51_Δ::URA3) or pJH1079 (_rad51_Δ::LEU2); pJH573-(pXRAD) (_rad54_Δ::LEU2), a gift of L. Symington (Columbia University); pSTL11 (_rad55_Δ::LEU2) (35); pSM57 (_rad57_Δ::LEU2), a gift of David Schild (University of California, Berkeley); pL962 (_rad1_Δ::LEU2), a gift of Ralph Keil (Pennsylvania State University Medical School, Hershey). pJH1340 (_sgs1_Δ::URA3) was disrupted by F. Pâques. _tid1_Δ::URA3 was disrupted as previously described for _tid1_Δ::HIS3 (27), and _rad59_Δ::KAN was created in strain JKM179 by Qijun Chen and Carol Greider.
All rad deletion strains were checked for UV or methyl methanesulfonate (MMS) sensitivity and were verified by Southern blot analysis. When double mutants were constructed with _rad51_Δ by transformation, the two haploid parents were first subjected to deletion of the second gene and then RAD51 was deleted. In other cases, the double mutants were obtained from meiotic segregants of appropriate crosses. The _rad50_Δ _rad51_Δ double mutant (MLN154) was obtained by a cross of two segregants (AM484 and AM483) from a cross of a _rad50_Δ derivative of EI515 and a _rad51_Δ derivative of AM133. The _rad51_Δ _rad59_Δ diploid MLN128 was obtained from a cross of two segregants (AM482 and MN130) from crosses between _rad59_Δ derivatives of strain JKM111, a _MAT_α strain otherwise identical to EI515 (38, 40), and a _rad51_Δ derivative of AM133.
Media and growth conditions.
Rich medium (yeast extract-peptone-dextrose [YEP-dextrose]) and synthetic complete medium with bases and amino acids omitted as specified were used as described previously (25). YEP-glycerol and YEP-galactose (YEP-Gal) consisted of 1% yeast extract–2% Bacto Peptone medium supplemented with 3% (vol/vol) glycerin and 2% (wt/vol) galactose, respectively. YEP-dextrose medium containing 0.015% (vol/vol) MMS was used to assess Rad− phenotypes. Cultures were incubated at 30°C.
Analysis of DNA repair.
Logarithmically growing cells grown in YEP-glycerol were plated on YEP-Gal and grown into colonies. The colonies were then replica plated onto nutritional dropout media. Colonies containing Ade+ Thr− sectors against an Ade− Thr− background were counted in two different ways during the course of this work. In experiments shown in Table 2, group B and C Ade+ Thr− sectors smaller than one-fourth of the total colony were not included in this category but were classified as Ade− Thr− colonies. In other experiments, such colonies were included. There is thus a quantitative but not qualitative difference in the number of sectored colonies enumerated.
TABLE 2.
Gene conversion, BIR, and chromosome loss following an HO-induced DSBa
| Strain | Temp tested (°C) | No. tested | % Event for phenotype of colonies | |||||
|---|---|---|---|---|---|---|---|---|
| Ade+ Thr+ (GC) | Ade+ Thr+ Ade+ Thr− (GC + CO, GC + BIR) | Ade+ Thr− (BIR) | Ade+ Thr− Ade− Thr− (BIR + chrom. loss) | Ade− Thr− (chrom. loss) | Ade+ Thr+ Ade− Thr− (GC + chrom. loss) | |||
| Group A | ||||||||
| Wild-type YLS100 | 30 | 638 | 70.6 | 28.0 | 1.0 | 0.3 | 0 | 0.1 |
| 18 | 314 | 87.0 | 12.0 | 1.0 | 0 | 0 | 0 | |
| _rad51_Δ YLS101 | 30 | 706 | 2.0 | 0 | 13.0 | 70.0 | 15.0 | 0 |
| 18 | 205 | 3.0 | 0 | 20.0 | 42.0 | 35.0 | 0 | |
| _rad54_Δ YLS102 | 30 | 383 | 1.0 | 0 | 14.0 | 30.0 | 55.0 | 0 |
| 18 | 97 | 2.0 | 0 | 3.0 | 66.0 | 29.0 | 0 | |
| _rad55_Δ YLS103 | 30 | 78 | 55.0 | 10.0 | 3.0 | 10.0 | 5.0 | 17.0 |
| 18 | 119 | 3.0 | 0 | 15.0 | 55.0 | 27.0 | 0 | |
| _rad57_Δ YLS104 | 30 | 764 | 52.0 | 13.0 | 4.0 | 8.0 | 8.0 | 15.0 |
| 18 | 84 | 8.0 | 0 | 13.0 | 51.0 | 27.0 | 0 | |
| _rad52_Δ EI524b | 30 | 3.0 | 97.0 | |||||
| Group B | ||||||||
| _sgs1_Δ JZ604 | 30 | 155 | 90.0 | 3.9 | 4.1 | 2.0 | ||
| _sgs1_Δ _rad51_Δ JZ605 | 30 | 187 | 5.9 | 34.2 | 26.7 | 33.2 | ||
| Group C | ||||||||
| _rad1_Δ YLS105 | 30 | 977 | 94.7 | 4.5 | 0.8 | |||
| _rad1_Δ _rad51_Δ YLS106 | 30 | 641 | 2.0 | 27.9 | 21.9 | 45.2 |
Induction of recombination.
The induction of DSBs by HO and the analysis of colonies were carried out as described previously (38). Cells were grown overnight in 50 ml of YEP-glycerol to a density of 1 × 107 to 5 × 107 cells per ml. Appropriate dilutions of cells were plated on YEP-Gal, grown to colonies, and analyzed.
RESULTS
_rad54_Δ, _rad55_Δ, and _rad57_Δ resemble _rad51_Δ, preventing gene conversions but allowing BIR.
The ability of diploids homozygous for deletion of _rad54_Δ, _rad55_Δ, or _rad57_Δ to repair an HO-induced DSB was compared with that of wild-type strains. As illustrated in Fig. 1, HO endonuclease will cleave the MATa locus but not the _MAT_α-inc allele, so that a single broken chromosome is created. If there is no repair, this broken chromosome will be lost, creating an Ade1− Thr4− colony that is 2N − 1 monosomic for chromosome III (Fig. 1E). If repair occurs by BIR, then the cells will be Ade1+ but Thr4− (Fig. 1D). If the DSB is repaired by gene conversion without crossing over, the colony will be Ade1+ and Thr4+ (Fig. 1A). Crossing over of a cell in the G2 stage of the cell cycle may also produce colonies fully Ade1+ but sectored for Thr4+ and Thr4− (Fig. 1B), but similar colonies could arise if there were two independent repair events, one leading to gene conversion and one to BIR (Fig. 1C). Some colonies are expected to be sectored because recombination was initiated in asynchronous cells, so that G2 cells will have two genomes that can be repaired independently. Furthermore, in the case of repair-defective mutant cells, sectored colonies may also arise if a broken chromosome is replicated and segregated, so that repair occurs only in a later generation (33, 52, 63).
As shown in Table 2, _rad51_Δ cells gave rise to sectored colonies displaying a mixture of Ade− Thr− and Ade+ Thr− sectors that, as researchers previously demonstrated, corresponded to chromosome loss and BIR events, respectively (38). In nearly all cases, the mating type of the colonies arising in _rad51_Δ strains was changed from nonmating (MATa/_MAT_α-inc) to α-mating, either hemizygous or homozygous for _MAT_α-inc. Indeed, this is the predominant type of repair seen for _rad51_Δ strains (Table 2). These results are very different from what is found in cells lacking RAD52, which produce almost exclusively Ade− Thr− colonies, indicative of chromosome loss (38). The small proportion of Ade+ Thr− cells in _rad52_Δ strains proved to result from nonreciprocal crossover events associated with a loss of the other chromosome, different from what is seen in _rad51_Δ cells (38). We note that in the absence of HO induction, more than 99% remain Ade+ Thr+ and that there are occasional chromosome loss events that do not favor the MATa-containing chromosome compared to the _MAT_α-inc chromosome (data not shown).
In _rad51_Δ diploids and in the _rad54_Δ, _rad55_Δ, and _rad57_Δ diploids discussed below, there were a small number of α-mating Ade+ Thr+ colonies that could be the result of authentic Rad51p-independent gene conversions (Table 2). These might occur either by a conventional gene conversion pathway or by a combination of two _RAD51_-independent pathways, an interrupted BIR event that would copy across _MAT_α-inc but would then dissociate, followed by SSA (26). It is also possible that these represent large deletions of the HO-cleaved MATa locus, so that only _MAT_α-inc on the other chromosome is expressed. This seems likely, because there were an equal number of nonmating (but no longer HO-cleavable) Ade+ Thr+ cells appearing to represent nonhomologous end-joinings that remove the HO cleavage site of MATa without deleting the MATa1 gene (40). In any case, these represent a very small proportion of recombinants in _rad51_Δ.
Diploids homozygous for _rad54_Δ behaved nearly identically to _rad51_Δ (Table 2). As with _rad51_Δ, the _rad54_Δ diploids produced mostly colonies that had either completely lost the broken chromosome or repaired the break by BIR (Ade+ Thr−). At 30°C the _rad54_Δ strain produced fewer sectored colonies that had undergone both BIR and chromosome loss events, and there were more colonies with only chromosome loss than found with _rad51_Δ. The small number of gene conversions in _rad54_Δ strains is consistent with the finding of rare gene conversion repair of HO-induced DSBs during MAT switching (53). Thus BIR is RAD54 independent as well as RAD51 independent in a situation where efficient gene conversion requires both genes.
Diploids homozygous for _rad55_Δ and _rad57_Δ were tested at both 18 and 30°C, because in some assays, such as MMS treatment or gamma irradiation, the repair defects of these mutants are seen only at lower temperatures (17, 35). Indeed, both of these mutants showed a striking temperature dependence in terms of the types of repair that were obtained. At 30°C, both mutants were nearly wild type in their outcomes, as more than half of the cells plated gave rise to gene conversion (Ade+ Thr+) events (Table 2). However, in a significant number of cases, the cell that was plated, which may have been in G2, gave rise to a mixed colony containing one half in which gene conversion was successful and the other half in which loss of the broken chromosome had apparently occurred. Thus, the two mutants were not fully wild type at 30°C. In contrast, at 18°C, _rad55_Δ and _rad57_Δ strongly resembled _rad51_Δ and _rad54_Δ (Table 2).
_RAD51_-independent DSB repair mostly depends on RAD50.
A recent study showed that the maintenance of telomeres in the absence of the telomerase TLC1 gene could occur in the absence of RAD51, RAD54, RAD55, and RAD57, as well as without RAD50, MRE11, or XRS2 (32). However, survivors that could maintain telomeres without telomerase were prevented both in a _rad52_Δ strain and in a _rad51_Δ _rad50_Δ double mutant. By itself, a _rad50_Δ mutation behaved very similarly to the wild-type diploid (Table 3). More than 80% of the repair events were gene conversions, although there were a small number of instances of chromosome loss to produce Ade− Thr− sectors of colonies. When we compared a _rad51_Δ _rad50_Δ double mutant isogenic with the _rad51_Δ strain discussed above, we found that BIR was severely reduced and the great majority of colonies showed only chromosome loss. The Ade+ Thr− colonies, which we presume to arise by BIR, were reduced from 85% of colonies in _rad51_Δ to 20% in _rad51_Δ _rad50_Δ (Table 3). In contrast to _rad52_Δ diploids, where BIR was eliminated (38), 20% of the colonies derived from the HO-induced _rad51_Δ _rad50_Δ diploid still were Ade+ Thr−. We confirmed that all nine out of nine colonies tested were still heterozygous for the ADE1 gene inserted on the left arm of chromosome III; hence these appear to be BIR events and not a nonreciprocal exchange event associated with chromosome loss that is seen in a small proportion of _rad52_Δ cells (38). This suggests that there is yet another pathway, which is RAD52 dependent but independent of RAD51 and RAD50, to generate these events (see Discussion).
TABLE 3.
Effects of recombination mutations on repair of HO-induced DSBs.
| Strain | No. tested | % Event for phenotype of coloniesa | ||||||
|---|---|---|---|---|---|---|---|---|
| Ade+ Thr+ (GC) | Ade+ Thr+ Ade+ Thr− (GC + CO, GC + BIR) | Ade+ Thr− (BIR) | Ade+ Thr− Ade− Thr− (BIR + chrom. loss) | Ade− Thr− (chrom. loss) | Ade+ Thr+ Ade− Thr− (GC + chrom. loss) | Ade− Thr+ (not analyzed) | ||
| Wild type MLN155 | 60 | 78.3 | 15.0 | 1.7 | 1.7 | 3.3 | ||
| _rad51_Δ MLN123 | 732 | 1.2 | 0.5 | 27.9 | 57.1 | 12.4 | 0.7 | 0.2 |
| _rad59_Δ MLN147 | 135 | 94.8 | 2.2 | 1.5 | 1.5 | |||
| _rad50_Δ MLN113 | 490 | 83.4 | 13.9 | 2.3 | 0.2 | 0.2 | 0.2 | |
| _rad51_Δ _rad50_Δ MLN114 | 157 | 2.5 | 8.9 | 11.5 | 77.1 | |||
| _rad51_Δ rad59Δ MLN128 | 169 | 3.6 | 1.2 | 4.7 | 5.9 | 84.0 | 0.6 | |
| _rad54_Δ YLS102 | 383 | 1.0 | 0 | 14.0 | 30.0 | 55.0 | 0 | |
| _tid1_Δ MLN126 | 1,059 | 70.4 | 19.6 | 1.3 | 0.1 | 0.5 | 2.0 | 0.2 |
| _rad54_Δ _tid1_Δ MLN127 | 576 | 1.0 | 13.7 | 6.3 | 78.6 | 0.2 | 0.2 | |
| _rad51_Δ _tid1_Δ MLN120 | 1,110 | 2.2 | 1.0 | 15.9 | 36.1 | 44.1 | 0.7 |
_RAD51_-independent DSB repair involves RAD59.
Recently Bai and Symington (1) showed that a _rad51_Δ _rad59_Δ strain was nearly as impaired in spontaneous heteroallelic recombination as _rad52_Δ and much more severely impaired than either single mutant. By itself, a _rad59_Δ derivative of our diploid yielded results very similar to those for the wild-type isogenic strain; nearly all repair events were gene conversions (Table 3). However, DSB repair in a _rad51_Δ _rad59_Δ double mutant is much more severely affected than in _rad51_Δ alone (Table 3). Only 11% of colonies were fully or partially Ade+ Thr−, with the rest being Ade− Thr−, reflecting the failure to repair the broken chromosome in almost 85% of cases. Thus, RAD59 appears to play an important role in the _RAD51_-independent repair of DSBs by BIR. As with spontaneous recombination (1), however, the _rad51_Δ _rad59_Δ double mutant was not as severely blocked in recombination as a _rad52_Δ strain. Indeed, 12 of 14 of the remaining Ade+ Thr− colonies recovered from the _rad51_Δ _rad59_Δ diploid were shown by Southern blot analysis to have undergone BIR events. The remaining two were likely to have undergone chromosome loss events in which there was a reversion of the ade1 mutation on chromosome I.
A TID1 deletion impairs BIR in _rad51_Δ and _rad54_Δ strains.
Recent studies of spontaneous recombination have shown that deletion of TID1 affects spontaneous interchromosomal gene conversions between heteroalleles but not intrachromosomal or sister chromatid interactions. In some cases the inhibition of interchromosomal recombination by deleting TID1 could be seen in comparison to wild-type strains (27); in other strains, the _tid1_Δ effect was evident only when RAD54 was also deleted (56). We therefore assessed how a _tid1_Δ diploid would carry out repair of an HO-induced DSB. We found that the absence of Tid1p had no significant effect on the repair of DSBs (Table 2). The great majority of events were gene conversions (Ade1+ Thr4+). Thus, in our strains Tid1p does not play a key role in interchromosomal DSB-induced gene conversions in an otherwise wild-type strain.
A _rad54_Δ _tid1_Δ strain was more severely blocked in DSB repair than was either single mutant alone (Table 3). The _rad54_Δ _tid1_Δ double mutant gave results virtually identical to those for _rad51_Δ _rad50_Δ, reducing but not completely eliminating Ade+ Thr− recombinants. Southern blot analysis showed that 10 of 19 derivatives were consistent with BIR events. Four may be chromosome losses with a reversion of ade1, and four may be similar to _rad52_Δ-independent events in which there is a crossover chromosome associated with loss of the reciprocal partner (15, 38).
Interestingly, the _rad51_Δ _tid1_Δ double mutant showed intermediate behavior compared to _rad51_Δ alone or _rad54_Δ _tid1_Δ (Table 3). The number of colonies in which there was no repair (Ade− Thr−) increased approximately fourfold, from 11% in _rad51_Δ to 44%, still much less than the almost 80% lack of repair in _rad54_Δ _tid1_Δ. One explanation for this result is that Rad54p is able to substitute in part for Tid1p in a _RAD51_-independent pathway of BIR.
SGS1 does not play a significant role in BIR.
The _sgs1_Δ mutation has been shown to increase spontaneous mitotic recombination both in ribosomal DNA and for sister chromatid exchange (45, 55, 67). The Sgs1 helicase has also been implicated as an important helicase during DNA replication (34). Hence it was of interest to know if an _sgs1_Δ mutation would affect HO-induced recombination. As shown in Table 2, group B, the absence of Sgs1p had no effect on predominantly gene conversion repair compared to what was found with wild-type cells. Likewise, a _rad51_Δ _sgs1_Δ strain was able to carry out BIR in a manner similar to that of _rad51_Δ alone. Hence it appears that _RAD51_-independent BIR does not require Sgs1p.
RAD1 is not required for _RAD51_-independent BIR.
The Rad1-Rad10 endonuclease has been shown to play an important role in recombination in which nonhomologous, 3′-ended single-stranded tails at the ends of a DSB must be excised before a 3′ end can be generated to prime new DNA synthesis (7, 10, 47). When there is nonhomology on both DNA ends, RAD1 is essential, but when there is nonhomology on only one side, as there is when HO cleaves MATa and _MAT_α-inc is the donor, a rad1 deletion delays but allows the completion of the process (7, 18). We investigated whether _RAD51_-independent BIR also requires RAD1. In an otherwise wild-type strain, _rad1_Δ has no significant effect: most repair events are gene conversions, replacing MATa with _MAT_α-inc. Perhaps more surprising, in a _rad51_Δ _rad1_Δ double mutant, there was also no significant effect relative to _rad51_Δ alone. Thus, even though at least 700 bp of the Ya region in MATa must be removed before a DNA end is homologous to the template chromosome and BIR can be initiated, Rad1p does not appear to be critical for this process (Table 2, group C). Previously, it has been shown that there is an alternative, though less efficient, mechanism for removing nonhomologous tails from DNA ends (6, 38), but the genes encoding elements of this process have yet to be identified.
DISCUSSION
From bacteriophages to eukaryotes, recombination-dependent DNA replication plays an important role in the repair of DSBs. Break-induced DNA replication was first invoked to account for recombination in phage λ (56) and to explain late DNA replication in phage T4 (36, 43). Recently, extensive DNA replication during recombination has been observed by George and Kreuzer (12) in phage T4-mediated recombination and by Kuzminov and Stahl (31) and Motamedi et al. (44) while studying recombination in phage λ. The work of Kogoma (29) extended this concept to account for recombination-dependent, origin-independent replication in E. coli. More recently, BIR has been proposed to explain how broken replication forks can be restarted (reviewed in references 14 and 30). BIR has also been proposed to explain how the ends of a linear transforming DNA segment can establish the complete replication of a copy of the E. coli genome (8), and a similar proposal has been made by Morrow et al. (41) to account for the duplication of an entire yeast chromosome that received a linearized fragment of transforming DNA.
BIR also appears to account for very long gene conversion tracts, apparently extending to the end of a chromosome, which have been observed in several studies of meiotic and mitotic recombination in S. cerevisiae (9, 24, 65). A demonstration of such repair following a DSB in mitotic cells was provided by Bosco and Haber (3), who used HO endonuclease to cleave one chromosome in such a way that it shared extensive homology with a homologous chromosome only centromere proximal to the DSB. This study also suggested that BIR could occur prior to normal S phase, so that both progeny of a cell suffering a broken chromosome in G1 survived with the same BIR repair of the chromosome.
One key process in which BIR appears to be of critical importance in eukaryotic cells is in the repair of chromosomes with telomeres that are too short to retain their normally end-protected state. Lundblad and Blackburn (37) first showed that survivors in S. cerevisiae that lacked the telomerase component Est1p required the key recombination gene RAD52. A similar observation was made by McEachern and Blackburn (39) for telomerase-defective Kluyveromyces lactis. These observations were extended by Le et al. (32) to show that there seem to be two distinct pathways for survival in the absence of telomerase, one apparently requiring RAD51, RAD54, and RAD57 (and presumably RAD55) and the other involving RAD50, MRE11, and XRS2. Survivors after deletion of the telomerase TLC1 gene failed to arise in both _rad52_Δ strains and in _rad51_Δ _rad50_Δ double mutants. The idea that there may be two different pathways of telomere repair in the absence of telomerase was supported by the work of Teng and Zakian (62), who found two distinct types of recombination events at telomeres, both RAD52 dependent, and by more recent work showing that the two different types are eliminated in _rad51_Δ and _rad50_Δ mutants (61).
Because it is not possible to examine individual telomere repair events in detail, for example to determine if they yield nonreciprocal translocations, we have examined BIR repair of a single DSB in the middle of a chromosome. We showed that while an efficient gene conversion pathway requires RAD51, RAD54, RAD55, and RAD57, BIR can occur in the absence of any of these genes. Moreover, this _RAD51_- and _RAD54_-independent BIR process is dependent on RAD59, TID1, RAD50, and presumably MRE11 and XRS2.
In this paper we have focused on the _RAD51_- and _RAD54_-independent pathway of BIR that can be seen because the more efficient gene conversion pathway has been eliminated. But we have several indications that there is also a more efficient _RAD51_-dependent pathway of BIR that is usually masked by gene conversion. First, we have examined BIR events in RAD51 cells, but not at the same location where these studies have been conducted (3). In that study it appeared that BIR in the presence of RAD51 is more efficient than in _rad51_Δ cells, where repair may not occur for several generations after initiation of the DSB (38). Second, researchers have carried out preliminary experiments, using a diploid with the HO-induced DSB at the same site used in these studies but with only a small amount of homology distal to the cleavage site. Here, where gene conversion is nearly absent, it appears that _RAD51_-dependent BIR is indeed more efficient than _rad51_Δ events (M. L. Naylor, A. Malkova, and J. E. Haber, unpublished observations).
One suggestion that Rad54p may also be important for BIR comes from the fact that a _rad51_Δ _tid1_Δ strain is much less severely affected than _rad51_Δ _rad59_Δ, _rad51_Δ _rad50_Δ, or _rad54_Δ _tid1_Δ. The simplest interpretation of the fact that this particular double mutant is less severe is that Rad54p can substitute for Tid1p in Rad51p-independent BIR. Moreover, a _rad54_Δ mutant had a higher incidence of chromosome loss and fewer BIR events that did _rad51_Δ (Table 2). Since none of the double mutations that we have tested are as severe as _rad52_Δ, our findings might further suggest that there are still other redundancies to be revealed by further multiple mutant analysis.
The idea that there are two alternative BIR pathways is supported by studies of telomerase-independent telomere maintenance (6, 61). In the absence of RAD51, the great majority of survivors prove to be one of the two types (type II) described by Teng and Zakian (62), in which the TG1–3 telomere ends themselves are lengthened by recombination. In the absence of RAD59, type I prevails, in which tandem arrays of Y′ subtelomeric genes separated by short stretches of telomere sequence are found at most chromosome ends. One might suggest that type I events involve BIR events in which a telomere end is resected into subtelomeric regions, such as X or Y′, and that there is extensive homology shared between that single-stranded region and other X or Y′ elements to promote BIR. In contrast, type II events appear to involve the invasion of TG1–3 telomeric sequences into the same other TG1–3 telomere sequences. This might occur by an intrachromosomal strand invasion, forming a T loop (13, 62, 66), and leading to rolling-circle replication.
One additional interesting finding is that _rad55_Δ and _rad57_Δ mutants are cold sensitive for the interchromosomal repair of an HO-induced DSB in a diploid, the same phenotype seen for X-ray or MMS sensitivity for these deletions (35). At 30°C or above, the _rad55_Δ and _rad57_Δ strains are resistant to ionizing radiation, whereas they are sensitive at 23°C. It is thought that Rad55p and Rad57p act as auxiliary factors to help load Rad51p onto single-stranded DNA created at DSB ends (11, 23, 60). However, not all HO-induced recombination events show cold sensitivity. In HO-induced MAT switching (where the donor sequences are heterochromatic), _rad55_Δ and _rad57_Δ strains are unable to complete recombination at 30 or even 34°C. Alternatively, HO-induced gene conversion of inverted repeated LacZ sequences on a plasmid proved to be independent of RAD51, RAD54, RAD55, and RAD57 at 23°C (20, 58). The repair event that we are studying here, an interchromosomal recombination gene conversion repair of a DSB, is the only HO-induced event that we have examined in which _rad55_Δ and _rad57_Δ mutants have the same cold sensitivity which they display for ionizing radiation and MMS sensitivity. This suggests to us that the system which we are using is in fact an excellent model system for the consequences of ionizing radiation.
How does BIR occur without Rad51p?
How BIR occurs in the absence of the strand invasion protein Rad51p remains a mystery. Based on our previous studies it seems very likely that the ends of the DSB are resected by a 5′-to-3′ exonuclease, producing long 3′-ended single-strand-DNA (ssDNA) tails (68). Special, accessible sites where Rad52p mediates strand annealing in the absence of Rad51p and Rad54p (43, 51, 59) between the ssDNA tail and a partially denatured region of the template chromosome would permit the necessary formation of heteroduplex DNA that could be used to prime new DNA replication. SSA in S. cerevisiae does not require Rad51p, Rad54p, Rad55p, or Rad57p (20) but does in fact require both Rad52p and Rad59p (57). However, in SSA the absence of Rad50p delays the event without preventing it (20). The role of Tid1p in this process has not been assessed. It is possible that these proteins play a role in the creation of a stable intermediate that can be converted into a replication fork. Support for the general idea that _RAD51_-independent BIR can occur only at particularly accessible sites comes from our recent discovery that BIR does not initiate anywhere (and hence does not retain genetic markers) in the first 10 kb centromere proximal to MAT (A. Malkova, L. Signon, C. B. Schaefer, M. L. Naylor, J. F. Theis, C. S. Newlon, and J. E. Haber, unpublished observations). Preferential sites of repair could also represent sites where the repair-replication fork could acquire processivity factors that would permit the repair-replication fork to move more than 130 kb down the chromosome.
In the experiments reported here, with the use of _rad51_Δ and other mutant cells, the efficiency of BIR is high enough that most colonies derived after plating single cells onto medium to induce HO cleavage can complete BIR; however, in the majority of cases, one observes one or more Ade+ Thr− sectors against a background of Ade− Thr− cells that have lost the broken chromosome. In many colonies there are multiple Ade+ sectors, suggesting that DNA repair occurred several generations after the creation of the DSB. It is now well established that cells carrying a single DSB will arrest but then adapt and resume cell cycle progression even though they carry a broken chromosome (33, 52). The experiments of Malkova et al. (38) and those reported here demonstrate that this broken chromosome can then be repaired in approximately 10 to 20% of cell divisions. Whether BIR becomes an even more efficient event after cells have adapted, where some normal DNA damage checkpoint controls have apparently been turned off, is an important question that we are pursuing.
Finally, we note that BIR shares many common features with gene conversion. _RAD51_-dependent gene conversion initiated by a DSB is likely to involve both leading- and lagging-strand DNA polymerases (18), and scholars have suggested that the ways in which new DNA synthesis is initiated in gene conversion and in BIR are likely to be very similar or identical (18, 46). Gene conversion would occur if the second end of a DSB becomes engaged in the recombination event, whereas in the absence of a second end, the repair-replication fork would proceed to the chromosome end.
ACKNOWLEDGMENTS
We thank Carol Greider and members of the Haber lab for their comments and suggestions. Qijun Chen and Carol Greider kindly provided _rad59_Δ strains, and we obtained plasmids from Ralph Keil, David Schild, Susan Lovett and Frédéric Pâques.
H.K. was supported by National Institutes of Health grant GM53738. J.E.H. was supported by National Institutes of Health grant GM20056 and National Science Foundation grant MCB-9724086. M.L.N. was a Howard Hughes Medical Institute undergraduate summer research scholar at Brandeis University.
L.S., A.M., and M.L.N. made equal contributions to this work.
REFERENCES
- 1.Bai Y, Symington L S. A RAD52 homolog is required for RAD51-independent mitotic recombination in Saccharomyces cerevisiae. Genes Dev. 1996;10:2025–2037. doi: 10.1101/gad.10.16.2025. [DOI] [PubMed] [Google Scholar]
- 2.Bartsch S, Kang L E, Symington L S. RAD51 is required for the repair of plasmid double-stranded DNA gaps from either plasmid or chromosomal templates. Mol Cell Biol. 2000;20:1194–1205. doi: 10.1128/mcb.20.4.1194-1205.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosco G, Haber J E. Chromosome break-induced DNA replication leads to non-reciprocal translocations and telomere capture. Genetics. 1998;150:1037–1047. doi: 10.1093/genetics/150.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bressan D A, Baxter B K, Petrini J H J. The Mre11-Rad50-Xrs2 protein complex facilitates homologous recombination-based double-strand break repair in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:7681–7687. doi: 10.1128/mcb.19.11.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen D C, Yang B C, Kuo T T. One-step transformation of yeast in stationary phase. Curr Genet. 1992;21:83–84. doi: 10.1007/BF00318659. [DOI] [PubMed] [Google Scholar]
- 6.Chen Q, Ijpma A, Greider C W. Two survivor pathways that allow growth in the absence of telomerase are generated by distinct telomere recombination events. Mol Cell Biol. 2001;21:1819–1827. doi: 10.1128/MCB.21.5.1819-1827.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colaiácovo M P, Pâques F, Haber J E. Removal of one nonhomologous DNA end during gene conversion by a RAD1- and MSH2-independent pathway. Genetics. 1999;151:1409–1423. doi: 10.1093/genetics/151.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dabert P, Smith G R. Gene replacement with linear DNA fragments in wild-type Escherichia coli: enhancement by Chi sites. Genetics. 1997;145:877–889. doi: 10.1093/genetics/145.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esposito M S. Evidence that spontaneous mitotic recombination occurs at the two-strand stage. Proc Natl Acad Sci USA. 1978;75:4436–4440. doi: 10.1073/pnas.75.9.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fishman-Lobell J, Haber J E. Removal of nonhomologous DNA ends in double-strand break recombination: the role of the yeast ultraviolet repair gene RAD1. Science. 1992;258:480–484. doi: 10.1126/science.1411547. [DOI] [PubMed] [Google Scholar]
- 11.Gasior S L, Wong A K, Kora Y, Shinohara A, Bishop D K. Rad52 associates with RPA and functions with Rad55 and Rad57 to assemble meiotic recombination complexes. Genes Dev. 1998;12:2208–2221. doi: 10.1101/gad.12.14.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George J W, Kreuzer K N. Repair of double-strand breaks in bacteriophage T4 by a mechanism that involves extensive DNA replication. Genetics. 1996;143:1507–1520. doi: 10.1093/genetics/143.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffith J D, Comeau L, Rosenfield S, Stansel R M, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 14.Haber J E. DNA recombination: the replication connection. Trends Biochem Sci. 1999;24:271–275. doi: 10.1016/s0968-0004(99)01413-9. [DOI] [PubMed] [Google Scholar]
- 15.Haber J E, Hearn M. RAD52-independent mitotic gene conversion in Saccharomyces cerevisiae frequently results in chromosomal loss. Genetics. 1985;111:7–22. doi: 10.1093/genetics/111.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haber J E, Thorburn P C. Healing of broken linear dicentric chromosomes in yeast. Genetics. 1984;106:207–226. doi: 10.1093/genetics/106.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hays S L, Firmenich A A, Berg P. Complex formation in yeast double-strand break repair: participation of Rad51, Rad52, Rad55, and Rad57 proteins. Proc Natl Acad Sci USA. 1995;92:6925–6929. doi: 10.1073/pnas.92.15.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes A, Haber J E. Double-strand break repair in yeast requires both leading and lagging strand DNA polymerases. Cell. 1999;96:415–424. doi: 10.1016/s0092-8674(00)80554-1. [DOI] [PubMed] [Google Scholar]
- 19.Ivanov E L, Korolev V G, Fabre F. XRS2, a DNA repair gene of Saccharomyces cerevisiae, is needed for meiotic recombination. Genetics. 1992;132:651–664. doi: 10.1093/genetics/132.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivanov E L, Sugawara N, Fishman L J, Haber J E. Genetic requirements for the single-strand annealing pathway of double-strand break repair in Saccharomyces cerevisiae. Genetics. 1996;142:693–704. doi: 10.1093/genetics/142.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivanov E L, Sugawara N, White C I, Fabre F, Haber J E. Mutations in XRS2 and RAD50 delay but do not prevent mating-type switching in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:3414–3425. doi: 10.1128/mcb.14.5.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jablonovich Z, Liefshitz B, Steinlauf R, Kupiec M. Characterization of the role played by the RAD59 gene of Saccharomyces cerevisiae in ectopic recombination. Curr Genet. 1999;36:13–20. doi: 10.1007/s002940050467. [DOI] [PubMed] [Google Scholar]
- 23.Johnson R D, Symington L S. Functional differences and interactions among the putative RecA homologs Rad51, Rad55, and Rad57. Mol Cell Biol. 1995;15:4843–4850. doi: 10.1128/mcb.15.9.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Judd S R, Petes T D. Physical lengths of meiotic and mitotic gene conversion tracts in Saccharomyces cerevisiae. Genetics. 1988;118:401–410. doi: 10.1093/genetics/118.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaiser C, Michaelis S, Mitchell A. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 26.Kang L E, Symington L S. Aberrant double-strand break repair in rad51 mutants of Saccharomyces cerevisiae. Mol Cell Biol. 2000;20:9162–9172. doi: 10.1128/mcb.20.24.9162-9172.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein H L. RDH54, a RAD54 homologue in Saccharomyces cerevisiae, is required for mitotic diploid-specific recombination and repair and for meiosis. Genetics. 1997;147:1533–1543. doi: 10.1093/genetics/147.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kogoma T. Recombination by replication. Cell. 1996;85:625–627. doi: 10.1016/s0092-8674(00)81229-5. [DOI] [PubMed] [Google Scholar]
- 29.Kogoma T. Stable DNA replication: interplay between DNA replication, homologous recombination, and transcription. Microbiol Mol Biol Rev. 1997;61:212–238. doi: 10.1128/mmbr.61.2.212-238.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuzminov A. Collapse and repair of replication forks in Escherichia coli. Mol Microbiol. 1995;16:373–384. doi: 10.1111/j.1365-2958.1995.tb02403.x. [DOI] [PubMed] [Google Scholar]
- 31.Kuzminov A, Stahl F W. Double-strand end repair via the RecBC pathway in Escherichia coli primes DNA replication. Genes Dev. 1999;13:345–356. doi: 10.1101/gad.13.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le S, Moore J K, Haber J E, Greider C. RAD50 and RAD51 define two different pathways that collaborate to maintain telomeres in the absence of telomerase. Genetics. 1999;152:143–152. doi: 10.1093/genetics/152.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S E, Moore J K, Holmes A, Umezu K, Kolodner R, Haber J E. Saccharomyces Ku70, Mre11/Rad50 and RPA proteins regulate adaptation to G2/M arrest after DNA damage. Cell. 1998;94:399–409. doi: 10.1016/s0092-8674(00)81482-8. [DOI] [PubMed] [Google Scholar]
- 34.Lee S K, Johnson R E, Yu S L, Prakash L, Prakash S. Requirement of yeast SGS1 and SRS2 genes for replication and transcription. Science. 1999;286:2339–2342. doi: 10.1126/science.286.5448.2339. [DOI] [PubMed] [Google Scholar]
- 35.Lovett S T, Mortimer R K. Characterization of null mutants of the RAD55 gene of Saccharomyces cerevisiae: effects of temperature, osmotic strength and mating type. Genetics. 1987;116:547–553. doi: 10.1093/genetics/116.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luder A, Mosig G. Two alternative mechanisms for initiation of DNA replication forks in bacteriophage T4: priming by RNA polymerase and by recombination Proc. Natl Acad Sci USA. 1982;79:1101–1105. doi: 10.1073/pnas.79.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundblad V, Blackburn E H. An alternative pathway for yeast telomere maintenance rescues est1-senescence. Cell. 1993;73:347–360. doi: 10.1016/0092-8674(93)90234-h. [DOI] [PubMed] [Google Scholar]
- 38.Malkova A, Ivanov E L, Haber J E. Double-strand break repair in the absence of RAD51 in yeast: a possible role for break-induced DNA replication. Proc Natl Acad Sci USA. 1996;93:7131–7136. doi: 10.1073/pnas.93.14.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McEachern M J, Blackburn E H. Cap-prevented recombination between terminal telomeric repeat arrays (telomere CPR) maintains telomeres in Kluyveromyces lactis lacking telomerase. Genes Dev. 1996;10:1822–1834. doi: 10.1101/gad.10.14.1822. [DOI] [PubMed] [Google Scholar]
- 40.Moore J K, Haber J E. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2164–2173. doi: 10.1128/mcb.16.5.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrow D M, Connelly C, Hieter P. “Break copy” duplication: a model for chromosome fragment formation in Saccharomyces cerevisiae. Genetics. 1997;147:371–382. doi: 10.1093/genetics/147.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mortensen U H, Bendixen C, Sunjevaric I, Rothstein R. DNA strand annealing is promoted by the yeast Rad52 protein. Proc Natl Acad Sci USA. 1996;93:10729–10734. doi: 10.1073/pnas.93.20.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosig G. Recombination and recombination-dependent DNA replication in bacteriophage T4. Annu Rev Genet. 1998;32:379–413. doi: 10.1146/annurev.genet.32.1.379. [DOI] [PubMed] [Google Scholar]
- 44.Motamedi M R, Szigety S K, Rosenberg S M. Double-strand-break repair recombination in Escherichia coli: physical evidence for a DNA replication mechanism in vivo. Genes Dev. 1999;13:2889–2903. doi: 10.1101/gad.13.21.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onoda F, Seki M, Miyajima A, Enomoto T. Elevation of sister chromatid exchange in Saccharomyces cerevisiae sgs1 disruptants and the relevance of the disruptants as a system to evaluate mutations in Bloom's syndrome gene. Mutat Res. 2000;459:203–209. doi: 10.1016/s0921-8777(99)00071-3. [DOI] [PubMed] [Google Scholar]
- 46.Pâques F, Haber J E. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pâques F, Haber J E. Two pathways for removal of nonhomologous DNA ends during double-strand break repair in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:6765–6771. doi: 10.1128/mcb.17.11.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petukhova G, Stratton S A, Sung P. Single strand DNA binding and annealing activities in the yeast recombination factor Rad59. J Biol Chem. 1999;274:33839–33842. doi: 10.1074/jbc.274.48.33839. [DOI] [PubMed] [Google Scholar]
- 49.Rattray A J, Symington L S. Multiple pathways for homologous recombination in Saccharomyces cerevisiae. Genetics. 1995;139:45–56. doi: 10.1093/genetics/139.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rattray A J, Symington L S. Use of a chromosomal inverted repeat to demonstrate that the RAD51 and RAD52 genes of Saccharomyces cerevisiae have different roles in mitotic recombination. Genetics. 1994;138:587–595. doi: 10.1093/genetics/138.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reddy G, Golub E I, Radding C M. Human Rad52 protein promotes single-strand DNA annealing followed by branch migration. Mutat Res. 1997;377:53–59. doi: 10.1016/s0027-5107(97)00057-2. [DOI] [PubMed] [Google Scholar]
- 52.Sandell L L, Zakian V A. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. 1993;75:729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- 53.Schmuckli-Maurer J, Heyer W D. The Saccharomyces cerevisiae RAD54 gene is important but not essential for natural homothallic mating-type switching. Mol Gen Genet. 1999;260:551–558. doi: 10.1007/s004380050928. [DOI] [PubMed] [Google Scholar]
- 54.Shinohara M, Shita Y E, Buerstedde J M, Shinagawa H, Ogawa H, Shinohara A. Characterization of the roles of the Saccharomyces cerevisiae RAD54 gene and a homologue of RAD54, RDH54/TID1, in mitosis and meiosis. Genetics. 1997;147:1545–1456. doi: 10.1093/genetics/147.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinclair D A, Guarente L. Extrachromosomal rDNA circles—a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 56.Skalka A. A replicator's view of recombination (and repair). New York, N.Y: Plenum Press; 1974. [Google Scholar]
- 57.Sugawara N, Ira G, Haber J E. DNA length dependence of the single-strand annealing pathway and the role of Saccharomyces cerevisiae RAD59 in double-strand break repair. Mol Cell Biol. 2000;20:5300–5309. doi: 10.1128/mcb.20.14.5300-5309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sugawara N, Ivanov E L, Fishman L J, Ray B L, Wu X, Haber J E. DNA structure-dependent requirements for yeast RAD genes in gene conversion. Nature. 1995;373:84–86. doi: 10.1038/373084a0. [DOI] [PubMed] [Google Scholar]
- 59.Sugiyama T, New J H, Kowalczykowski S C. DNA annealing by Rad52 protein is stimulated by specific interaction with the complex of replication protein A and single-stranded DNA. Proc Natl Acad Sci USA. 1998;95:6049–6054. doi: 10.1073/pnas.95.11.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sung P. Yeast Rad55 and Rad57 proteins form a heterodimer that functions with replication protein A to promote DNA strand exchange by Rad51 recombinase. Genes Dev. 1997;11:1111–1121. doi: 10.1101/gad.11.9.1111. [DOI] [PubMed] [Google Scholar]
- 61.Teng S, Chang J, McCowan B, Zakian V A. Telomerase-independent lengthening of yeast telomeres occurs by an abrupt Rad50p-dependent, Rif-inhibited recombinational process. Mol Cell. 2000;6:947–952. doi: 10.1016/s1097-2765(05)00094-8. [DOI] [PubMed] [Google Scholar]
- 62.Teng S C, Zakian V A. Telomere-telomere recombination is an efficient bypass pathway for telomere maintenance in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:8083–8093. doi: 10.1128/mcb.19.12.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toczyski D P, Galgoczy D J, Hartwell L H. CDC5 and CKII control adaptation to the yeast DNA damage checkpoint. Cell. 1997;90:1097–1106. doi: 10.1016/s0092-8674(00)80375-x. [DOI] [PubMed] [Google Scholar]
- 64.Usui T, Ohta T, Oshumi H, Tsubouchi H, Tomizawa J-I, Ogawa H, Ogawa T. Complex formation and functional versatility of Mre11 of budding yeast in recombination. Cell. 1998;95:705–716. doi: 10.1016/s0092-8674(00)81640-2. [DOI] [PubMed] [Google Scholar]
- 65.Voelkel-Meiman K, Roeder G S. Gene conversion tracts stimulated by HOT1-promoted transcription are long and continuous. Genetics. 1990;126:851–867. doi: 10.1093/genetics/126.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walmsley R W, Chan C S, Tye B K, Petes T D. Unusual DNA sequences associated with the ends of yeast chromosomes. Nature. 1984;310:157–160. doi: 10.1038/310157a0. [DOI] [PubMed] [Google Scholar]
- 67.Watt P M, Hickson I D, Borts R H, Louis E J. SGS1, a homologue of the Bloom's and Werner's syndrome genes, is required for maintenance of genome stability in Saccharomyces cerevisiae. Genetics. 1996;144:935–945. doi: 10.1093/genetics/144.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.White C I, Haber J E. Intermediates of recombination during mating type switching in Saccharomyces cerevisiae. EMBO J. 1990;9:663–673. doi: 10.1002/j.1460-2075.1990.tb08158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]