Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer (original) (raw)
. Author manuscript; available in PMC: 2022 Feb 28.
Published in final edited form as: Science. 2020 Dec 11;370(6522):1328–1334. doi: 10.1126/science.abb9847
Abstract
Adoptive T cell therapy (ACT) utilizes _ex vivo_-expanded autologous tumor infiltrating lymphocytes (TILs) to target cancer. The impact of T cell phenotype on clinical success of ACT in humans has not been elucidated. Using high-dimensional analysis of patient ACT products, we identified two CD8+ TIL populations associated with clinical outcomes: a memory-progenitor stem-like TIL associated with complete cancer regression and T cell persistence, and a highly differentiated state associated with poor TIL persistence. Although most anti-tumor neoantigen-reactive T cells existed in the differentiated state, ACT responders retained a pool of stem-like neoantigen-specific TILs that was absent in ACT non-responders. Tumor-reactive stem-like TILs were capable of self-renewal, superior expansion, and anti-tumor response in vitro and in vivo. Our study underscores the importance of T cell phenotypes in ACT response and provides a potential strategy for designing effective immunotherapies.
One Sentence Summary:
This study highlights the importance of self-renewing tumor-specific mutation-reactive T cells in mediating anti-cancer response of cellular immunotherapies.
Cancer immunotherapies such as immune checkpoint blockade (ICB), adoptive cell therapy (ACT), and chimeric antigen receptor (CAR) therapy rely on the targeted destruction of cancer cells by potent anti-tumor T cells (1–4). In ACT and ICB, it is now established that CD8+ T cells target cancer cells through the recognition of mutated neoantigens presented on human leukocyte antigen (HLA) class I molecules (1, 2, 5, 6). In multiple phase 2 clinical trials, ACT using autologous _in vitro_-expanded TILs has been shown to mediate complete durable responses in patients with metastatic melanomas as well as against epithelial cancers that are conventionally considered to be weakly immunogenic (7–11).
Successful immunotherapy is influenced by several tumor-intrinsic factors including tumor mutational burden, neoantigen burden, HLA type and expression, DNA damage repair capacity, and programmed death ligand-1 expression (2, 3, 12–14). Recently, T cell-intrinsic factors have also been associated with immunotherapy response in murine and human ICB studies (15–18). This subset of self-renewing stem-cell-like TILs has been reported to exist in unique intratumoral structures and is characterized by the expression of transcription factor 7 (TCF7), along with the lack of cell surface inhibitory markers such as CD39 or TIM3 (15, 19). Paradoxically, analyses of tumor-reactive populations have concluded that anti-tumor neoantigen-specific TILs are enriched in subsets defined by the expression of PD-1 or CD39 (20–22). Thus, there is a lack of consensus regarding the tumor-reactive TIL subset that is directly responsible for successful immunotherapy.
Although the influence of the state of T cell differentiation on ACT has been evaluated in murine studies, the phenotypic fitness landscape of tumor-reactive TILs associated with ACT response against human cancer is unclear (23). ACT using TILs in lymphodepleted human patients provides a unique opportunity to investigate the T cell states responsible for cancer regression, as the T cell-enriched treatment product can be isolated and studied. To this end, we compared the phenotypic differences that could distinguish ACT infusion products (I.P.) administered to patients who had complete response to therapy (complete responders, CRs, N = 24) from those whose disease progressed following ACT (non-responders, NRs, N = 30) (Fig. 1A). These ACT I.P. were from a cohort of stage IV metastatic melanoma patients who had been treated with autologous _in vitro_-expanded TILs and had not experienced prior immunotherapies in the form of genetically engineered T cell therapy or anti-PD-1 blockade (Table S1 and Cohort description in materials and methods) (24, 25).
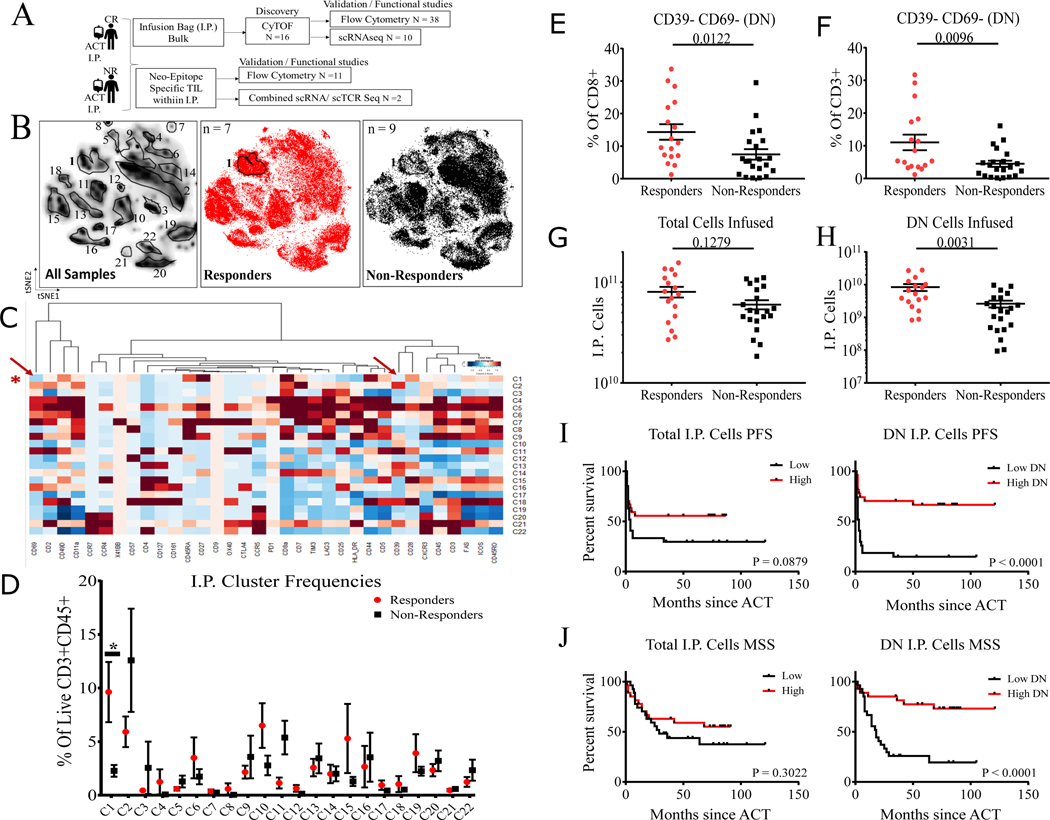
Figure 1. Phenotypic landscape of TIL infusion products from patients with metastatic melanoma treated with adoptive T cell therapy.
(A) Melanoma cohort patient I.P. and schema used for this study. (B) t-SNE (t-distributed stochastic neighbor embedding) plots of live CD45+CD3+ cell clusters from patient I.P. showing all patient I.P. (left), clustering showing CR I.P. only in red (middle), and NR I.P. only in black (right). (C) Heatmap of scaled protein expression (columns) per each cluster (rows), red arrows indicate CD69 and CD39 expression. (D) Plot showing % of cells within each cluster derived from CR and NR I.P. *P = 0.0264 from two-sided Wilcoxon rank-sum test adjusted by bonferroni correction for all clusters. (E) Independent validation samples (N = 38) analyzed by flow cytometry of CR I.P. compared to NR I.P. by % of CD39-CD69- (DN) cells displayed as % of total CD8+ and (F) displayed as a % of total CD3+ cells in the I.P. (G) CR I.P. compared to NR I.P. by total cells infused (N = 38). (H) CR I.P. compared to NR I.P. by total CD8+CD39-CD69- (DN) cells infused in the I.P. (N = 38). Numbers indicate P-values by two-sided Wilcoxon rank-sum test. I) Progression-free survival (PFS) of all I.P. samples (N = 54) separated by median cell numbers infused (left) and median CD39-CD69- cell numbers infused (right). I) Melanoma-specific survival (MSS) of all I.P. samples (N = 54) separated by median cell number infused (left) and median CD39-CD69- cell number infused (right). “Low” indicates patients with I.P. cells less than median of the sub-group analyzed and “High” indicates patients with I.P. cells greater than median of the sub-group analyzed. Numbers indicate P-values by Log-rank Mantel-Cox test.
Initial single-cell analysis of a discovery set of 4.8 million I.P. TILs from 7 CRs and 9 NRs by mass cytometry (CyTOF) revealed heterogeneous expression of 34 T cell surface markers (Fig. S1). We applied machine learning-based unsupervised clustering to define T cell clusters with activation/exhaustion states to classify patients according to their clinical outcome (Fig. S2): this approach exposed CD69 and CD39 expression as two significant features of most clinical relevance. Manual visualization of TIL CyTOF profiles identified clusters that appeared prevalent in either CRs or NRs (Fig. 1B–C). We found that cluster 1, which was four-fold more abundant in CR I.P. relative to NR I.P. (corrected P = 0.0264, Fig. 1D), corresponded to CD8+ T cells with abundant CD44, CD27, CD28, and low expression of TIM3, characterized in prior studies as memory-like (15) and stem-like (19) T cells (Fig. 1C). Notably, cluster 1 also had low expression of inhibitory marker CD39 and T cell activation marker CD69 (Fig. 1C) identified as relevant features from our machine learning classifier (Fig. S2). Of note, cluster 2, with high expression of CD39 and CD69 but lower levels of CD44, CD27, and CD28 (Fig. 1C) trended higher in NR I.P. than in CR I.P., although this difference did not reach statistical significance (Fig. 1D).
Flow cytometric analysis of the 16 discovery samples defining CD8+CD39-CD69- cells as members of cluster 1 recapitulated mass cytometry data with high confidence (Fig, S3A–B). An evaluation of an independent set of 38 I.P. (CRs n =17, NRs n=21) by multi-parameter flow cytometry revealed that the frequency of the CD8+CD39-CD69- TIL population was 2.5-fold higher in CR I.P. relative to NR I.P. (P = 0.0096, % of CD3) supporting the association between this subset and response to ACT (Fig. 1E–F). While the total number of infused T cells did not differ significantly between CR and NR I.P. in this set of 38 patient samples, the total number of infused CD8+CD39-CD69- cells was four-fold higher in CR I.P. than NR I.P. (P = 0.0031, Fig. 1G–H). Querying individual markers, there was a modest trend towards an association between CD8+ TIM3+ TILs and non-response to ACT (Fig. S3C). Analyzing patient survival from all 54 patients based on I.P. cell numbers, we found that higher numbers of CD8+CD39-CD69- cells in the I.P. were significantly associated with improved progression-free survival (PFS, P < 0.0001, HR = 0.255, 95% CI 0.1257 to 0.5186, Fig. 1I) and melanoma-specific survival (MSS, P < 0.0001, HR = 0.217, 95% CI 0.101 to 0.463, Fig. 1J) in a dose-dependent manner (Fig. S4 tertile analysis).
To further explore the potential significance of the CD39-CD69- (cluster 1, double-negative: DN) and the I.P. predominant CD39+CD69+ (cluster 2, double-positive: DP) subsets, we evaluated the transcriptome profile of these two subsets (Fig. S5A). DN TILs had increased expression of quiescent T-stem-cell markers KLF2, TCF7, S1PR1, LEF1, IL7R, CD27, and SELL (CD62L) while DP TILs expressed CD38, GITR, GZMA, TNF, and IFNG found in differentiated, activated T cells (Fig. S5B, Table S3). Unsupervised clustering of 18,624 CD8+ TILs by single-cell transcriptome analysis (scRNA) from 10 patient I.P. (5 CRs, 5 NRs) defined 8 clusters (Fig. 2A–B). Analysis of the distribution of responder and non-responder cells indicated that they segregated into a two-cluster solution (15) defined by S.Cluster.A (Super Cluster A, comprising clusters C0, C2, C5, C6, C7) and S.Cluster.B (Super Cluster B, comprising clusters C1, C3, C4, C8). Responder TILs were distributed more in S.Cluster.A (P = 0.0317), while S.Cluster.B largely comprised non-responder TILs (P = 0.03, Fig. 2B–C). TILs in S.Cluster.A were enriched for DN gene signature (81% overlap, Fig, S5C, Table S4) while TILs in S.Cluster.B were enriched for DP gene signature (Fig. 2D–E). Consistent with these analyses, CR TILs and NR TILs scored by single cell gene set enrichment analysis (scGSEA) of DN and DP signatures indicated that CR TILs had higher DN scores on average while NR I.P. TILs had higher DP scores (P < 4.5 × 10−12 for both, Fig. 2F).
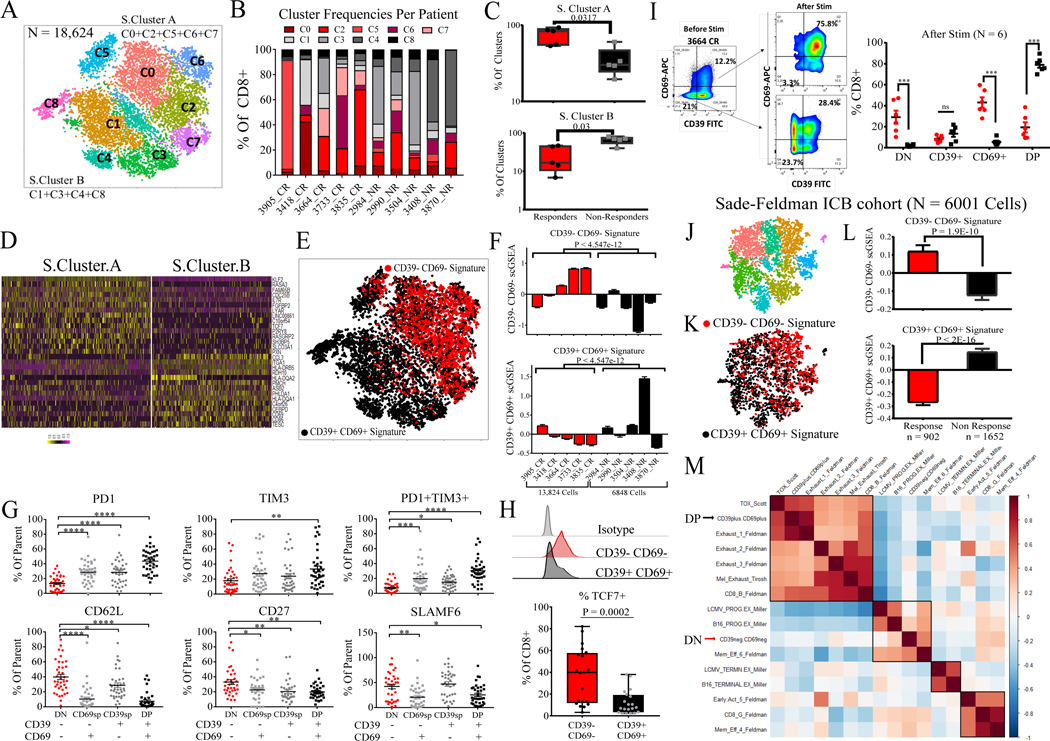
Figure 2. CD39-CD69- CD8+ TILs in infusion product are in a memory progenitor stem-like state.
(A) t-SNE plot of all CD8+ TILs from CR and NR I.P. (5 CRs, 5 NRs). (B) Frequency distribution of each cluster (1 through 8) expressed as a % of total CD8+ T cells in the I.P. for each patient. (C) Data points representing % of S.Cluster.A (top) and % of S.Cluster.B (bottom) per each patient I.P. between CRs and NRs. S.Cluster.A encompasses clusters C0, C2, C5, C6, and C7; S.Cluster.B comprises clusters C1, C3, C4, and C8. P-values by two-sided Wilcoxon rank-sum test are shown. (D) Heatmap of top 15 discriminating genes between S.Cluster.A and S.Cluster.B displayed for each cell. All discriminating genes are listed in Table S4. (E) t-SNE plot of clustered cells displayed by the top two quartiles of CD39-CD69- (DN) gene signature expression, and top two quartiles of CD39+CD69+ (DP) gene signature expression. (F) Each patient I.P. was scored by DN and DP gene signature scores and their mean scGSEA values are plotted. CRs are in red, and NRs are in black. P-values by two-sided Wilcoxon rank-sum test comparing the mean DN and DP signature scores between CRs and NRs are shown. (G) Flow cytometric analysis of inhibitory and memory markers within each subset (DN [CD39-CD69-], SP [CD39-CD69+, CD39+CD69-] and DP [CD39+CD69+] of patient I.P. in the validation set (n=38) expressed as % of each subset (parent gate). * P < 0.05 **P < 0.01 ***P < 0.001 ****P < 0.0001 by Tukey’s multiple comparison test. (H) Flow cytometry of intracellular TCF7 expression for DN and DP subsets showing representative patient I.P. sample (top) and quantitation for 18 I.P. (bottom). P-value by two-sided Wilcoxon rank-sum test is shown. (I) Phenotypes of DN, SP, and DP states before and after CD3/CD28 stimulation at 48 hours showing flow cytometry plot in a representative patient sample (left) and summary of daughter cells in each state after stimulation of 6 patient I.P. (right). ***P < 0.001 by two-sided Wilcoxon rank-sum test. (J) t-SNE clusters of CD8+ TILs from Melanoma ICB cohort (15) (K) t-SNE plot colored by top two quartiles of DN and DP gene signatures, DN: red, DP: black (L) TILs from pre ICB therapy were scored by the top DN and DP gene signature scores and mean scGSEA scores are plotted. Cells from responding lesions are in red, and cells from progressing lesions are in black. Cell numbers and P-values by two-sided Wilcoxon rank-sum test are shown. (M) Clustered correlation matrix of gene signatures from other studies (Table S5) along with DN and DP scGSEA scores on pre-ICB cells from the cohort.
We analyzed cell surface expression of inhibitory and memory markers by flow cytometry within CD39/CD69 TIL subsets from the validation samples (n = 38 I.P., Fig. 2G). We observed an increase in the expression and coexpression of exhaustion markers PD-1 and TIM3 from DN subset relative to single positive (SP, CD39+CD69- and CD39-CD69+) and DP subsets (Fig. 2G, Fig. S6). Conversely, expression of memory markers CD62L, CD27, and SLAMF6 decreased from DN to DP subsets while the SP population varied in its expression of these markers (Fig. 2G, Fig. S6). Protein expression of progenitor stem-like T cell marker TCF7 was five-fold higher in DN TILs relative to DP TILs (P = 0.0002, n=18 I.P., Fig. 2H) consistent with our scRNA analysis and other studies (15, 16). To assess if CD39-CD69- I.P. TILs were progenitor cells, we isolated and stimulated the DN and DP subsets using plate-bound CD3/CD28. Upon T cell receptor (TCR) stimulation, DN TILs underwent self-renewal and gave rise to both single positive and CD39+CD69+ DP populations, while stimulated DP TILs largely remained in the same DP state, indicating a DN-progenitor state (n = 6 I.P., Fig. 2I). In addition, while DP and DN TILs secreted similar levels of IL-2, IFN-g, GZMA, GZMB, and perforin in response to polyclonal stimulation, DN TIL stimulation resulted in higher levels of secreted IL-17A, TNF-a, IL-4, sFasL, and granulysin than DP TILs (Fig. S7).
To understand if TIL states in the I.P. corresponded to those within fresh tumor, we re-analyzed the transcriptome profiles of 6001 CD8+ T cells from a previous melanoma ICB response study (15) using DN and DP gene signatures described above (Fig. 2J–M). We found that prior to immune checkpoint therapy, TILs from ICB-responding lesions had higher CD39-CD69- signature scores (P = 1.9 × 10−10) than non-responding lesions, while TILs from lesions that progressed during ICB had higher CD39+CD69+ signature scores (P < 2.2 × 10−16). Gene set enrichment analysis of published signatures (Fig. S8) and hierarchical clustering correlation analysis with T cell dysfunctional and progenitor gene signatures from other recent studies (listed in Table S5) revealed that the signatures of CD39-CD69- TILs were most similar to ICB response-associated memory and progenitor-exhausted TILs, while the gene signatures of CD39+CD69+ TILs were highly correlated with TOX+ and terminally-exhausted TILs associated with poor ICB response (Fig. 2M) (15, 16, 26, 27). We developed a phenotypic fitness score, defined as the difference between DN stem-like signature and DP differentiated signature (DN-DP), and scored scRNA from ACT I.P. and melanoma ICB TILs. Single-cell fitness scores re-confirmed that responder TILs have on average more stem-like fitness compared to non-responder TILs (Fig. S9). Taken together, these results indicate that response-associated CD39-CD69- TILs are in a progenitor stem-like state while CD39+CD69+ TILs are in a terminally differentiated state.
Previous studies on stem-like and differentiated TIL subsets were performed on bulk TIL populations lacking specific analyses of anti-tumor T cells (15, 19). Given recent findings suggesting that neoantigen-specific TILs are nearly exclusively CD39+ whereas CD39- TILs represent bystander cells (20, 21), we sought to determine whether neoantigen-reactive T cells present in I.P. TIL exist in the CD39-CD69- stem-like state associated with response to ACT. Using our previously described tandem minigene neoantigen screening platform (9), we defined 26 HLA class I restricted neoantigens from I.P. for phenotypic evaluation (n = 11 patients, Fig. S10–S11, Table S6). Stem-like and differentiated TIL subsets defined by their CD39/CD69 expression were readily detectable within HLA-neoantigen tetramer+ TILs as shown in representative CR and NR (Fig. 3A–B). Combined analysis of all 26 neoantigen-specific TILs indicated that they largely existed in the CD39+CD69+ differentiated state, consistent with other studies reporting CD39 enrichment of neoantigen-specific T cells (mean DP = 62.7%, Fig. 3C) (20). Stratification of the data by ACT response status, however, revealed that the frequency of neoantigen-specific DN cells was significantly higher in CR I.P. (mean = 9.24%) than in NR I.P. (mean = 1.02%, P = 1.2 × 10−4, Fig. 3D). CD39 as a single marker indicated that CD39+ neoantigen-reactive TILs were significantly lower in CR TILs relative to NR TILs (P = 0.0001, Fig. 3E). These results indicate that at least in the context of melanoma ACT, not all CD39-negative (CD39-) TILs are bystander T cells as previously reported, and that responders retain a detectable proportion of tumor-reactive TILs in a CD39- stem-like state.
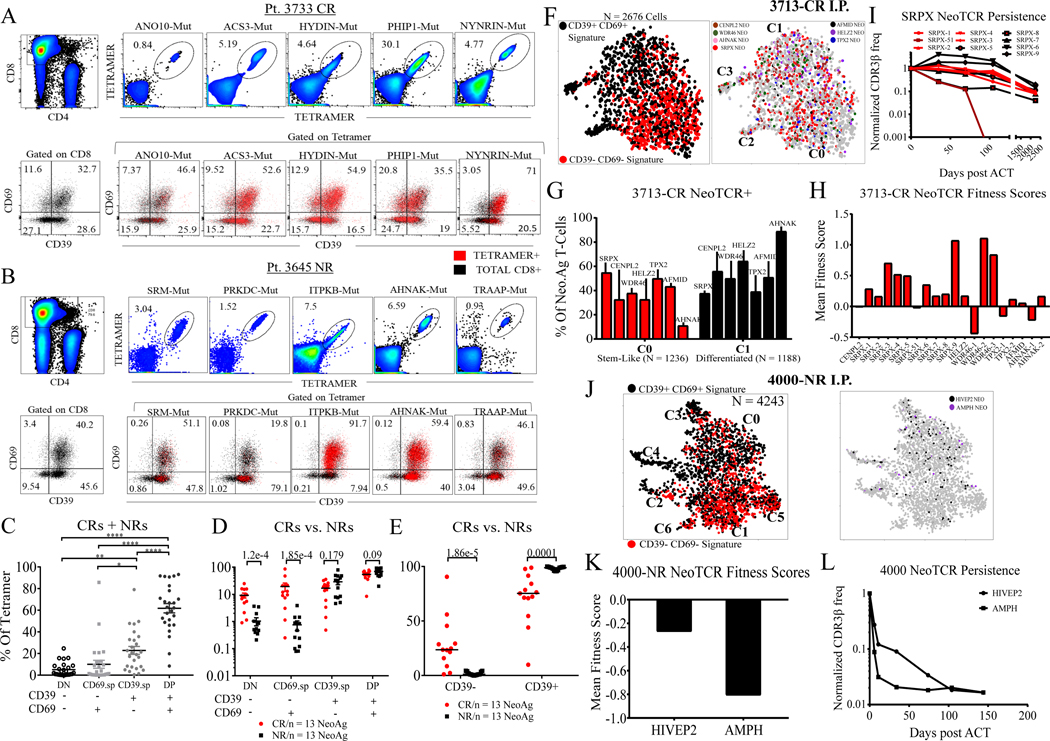
Figure 3. Stem-like neoantigen-specific TILs from responder and non-responder I.P.
(A) Representative CR I.P. showing detection of neoantigen-specific tetramers (top) and CD39/CD69 phenotypes of bulk CD8+ TILs and CD8+ tetramer+ TILs (bottom) (B) NR I.P. with comparable number of neoantigens (top) and CD39/CD69 phenotypes of bulk CD8+ TILs and CD8+ tetramer+ TILs (bottom). Numbers within quadrants for A and B, listed under tetramer category represent % of the sub-population within CD8+ tetramer+ gate (C) DN, SP, and DP phenotypes of all 26 neoantigen-specific TILs expressed as a % of tetramer+ cells from 11 patient I.P. CRs and NRs are combined for this data analysis. *P < 0.05 **P < 0.01 ***P < 0.001 ****P < 0.0001 by Tukey’s multiple comparison test. (D) DN, SP, and DP phenotypes of 26 neoantigen-specific TILs sub-divided by response status (CRs versus NRs). P-values by two-sided Wilcoxon rank-sum test between CRs and NR tetramer+ cells for each subset are shown. (E) CD39-positive and CD39-negative neoantigen-specific tetramer+ TILs (CD39 as a single marker) sub-divided by response status (CRs versus NRs). P-values by two-sided Wilcoxon rank-sum test between CR and NR tetramer+ TILs are shown. (F) t-SNE plot of CD8+ TILs from 3713-CR I.P. showing projection of stem-like DN and differentiated DP gene signatures (left), projection of neoantigen-specific TCRs colored by antigen specificity (right) (G) Pt. 3713 NeoTCR+ cells from stem-like C0 cluster and differentiated C1 cluster displayed as a % of all NeoTCR+ cells for each neoantigen specificity. (H) Each NeoTCR+ clonotype scored by their mean fitness score (value of DN minus DP scores). Positive values indicate clonotype enrichment in stem-like phenotypes and negative scores indicate clonotype enrichment in differentiated state. (I) Post-ACT persistence of the immunodominant SRPX NeoTCR clonotypes from Pt. 3713-CR I.P. in peripheral blood normalized to their initial frequency in the infusion product (day 0). (J) t-SNE plot of CD8+ TILs from Pt. 4000-NR I.P. showing projection of stem-like DN and differentiated DP gene signatures (left), projection of neoantigen-specific TCRs colored by antigen-specificity (right). (K) Each NeoTCR+ clonotype (HIVEP2, AMPH) scored by their mean fitness scores as described above (L) Post-ACT persistence of the HIVEP2 and AMPH NeoTCR clonotypes from Pt. 4000-NR in peripheral blood normalized to their frequency in the infusion product. Patient follow-up was stopped at 150 days due to disease progression.
In order to explore the heterogeneity in stem-like and differentiated states of neoantigen-specific TILs at the transcriptomic level, we performed combined scRNA and scTCR sequencing on I.P. from a CR (Pt. 3713) and an NR (Pt. 4000), with defined neoantigen-reactive TCRs (Fig. 3F–L, Table S7, refs (28–31)).
The CR 3713 I.P. was dominated by two major clusters C0 and C1, where C0 represented the stem-like DN state and C1, the differentiated DP state (Fig. 3F, Fig. S12A–B). Projection of the 20 neoantigen-specific TCR+ (NeoTCR+) clonotypes showed a broad distribution among the two clusters (Fig. 3F–G). The immunodominant SRPX-mutation-specific NeoTCRs were enriched in cluster C0 while other NeoTCRs varied in their prevalence within the two clusters (Fig. 3G), in line CDR3β sequencing of FACS-sorted DN and DP TILs from I.P. (Fig. S12C–D). Finer analysis of SRPX NeoTCR+ single cells indicated that 9/10 SRPX NeoTCR clonotypes (and 15/20 of all NeoTCR clonotypes) were enriched for positive phenotypic fitness scores (Fig, 3H). As prior studies have suggested that T cell-intrinsic differences potentially impact post-ACT clonotypic persistence (32, 33), we analyzed the post-ACT peripheral blood of the patient 3713. TCR repertoire sequencing revealed that the majority of NeoTCR clonotypes (18/20) persisted up to 75 months consistent with the idea of TIL expansion from the progenitor stem-like state (SRPX: Fig.3I, other NeoTCRs: Fig. S12E). Interestingly, among the 10 SRPX-NeoTCRs targeting the same neo-epitope, one clonotype (TCR-51) with the lowest phenotypic fitness score declined in frequency and became undetectable only 3 months post treatment (Fig. 3H–I).
Combined scRNA and scTCR sequencing of the non-responder Pt. 4000 I.P. showed two major stem-like clusters (C1, C5) and one major differentiated cluster (C0) (Fig. 3J, S13A–B). However, NeoTCR+ cells were largely concentrated in the differentiated C0 cluster (~60%, Fig. 3J, S13C–E). Single-cell gene signature analysis indicated that both NeoTCR clonotypes from patient 4000 had negative phenotypic fitness scores (Fig. 3K). Importantly, in stark contrast to the 3713-CR persistent clonotypes, Pt. 4000 NeoTCRs did not persist post-ACT expansion; rather both infused NeoTCRs clonotypes rapidly declined in peripheral blood following treatment (Fig. 3L). These results are largely in line with our own prior results and other studies that have linked cell therapy response to post-treatment TCR persistence (33–35).
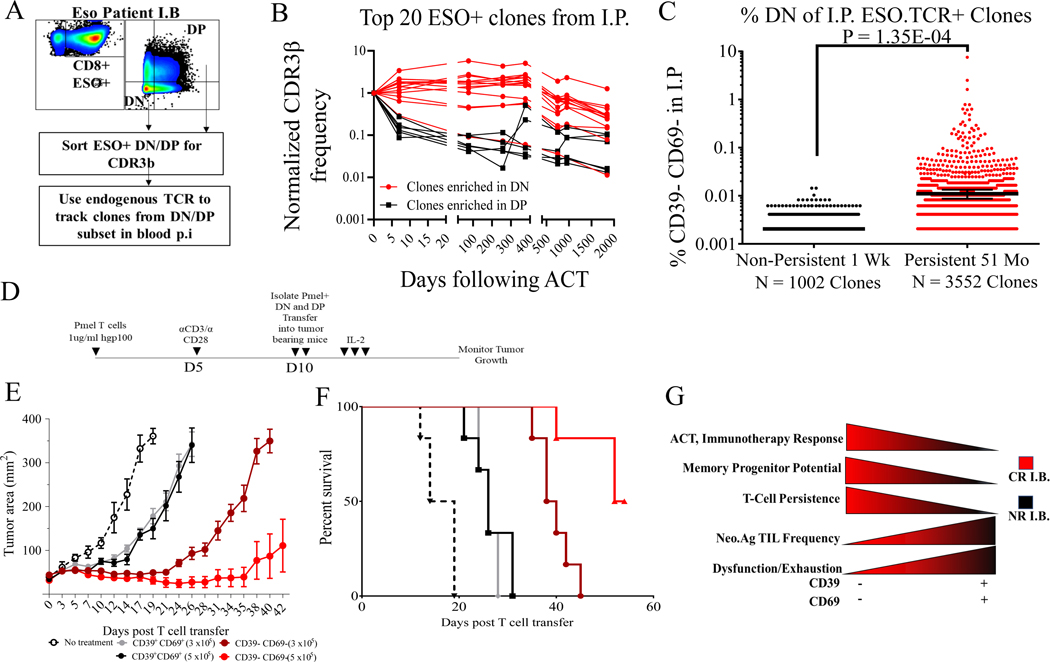
While our results indicate that T cell intrinsic phenotypic differences are associated with persistence following ACT, additional factors such as TCR avidity against variable tumor antigens may also play a profound role in T cell persistence. To address this issue, we performed an exploratory analysis of T cell persistence in a metastatic synovial cell sarcoma patient who experienced a CR following the adoptive transfer of autologous PBMC that had been transduced with a TCR directed against a single HLA-A*02:01-restricted NY-ESO-1 epitope. We used endogenous human TCRβ as barcodes to track 5356 stem-like DN clones and 6715 differentiated DP clones within TCR-transduced population in post-treatment blood (Fig. 4A) (36). Fourteen out of the top 20 ESO TCR+ I.P. clonotypes were enriched in stem-like DN state while six were enriched in the differentiated DP state. Stem-like clones demonstrated a gradual decrease in their frequency over a period of five years following TCR transfer, whereas the differentiated clones declined rapidly to minor frequencies in post-treatment blood of the patient (Fig. 4B). Overall, long-term persistent clones (detectable at 51 months post-ACT, n=3552) were present at significantly higher frequencies in the stem-like state in the I.P. when compared to poorly persistent clones (those undetectable at 7 days post treatment, n = 1002) (Fig. 4C). These analyses indicate that intrinsic stem-like phenotypes can modulate the behavior of T cell clonotypes following ACT, although further studies in larger patient cohorts and in TCR and CAR trials targeting other antigens are needed to test these hypotheses.
Fig. 4. Stem-like TILs mediate anti-tumor activity and TCR persistence.
(A) Schema for using endogenous human TCR to track NY-ESO TCR-transduced infusion products in post-treatment peripheral blood of a complete responder to NY-ESO TCR therapy (B) Post-treatment peripheral blood persistence of top 20 ESO.TCR+ I.P. clones using endogenous human TCR according to enrichment in DN (in red) and DP (in black) phenotypes in TCR infusion product. Clones with DN / DP > median DN frequency are defined as enriched in DN state; clones with DN / DP < median DN frequency are defined as enriched in DP state. (C) Non-persistent clones at day 7 post-ACT were compared to long-term persistent clones at day 1846 post-ACT by their clonotypic frequency in the CD39-CD69- stem-like state in the I.P. P-value by two-sided Wilcoxon rank-sum test between CRs and NRs is shown. (D) Schema of adoptive transfer of sorted DN and DP pmel-transgenic T cells into mice bearing established B16 melanoma tumors. (E) Tumor growth curves and (F) survival curves of mice bearing B16 tumors treated with pmel DN or DP T cells in two doses. N = 6 mice per group. (G) Illustration of the role of stem-like T cells in immunotherapy and ACT success and paradoxical nature of tumor mutation-reactive T cells in stem-like and terminally differentiated states.
Our findings suggested that treatment with CD39-CD69- tumor-reactive TIL might result in superior tumor control. We first tested this hypothesis in vitro by isolating tumor-reactive DN and DP populations from a CR I.P. (Pt. 3733) by co-culture with autologous 3733-mel tumor line, followed by multiple rounds of rapid expansion to evaluate for their proliferative potential and tumor recognition (Fig. S14A). We found that the expansion of the tumor-reactive stem-like DN subsets was approximately one thousand-fold higher than the differentiated DP subset (Fig. S14B). Crucially, the stem-like DN subset maintained tumor recognition while the differentiated DP subset lost tumor reactivity following subsequent rounds of expansion (Fig. S14C–D).
To assess the in vivo anti-tumor effects of stem-like DN and differentiated DP subsets, we isolated CD39-CD69- and CD39+CD69+ CD8+ T cell populations from Pmel transgenic TCR splenocytes and performed adoptive transfer of the isolated subsets into mice implanted with B16-F10 melanoma expressing the human gp100 antigen (Fig. 4D) (37). While the adoptive transfer of either 3×105 or 5×105 differentiated DP Pmel T cells had a modest effect on tumor control and mouse survival, the transfer of the same numbers of stem-like DN T cells led to substantial tumor regression and improved survival in a dose-dependent manner (Fig. 4E–F).
Harnessing anti-tumor T cell reactivity has accelerated immunotherapy treatment strategies against multiple human cancer types (1, 11, 38), but many disease settings currently exist outside the realm of approved immunotherapy modalities. In this report, we have explored the phenotypic diversity of anti-tumor neoantigen-specific T cells in a setting of successful immunotherapy. Our results indicate that responders to ACT received I.P. containing a minor pool of stem-like neoantigen-specific TILs that are able to undergo prolific expansion, give rise to differentiated subsets, and mediate long-term tumor control and T cell persistence, in line with recent murine ICB studies mediated by TCF+ progenitor T cells (17, 18). In contrast, non-responders’ I.P., despite containing anti-tumor neoantigen-specific TIL subsets enriched for tumor reactivity (e.g. CD39+) are likely terminally differentiated TILs with a relatively poor proliferative potential and therefore are not effective at mediating tumor control (Fig. 4G). Among tumor-reactive TILs, the relatively lower frequency of stem-like TILs compared to their largely differentiated counterparts, even in complete responders, highlights the paradox and challenges of mounting an effective anti-tumor immune response. Our findings suggest that strategies aimed at the isolation and expansion of stem-like neoantigen-specific T cells, or the engineering of T cells to have stem-like attributes, might provide opportunities for the development of more effective T cell-based immunotherapies.
Supplementary Material
Supp materials
Table S1
Table S3
Table S4
Table S5
Acknowledgments
We thank Don White for curating the melanoma patient cohort, and J. Panopoulos (Flowjo) for helpful discussions on high-dimensional analysis. We thank A. Mixon and S. Farid for flow cytometry support, and R. Yosseph, S. Vodnala, K. Hanada and other members of the Surgery Branch for helpful discussions and technical support. We also thank Support from CCR Single Cell Analysis Facility was funded by FNLCR Contract HHSN261200800001E. Sequencing was performed with the CCR Genomics Core. This work utilized the computational resources of the NIH HPC Biowulf cluster. (http://hpc.nih.gov).
Funding: This research was supported by the Center for Cancer Research intramural research program of the National Cancer Institute. S. Krishna acknowledges funding support from NCI Director’s Innovation Award
Footnotes
Competing interests: competing interests (including but not limited to patents, financial holdings, professional affiliations, advisory positions, and board memberships) of any of the authors must be listed (all authors must also fill out a separate, internal Conflict of Interest form). Where authors have no competing interests, this should also be declared (e.g., “Authors declare no competing interests.”)
Data and materials availability: All data is available in the main text or the supplementary materials.
References and Notes
- 1.Tran E, Robbins PF, Rosenberg SA, “Final common pathway” of human cancer immunotherapy: targeting random somatic mutations. Nat. Immunol. 18, 255–262 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA, Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA, Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 348, 124–128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA, Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robbins PF, Lu Y-C, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, Samuels Y, Rosenberg SA, Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 19, 747–752 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parkhurst MR, Robbins PF, Tran E, Prickett TD, Gartner JJ, Jia L, Ivey G, Li YF, El-Gamil M, Lalani A, Crystal JS, Sachs A, Groh E, Ray S, Ngo LT, Kivitz S, Pasetto A, Yossef R, Lowery FJ, Goff SL, Lo W, Cafri G, Deniger DC, Malekzadeh P, Ahmadzadeh M, Wunderlich JR, Somerville RPT, Rosenberg SA, Unique Neoantigens Arise from Somatic Mutations in Patients with Gastrointestinal Cancers. Cancer Discov. 9, 1022–1035 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Restifo NP, Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 348 (2015), pp. 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goff SL, Dudley ME, Citrin DE, Somerville RP, Wunderlich JR, Danforth DN, Zlott DA, Yang JC, Sherry RM, Kammula US, Klebanoff CA, Hughes MS, Restifo NP, Langhan MM, Shelton TE, Lu L, Kwong MLM, Ilyas S, Klemen ND, Payabyab EC, Morton KE, Toomey MA, Steinberg SM, White DE, Rosenberg SA, Randomized, Prospective Evaluation Comparing Intensity of Lymphodepletion Before Adoptive Transfer of Tumor-Infiltrating Lymphocytes for Patients With Metastatic Melanoma. J. Clin. Oncol. 34, 2389–2397 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran E, Turcotte S, Gros A, Robbins PF, Lu Y-C, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, Parkhurst MR, Yang JC, Rosenberg SA, Cancer Immunotherapy Based on Mutation-Specific CD4 T Cells in a Patient with Epithelial Cancer. Science. 344 (2014), pp. 641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran E, Robbins PF, Lu Y-C, Prickett TD, Gartner JJ, Jia L, Pasetto A, Zheng Z, Ray S, Groh EM, Kriley IR, Rosenberg SA, T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N. Engl. J. Med. 375, 2255–2262 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zacharakis N, Chinnasamy H, Black M, Xu H, Lu Y-C, Zheng Z, Pasetto A, Langhan M, Shelton T, Prickett T, Gartner J, Jia L, Trebska-McGowan K, Somerville RP, Robbins PF, Rosenberg SA, Goff SL, Feldman SA, Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat. Med. 24, 724–730 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, Kuo F, Kendall SM, Requena D, Riaz N, Greenbaum B, Carroll J, Garon E, Hyman DM, Zehir A, Solit D, Berger M, Zhou R, Rizvi NA, Chan TA, Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 359 (2018), pp. 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA, Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment with Response to Anti-PD-1 Therapy. Clinical Cancer Research. 20 (2014), pp. 5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandal R, Samstein RM, Lee K-W, Havel JJ, Wang H, Krishna C, Sabio EY, Makarov V, Kuo F, Blecua P, Ramaswamy AT, Durham JN, Bartlett B, Ma X, Srivastava R, Middha S, Zehir A, Hechtman JF, Morris LGT, Weinhold N, Riaz N, Le DT, Diaz LA, Chan TA, Genetic diversity of tumors with mismatch repair deficiency influences anti–PD-1 immunotherapy response. Science. 364 (2019), pp. 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, Lieb DJ, Chen JH, Frederick DT, Barzily-Rokni M, Freeman SS, Reuben A, Hoover PJ, Villani A-C, Ivanova E, Portell A, Lizotte PH, Aref AR, Eliane J-P, Hammond MR, Vitzthum H, Blackmon SM, Li B, Gopalakrishnan V, Reddy SM, Cooper ZA, Paweletz CP, Barbie DA, Stemmer-Rachamimov A, Flaherty KT, Wargo JA, Boland GM, Sullivan RJ, Getz G, Hacohen N, Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell. 176, 404 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, Yates KB, Lako A, Felt K, Naik GS, Manos M, Gjini E, Kuchroo JR, Ishizuka JJ, Collier JL, Griffin GK, Maleri S, Comstock DE, Weiss SA, Brown FD, Panda A, Zimmer MD, Manguso RT, Hodi FS, Rodig SJ, Sharpe AH, Haining WN, Subsets of exhausted CD8 T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddiqui I, Schaeuble K, Chennupati V, Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, Carmona SJ, Scarpellino L, Gfeller D, Pradervand S, Luther SA, Speiser DE, Held W, Intratumoral Tcf1PD-1CD8 T Cells with Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy. Immunity. 50, 195–211.e10 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Kurtulus S, Madi A, Escobar G, Klapholz M, Nyman J, Christian E, Pawlak M, Dionne D, Xia J, Rozenblatt-Rosen O, Kuchroo VK, Regev A, Anderson AC, Checkpoint Blockade Immunotherapy Induces Dynamic Changes in PD-1CD8 Tumor-Infiltrating T Cells. Immunity. 50, 181–194.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansen CS, Prokhnevska N, Master VA, Sanda MG, Carlisle JW, Bilen MA, Cardenas M, Wilkinson S, Lake R, Sowalsky AG, Valanparambil RM, Hudson WH, McGuire D, Melnick K, Khan AI, Kim K, Chang YM, Kim A, Filson CP, Alemozaffar M, Osunkoya AO, Mullane P, Ellis C, Akondy R, Im SJ, Kamphorst AO, Reyes A, Liu Y, Kissick H, An intratumoral niche maintains and differentiates stem-like CD8 T cells. Nature. 576 (2019), pp. 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simoni Y, Becht E, Fehlings M, Loh CY, Koo S-L, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, Duan K, Ang N, Poidinger M, Lee YY, Larbi A, Khng AJ, Tan E, Fu C, Mathew R, Teo M, Lim WT, Toh CK, Ong B-H, Koh T, Hillmer AM, Takano A, Lim TKH, Tan EH, Zhai W, Tan DSW, Tan IB, Newell EW, Bystander CD8 T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 557, 575–579 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, Goodall CP, Blair TC, Fox BA, McDermott JE, Chang S-C, Grunkemeier G, Leidner R, Bell RB, Weinberg AD, Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 9, 2724 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, Hanada K-I, Almeida JR, Darko S, Douek DC, Yang JC, Rosenberg SA, PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J. Clin. Invest. 124, 2246–2259 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klebanoff CA, Gattinoni L, Palmer DC, Muranski P, Ji Y, Hinrichs CS, Borman ZA, Kerkar SP, Scott CD, Finkelstein SE, Rosenberg SA, Restifo NP, Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin. Cancer Res. 17, 5343–5352 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goff SL, Dudley ME, Citrin DE, Somerville RP, Wunderlich JR, Danforth DN, Zlott DA, Yang JC, Sherry RM, Kammula US, Klebanoff CA, Hughes MS, Restifo NP, Langhan MM, Shelton TE, Lu L, Kwong MLM, Ilyas S, Klemen ND, Payabyab EC, Morton KE, Toomey MA, Steinberg SM, White DE, Rosenberg SA, Randomized, Prospective Evaluation Comparing Intensity of Lymphodepletion Before Adoptive Transfer of Tumor-Infiltrating Lymphocytes for Patients With Metastatic Melanoma. J. Clin. Oncol. 34, 2389–2397 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dudley ME, Gross CA, Langhan MM, Garcia MR, Sherry RM, Yang JC, Phan GQ, Kammula US, Hughes MS, Citrin DE, Restifo NP, Wunderlich JR, Prieto PA, Hong JJ, Langan RC, Zlott DA, Morton KE, White DE, Laurencot CM, Rosenberg SA, CD8+ enriched “young” tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin. Cancer Res. 16, 6122–6131 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott AC, Dündar F, Zumbo P, Chandran SS, Klebanoff CA, Shakiba M, Trivedi P, Menocal L, Appleby H, Camara S, Zamarin D, Walther T, Snyder A, Femia MR, Comen EA, Wen HY, Hellmann MD, Anandasabapathy N, Liu Y, Altorki NK, Lauer P, Levy O, Glickman MS, Kaye J, Betel D, Philip M, Schietinger A, TOX is a critical regulator of tumour-specific T cell differentiation. Nature. 571, 270–274 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tirosh I, Izar B, Prakadan SM, Wadsworth MH 2nd, Treacy D, Trombetta JJ, Rotem A, Rodman C, Lian C, Murphy G, Fallahi-Sichani M, Dutton-Regester K, Lin J-R, Cohen O, Shah P, Lu D, Genshaft AS, Hughes TK, Ziegler CGK, Kazer SW, Gaillard A, Kolb KE, Villani A-C, Johannessen CM, Andreev AY, Van Allen EM, Bertagnolli M, Sorger PK, Sullivan RJ, Flaherty KT, Frederick DT, Jané-Valbuena J, Yoon CH, Rozenblatt-Rosen O, Shalek AK, Regev A, Garraway LA, Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 352, 189–196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prickett TD, Crystal JS, Cohen CJ, Pasetto A, Parkhurst MR, Gartner JJ, Yao X, Wang R, Gros A, Li YF, El-Gamil M, Trebska-McGowan K, Rosenberg SA, Robbins PF, Durable Complete Response from Metastatic Melanoma after Transfer of Autologous T Cells Recognizing 10 Mutated Tumor Antigens. Cancer Immunology Research. 4 (2016), pp. 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasetto A, Gros A, Robbins PF, Deniger DC, Prickett TD, Matus-Nicodemos R, Douek DC, Howie B, Robins H, Parkhurst MR, Gartner J, Trebska-McGowan K, Crystal JS, Rosenberg SA, Tumor- and Neoantigen-Reactive T-cell Receptors Can Be Identified Based on Their Frequency in Fresh Tumor. Cancer Immunol Res. 4, 734–743 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkhurst M, Gros A, Pasetto A, Prickett T, Crystal JS, Robbins P, Rosenberg SA, Isolation of T-Cell Receptors Specifically Reactive with Mutated Tumor-Associated Antigens from Tumor-Infiltrating Lymphocytes Based on CD137 Expression. Clin. Cancer Res. 23, 2491–2505 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen CJ, Gartner JJ, Horovitz-Fried M, Shamalov K, Trebska-McGowan K, Bliskovsky VV, Parkhurst MR, Ankri C, Prickett TD, Crystal JS, Li YF, El-Gamil M, Rosenberg SA, Robbins PF, Isolation of neoantigen-specific T cells from tumor and peripheral lymphocytes. J. Clin. Invest. 125, 3981–3991 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP, A human memory T cell subset with stem cell-like properties. Nat. Med. 17, 1290–1297 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME, Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 17, 4550–4557 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Differentiation and Persistence of Memory CD8+ T Cells Depend on T Cell Factor 1. Immunity. 33, 229–240 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH, T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 3, 95ra73 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Angelo SP, Melchiori L, Merchant MS, Bernstein D, Glod J, Kaplan R, Grupp S, Tap WD, Chagin K, Binder GK, Basu S, Lowther DE, Wang R, Bath N, Tipping A, Betts G, Ramachandran I, Navenot J-M, Zhang H, Wells DK, Van Winkle E, Kari G, Trivedi T, Holdich T, Pandite L, Amado R, Mackall CL, Antitumor Activity Associated with Prolonged Persistence of Adoptively Transferred NY-ESO-1 T Cells in Synovial Sarcoma. Cancer Discov. 8, 944–957 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanada K-I, Yu Z, Chappell GR, Park AS, Restifo NP, An effective mouse model for adoptive cancer immunotherapy targeting neoantigens. JCI Insight. 4 (2019), doi: 10.1172/jci.insight.124405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishna S, Ulrich P, Wilson E, Parikh F, Narang P, Yang S, Read AK, Kim-Schulze S, Park JG, Posner M, Wilson Sayres MA, Sikora A, Anderson KS, Human Papilloma Virus Specific Immunogenicity and Dysfunction of CD8 T Cells in Head and Neck Cancer. Cancer Res. 78, 6159–6170 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Tran KQ, Zhou J, Durflinger KH, Langhan MM, Shelton TE, Wunderlich JR, Robbins PF, Rosenberg SA, Dudley ME, Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J. Immunother. 31, 742–751 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA, Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J. Immunother. 26, 332–342 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin J, Sabatino M, Somerville R, Wilson JR, Dudley ME, Stroncek DF, Rosenberg SA, Simplified method of the growth of human tumor infiltrating lymphocytes in gas-permeable flasks to numbers needed for patient treatment. J. Immunother. 35, 283–292 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bendall SC, Simonds EF, Qiu P, Amir E-AD, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, Balderas RS, Plevritis SK, Sachs K, Pe’er D, Tanner SD, Nolan GP, Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 332, 687–696 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finck R, Simonds EF, Jager A, Krishnaswamy S, Sachs K, Fantl W, Pe’er D, Nolan GP, Bendall SC, Normalization of mass cytometry data with bead standards. Cytometry A. 83, 483–494 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Maaten L, van der Maaten L, Hinton G, Visualizing non-metric similarities in multiple maps. Machine Learning. 87 (2012), pp. 33–55. [Google Scholar]
- 45.R. C. Team, R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014 (2014). [Google Scholar]
- 46.Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joseph-Pietras D, Welters MJP, van der Burg S, Kapiteijn E, Michielin O, Romano E, Linnemann C, Speiser D, Blank C, Haanen JB, Schumacher TN, Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci. Transl. Med. 6, 254ra128 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Lun ATL, Bach K, Marioni JC, Pooling across cells to normalize single-cell RNA sequencing data with many zero counts. Genome Biol. 17, 75 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halko N, Martinsson PG, Tropp JA, Finding Structure with Randomness: Probabilistic Algorithms for Constructing Approximate Matrix Decompositions. SIAM Review. 53 (2011), pp. 217–288. [Google Scholar]
- 49.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R, Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Godec J, Tan Y, Liberzon A, Tamayo P, Bhattacharya S, Butte AJ, Mesirov JP, Haining WN, Compendium of Immune Signatures Identifies Conserved and Species-Specific Biology in Response to Inflammation. Immunity. 44, 194–206 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Şenbabaoğlu Y, Gejman RS, Winer AG, Liu M, Van Allen EM, de Velasco G, Miao D, Ostrovnaya I, Drill E, Luna A, Weinhold N, Lee W, Manley BJ, Khalil DN, Kaffenberger SD, Chen Y, Danilova L, Voss MH, Coleman JA, Russo P, Reuter VE, Chan TA, Cheng EH, Scheinberg DA, Li MO, Choueiri TK, Hsieh JJ, Sander C, Hakimi AA, Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome Biol. 17, 231 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp materials
Table S1
Table S3
Table S4
Table S5