Identification of Rifampin-Resistant Mycobacterium tuberculosis Strains by Hybridization, PCR, and Ligase Detection Reaction on Oligonucleotide Microchips (original) (raw)
Abstract
Three new molecular approaches were developed to identify drug-resistant strains of Mycobacterium tuberculosis using biochips with oligonucleotides immobilized in polyacrylamide gel pads. These approaches are significantly faster than traditional bacteriological methods. All three approaches—hybridization, PCR, and ligase detection reaction—were designed to analyze an 81-bp fragment of the gene rpoB encoding the β-subunit of RNA polymerase, where most known mutations of rifampin resistance are located. The call set for hybridization analysis consisted of 42 immobilized oligonucleotides and enabled us to identify 30 mutant variants of the rpoB gene within 24 h. These variants are found in 95% of all mutants whose rifampin resistance is caused by mutations in the 81-bp fragment. Using the second approach, allele-specific on-chip PCR, it was possible to directly identify mutations in clinical samples within 1.5 h. The third approach, on-chip ligase detection reaction, was sensitive enough to reveal rifampin-resistant strains in a model mixture containing 1% of resistant and 99% of susceptible bacteria. This level of sensitivity is comparable to that from the determination of M. tuberculosis drug resistance by using standard bacteriological tests.
Tuberculosis (TB), one of the most deadly and common infectious diseases, claims 3,000,000 lives a year worldwide (24). Although the disease is found mostly in developing countries, a growing number of cases are diagnosed in the industrialized world as well. The global spread of the disease is further complicated by the ubiquitous appearance of drug-resistant (6) and especially multidrug-resistant strains that, by definition, are resistant to at least rifampin (RIF) and isoniazid. Thus, in 1996 13% of all newly diagnosed cases of TB in the United States were resistant (primary resistance) to at least one first-line drug and 1.6% were multidrug resistant (19).
After radical political changes in the former Soviet Union, the incidence of TB and related mortality in Russia increased to levels that are among the highest in the world. The incidence of TB in Russia in the period between 1991 and 1997 increased from 34 to 82 per 100,000 adults (10), four to seven times higher than that in most European countries. In view of the potential global spread of multidrug-resistant forms of TB, special concerns arise regarding the Russian penitentiary system. Its population presently exceeds 1,000,000, and 10% of the prisoners have active TB (for a review, see reference 10). Among the prisoners, about 25% of all new cases and 92% of nonresponding cases are drug resistant (7); according to another report, 66% of all cases are drug resistant, 50% of which are RIF-resistant (Rifr) forms (29).
According to the recommendations of the Centers for Disease Control and Prevention, bacteriological laboratories must determine the resistance of all submitted samples of M. tuberculosis to all first-line antibiotics. This has to be done using the fastest methods available, and final results must be reported within 30 days after receiving the sample (32). Most laboratories incubate solid medium cultures for 8 weeks to achieve a better sensitivity (9). Several liquid-culture-based strategies, such as MB/BacT (Organon-Teknika), MGIT, BACTEC 460TB, and BACTEC 9000 MB (Becton Dickinson), have been developed that allow the mean time required for detection of Mycobacterium tuberculosis to be reduced to 8 to 18 days (1, 3, 22). Subsequent assessment of drug resistance may take another 3 weeks. Even with the most advanced methods the turnaround times are 3 to 15 days for the BACTEC 460TB system (23, 27), 5 to 11 days for the MB/BacT system (5), and 3 to 14 days for MGIT (26). Therefore, there is an urgent need to develop simple, fast, and cost-effective methods to identify drug resistance in mycobacteria that could be used for large-scale population screening, particularly for the screening of prisoners.
We focused our efforts on the resistance of M. tuberculosis to RIF, probably the most efficient anti-TB drug and a key component of modern chemotherapeutic cocktails of three to four drugs. RIF resistance could be considered a surrogate marker for multidrug-resistant TB strains (31) and should therefore be subject to the most rapid test performed.
The resistance of M. tuberculosis to RIF is caused by a number of mutations. About 95% of these mutations are confined to a short 81-bp-long DNA region in the gene rpoB encoding the β-subunit of RNA polymerase B, the so-called RIF-resistance-determining region (RRDR) (20, 31). A number of protocols have been developed to analyze the underlying sequence polymorphisms of RRDR, including direct sequencing (16), dideoxy fingerprinting (11), PCR-heteroduplex analysis (36), single-strand conformation polymorphism (28, 31), DNA probe arrays (13, 34), hybridization of PCR-amplified products to a limited set of probes (8, 15, 25, 35), and RNA mismatch analysis (21).
In the first approach we propose to use hybridization on an oligonucleotide microarray—MAGIChips (38)—to identify Rifr strains of M. tuberculosis in clinical samples within 24 h. This method is based on the difference in stability between perfect and imperfect duplexes formed by the fluorescently labeled target DNA and the probes immobilized in gel pads of the dedicated TB biochip. The relative intensities of fluorescence of the pads indicate the presence and the nature of the mutations.
The second approach involves PCR amplification on MAGIChips. Using this method, the time to identify Rifr strains is further shortened to 1.5 h. In addition, it does not require any special probe preparation and can be applied to simultaneous analysis of several variable segments of the bacterial genome.
Early detection of low-copy-number mutant DNA against a high background of wild-type DNA is an important practical goal that requires high sensitivity. The third approach, ligase detection reaction (LDR), is applied to identify about 1% of mutant sequences in model samples consisting of mixtures of DNA from wild-type and resistant strains.
MATERIALS AND METHODS
Oligonucleotides.
Oligonucleotides for hybridization analysis and on-chip PCR were designed using the programs Oligo 5 (Molecular Biology Insights, Cascade, Colo.) and Primer Calculator. The procedure included the following steps. First, melting temperatures for perfect matches were determined for prospective oligonucleotides. Second, the length of the oligonucleotides was adjusted to maintain the range of melting temperatures within 3 to 4°C for hybridization probes and 2 to 3°C for PCR primers.
Oligonucleotides were synthesized on an ABI-394 DNA/RNA synthesizer (Applied Biosystems, Foster City, Calif.) using standard phosphoramidite chemistry. Oligonucleotides were purified by reverse-phase high-performance liquid chromatography on C18-Nucleosil columns (Sigma, St. Louis, Mo.) for hybridization and on-chip PCR and in denaturing polyacrylamide gel electrophoresis for on-chip LDR. To immobilize oligonucleotides in gel pads or to attach the fluorescent label Texas Red (Molecular Probes, Inc., Eugene, Oreg.), an amino group was introduced during synthesis using 3′-Amino-Modifier C7 CPG 500 or 5′-Amino-Modifier C6 (Glen Research, Sterling, Va.). The attachment of the fluorescent group to the amino group in oligonucleotides was carried out according to the manufacturer's instructions. Interrogating oligonucleotides were immobilized through their 3′ ends; oligonucleotide primers were immobilized or fluorescently labeled at their 5′ ends; fluorescent oligonucleotides that imitated target DNA with rare mutations were labeled at their 3′ ends.
M. tuberculosis strains.
M. tuberculosis strains were isolated from sputum, pleural exudate, bronchial lavage, urine, and cerebrospinal fluid of patients in central Russia (Moscow City and Moscow and Kaluga regions), in the Siberian regions of Russia (Novosibirsk and Tomsk), and in St. Petersburg, Russia. All cultures were grown on Löwenstein-Jensen (LJ) agar slants at 37°C for 8 weeks and were examined for growth rate, gross and microscopic colony morphology, and pigmentation and also were tested for niacin accumulation, nitrate reduction, and catalase and urease activity (18). All cultures underwent standard tests for RIF resistance using the absolute concentration method (14) as follows. The cultures were grown on LJ agar slants, and then the cells were resuspended in sterile saline (0.85% NaCl) to turbidity corresponding to a cell density of 5 × 108 cells per ml. The suspension was further diluted 10-fold with sterile saline, and a 0.2-ml aliquot was plated on LJ solid medium (control) and on the same medium containing 40 μg of RIF/ml. The samples that developed fewer than 20 colonies on RIF-containing medium and showed normal growth on control medium by the end of 4 weeks were considered RIF susceptible.
Preparation of DNA samples from M. tuberculosis cultures.
One or two colonies of M. tuberculosis, 2 to 3 mm in diameter, were resuspended in 0.5 ml of TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]) and centrifuged at 12,000 × g for 10 min at 4°C. The pellet was resuspended in 30 μl of TE buffer containing 1% Triton X-100 and incubated for 20 min at 95°C. The extracts were cooled on ice and centrifuged at 12,000 × g for 10 min, and 2-μl aliquots of clear supernatant were used for PCR.
Preparation of DNA samples from clinical specimens.
Specimens of sputum, pleural exudate, and bronchial lavage (approximately 10 ml each) were decontaminated by treatment with _N_-acetylcysteine and 3% NaOH (17) for 40 min at room temperature. Cell extracts were then obtained by centrifugation and lysis as described above. Other bodily fluids (1.5 ml of urine or 0.5 ml of cerebrospinal fluid) were centrifuged at 10,000 × g for 10 min at 4°C. Cell extracts were prepared similarly to the procedure used for pure bacterial culture as described above. Two-microliter aliquots of clear supernatants were used for PCR.
Preparation of target DNA.
Target samples of DNA from M. tuberculosis were prepared by two-stage PCR. At the first stage, a 193-bp-long fragment of the rpoB gene (nucleotides 2288 to 2480; GenBank accession no. L27989) was amplified using primers F105 (5′-CGT GGA GGC GAT CAC ACC GCA GAC GTT G-3′) and R273 (5′-GAC CTC CAG CCC GGC ACG CTC ACG T-3′). The reaction mixture contained 1.5 mM MgCl2, 10 mM KCl, 10 mM Tris-HCl (pH 8.3), 2.5 U of AmpliTaq DNA polymerase (PE Corporation, Norwalk, Conn.), 0.5 U of uracyl-DNA-glycosylase (Medigen, Moscow, Russia), 100 nM concentrations of each primer, 2 μl of DNA sample, and 0.2 mM (each) dATP, dCTP, dGTP, and dUTP. The reaction was carried out in a MiniCycler (MJ Research, Waltham, Mass.) as follows: 10 min at room temperature; 5 min at 95°C; 30 cycles of 30 s at 95°C and 40 s at 72°C; and 5 min at 72°C. Two microliters of the reaction mixture obtained after the first reaction was used for the second PCR.
In the second reaction, an internal 126-bp-long fragment (nucleotides 2336 to 2461 of the rpoB gene) of the first product was amplified using primers F1272 (5′-CGC CGC GAT CAA GGA GTT CT-3′) and R1398 (5′-TCA CGT GAC AGA CCG CCG GG-3′). The reaction mixture contained dTTP instead of the dUTP used in the first reaction mixture, no uracyl-DNA-glycosylase, a 100 nM concentration of fluorescently labeled F1272, and a 10 nM concentration of R1398 in 100 μl. Because of the difference in the concentrations of primers, the reaction yielded predominantly single-stranded DNA. Amplification was carried out as follows: 5 min at 95°C; 35 cycles of 20 s at 95°C, 30 s at 65°C, and 30 s at 72°C; and 5 min at 72°C.
For on-chip LDR, predominantly single-stranded lower-strand (antisense) DNA was amplified in a similar reaction. The F1272 primer was not labeled and was used at 10 nM, while R1398 was used at 100 nM. DNA-polymerase was removed by adding 1 μg of proteinase K (Sigma)/ml. The mixture was incubated for 15 min at 37°C, 10 min at 55°C, and then 10 to 15 min at 95°C to inactivate the proteinase.
PCR products were analyzed by electrophoresis in agarose gels.
Sequencing.
The fragment of the rpoB gene that determines RIF resistance was amplified with primers F105 and R273 and subjected to automated dideoxy sequencing using one of the terminal primers, a commercial kit (Dye Deoxy Terminator ABI Sequencing Kit with _Taq_-Polymerase FS; PE Corporation), and an ABI-373A automatic sequencer (Applied Biosystems).
TB-MAGIChip with immobilized oligonucleotides.
The MAGIChips were prepared as described earlier (38). Each chip consisted of a microscope slide with 169 (13 by 13) polyacrylamide gel pads created on its surface by photopolymerization. Each gel pad is a 100- by 100- by 20-μm block separated from adjacent blocks by a 200-μm-wide strip of hydrophobic surface. Each pad contained 1 pmol of an immobilized oligonucleotide probe. For hybridization, oligonucleotides were immobilized through their 3′ ends; for on-chip PCR and LDR they were immobilized through their 5′ ends.
On-chip hybridization.
A hybridization mixture was prepared by adding 12 μl of the second-stage asymmetric PCR mixture to 24 μl of a solution containing 1.5 M guanidine thiocyanate (GuCNS), 0.075 M HEPES (pH 7.5), and 7.5 mM EDTA (unless noted otherwise). Twenty-eight microliters of the hybridization mixture was loaded on the chip and sealed in a hybridization chamber (10 by 10 mm) (in situ frame; Eppendorf Scientific, Westbury, N.Y.). The chamber was incubated for 18 h at 37°C. After hybridization the chip was washed three times at 37°C with 6.67× SSPE buffer, pH 7.4, containing 10% Tween 20, and was air dried.
On-chip PCR.
On-chip PCR was described earlier (30). The reaction mixture contained 2.5 mM MgCl2, 10 mM KCl, 10 mM Tris-HCl (pH 8.3), 1 mg of bovine serum albumin/ml, 0.2 mM concentrations of each deoxynucleoside triphosphate, 5 U of the Stoffel fragment of Taq DNA-polymerase (PE Corporation), a 33 nM concentration of unlabeled forward primer (5′-CGC GAT CAA GGA GTT CTT CGG CAC C-3′), and a 330 nM concentration of fluorescently labeled reverse primer (5′-CCC GGC GGT CTG TAC GTG A-3′). This pair of primers amplified a 133-bp-long fragment of the rpoB gene (nucleotides 2339 to 2471). PCR was carried out as follows: 2 min at 95°C and 25 to 35 cycles of 30 s at 95°C, 60 s at 63°C, and 40 s at 72°C. The reaction was monitored in real time in all microchip elements with a fluorescence microscope equipped with a Peltier element (12).
On-chip LDR.
For LDR, two interrogating oligonucleotide probes were immobilized through their 5′ ends in separate gel pads. They were designed to discriminate between the wild-type gene with His in position 526 (codon CAC; corresponding probe, 5′-CTA CCC GCT GTC GTG GTT GAC CCA-3′) and a mutant gene with the substitution His-526-Leu (codon CTC; corresponding probe, 5′-CTA CCC GCT GTC GTG GTT GAC CCT-3′ ). The reaction mixture contained 20 mM Tris-HCl (pH 8.3), 25 mM KCl, 10 mM MgCl2, 0.5 mM NAD, 0.01% Triton X-100, 1 mg of acetylated bovine serum albumin (Sigma)/ml, 40 to 240 nM single-stranded target DNA, 15 U of thermostable Tth DNA-ligase (Ampligase; Epicentre Technologies, Madison, Wis.), and 600 to 720 nM detecting oligonucleotide 5′-pCAA GCG CCG ACT GTA GGC ACT GGG-TR-3′. The latter was phosphorylated on the 5′ end and fluorescently labeled with Texas Red on the 3′ end.
The reaction chamber was incubated for 50 to 60 min at 40 to 42°C and then was placed on a thermal table, and five to eight cycles were carried out as follows: 10 s at 92°C, 16 min at 48 to 50°C. The chamber was rinsed with several changes of 0.15 M NaCl for 5 min at 92°C. The reaction chamber was then disassembled and the chip was rinsed with water and air dried, and its fluorescence was recorded.
Fluorescence measurement.
All measurements were taken in real time using a setup of a fluorescence microscope equipped with a CCD camera, a thermal table with step motors and movement controller, and a computerized data acquisition system (12). Data collection and processing were performed using dedicated software, Special Hybridization Experiment Software, based on a LabVIEW interface (National Instruments, Austin, Tex.).
Statistical analysis of the data.
The discriminating ability of each pair of oligonucleotides immobilized on the MAGIChip was assessed by the ratio r = Im/Ip, where Im is the fluorescence of the gel pad corresponding to an imperfect duplex and Ip is the fluorescence of the gel pad corresponding to a perfect duplex. Wild-type oligonucleotide probes were always located in the upper row of the array. Therefore, for wild-type target DNA, fluorescence of the upper gel pad in each column equaled Ip. For mutant target DNA, on the contrary, fluorescence of the gel pad corresponding to the mutation was assumed to equal Ip, while fluorescence of the corresponding wild-type gel pad equaled Im.
After a series of measurements, a median value (M) of all r values was determined and the scattering of data (L) was assessed as the following quartile deviation (4):
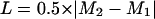
where _M_1 is the median for all values below M and _M_2 is the median for all values above M.
RESULTS
Hybridization on TB-MAGIChip.
The method for identification of Rifr mutants of M. tuberculosis by hybridization on a dedicated MAGIChip includes three successive steps: (i) PCR amplification of clinical sample DNAs, (ii) asymmetric PCR to yield a fluorescently labeled predominantly single-stranded target DNA, and (iii) hybridization of the labeled product to the chip with gel pads carrying immobilized oligonucleotides. These oligonucleotides correspond to either the wild-type or the mutant sequence. As they form, correspondingly, a perfect or an imperfect match, the difference in these structures' stability enables one to discriminate between positive and negative hybridization signals by the intensity of fluorescence.
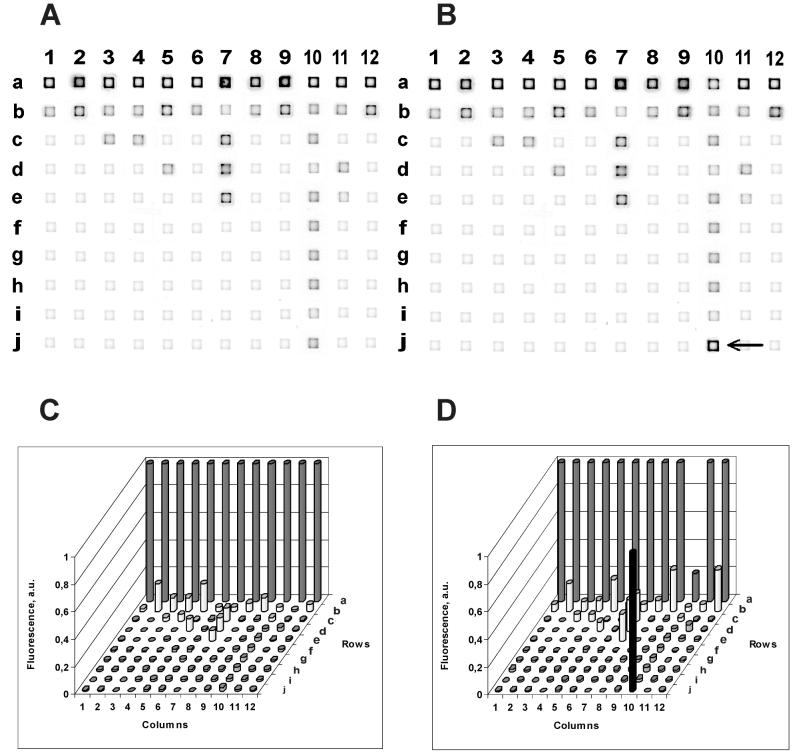
The TB-MAGIChip for identification of Rifr strains of M. tuberculosis contains a call set of 42 oligonucleotides (Table 1). It can detect 30 of the most common mutations in the rpoB gene responsible for the resistance. Gel pads with immobilized oligonucleotides are arrayed in 12 columns (Fig. 1), each column corresponding to a single variable amino acid position. The upper gel pad in each column (a1 through a12) matches the wild-type sequence, i.e., forms a perfect duplex with the wild-type target DNA. Oligonucleotides immobilized in the gel pads below form perfect duplexes with different mutant variants of the same codon.
TABLE 1.
Immobilized oligonucleotides for TB hybridization technique
| Oligonucleotidea | Amino acid position | Amino acid substitutionb | Nucleotide substitution | Sequence 5′→3′c | Nucleotide positions |
|---|---|---|---|---|---|
| a1 | 507 | WT | GCT GTC TGG TGC CGA AGA | 2370–2353 | |
| b1 | 507 | DEL (2) | GGCACC | GGC TCA GCT GGC T-- --- -GA AGA A | 2376–2352 |
| a2 | 510 | Gln (WT) | CAG | GCT CAG CTG GCT GGT GC | 2375–2359 |
| b2 | 510 | Gln→His | CAG→CAT | TG GCT CAG ATG GCT GGT G | 2377–2360 |
| a3 | 511 | Leu (WT) | CTG | AA TTG GCT CAG CTG GCT G | 2380–2364 |
| b3 | 511 | Leu→Pro | CTG→CCG | AA TTG GCT CGG CTG GCT | 2380–2364 |
| c3 | 511 | Leu→Arg | CTG→CGG | AA TTG GCT CCG CTG GCT | 2380–2364 |
| a4 | 512 | Ser (WT) | AGC | AT GAA TTG GCT CAG CTG GC | 2383–2365 |
| b4 | 512 | Ser→Thr | AGC→ACC | AT GAA TTG GGT CAG CTG GC | 2383–2365 |
| c4 | 512 | Ser→Arg | AGC→CGC | AT GAA TTG GCG CAG CTG | 2383–2367 |
| a5 | 513 | Gln (WT) | CAA | TC CAT GAA TTG GCT CAG CT | 2386–2368 |
| b5 | 513 | Gln→Leu | CAA→CTA | TC CAT GAA TAG GCT CAG CT | 2386–2368 |
| c5 | 513 | Gln→Lys | CAA→AAA | TC CAT GAA TTT GCT CAG CT | 2386–2368 |
| d5 | 513 | Gln→Pro | CAA→CCA | TC CAT GAA TGG GCT CAG CT | 2386–2368 |
| a6 | 515 | Met (WT) | ATG | GTT GTT CTG GTC CAT GAA TTG GC | 2396–2374 |
| b6 | 515 | Met→Ile | ATG→ATA | GTT GTT CTG GTC TAT GAA TTG GC | 2396–2374 |
| a7 | 516 | Asp (WT) | GAC | TT GTT CTG GTC CAT GAA T | 2397–2376 |
| b7 | 516 | Asp→Tyr | GAC→TAC | TT GTT CTG GTA CAT GAA T | 2397–2376 |
| c7 | 516 | Asp→Gly | GAC→GGC | TT GTT CTG GCC CAT GAA T | 2397–2376 |
| d7 | 516 | Asp→Val | GAC→GTC | TT GTT CTG GAC CAT GAA T | 2397–2376 |
| e7 | 516 | Asp→Glu | GAC→GAG | TT GTT CTG CTC CAT GAA T | 2397–2376 |
| a8 | 522 | Ser (WT) | TCG | T CAA CCC CGA CAG CG | 2414–2398 |
| b8 | 522 | Ser→Leu | TCG→TTG | T CAA CCC CAA CAG CG | 2414–2398 |
| a9 | 524 | Leu (WT) | TTG | G CTT GTG GGT CAA CCT CGA | 2421–2403 |
| b9 | 524 | Leu→Ser | TTG→TCC | G CTT GTG GGT GGA CCT CGA | 2421–2403 |
| a10 | 526 | His (WT) | CAC | G GCG CTT GTG GGT CAA C | 2424–2408 |
| b10 | 526 | His→Asp | CAC→GAC | G GCG CTT GTC GGT CAA C | 2424–2408 |
| c10 | 526 | His→Leu | CAC→CTC | G GCG CTT GAG GGT CAA C | 2424–2408 |
| d10 | 526 | His→Gln2 | CAC→CAA | G GCG CTT TTG GGT CAA C | 2424–2408 |
| e10 | 526 | His→Gln | CAC→CAG | G GCG CTT CTG GGT CAA C | 2424–2408 |
| f10 | 526 | His→Cys | CAC→TGC | G GCG CTT GCA GGT CAA C | 2424–2408 |
| g10 | 526 | His→Asn | CAC→AAC | G GCG CTT GTT GGT CAA C | 2424–2408 |
| h10 | 526 | His→Arg | CAC→CGC | G GCG CTT GCG GGT CAA C | 2424–2408 |
| i10 | 526 | His→Pro | CAC→CCC | G GCG CTT GGG GGT CAA C | 2424–2408 |
| j10 | 526 | His→Tyr | CAC→TAC | G GCG CTT GTA GGT CAA C | 2424–2408 |
| a11 | 531 | Ser (WT) | TCG | CAG CGC CGA CAG TCG | 2438–2424 |
| b11 | 531 | Ser→Leu | TCG→TTG | C CAG CGC CAA CAG TCG | 2439–2424 |
| c11 | 531 | Ser→Trp | TCG→TGG | CAG CGC CCA CAG TCG | 2438–2424 |
| d11 | 531 | Ser→Gln | TCG→CAG | CAG CGC CTG CAG TCG | 2438–2424 |
| e11 | 531 | Ser→Cys | TCG→TGT | CAG CGC ACA CAG TCG | 2438–2424 |
| a12 | 533 | Leu (WT) | CTG | TG CCC CAG CGC CGA CAG | 2443–2427 |
| b12 | 533 | Leu→Pro | CTG→CCG | TG CCC CGG CGC CGA CAG | 2443–2427 |
FIG. 1.
The images (A and B) and intensities (C and D) of hybridization. The immobilized probes are listed in Table 1. (A and C) Wild-type target DNA; (B and D) His-526-Tyr mutant target DNA. The fluorescence intensities within each column were normalized to a maximal fluorescence signal corresponding to a perfect hybridization duplex. a. u., arbitrary units. The arrow in panel B points to the only gel pad where a perfect duplex formed, resulting in a higher level of fluorescence.
Figure 1A and C illustrate the hybridization of wild-type target DNA with the TB-MAGIChip. The fluorescence of the gel pads in the upper row is the brightest in each individual column. Only the oligonucleotides in these pads form perfect duplexes with the target DNA.
A mutation in the RRDR segment of the rpoB gene results in the formation of a perfect duplex and, therefore, bright fluorescence in a gel pad located in rows b through j. An example of hybridization of a mutant target DNA (His-526-Tyr) is shown in Fig. 1B and D. The fluorescence of the gel pad j10 is much higher than the fluorescence of a10, where the wild-type probe is immobilized, because this is the only pad in the column where a perfect duplex has formed.
Optimization of hybridization on MAGIChip.
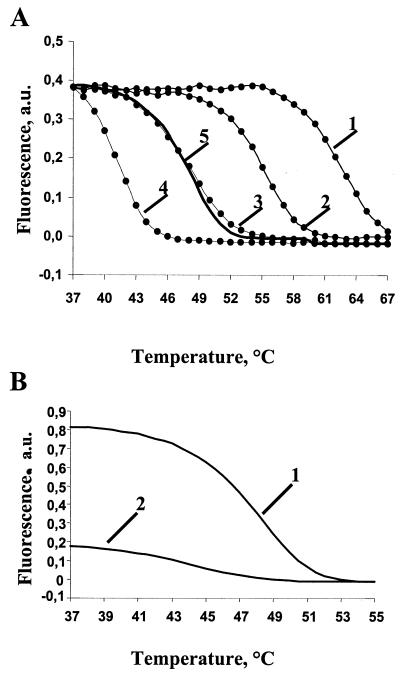
Melting temperatures of perfect duplexes formed with oligonucleotides immobilized on the TB-MAGIChip in 1 M NaCl are within the range of 58 to 63°C (Fig. 2A). To adapt hybridization to be performed at 37°C, 1 M GuCNS (or 20% formamide) was added to the hybridization buffer, which decreased the melting temperature to about 47°C (Fig. 2A). However, the mechanical strength of the gel pads incubated in the presence of GuCNS is higher than that in formamide.
FIG. 2.
Melting curves of hybridization duplexes. (A) Effect of buffer composition on the melting temperature of a perfect duplex formed between oligonucleotide probe a3 (Table 1) and a PCR-amplified fragment of the wild-type rpoB gene (labeled target DNA). The samples were melted in 1 M NaCl (curve 1), 1 M NaCl with 10% formamide (curve 2), 20% formamide (curve 3), 30% formamide (curve 4), or 1 M GuCNS (curve 5). (B) Melting curves of perfect (curve 1) and imperfect (curve 2) duplexes in 1 M GuCNS. The duplexes were formed by a PCR-amplified fragment of the wild-type rpoB gene and oligonucleotide probe a3 (perfect duplex) or b3 (imperfect duplex). a. u., arbitrary units.
The difference in the intensity of fluorescence between perfect and imperfect duplexes after hybridization reactions performed at different temperatures (Fig. 2B) was determined. This parameter defines the discrimination ability of the procedure, and it was at its highest level when the hybridization was performed at 37°C. Therefore, the optimal hybridization temperature in 1 M GuCNS was 37°C, 8 to 10°C below the melting temperature, and partial equilibrium was achieved after 14 h of incubation.
Every discriminating pair of oligonucleotides underwent control hybridization with a previously characterized DNA sample carrying the corresponding mutation. Some probes on the microchip corresponded to the mutations that were not available in clinical samples. These probes were successfully tested using synthetic DNA samples.
Analysis of mycobacterial DNA samples on TB-MAGIChip.
DNA samples were isolated from 130 Rifr strains of M. tuberculosis and hybridized with the TB-MAGIChip. In 128 of them, mutations in the RRDR were identified. The results of this experiment are summarized in Table 2. The results clearly demonstrate that Ser-531 and His-526 amino acid substitutions are found most frequently, in agreement with the published data (for a review, see reference 20). Nineteen samples representing different mutations as judged from their on-chip hybridization patterns were sequenced; the results of the sequencing fully confirmed the hybridization data. In one of the samples that showed a wild-type pattern when hybridized with the chip, no mutations in the RRDR were found by sequencing (see Discussion).
TABLE 2.
Distribution of rpoB mutations in Rifr M. tuberculosis strains
| Mutation type(s) | No. of strains |
|---|---|
| Leu511→Pro | 1 |
| Gln513→Leu | 1 |
| Asp516→Tyr | 2 |
| Asp516→Val | 5 |
| Ser522→Leu | 2 |
| His526→Arg | 1 |
| His526→Asp | 7 |
| His526→Asn | 5 |
| His526→Cys | 1 |
| His526→Leu | 1 |
| His526→Tyr | 5 |
| Ser531→Leu | 85 |
| Ser531→Trp | 1 |
| Leu533→Pro | 10 |
| Leu511→Pro, His526→Asn | 1 |
| Wild type | 2 |
| Total | 130 |
Comparison between detection of RIF resistance in sputum by hybridization with TB-MAGIChip and by conventional drug susceptibility testing.
Thirty-one samples of sputum from patients with clinically confirmed TB were each divided in two parts. One part was used to prepare target DNA; the amplified DNA was then hybridized with the TB-MAGIChip. The other part was used to isolate and identify M. tuberculosis by using standard clinical methods and then to determine its RIF resistance by the absolute concentration method.
The results of this experiment are summarized in Table 3. According to the hybridization results, 20 samples were categorized as RIF susceptible, while 11 contained mutations (Ser-531-Leu, 8 samples; Asp-516-Val, 2 samples; His-526-Arg, 1 sample).
TABLE 3.
Comparison of RIF susceptibilities of M. tuberculosis in sputum specimens as assessed by on-chip hybridization and conventional drug susceptibility testing
| On-chip hybridization result | Standard drug susceptibility testing resulta | ||
|---|---|---|---|
| No. resistant | No. susceptible | Total | |
| Resistant | 11 | 0 | 11 |
| Susceptible | 2 | 18 | 20 |
| Total | 13 | 18 | 31 |
Statistical evaluation of the hybridization results.
The discrimination ability of the TB-MAGIChip was assessed by the r value (see Materials and Methods) for 18 pairs of wild-type and mutant oligonucleotide probes. In six independent series of experiments, hybridization was carried out with wild-type DNA and DNA from five different mutants: His-526-Tyr (CAC→TAC); Ser-522-Leu (TCG→TTG); Leu-533-Pro (CTG→CCC); His-526-Asn (CAC→AAC); and double mutant Leu-511-Arg (CTG→CGG) and Asp-516-Tyr (GAC→TAC). Each hybridization was performed eight times. Data for each individual pair were then grouped together, and median values and quartile deviations were determined (Table 4).
TABLE 4.
Discriminatory ability of the TB-MAGIChip for different mutations in the rpoB gene
| Amino acid substitution | Nucleotide sequences of the probesa | Statistical analysis of r valuesb | |
|---|---|---|---|
| M | L | ||
| Gln510→His | GCT CAG (C/A)TG GCT GGT GC | 0.41 | 0.09 |
| Leu511→Arg | AAT TGG CTC (A/C)GC TGG CT | 0.03 | <0.01 |
| Leu511→Pro | AAT TGG CTC (A/G)GC TGG CT | 0.33 | 0.02 |
| Asp516→Val | GGT TGT TCT GG(T/A) CCA TGA ATT G | 0.23 | 0.02 |
| Asp516→Glu | GGT TGT TCT G(G/C)T CCA TGA ATT G | 0.01 | <0.01 |
| Asp516→Tyr | GGT TGT TCT GGT (C/A)CA TGA ATT G | 0.03 | 0.06 |
| Asp516→Gly | GGT TGT TCT GG(T/C) CCA TGA ATT G | 0.02 | 0.04 |
| Ser522→Leu | GGT CAA CCC C(G/A)A CAG CG | 0.06 | 0.02 |
| His526→Asp | GGC GCT TGT (G/C)GG TCA AC | 0.01 | <0.01 |
| His526→Leu | GGC GCT TG(T/A) GGG TCA AC | 0.16 | <0.01 |
| His526→Gln2 | GGC GCT T(G/T)T GGG TCA AC | 0.15 | 0.02 |
| His526→Gln1 | GGC GCT T(G/C)T GGG TCA AC | 0.01 | <0.01 |
| His526→Cys | GGC GCT TG(T/C) (G/A)GG TCA AC | 0.01 | <0.01 |
| His526→Asn | GGC GCT TGT (G/T)GG TCA AC | 0.01 | 0.01 |
| His526→Arg | GGC GCT TG(T/C) GGG TCA AC | 0.02 | <0.01 |
| His526→Pro | GGC GCT TG(T/G) GGG TCA AC | 0.08 | 0.01 |
| His526→Tyr | GGC GCT TGT (G/A)GG TCA AC | 0.01 | <0.01 |
| Leu533→Pro | TGC CCC (A/G)GC GCC GAC AG | 0.01 | 0.01 |
Identification of Rifr mutants by on-chip PCR.
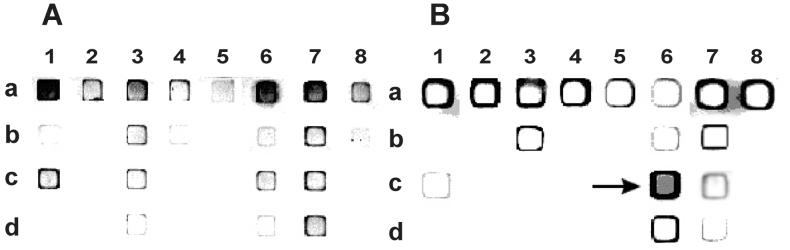
The on-chip PCR approach is a modification of allele-specific PCR that had been considered as a tool for the detection of point mutations (30). The sequences of allele-specific immobilized primers and corresponding nucleotide and amino acid substitutions are listed in Table 5. During the first cycles, asymmetric PCR occurs in the liquid covering the chip and results in a single-stranded product with a fluorescently labeled primer at its 5′ end. Accumulation of this product leads to its hybridization to specific primers immobilized inside the gel pads and their extension. Long perfect duplexes formed during this process have significantly higher melting temperatures than do the short duplexes between the single-stranded PCR product and immobilized primers. Therefore, when the temperature exceeds that of primer annealing, fluorescence can be observed only in those gel pads in which the immobilized primer has been extended. Similar to the setup described for hybridization experiments, the interrogating sets for individual codons of RRDR were placed in columns with wild-type primers on top. For wild-type target DNA, the upper gel pads must be the brightest; for mutant samples, the most intense fluorescence was seen in one of the pads described below. An example of on-chip PCR with DNA from wild-type M. tuberculosis DNA and from the His-526-Asp (CAC→GAC) mutant is shown in Fig. 3. In further experiments, we tested DNA from 30 different mutant strains of M. tuberculosis, and the results were fully concordant with the hybridization analysis of the same samples.
TABLE 5.
Immobilized primers for on-chip PCR amplification
| Oligonucleotidea | Amino acid position | Amino acid substitutionb | Nucleotide substitution | Sequence (5′→3)c | Sequence position |
|---|---|---|---|---|---|
| a1pcr | 513 | Gln (WT) | CAA | TTCGGCACCAGCCAGCCAGCTGAGCCA | 2355–2378 |
| b1pcr | 513 | Gln→Leu | CAA→CTA | TTCGGCACCAGCCAGCCAGCTGAGCCt | 2355–2377 |
| c1pcr | 513 | Gln→Lys | CAA→AAA | TTCGGCACCAGCCAGCCAGCTGAGCa | 2355–2376 |
| d1pcr | 513 | Gln→Pro | CAA→CCA | TTCGGCACCAGCCAGCCAGCTGAGCCc | 2355–2377 |
| a2pcr | 515 | Met (WT) | ATG | TCGGCACCAGCCAGCTGAGACAATTCATG | 2356–2384 |
| b2pcr | 515 | Met→Ile | ATG→ATA | TCGGCACCAGCCAGCTGAGACAATTCATa | 2356–2384 |
| a3pcr | 516 | Asp (WT) | GAC | GCACCAGCCAGCTGAGACAATTCATGGAC | 2359–2387 |
| b3pcr | 516 | Asp→Tyr | GAC→TAC | GCACCAGCCAGCTGAGCCAATTCATGt | 2359–2385 |
| c3pcr | 516 | Asp→Gly | GAC→GGC | GCACCAGCCAGCTGAGACAATTCATGGg | 2359–2386 |
| d3pcr | 516 | Asp→Val | GAC→GTC | GCACCAGCCAGCTGAGACAATTCATGGt | 2359–2386 |
| e3pcr | 516 | Asp→Glu | GAC→GAG | GCACCAGCCAGCTGAGACAATTCATGGAg | 2359–2387 |
| a4pcr | 522 | Ser (WT) | TCG | GCCAATTCATGGACCAGAACAACCCGCTGTC | 2374–2404 |
| b4pcr | 522 | Ser→Leu | TCG→TTG | GCCAATTCATGGACCAGAACAACCCGCTGTt | 2374–2404 |
| a5pcr | 524 | Leu (WT) | TTG→TCC | CATGGACCAGAACAACCCGCTGTCGAGTTG | 2381–2410 |
| b5pcr | 524 | Leu→Ser | TTG→TCC | CATGGACCAGAACAACCCGCTGTCGAGTTc | 2381–2420 |
| a6pcr | 526 | His (WT) | CAC | CCAGAACTACCCGCTGTCGTGGTTGACCC | 2387–2415 |
| b6pcr | 526 | His→Leu | CAC→CTC | CCAGAACTACCCGCTGTCGTGGTTGACCCt | 2387–2416 |
| c6pcr | 526 | His→Asp | CAC→GAC | CCAGAACTACCCGCTGTCGTGGTTGACCg | 2387–2415 |
| d6pcr | 526 | His→Gln2 | CAC→CAA | CCAGAACTACCCGCTGTCGTGGTTGACCCAa | 2387–2417 |
| e6pcr | 526 | His→Gln | CAC→CAG | CCAGAACTACCCGCTGTCGTGGTTGACCCAg | 2387–2417 |
| f6pcr | 526 | His→Cys | CAC→TGC | CCAGAACTACCCGCTGTCGTGGTTGACCTg | 2387–2416 |
| g6pcr | 526 | His→Asn | CAC→AAC | CCAGAACTACCCGCTGTCGTGGTTGACCa | 2387–2415 |
| h6pcr | 526 | His→Arg | CAC→CGC | CCAGAACTACCCGCTGTCGTGGTTGACCCg | 2387–2416 |
| i6pcr | 526 | His→Pro | CAC→CCC | CCAGAACTACCCGCTGTCGTGGTTGACCCc | 2387–2416 |
| j6pcr | 526 | His→Tyr | CAC→TAC | CCAGAACTACCCGCTGTCGTGGTTGACCt | 2387–2415 |
| a7pcr | 531 | Ser (WT) | TCG | GGTTGACCAACAAGCGCCGACTGTC | 2407–2431 |
| b7pcr | 531 | Ser→Leu | TCG→TTG | GGTTGACCAACAAGCGCCGACTGTt | 2407–2431 |
| c7pcr | 531 | Ser→Trp | TCG→TGG | GGTTGACCAACAAGCGCCGACTGTGg | 2407–2431 |
| d7pcr | 531 | Ser→Gln | TCG→CAG | GGTTGACCAACAAGCGCCGACTGca | 2407–2431 |
| e7pcr | 531 | Ser→Cys | TCG→TGT | GGTTGACCAACAAGCGCCGACTGTGt | 2407–2432 |
| a8pcr | 533 | Leu (WT) | CTG | TGACCCACAAGCGCCGACTGTCGGAGCT | 2413–2437 |
| b8pcr | 533 | Leu→Pro | CTG→CCG | TGACCCACAAGCGCCGACTGTCGGAGc | 2413–2437 |
FIG. 3.
Allele-specific on-chip PCR. A strong fluorescence signal is observed when the 3′ nucleotide of the immobilized primer is complementary to target DNA, extended by Taq polymerase, and forms a stable duplex with the fluorescently labeled target DNA. Immobilized primers are listed in Table 5. (A) Wild-type target DNA; (B) His-526-Asp mutant target DNA (CAC→GAC); the corresponding gel pad is marked with an arrow.
Identification of Rifr mutants by on-chip LDR.
To test the LDR-based approach to identify Rifr mutations, a chip was designed with two interrogating immobilized oligonucleotides. They discriminated between a wild-type sequence encoding His-526 (codon CAC) and a mutant sequence encoding Leu (codon CTC). The interrogating oligonucleotides had T or A in the 3′ position, resepectively. The reaction mixture contained the detecting oligonucleotide which hybridized with the target DNA immediately adjacent to the immobilized oligonucleotides and carried 5′ phosphate to make the ligation reaction possible and 3′-fluorescent label for detection. When the target DNA formed a perfect hybridization duplex with the immobilized interrogating nucleotide, the ligase covalently linked its 3′-terminal base to the 5′-terminal base of the detecting oligonucleotide which became an integral part of the immobilized oligonucleotide. To enhance the positive fluorescence signal, the reaction was carried out at elevated temperature and all participating compounds underwent multiple cycles of annealing, ligation, and melting. Therefore, thermostable ligase has been used.
The results of on-chip LDR are shown in Fig. 4. The gel pads on the right contained the oligonucleotide corresponding to the wild-type sequence, and those on the left contained the oligonucleotide corresponding to the mutant sequence. Using either wild-type or mutant DNA, the signal accumulated almost exclusively in the corresponding pads (rows a and e). When the samples were mixed in different proportions, various levels of fluorescence were detected in both gel pads. In particular, there was a definite signal in the left (mutant) gel pad when mutant DNA made up just 1% of the total target DNA (row b). In control experiments, neither variations in the amount of oligonucleotides immobilized in the gel pads nor changes in the concentration of detecting oligonucleotide and target DNA resulted in false-positive or -negative signals (not shown).
FIG. 4.

Detection of mutant DNA by on-chip LDR. Each reaction mixture contained a total of 3 pmol of single-stranded DNA. Reaction a was performed with wild-type DNA; reaction e was performed with His-526-Leu mutant DNA; other reactions contained 3 pmol of wild-type DNA with a mixture of 1% (b), 2% (c), or 10% (d) of the mutant DNA.
DISCUSSION
Molecular analysis of M. tuberculosis is a promising alternative to bacteriological assays. Using the state-of-the-art technology of oligonucleotide microchips, we developed three approaches to the detection of M. tuberculosis resistance to RIF in both cultures and clinical samples: hybridization, on-chip PCR, and on-chip LDR on dedicated TB-MAGIChips.
The use of the MAGIChip offers several advantages for RIF resistance testing over alternative microchip technologies (34), e.g., GeneChips developed by Affymetrix, Inc. (Santa Clara, Calif.), and manufactured by solid-phase chemical synthesis of oligonucleotides with photolithographic fabrication techniques. The MAGIChips are lower in cost, simpler to use, and more efficient in discrimination of the perfect and mismatched duplexes (12). Because of the better discrimination capacity, the analysis of specific sequences can be performed using a very limited call set of oligonucleotides necessary and sufficient for identification of known mutations. The latter consideration dramatically decreases the cost of chip manufacturing and eliminates the need for cumbersome mathematical interpretation of the results.
Furthermore, the readout of the results may be carried out not only by using the fluorescence microscope setup described earlier (12) but also by a simplified version of the chip analyzer developed in our laboratory (2). The latter device can be equipped with either a CCD camera or a Polaroid camera and costs under $2,000. In particular, the use of a Polaroid camera enables one to analyze the results visually without any computations.
Finally, three-dimensional polyacrylamide gel pads offer much higher capacity and therefore a stronger fluorescence signal than the two-dimensional glass surface. Although there are certain steric hindrances that limit even distribution of the reacting molecules throughout the gel pads and result in higher intensity of the fluorescence at their periphery, this does not affect signal discrimination. Presently we are investigating ways to increase the porosity of the gel in order to make it more accessible for various components of chemical reactions, particularly larger DNA molecules.
Optimization of hybridization on TB-MAGIChip for clinical laboratories.
The conditions were optimized to perform hybridization at 37°C (Fig. 2), which is convenient for clinical laboratories.
Initially, we found that the absolute fluorescence intensities in individual gel pads of the TB-MAGIChip varied widely, and this made visual interpretation of the results rather difficult. We attempted to equalize the intensity of positive signals by adjusting the length of the probes. However, for some probes this still did not help. Apparently, under nonequilibrium conditions the formation of secondary structures by both target DNA and immobilized oligonucleotides affects the yield of annealed duplexes and therefore the intensity of the corresponding fluorescence signals.
Sensitivity and specificity of hybridization and on-chip PCR.
In our experiments with Rifr strains, 128 out of 130 samples were mutant as judged by hybridization with the TB-MAGIChip; therefore, in a real clinical situation they would be correctly classified as resistant. Hence, the accuracy of the hybridization protocol was better than 98%. Sequencing of the RRDR from one of the Rifr samples that gave a wild-type hybridization pattern on the TB-MAGIChip confirmed the absence of any mutations. This is not surprising, since in about 4% of all Rifr M. tuberculosis strains the resistance is not determined by mutations in RRDR (20).
In primary specimens, the detection of RIF susceptibility by hybridization with the MAGIChip showed good correlation with bacteriological testing obtained by the absolute concentration method (accuracy was better than 93%). The limited number of tested specimens (31 altogether) calls for cautious interpretation of the results. Nevertheless, the specificity of the procedure (i.e., the ability to detect true susceptibility) was 100% and the sensitivity of the procedure (i.e., the ability to detect true resistance) was reasonably high (85%). The positive predictive value of a resistant result was 100%, while the negative predictive value was 90%.
These data indicate that hybridization with the TB-MAGIChip could be a promising approach to fast detection of RIF sensitivity of TB pathogens directly in clinical samples. The analysis takes less than 24 h and is much faster than the most advanced bacteriological methods. False-negative results obtained by hybridization with the TB-MAGIChip may be caused by several factors: low content of Rifr mycobacteria in the primary population, resistance independent of mutations in RRDR, and rare mutations that do not have complementary probes in the standard call set on the chip. Despite all these drawbacks, the high positive predictive value of the hybridization results enables physicians to exclude RIF from the treatment regimen and seek an alternative drug.
There was full concordance between the analysis by hybridization on TB-MAGIChip and on-chip PCR; in addition, the latter protocol can be carried out in just 1.5 to 2 h.
Reproducibility of hybridization results.
For statistical purposes, the r values were averaged using the median, which is less sensitive to random deviations than other averages (4). One of the reasons for observed experimental deviations could be minor differences in the process of chip manufacturing. Even for mutations with the most significant scattering of data (substitutions Gln-510-His and Leu-511-Pro), the quartile deviation was well within the limit of confidence (Table 4), i.e., it allowed for reliable discrimination between positive and negative signals. Indeed, positive signals in these cases were still 2.5 and 3 times stronger than negative signals, respectively. This is sufficient to identify mutations visually without any special equipment. In other words, in no case did chip-to-chip or probe-to-probe variations result in the inversion of positive and negative readings, regardless of the observed scattering of data and fluctuations of fluorescence intensity for individual oligonucleotide probes.
On-chip LDR.
The method of on-chip LDR is especially attractive for simultaneously identifying several variants of the rpoB gene against a high background of the wild-type gene. We succeeded in detecting a variant gene that made up only 1% of the target sample. It has to be noted that this level of sensitivity has been chosen as an arbitrary threshold for the bacteriological definition of RIF sensitivity (37).
Several advantages offered by TB-MAGIChips and their potentially low cost make them attractive enough for large-scale commercial production. The on-chip PCR technique can also be extended to analysis of genes responsible for resistance to other drugs for which genetic determinants of resistance are known. One such method was described recently (33). It enables one to simultaneously analyze several mutations in three different genes responsible for resistance to RIF, isoniazid, and streptomycin.
ACKNOWLEDGMENTS
This work was supported by the Moscow Government (a262), by grant no. 5/2000 from the Russian Human Genome Program, and by the U.S. Department of Energy at the initial stage of research.
We are grateful to V. Chupeeva and E. Kreindlin for the manufacturing of microchips, to I. Taran, S. Surzhikov, and B. Chernov for the synthesis of oligonucleotides, to E. Vishnevskaja and S. Tatkov for bacterial DNA samples, and to V. Barsky for kindly providing the portable chip analyzer prior to publication of the manuscript. The assistance of Health Front Line, Ltd. (Champaign, Ill.), in the preparation of the manuscript is appreciated.
REFERENCES
- 1.Badak F Z, Kiska D L, Setterquist S, Hartley C, O'Connell M A, Hopfer R L. Comparison of mycobacteria growth indicator tube with BACTEC 460 for detection and recovery of mycobacteria from clinical specimens. J Clin Microbiol. 1996;34:2236–2239. doi: 10.1128/jcm.34.9.2236-2239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bavykin S G, Akowski J P, Zakhariev V M, Barsky V E, Perov A N, Mirzabekov A D. Portable system for microbial sample preparation and oligonucleotide microarray analysis. Appl Environ Microbiol. 2001;67:922–928. doi: 10.1128/AEM.67.2.922-928.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin W H, Jr, Waites K B, Beverly A, Gibbs L, Waller M, Nix S, Moser S A, Willert M. Comparison of MB/BacT system with a revised antibiotic supplement kit to the BACTEC 460 system for detection of mycobacteria in clinical specimens. J Clin Microbiol. 1998;36:3234–3238. doi: 10.1128/jcm.36.11.3234-3238.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bocharov P P. Series criterion based on median. In: Pechinkin A B, editor. Mathematical statistics. 1994. p. 164. RUDN, Moscow, Russia. [Google Scholar]
- 5.Brunello F, Fontana R. Reliability of the MB/BacT system for testing susceptibility of Mycobacterium tuberculosis complex isolates to antituberculosis drugs. J Clin Microbiol. 2000;38:872–873. doi: 10.1128/jcm.38.2.872-873.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohn D L, Bustreo F, Raviglione M C. Drug-resistant tuberculosis: review of the worldwide situation and the W. H. O./IUATLD global surveillance project. Clin Infect Dis. 1997;24:S121–S130. doi: 10.1093/clinids/24.supplement_1.s121. [DOI] [PubMed] [Google Scholar]
- 7.Coninx R, Pfyffer G E, Mathieu C, Savina D, Debacker M, Jafarov F, Jabrailov I, Ismailov A, Mirzoev F, de Haller R, Portaels F. Drug resistant tuberculosis in prisons in Azerbaijan: case study. Br Med J. 1998;316:1423–1425. doi: 10.1136/bmj.316.7142.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Beenhouwer H, Lhiang Z, Jannes G, Mijs W, Machtelinckx L, Rossau R, Traore H, Portaels F. Rapid detection of rifampin resistance in sputum and biopsy specimens from tuberculosis patients by P. C. R. and line probe assay. Tuber Lung Dis. 1995;76:425–430. doi: 10.1016/0962-8479(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 9.Drobniewski F A, Watt B, Smith E G, Magee J G, Williams R, Holder J, Ostrowski J. A national audit of the laboratory diagnosis of tuberculosis and other mycobacterial diseases within the United Kingdom. J Clin Pathol. 1999;52:334–337. doi: 10.1136/jcp.52.5.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farmer P E, Kononets A S, Borisov S E, Goldfarb A, Healing T, McKee M. The global impact of drug resistant tuberculosis. 1999. Recrudescent tuberculosis in the Russian Federation; pp. 41–83. Program in Infectious Disease and Social Change. Harvard Medical School, Boston, Mass. [Google Scholar]
- 11.Felmlee T A, Lin Q, Whelen A C. Genomic detection of Mycobacterium tuberculosis rifampin resistance: comparison of single-strand conformation polymorphism and dideoxy fingerprinting. J Clin Microbiol. 1995;33:1617–1623. doi: 10.1128/jcm.33.6.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fotin A, Drobyshev A, Proudnikov D, Perov A, Mirzabekov A. Parallel thermodynamic analysis of duplexes on oligodeoxyribonucleotide microchips. Nucleic Acids Res. 1998;26:1515–1521. doi: 10.1093/nar/26.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gingeras T R, Ghandour G, Wang E, Berno A, Small P M, Drobniewski F, Alland D, Desmond E, Holodniy M, Drenkow J. Simultaneous genotyping and species identification using hybridization pattern recognition analysis of generic Mycobacterium DNA arrays. Genome Res. 1998;8:435–448. doi: 10.1101/gr.8.5.435. [DOI] [PubMed] [Google Scholar]
- 14.Heifets L B. Drug susceptibility tests in the management of chemotherapy of tuberculosis. In: Heifets L B, editor. Drug susceptibility of mycobacterial infections. Boca Raton, Fla: CRC Press; 1991. pp. 90–121. [Google Scholar]
- 15.Hirano K, Abe C, Takahashi M. Mutations in the rpoB gene of rifampin-resistant Mycobacterium tuberculosis strains isolated mostly in Asian countries and their rapid detection by line probe assay. J Clin Microbiol. 1999;37:2663–2666. doi: 10.1128/jcm.37.8.2663-2666.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapur V, Li L, Iordanescu S, Hamrick M R, Wanger A, Kreiswirth B N, Musser J M. Characterization by automated DNA sequencing of mutations in the gene (rpoB) encoding the RNA polymerase B subunit in rifampin-resistant Mycobacterium tuberculosis strains from New York City and Texas. J Clin Microbiol. 1994;32:1095–1098. doi: 10.1128/jcm.32.4.1095-1098.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kent P T, Kubica G P. Public health mycobacteriology. A guide for the level III laboratory. U. S. Atlanta, Ga: Department of Health and Human Services. Centers for Disease Control and Prevention; 1985. [Google Scholar]
- 18.Metchok B, Nolte F S, Wallace R J., Jr . Mycobacterium. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 399–437. [Google Scholar]
- 19.Moore M, Onorato I M, McCray E, Castro K G. Trends in drug-resistant tuberculosis in the United States, 1993–1996. JAMA. 1997;278:833–837. [PubMed] [Google Scholar]
- 20.Musser J M. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin Microbiol Rev. 1995;8:496–514. doi: 10.1128/cmr.8.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nash K A, Gaytan A, Inderlied C B. Detection of rifampin resistance in Mycobacterium tuberculosis by means of a rapid, simple, and specific RNA/RNA mismatch assay. J Infect Dis. 1997;176:533–536. doi: 10.1086/517283. [DOI] [PubMed] [Google Scholar]
- 22.Pfyffer G E, Cieslak C, Welscher H-M, Kissling P, Ruesch-Gerdes S. Rapid detection of Mycobacteria in clinical specimens by using the automated BACTEC 9000 MB system and comparison with radiometric and solid-culture systems. J Clin Microbiol. 1997;35:2229–2234. doi: 10.1128/jcm.35.9.2229-2234.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rastogi N, Goh K S, David H L. Drug susceptibility testing in tuberculosis: a comparison of the proportion method using Löwenstein-Jensen, Middlebrook 7H10 and 7H11 agar media and a radiometric method. Res Microbiol. 1989;140:405–417. doi: 10.1016/0923-2508(89)90016-8. [DOI] [PubMed] [Google Scholar]
- 24.Raviglione M C, Snider D E, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 25.Rossau R, Traore H, de Beenhouwer H, Mijs W, Jannes G, de Rijk P, Portaels F. Evaluation of the INNO-LIPA Rif TB assay, a reverse hybridization assay for the simultaneous detection of Mycobacterium tuberculosis complex and its resistance to rifampin. Antimicrob Agents Chemother. 1997;41:2093–2098. doi: 10.1128/aac.41.10.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rüsch-Gerdes S, Domehl C, Nardi G, Gismondo M R, Welscher H-M, Pfyffer G E. Multicenter evaluation of the mycobacteria growth indicator tube for testing susceptibility of Mycobacterium tuberculosis to first-line drugs. J Clin Microbiol. 1999;37:45–48. doi: 10.1128/jcm.37.1.45-48.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siddiqi S H, Hawkins J E, Laszlo A. Interlaboratory drug susceptibility testing of Mycobacterium tuberculosis by radiometric procedure and two conventional methods. J Clin Microbiol. 1985;22:919–923. doi: 10.1128/jcm.22.6.919-923.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sreevatsan S, Bookout J B, Ringpis F M, Mogazeh S L, Kreiswirth B N, Pottathil R R, Barathur R R. Comparative evaluation of cleavase fragment length polymorphism with PCR-SSCP and PCR-RFLP to detect antimicrobial agent resistance in Mycobacterium tuberculosis. Mol Diagn. 1998;3:81–91. doi: 10.154/MODI00300081. [DOI] [PubMed] [Google Scholar]
- 29.Stepanshina V N, Panfertsev E A, Korobova O V, Shemyakin I G, Stepanshin Y G, Medvedeva I M, Dorozhkova I R. Drug-resistant strains of Mycobacterium tuberculosis isolated in Russia. Int J Tuberc Lung Dis. 1999;3:149–152. [PubMed] [Google Scholar]
- 30.Strizhkov B N, Drobyshev A L, Mikhailovich V M, Mirzabekov A D. PCR amplification on a microarray of gel-immobilized oligonucleotides: detection of bacterial toxin- and drug-resistant genes and their mutations. BioTechniques. 2000;29:844–854. doi: 10.2144/00294rr01. [DOI] [PubMed] [Google Scholar]
- 31.Telenti A, Imboden P, Marchesi F, Lowrie D, Cole S, Colston M J, Matter L, Schopfer K, Bodmer T. Detection of rifampin-resistance mutations in Mycobacterium tuberculosis. Lancet. 1993;341:647–650. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- 32.Tenover F C, Crawford J T, Huebner R E, Geiter L J, Horsburgh C R, Jr, Good R C. The resurgence of tuberculosis: is your laboratory ready? J Clin Microbiol. 1993;31:767–770. doi: 10.1128/jcm.31.4.767-770.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tillib, S., B. Strizhkov, and A. Mirzabekov. Integration of multiple PCR amplifications and DNA mutation analysis by using oligonucleotide microchip. Anal. Biochem., in press. [DOI] [PubMed]
- 34.Troesch A, Nguyen H, Miyada C G, Desvarenne S, Gingeras T R, Kaplan P M, Cross P, Mabilat C. Mycobacterium species identification and rifampin resistance testing with high-density DNA probe arrays. J Clin Microbiol. 1999;37:49–55. doi: 10.1128/jcm.37.1.49-55.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Victor, T. C., A. M. Jordaan, A. van G. Rie, D. van der Spuy, M. Richardson, P. D. van Helden, and R. Warren. 1999. Detection of mutations in drug resistance genes of Mycobacterium tuberculosis by a dot-blot hybridization strategy. Tuber. Lung Dis. **79:**343–348. [DOI] [PubMed]
- 36.Williams D L, Waguespack C, Eisenach K, Crawford J T, Portaels F, Salfinger M, Nolan C M, Abe C, Sticht-Groh V, Gillis T P. Characterization of rifampin resistance in pathogenic mycobacteria. Antimicrob Agents Chemother. 1994;38:2380–2386. doi: 10.1128/aac.38.10.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization/IUATLD Global Working Group on Tuberculosis Drug Resistance Surveillance. Guidelines for surveillance of drug resistance in tuberculosis. World Health Organization publication WHO/TB/96.216. Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]
- 38.Yershov G, Barsky V, Belgovskiy A, Kirillov E, Kreindlin E, Ivanov I, Parinov S, Guschin D, Drobyshev A, Dubiley S, Mirzabekov A. DNA analysis and diagnostics on oligonucleotide microchips. Proc Natl Acad Sci USA. 1996;93:4913–4918. doi: 10.1073/pnas.93.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]