Cell-Based Fluorescence Assay for Human Immunodeficiency Virus Type 1 Protease Activity (original) (raw)
Abstract
The human immunodeficiency virus type 1 (HIV-1) protease is essential for production of infectious virus and is therefore a major target for the development of drugs against AIDS. Cellular proteins are also cleaved by the protease, which explains its cytotoxic activity and the consequent failure to establish convenient cell-based protease assays. We have exploited this toxicity to develop a new protease assay that relies on transient expression of an artificial protease precursor harboring the green fluorescent protein (GFP-PR). The precursor is activated in vivo by autocatalytic cleavage, resulting in rapid elimination of protease-expressing cells. Treatment with therapeutic doses of HIV-1 protease inhibitors results in a dose-dependent accumulation of the fluorescent precursor that can be easily detected and quantified by flow cytometric and fluorimetric assays. The precursor provides a convenient and noninfectious model for high-throughput screenings of substances that can interfere with the activity of the protease in living cells.
The protease encoded by human immunodeficiency virus type 1 (HIV-1) plays an essential role in the retroviral life cycle by processing the viral p55Gag and p160Gag-Pol polyprotein precursors into structural proteins and enzymes. The activity of the protease is required for conformational rearrangement of the immature virion and production of infectious virus particles, thus providing an attractive target for development of antiviral agents to treat AIDS and related disorders (29). Several potent HIV-1 protease inhibitors are widely used in clinics (7, 10). However, the constant emergence of resistant strains due to the additive effect of multiple amino acid substitutions within and outside the catalytic site motivates the continuous development of new protease inhibitors (26). The availability of reliable and convenient assays for protease activity is, in this context, of great importance.
The majority of assays available today are based on trans- or autocatalytic cleavage of reporter proteins in bacteria, in yeasts, or in vitro (1, 8, 17, 24) or on the in vitro hydrolysis of synthetic peptides encompassing the scissile bonds in p55Gag and p160Gag-Pol (3, 14, 23). However, none of these assays allows probing of all the native HIV-1 protease specificity sites under physiologic conditions, a situation for which a human cell environment would be required. An important reason for the lack of convenient mammalian cell-based assays is the cytotoxicity observed upon expression of the protease in cells. Thus, while this retroviral aspartic protease possesses unique structural and functional properties that distinguish it from its cellular counterparts (6), several cellular proteins are efficient substrates of the protease. Among those are cytoskeletal proteins such as vimentin, actin, troponin, and tropomyosin (20, 22), microtubule-associated proteins (30), bcl-2 (25), and precursors of NF-κB (19) providing a likely explanation for the capacity of the protease to induce apoptosis.
We report here on the development of a new reporter system that allows monitoring of HIV-1 protease activity and the effect of protease inhibitors in living cells. The reporter relies on transient expression of a nontoxic protease precursor harboring the green fluorescent protein (GFP). The reporter becomes toxic upon autocatalytic cleavage of the protease, and the consequent disappearance of fluorescence provides a simple means of quantifying protease activity and searching for inhibitors of this important enzyme.
MATERIALS AND METHODS
Plasmids.
The HIV-1 protease coding and flanking regions were amplified from the pK-HIV plasmid (12) using the sense primer 5′-AGCTGTACATTTGGGGAAGAGACAACAACTCCCT-3′ (_Ssp_BI site underlined) and the antisense primer 5′-CGAGATATCTTTTGGGCCATCCATTCCTGGCTTTA-3′ (_Eco_RV site underlined). The PCR product was cloned in frame with the GFP open reading frame from EGFP-N1 (Clontech, Palo Alto, Calif.) into the pcDNA3 vector (Invitrogen), yielding the pcDNA3/GFP-PR construct. For the pcDNA3/PR construct, the protease was amplified using the primers 5′-CCCAAGCTTATGGAATTCCCTCAGATCACTCTTTGGCAGCG-3′ (_Hin_dIII site underlined and start codon in bold) and 5′-CCCGCGGCCGCTTAAAAATTTAAAGTGCAGCCAATCTG-3′ (_Not_I site underlined and stop codon in bold) and cloned in pcDNA3. The identities of the plasmids were confirmed by DNA sequencing using dye terminator cycle sequencing (Applied Biosystems). The plasmid pT7-HIV1Gag was kindly provided by S. Schwartz (Uppsala University, Uppsala, Sweden).
Transfection and vaccinia infections.
HeLa (human cervical carcinoma cell line) and COS-1 (green monkey kidney cell line) cells were grown in Iscove's modified Eagle's medium supplemented with 10% fetal calf serum and antibiotics (Life Technologies, Grand Island, N.Y.). The cells were transfected with a mixture of plasmid DNA and Lipofectamine (Life Technologies) as recommended by the supplier. Stable sublines were generated by selection in 500 μg of G418 (Sigma, St. Louis, Mo.) ml−1 and screened by flow cytometry. The vaccinia virus-T7 RNA polymerase-based expression system was utilized for transient high-level expression of pT7-HIV1Gag as described previously (9). HeLa cells were infected with the recombinant virus vTF7-3 (provided by S. Schwartz) for 2 h before transfection. Where indicated, the transfected cells were treated with protease inhibitors for 24 h before harvesting. For protease inhibitor treatment of cultured cells, the inhibitors were initially dissolved in dimethyl sulfoxide and diluted to appropriate concentrations in Iscove's modified Eagle's medium supplemented with 10% fetal calf serum. Where indicated, the transfected cells were treated with protease inhibitors immediately after transfection until cells were harvested 24 h later.
Western blot analysis.
Lysates of 105 HeLa cells were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto Protan BA 85 nitrocellulose filters (Schleicher & Schuell, Keene, N.H.). The filters were probed with a rabbit polyclonal anti-GFP serum (Molecular Probes Europe, Leiden, The Netherlands) or anticapsid (p24) serum (kindly provided by H.-G. Krausslich, Heinrich-Pette-Institut, Hamburg, Germany) (15). The filters were developed by enhanced chemiluminescence (Amersham, Aylesbury, United Kingdom). Quantification of Western blot bands was performed by densitometry (Molecular Dynamics).
Flow cytometry, fluorescence microscopy, and fluorimetric analysis.
Expression of GFP was detected 24 h after transfection using a FACSort flow cytometer (Becton Dickinson, Mountain View, Calif.) and Cellquest software. For fluorescence microscopy, the cells were grown on coverslips and fixed with 4% paraformaldehyde in phosphate-buffered saline. A Leitz-BMRB fluorescence microscope (Leica, Heidelberg, Germany) was used with appropriate filter settings for GFP or Hoechst staining. Photographs were taken with a Hamamatsu 800 cooled charge-coupled device camera (Hamamatsu, Osaka, Japan) and processed with Adobe Photoshop software. Fluorimetric analyses were performed with an LS-50B luminescence spectrometer (Perkin-Elmer, Beaconsfield, United Kingdom), with excitation wavelengths at 480 nm and emission at 510 nm.
RESULTS
Construction of the GFP-PR reporter.
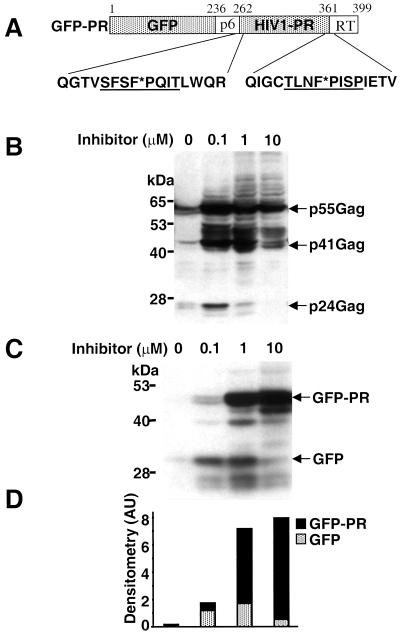
The toxicity of the HIV-1 protease has prevented the development of convenient assays for protease inhibitors in living cells. We reasoned that expression of a precursor in which the protease is fused to a reporter protein might circumvent this problem, since the chimera would be detected only when the activity of the protease is inhibited. As a reporter we chose the autofluorescent GFP from the jellyfish Aequorea victoria because its expression can be easily monitored and quantified in vivo (27). The GFP-PR chimera was generated by fusing a PCR product containing the HIV-1 protease open reading frame and the flanking sequences coding for 23 amino acids upstream and 20 amino acids downstream from the protease to the 3′ end of the GFP open reading frame (Fig. 1A). The flanking regions contain the endogenous p6/PR and PR/RT cleavage sites that are used for generation of an enzymatic active protease in virus-infected cells.
FIG. 1.
The GFP-PR chimera retains the capacity to cleave a natural viral substrate and is autocatalytically cleaved from the GFP-PR precursor. (A) Schematic representation of the GFP-PR chimera. The plasmid was constructed by linking the HIV-1 protease coding and flanking sequences to the 3′ end of the GFP open reading frame. The 5′ and 3′ flanking sequences of the protease are indicated (p6 and RT, respectively). In the amino acid sequences of the protease borders, cleavage sites are underlined and scissile bonds are marked by asterisks. (B) Lysates of HeLa cells cotransfected with GFP-PR and HIV-1 p55Gag were analyzed by Western blotting. Increasing concentrations of saquinavir were added to the culture medium immediately after transfection. The p55Gag precursor and the specific cleavage products p41Gag and p24Gag are indicated. Molecular masses are on the left. Results are from one representative experiment out of three. (C) Lysates of HeLa cells transiently transfected with GFP-PR were analyzed by Western blot using an anti-GFP antibody. The transfected cells were incubated with increasing concentrations of ritonavir for 24 h. The GFP-PR precursor and the GFP released upon autocatalytic cleavage are indicated. (D) Densitometric quantification of the GFP-PR and GFP bands in the Western blot. Results are from one representative experiment out of three.
The HIV-1 protease is activated in vivo by autocatalytic cleavage of the GFP-PR chimera.
In order to test whether the GFP-PR chimera retains the enzymatic properties of the HIV-1 protease, HeLa cells were cotransfected with the pcDNA3/GFP-PR plasmid and the plasmid pT7-Gag, which expresses the HIV-1 polyprotein p55Gag, a natural substrate of the protease (29). Processing of p55Gag is expected to yield the p41 (matrix and capsid protein) and p24 (capsid protein) products that can be detected in Western blots with a polyclonal antibody specific for p24 (15). High levels of unprocessed p55Gag were detected in HeLa cells transiently transfected with pT7-Gag and infected with a recombinant vaccinia virus expressing the T7 polymerase, as expected (Fig. 1B). In addition, some low-molecular-weight species were also detected by the anti-p24 antibody, probably due to processing of the overexpressed polyprotein by cellular proteases (S. Schwartz, personal communication). A characteristic band corresponding to the p24 capsid protein was readily detected in cells coexpressing the GFP-PR reporter (Fig. 1B). Similar results were obtained upon cotransfection with the pcDNA3/PR plasmid, which expresses an enzymatic active HIV-1 protease devoid of flanking sequences (data not shown). Furthermore, the generation of p24 from the p55Gag precursor was inhibited in GFP-PR-expressing cells by the HIV-1 protease inhibitors saquinavir, ritonavir, nelfinavir, and indinavir in a dose-dependent manner (Fig. 1B and data not shown), further confirming that the GFP-PR reporter harbors authentic protease activity.
The detection of HIV-1 protease activity in transfected cells indicates that processing of the chimera and activation of the protease occur in vivo. This was investigated by probing Western blots of transiently transfected HeLa cells with polyclonal antibodies to GFP, which should detect the intact chimera and its processed product GFP. The intact GFP-PR chimera was not detected while a weak band corresponding to the GFP moiety of the reporter could be identified (Fig. 1C). The failure to detect intact GFP-PR suggests that the reporter may be rapidly processed in the transfected cells. To test this possibility, increasing concentrations of the specific protease inhibitors saquinavir or ritonavir were added to the culture medium immediately after transfection (Fig. 1C and data not shown). A dose-dependent increase in the intensity of a band corresponding to the intact GFP-PR was observed, indicating that processing of the chimera is blocked by the inhibitor (Fig. 1C). Densitometric quantification of the GFP- and GFP-PR-specific bands confirmed that accumulation of the precursor was accompanied by disappearance of the GFP cleavage product (Fig. 1D). Taken together, these results indicate that the GFP-PR reporter behaves as a bona fide protease precursor which is activated in vivo by autocatalytic cleavage of the protease.
The HIV-1 protease is toxic in GFP-PR-expressing cells.
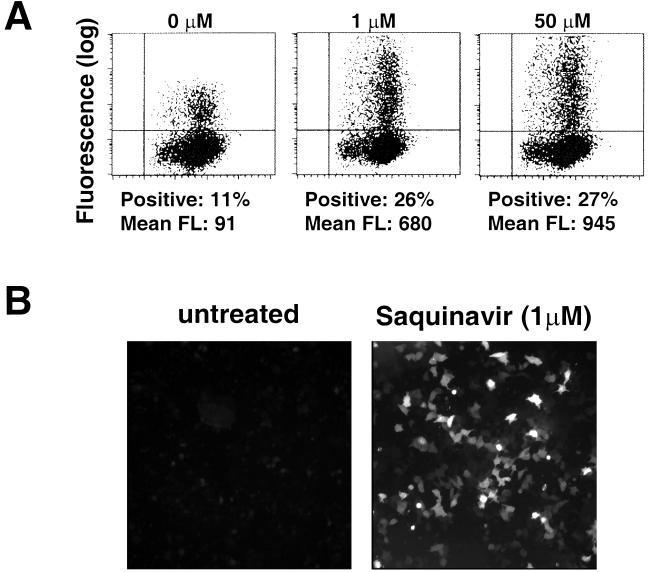
The GFP reporter allows accurate quantification in living cells. Thus, accumulation of GFP fluorescence should provide a convenient method for monitoring the presence of the HIV-1 protease in cells expressing GFP-PR. However, only a small number of weakly fluorescent cells were detected by fluorescence-activated cell sorting (FACS) analysis and fluorescence microscopy of HeLa cells transfected with the pcDNA3/GFP-PR plasmid. The poor fluorescence was not due to inefficient transfection, since dose-dependent increases in the number of fluorescent cells and fluorescence intensity of the positive cells were observed upon treatment with the HIV-1 protease inhibitor indinavir (Fig. 2A), and similar results were obtained on transfection of COS-1 cells (not shown). A dramatic increase in fluorescence intensity was also detected by fluorescence microscopic analysis of cells treated with 1 μM saquinavir (Fig. 2B).
FIG. 2.
Dose-dependent accumulation of GFP in cells treated with inhibitors of the HIV-1 protease. (A) HeLa cells transiently transfected with GFP-PR were treated for 24 h with increasing concentrations of indinavir, and the accumulation of GFP was monitored by flow cytometry. The percentage and mean fluorescence intensity (FL) of cells in the upper right quadrant are indicated. Data are from one representative experiment out of six. (B) Low-magnification fluorescence micrographs of HeLa cells transiently transfected with GFP-PR cultured with or without 1 μM saquinavir.
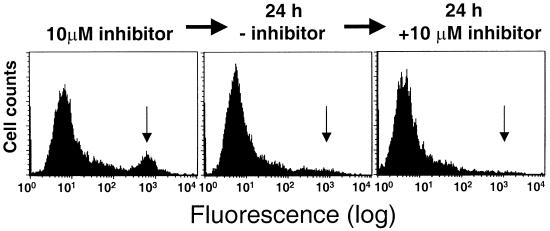
Failure to accumulate GFP in the absence of protease inhibitors could be due to inactivation of GFP or early elimination of the GFP-expressing cells by the cytotoxic effect of the protease. To discriminate between these possibilities, HeLa cells were transfected with plasmids encoding the GFP-PR chimera or GFP alone and the yield of fluorescent clones was compared after selection in G418. In anticipation of the cytotoxic effect of GFP-PR, a parallel selection was performed in continuous presence of the HIV-1 protease inhibitor saquinavir. Transfection with the GFP-expressing plasmid yielded a high proportion of clones that stably expressed high levels of GFP, as expected (Table 1). In contrast, only two poorly fluorescent clones out of 34 selected were obtained from GFP-PR-transfected cells. Inclusion of saquinavir throughout the selection procedure improved the yield of fluorescent clones and the percentage of fluorescent cells in each clone, but these remained in all cases far lower than those observed in cells transfected with GFP alone (Table 1). Furthermore, the fluorescence was gradually lost, and attempts to select stable populations of fluorescent cells by FACS sorting or cloning by limiting dilution failed, suggesting that the fluorescent cells may be progressively eliminated due to toxicity of the protease. This was confirmed by analysis of one fluorescent GFP-PR clone, which was originally selected with 1 μM saquinavir and later maintained in the presence of 10 μM ritonavir. At the time of first testing, the clone contained approximately 40% fluorescent cells, but the number decreased progressively and the fluorescent population was completely lost within a few weeks. Omission of ritonavir when only 12% of the cells remained fluorescent resulted in disappearance of the fluorescent cells within 24 h. The loss was irreversible, since subsequent administration of 10 μM ritonavir did not lead to reappearance of the fluorescent population (Fig. 3).
TABLE 1.
Comparison of yield of fluorescent clones
| Protein | No. of clones | ||||
|---|---|---|---|---|---|
| Analyzed | Fluorescent | With % fluorescent cells | |||
| <20 | 20–80 | >80 | |||
| GFP | 35 | 16 | 3 | 7 | 6 |
| GFP-PR | 34 | 2 | 2 | ||
| GFP-PR + inhibitor | 36 | 5 | 3 | 1a | 1b |
FIG. 3.
Irreversible loss of GFP-PR-expressing cells upon withdrawal of protease inhibitor. (Left) Flow-cytometric analysis of HeLa cells stably expressing GFP-PR after selection in the presence of 10 μM ritonavir. The subpopulation of highly fluorescent cells expressing GFP-PR is indicated with an arrow. The fluorescent cells disappear after culture for 24 h in ritonavir-free medium (middle) and do not reappear 24 h after reintroduction of 10 μM ritonavir into the culture medium (right).
Titration of HIV-1 protease inhibitors in GFP-PR-expressing cells.
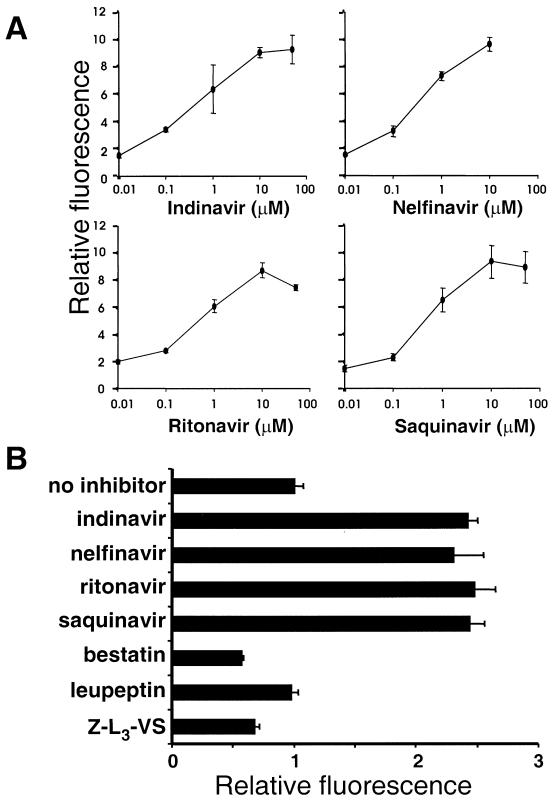
Since expression of the GFP-PR chimera can be detected only when the HIV-1 protease is suppressed, the reporter may provide a convenient tool for monitoring the inhibition of protease activity in vivo. To explore this possibility, we quantified the effects of indinavir, nelfinavir, ritonavir, and saquinavir in pcDNA3/GFP-PR-transfected HeLa cells. Increasing concentrations of the inhibitors were added immediately after transfection, and the cells were cultured for 24 h before analysis by flow cytometry. The inhibitors induced a dose-dependent increase in fluorescence intensity of the positive cells (Fig. 4A). Significant accumulation of the reporter was detected in cells treated with concentrations of the inhibitor as low as 0.01 μM, which is lower than the effective concentration achieved in plasma in protease inhibitor-treated patients (28). A maximum of approximately a ninefold increase in fluorescence was reached at 10 to 50 μM inhibitor (Fig. 4A). The possibility of using the GFP-PR reporter in fluorimetric assays would facilitate its employment in high-throughput screens. We therefore compared the fluorescence intensity of GFP-PR-expressing cells in response to the HIV-1 protease inhibitors indinavir, nelfinavir, saquinavir, and ritonavir and irrelevant inhibitors of aminopeptidases (bestatin), cysteine proteases (leupeptin), and proteasomes (carboxybenzyl-leucyl-leucyl-leucine vinyl sulfone [Z-L3-VS]) (5). An approximately threefold increase in total fluorescence was demonstrated by fluorimetric analysis of GFP-PR-transfected cells following treatment with the four HIV-1 protease inhibitors, whereas bestatin, leupeptin, and Z-L3-VS had no effect (Fig. 4B). The level of fluorescence accumulation detected by fluorimetry was well in line with the approximate ninefold increase in mean fluorescence intensity recorded in parallel experiments where the effect of HIV-1 protease inhibitors on the accumulation of GFP was monitored by FACS analysis. Although a stronger effect was measured by flow cytometry, the clear and highly reproducible increase detected by fluorimetric analysis in transiently transfected cells indicates that the GFP-PR reporter can be used in high-throughput screens of protease inhibitors using a multiwell plate fluorimeter.
FIG. 4.
Specific and sensitive assays for HIV-1 protease inhibitors based on the GFP-PR reporter. (A) HeLa cells transiently transfected with pcDNA3/GFP-PR were treated with increasing concentrations of indinavir, nelfinavir, ritonavir, and saquinavir for 24 h and analyzed by flow cytometry. Nelfinavir was toxic at concentrations higher then 10 μM. Relative fluorescence is expressed as fold induction over that of transfected cells cultured without inhibitor. Data are means ± standard deviations from three experiments. (B) Fluorimetric analysis of HeLa cells transiently transfected with pcDNA3/GFP-PR. Cells were treated for 24 h with the HIV-1 protease inhibitors indinavir (10 μM), nelfinavir (10 μM), ritonavir (10 μM), and saquinavir (10 μM), the aminopeptidase inhibitor bestatin (1 mM), the cysteine protease inhibitor leupeptin (1 mM), and the proteasome inhibitor Z-L3-VS. (8 μM). Data are means ± standard deviations from three experiments. Excitation and emission wavelengths were 480 and 510 nm, respectively.
DISCUSSION
Here we present a new and convenient method for monitoring HIV-1 protease activity in human cells, which is based on expression of a precursor protein harboring the viral protease fused to the reporter protein GFP. Expression of this reporter ensures a 1:1 stoichiometry between the viral protease and the reporter protein. Thus, quantification of the amount of GFP by its emitted fluorescence is directly correlated to the amount of protease in reporter-expressing cells. We have shown that the chimeric reporter is enzymatic active in vivo due to autocatalytic activation of the protease. As high intracellular levels of the protease are tolerated only when its enzymatic activity is inhibited, expression of GFP is a reliable parameter of intracellular HIV-1 protease activity. Indeed, we observed a clear inverse correlation between the emitted fluorescence and the activity of the HIV-1 protease in protease inhibitor-treated cells. Transient-transfection experiments showed that titration of HIV-1 protease inhibitors leads to an approximately ninefold increase in the mean fluorescence intensity, as measured by flow cytometry. Furthermore, measurement of the total fluorescence by fluorimetry revealed a reproducible approximately threefold increase, indicating that the reporter system can be adapted to high-throughput screenings of protease inhibitors using microtiter plate readers. The system was shown to be very sensitive, responding to concentrations of HIV-1 protease inhibitors in their therapeutic ranges. Importantly, no effect was observed when cells were treated with different classes of unrelated inhibitors, showing that this reporter is specific for HIV-1 protease inhibitors.
The GFP-PR reporter system offers several distinct advantages over the broad array of existing screening assays for HIV-1 protease activity. The currently available detection methods are performed mainly in yeast and bacterial cells or in vitro. The most simple in vivo assays exploit the inherent toxicity of the protease for bacterial cells (2), and more sophisticated strategies have involved the introduction of HIV-1 protease cleavage sites into selectable markers such as β-galactosidase (1), tetracycline resistance protein (4), thymidylate synthase (13), galactokinase (24), transcription factors of Saccharomyces cerevisiae (17), and the _c_I repressor of bacteriophage λ (21). These assays usually monitor only one or few of the native HIV-1 protease specificity sites. Furthermore, the activity of the protease is tested under nonphysiologic conditions that may modify the catalytic properties of the enzyme. Our assay monitors the activity of the protease where therapeutic interference is most desired, in human cells. The exact sequence of the proteolytic events in virus-infected cells is not known, but the protease is believed to act mainly during the budding process, when it is located at the intracellular face of the cell membrane. Moreover, the toxicity caused by the viral protease in infected cells is suspected to contribute to the complex pathogenesis of AIDS (11). Since the GFP readout is inversely correlated to the cytotoxic effect of the protease, the use of this system in various HIV-1-susceptible cells can provide detailed information on the extent to which different candidate inhibitors are able to suppress these cell-associated effects. Parameters that are expected to vary between different cell types, such as permeability to the inhibitor or metabolic stability, are conveniently evaluated by measuring GFP fluorescence. We therefore envision that the GFP-PR reporter could become a major tool in the identification of candidate HIV-1 protease inhibitors.
An important aspect of our work relates to the identification and analysis of HIV-1 protease variants. Treatment with protease inhibitors often results in accumulation of multiple mutations in the protease due to ongoing replication of incompletely suppressed virus (16). This consecutive accumulation of mutations that are often located in regions distant from the active site confers broad resistance to various protease inhibitors. In order to optimize treatment and to ensure a long-term antiviral response, it appears to be crucial to monitor antiviral resistance and to adjust the therapeutic regimen accordingly. Phenotypic assays are currently the only way to directly determine resistance and give a rationale for therapeutic adjustments, since they measure susceptibility of the actual viral strains to antiretrovirals (18). These assays are based on inserting PCR-amplified protease sequences from patient blood into a proviral clone defective in the protease gene, followed by transfection and analysis of the resulting virus population for susceptibility to various drugs. Although effective, these tests can be performed only in specialized laboratories, require several weeks for readout, and are quite expensive. Thus, there is still an urgent need for faster and cheaper assays which accurately monitor the development of resistance in vivo. The GFP-PR reporter assay may allow rapid evaluation of the sensitivity of different HIV-1 protease mutants by replacing the original protease open reading frame with the mutated variants. Thus, the GFP-PR system represents a step toward the development of a reliable, noninfectious system for analysis of viral resistance in AIDS patients treated with HIV-1 protease inhibitors.
It seems reasonable to assume that the strategy of expressing a toxic protease in the form of an artificial precursor harboring the GFP reporter could be applied to other viral proteases. The perfect stoichiometry between toxic protease and the reporter accomplished by the GFP-PR precursor is a prerequisite in this assay, since cotransfection of independent plasmids harboring GFP and protease open reading frames did not give similar results (K. Lindsten, unpublished results). Thus, the GFP-PR reporter can easily be adapted to other viral proteases, provided that overexpression of these proteases leads to cell death and that insertion of the GFP moiety does not disturb the autocatalytic release of the protease from the precursor. This new approach opens the possibility for a line of protease assays that allow monitoring the inhibitory effects of drugs in human cells.
ACKNOWLEDGMENTS
We thank Marianne Jellne for technical assistance, Stefan Schwartz for the pT7-HIV1Gag construct and vTF7-3 virus, Hans-Georg Krausslich for pK-HIV and anticapsid antibody, Hidde Ploegh for the Z-L3-VS inhibitor, and Bo Öberg for ritonavir, indinavir, and nelfinavir.
This work was supported by grants awarded by the Swedish Cancer Society, the Swedish Foundation of Strategy Research, the Hedlund Foundation, The Swedish Physicians against AIDS Research Foundation, and the Åke Wibergs Stiftelse, Stockholm, Sweden. N.P.D. was supported by a postdoctoral fellowship awarded by the European Commission Training and Mobility (ERBFMRXCT960026). J.K. and T.U. were supported by an International Research Scholar's Award from the Howard Hughes Medical Institute (HHMI 75195-540801, J.K.) and by the Grant Agency of the Czech Republic (303/98/1559).
REFERENCES
- 1.Baum E Z, Bebernitz G A, Gluzman Y. β-Galactosidase containing a human immunodeficiency virus protease cleavage site is cleaved and inactivated by human immunodeficiency virus protease. Proc Natl Acad Sci USA. 1990;87:10023–10027. doi: 10.1073/pnas.87.24.10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baum E Z, Bebernitz G A, Gluzman Y. Isolation of mutants of human immunodeficiency virus protease based on the toxicity of the enzyme in Escherichia coli. Proc Natl Acad Sci USA. 1990;87:5573–5577. doi: 10.1073/pnas.87.14.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billich A, Winkler G. Colorimetric assay of HIV-1 proteinase suitable for high-capacity screening. Pept Res. 1990;3:274–276. [PubMed] [Google Scholar]
- 4.Block T M, Grafstrom R H. Novel bacteriological assay for detection of potential antiviral agents. Antimicrob Agents Chemother. 1990;34:2337–2341. doi: 10.1128/aac.34.12.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogyo M, McMaster J S, Gaczynska M, Tortorella D, Goldberg A L, Ploegh H. Covalent modification of the active site threonine of proteasomal β subunits and the Escherichia coli homolog HslV by a new class of inhibitors. Proc Natl Acad Sci USA. 1997;94:6629–6634. doi: 10.1073/pnas.94.13.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies D R. The structure and function of the aspartic proteinases. Annu Rev Biophys Biophys Chem. 1990;19:189–215. doi: 10.1146/annurev.bb.19.060190.001201. [DOI] [PubMed] [Google Scholar]
- 7.De Clercq E. Toward improved anti-HIV chemotherapy: therapeutic strategies for intervention with HIV infections. J Med Chem. 1995;38:2491–2517. doi: 10.1021/jm00014a001. [DOI] [PubMed] [Google Scholar]
- 8.Deo S K, Lewis J C, Daunert S. Bioluminescence detection of proteolytic bond cleavage by using recombinant aequorin. Anal Biochem. 2000;281:87–94. doi: 10.1006/abio.2000.4539. [DOI] [PubMed] [Google Scholar]
- 9.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung H B, Kirschenbaum H L, Hameed R. Amprenavir: a new human immunodeficiency virus type 1 protease inhibitor. Clin Ther. 2000;22:549–572. doi: 10.1016/S0149-2918(00)80044-2. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan A H, Swanstrom R. Human immunodeficiency virus type 1 Gag proteins are processed in two cellular compartments. Proc Natl Acad Sci USA. 1991;88:4528–4532. doi: 10.1073/pnas.88.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konvalinka J, Litera J, Weber J, Vondrasek J, Hradilek M, Soucek M, Pichova I, Majer P, Strop P, Sedlacek J, Heuser A M, Kottler H, Krausslich H G. Configurations of diastereomeric hydroxyethylene isosteres strongly affect biological activities of a series of specific inhibitors of human-immunodeficiency-virus proteinase. Eur J Biochem. 1997;250:559–566. doi: 10.1111/j.1432-1033.1997.0559a.x. [DOI] [PubMed] [Google Scholar]
- 13.Kupiec J J, Hazebrouck S, Leste-Lasserre T, Sonigo P. Conversion of thymidylate synthase into an HIV protease substrate. J Biol Chem. 1996;271:18465–18470. doi: 10.1074/jbc.271.31.18465. [DOI] [PubMed] [Google Scholar]
- 14.Matayoshi E D, Wang G T, Krafft G A, Erickson J. Novel fluorogenic substrates for assaying retroviral proteases by resonance energy transfer. Science. 1990;247:954–958. doi: 10.1126/science.2106161. [DOI] [PubMed] [Google Scholar]
- 15.Mergener K, Facke M, Welker R, Brinkmann V, Gelderblom H R, Krausslich H G. Analysis of HIV particle formation using transient expression of subviral constructs in mammalian cells. Virology. 1992;186:25–39. doi: 10.1016/0042-6822(92)90058-w. [DOI] [PubMed] [Google Scholar]
- 16.Molla A, Korneyeva M, Gao Q, Vasavanonda S, Schipper P J, Mo H M, Markowitz M, Chernyavskiy T, Niu P, Lyons N, Hsu A, Granneman G R, Ho D D, Boucher C A, Leonard J M, Norbeck D W, Kempf D J. Ordered accumulation of mutations in HIV protease confers resistance to ritonavir. Nat Med. 1996;2:760–766. doi: 10.1038/nm0796-760. [DOI] [PubMed] [Google Scholar]
- 17.Murray M G, Hung W, Sadowski I, Das Mahapatra B. Inactivation of a yeast transactivator by the fused HIV-1 proteinase: a simple assay for inhibitors of the viral enzyme activity. Gene. 1993;134:123–128. doi: 10.1016/0378-1119(93)90185-6. [DOI] [PubMed] [Google Scholar]
- 18.Nijhuis M, Schuurman R, Boucher C A B. Homologous recombination for rapid phenotyping of HIV. Curr Opin Infect Dis. 1997;10:475–479. [Google Scholar]
- 19.Riviere Y, Blank V, Kourilsky P, Israel A. Processing of the precursor of NF-κB by the HIV-1 protease during acute infection. Nature. 1991;350:625–626. doi: 10.1038/350625a0. [DOI] [PubMed] [Google Scholar]
- 20.Shoeman R L, Honer B, Stoller T J, Kesselmeier C, Miedel M C, Traub P, Graves M C. Human immunodeficiency virus type 1 protease cleaves the intermediate filament proteins vimentin, desmin, and glial fibrillary acidic protein. Proc Natl Acad Sci USA. 1990;87:6336–6340. doi: 10.1073/pnas.87.16.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sices H J, Kristie T M. A genetic screen for the isolation and characterization of site-specific proteases. Proc Natl Acad Sci USA. 1998;95:2828–2833. doi: 10.1073/pnas.95.6.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snasel J, Shoeman R, Horejsi M, Hruskova-Heidingsfeldova O, Sedlacek J, Ruml T, Pichova I. Cleavage of vimentin by different retroviral proteases. Arch Biochem Biophys. 2000;377:241–245. doi: 10.1006/abbi.2000.1776. [DOI] [PubMed] [Google Scholar]
- 23.Stebbins J, Debouck C. A microtiter colorimetric assay for the HIV-1 protease. Anal Biochem. 1997;248:246–250. doi: 10.1006/abio.1997.2111. [DOI] [PubMed] [Google Scholar]
- 24.Stebbins J, Deckman I C, Richardson S B, Debouck C. A heterologous substrate assay for the HIV-1 protease engineered in Escherichia coli. Anal Biochem. 1996;242:90–94. doi: 10.1006/abio.1996.0433. [DOI] [PubMed] [Google Scholar]
- 25.Strack P R, Frey M W, Rizzo C J, Cordova B, George H J, Meade R, Ho S P, Corman J, Tritch R, Korant B D. Apoptosis mediated by HIV protease is preceded by cleavage of Bcl-2. Proc Natl Acad Sci USA. 1996;93:9571–9576. doi: 10.1073/pnas.93.18.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomasselli A G, Heinrikson R L. Targeting the HIV-protease in AIDS therapy: a current clinical perspective. Biochim Biophys Acta. 2000;1477:189–214. doi: 10.1016/s0167-4838(99)00273-3. [DOI] [PubMed] [Google Scholar]
- 27.Tsien R Y. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 28.van Heeswijk R P, Veldkamp A I, Mulder J W, Meenhorst P L, Lange J M, Beijnen J H, Hoetelmans R M. Once-daily dosing of saquinavir and low-dose ritonavir in HIV-1-infected individuals: a pharmacokinetic pilot study. AIDS. 2000;14:F103–F110. doi: 10.1097/00002030-200006160-00003. [DOI] [PubMed] [Google Scholar]
- 29.Vogt V M. Proteolytic processing and particle maturation. Curr Top Microbiol Immunol. 1996;214:95–131. doi: 10.1007/978-3-642-80145-7_4. [DOI] [PubMed] [Google Scholar]
- 30.Wallin M, Deinum J, Goobar L, Danielson U H. Proteolytic cleavage of microtubule-associated proteins by retroviral proteinases. J Gen Virol. 1990;71:1985–1991. doi: 10.1099/0022-1317-71-9-1985. [DOI] [PubMed] [Google Scholar]