Phylogenetic Affiliation of Soil Bacteria That Degrade Aliphatic Polyesters Available Commercially as Biodegradable Plastics (original) (raw)
Abstract
Thirty-nine morphologically different soil bacteria capable of degrading poly(β-hydroxyalkanoate), poly(ɛ-caprolactone), poly(hexamethylene carbonate), or poly(tetramethylene succinate) were isolated. Their phylogenetic positions were determined by 16S ribosomal DNA sequencing, and all of them fell into the classes Firmicutes and Proteobacteria. Determinations of substrate utilization revealed characteristic patterns of substrate specificities.
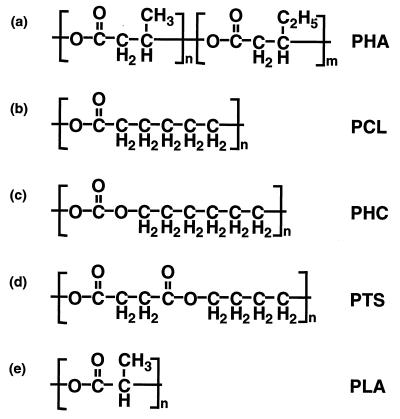
Aliphatic polyesters are currently considered the most promising materials for the production of biodegradable plastics. Poly(β-hydroxyalkanoate) (PHA) (Fig. 1a) is an aliphatic polyester known as one of the carbon storage materials in bacteria, and its industrial product is commercially distributed as BIOPOL (Monsanto, Billingham, United Kingdom). Poly(ɛ-caprolactone) (PCL) (Fig. 1b), poly(hexamethylene carbonate) (PHC) (Fig. 1c), poly(tetramethylene succinate) (PTS) (Fig. 1d), and poly(lactic acid) (PLA) (Fig. 1e) are chemically synthesized polymers that are distributed as TONE (Union Carbide), PCD (Toagosei Co., Tokyo, Japan), BIONOLLE (Showa High-Polymer, Tokyo, Japan), and LACTY (Shimadzu, Kyoto, Japan), respectively. These polymers are ready to replace some nondegradable plastics if the problem of their manufacturing costs can be solved.
FIG. 1.
Chemical structures of biodegradable polymers used in this study.
PHA- and PCL-degrading bacteria are known to be distributed widely in soils and water (7, 9, 10). In addition, the distribution of PTS- and PLA-degrading bacteria in the environment has been reported by Pranamuda et al. (11, 12). The presence of aliphatic polycarbonate-degrading bacteria has also been reported (10, 14). The percentages of degrading bacteria for PHA, PCL, PTS, and PLA in the soil environment have been estimated to be 0.5 to 9.6, 0.6 to 11.0, 0.2 to 6.0, and 0 to 0.04% of the total colonies, respectively (9–12). Although the numbers of PHC-degrading bacteria in the environment have not yet been estimated, poly(ethylene carbonate), another aliphatic polycarbonate, has been found to be degraded by bacteria which account for 0.2 to 5.7% of the total colonies (10). These studies have been made sporadically for each type of polymer or for each specific degrading strain. The enumeration and diversity of environmental bacteria capable of degrading these materials, therefore, have been studied nonsystematically.
The degradability of biodegradable plastics depends on the degrading organisms existing in the environment. However, little is known about what organisms are responsible for the degradation of biodegradable plastics in situ. Recently, chemotaxonomic approaches to the biotyping of PHA-degrading microbes have been attempted based on fatty acid composition analysis (6–8). Biotypes capable of degrading PHA, PTS, and PCL have also been reported (7), although these experiments were limited to streptomycete strains. These studies showed some characteristic patterns of biotypes and substrate specificities, but the correlation between the phylogenetic positions of bacteria and their degrading activities remained unclear. To obtain further insight into the environmental bacteria capable of degrading biodegradable plastics, we attempted to collect soil bacteria that degrade PHA, PCL, PHC, PTS, and PLA and to analyze their phylogenetic affiliations.
Isolation media for each substrate polymer were prepared by the method reported previously (9) with the following modification. The media contained (per liter) 1 g of emulsified polymer of PHA (BIOPOL-D400P-PB36; Monsanto), PCL (PLACCEL-230; Daicel Chemical Industries, Osaka, Japan), PHC (PCD-2000; Toagosei Co.), PTS (BIONOLLE-1020; Showa High-Polymer), or PLA (LACTY-9000-CSL-70718; Shimadzu); 0.8 g of nutrient broth (Difco); and 15 g of Bacto Agar (Difco).
Three different soil samples—ando soils (37.4% H2O, pH 4.5; from a woody area at Shima, Tsukuba, Japan), brown lowland soils (18.1% H2O, pH 6.0; from a sandy riverside at Hama, Mitsukaido, Japan), and muck soils (34.1% H2O, pH 5.8; from a muddy riverside at Shimokawarazaki, Tsukuba, Japan)—were collected. The samples were suspended and diluted with sterile water and inoculated onto the agar plates. Degradation activity for each polymer was determined by the formation of a clear zone around the colonies at 25°C.
Approximately 200 to 300 colonies appeared in 100 μl of soil suspension (10−4 g/ml) on each plate containing each polymer. More than 10 plates for each combination of the soil samples and the polymer substrates were observed, and the percentages of PHA-, PCL-, PHC-, and PTS-degrading colonies that appeared from the three soil samples were 2 to 18, 2 to 11, 1 to 7, and 0 to 1%, respectively, of the total colonies. Colonies degrading PLA were not detected. Thirty-nine strains with different colony morphotypes (Table 1) were chosen for further analysis.
TABLE 1.
Phylogenetic affiliations, colony morphologies, and polymer-degrading activities of the isolates
| Straina | Closest relative (% sequence similarity)b | Characteristic(s) of colony | Magnitude of degradationc | ||||
|---|---|---|---|---|---|---|---|
| PHA | PCL | PHC | PTS | PLA | |||
| High GC group of the Firmicutes | |||||||
| MB-14 | Terrabacter tumescens (97.1) | Yellowish white, soft, eroded agar surface | 3 | — | — | — | — |
| MB-15 | Terracoccus luteus (98.9) | Opaque, soft, eroded agar surface | 3 | — | — | — | — |
| Low GC group of the Firmicutes | |||||||
| YB-13 | Brevibacillus reuszeri (99.0) | White, small | 2 | — | — | — | — |
| YB-14 | Brevibacillus reuszeri (98.6) | White, faintly pink, small | 2 | nt | nt | nt | nt |
| Alpha subclass of the Proteobacteria | |||||||
| MB-11 | Agrobacterium tumefaciens (98.8) | White, hard | 2 | nt | nt | nt | nt |
| Beta subclass of the Proteobacteria | |||||||
| Burkholderia group | |||||||
| KS-1 | Burkholderia vietnamiensis (99.6) | Cream colored, motile, with swollen center | nt | nt | nt | 1 | nt |
| YS-1 | Burkholderia vietnamiensis (99.4) | Opaque, small | nt | nt | nt | 1 | nt |
| MB-9 | Duganella zoogloeoides (96.5) | Opaque, large | 3 | nt | nt | nt | nt |
| MB-13 | Duganella zoogloeoides (99.3) | Opaque, small, soft, viscous | 3 | nt | nt | nt | nt |
| MB-17 | Duganella zoogloeoides (92.7) | Clear, small | 3 | nt | nt | nt | nt |
| MC-9 | Duganella zoogloeoides (91.9) | Opaque, small | 3 | 3 | 2 | 2 | — |
| MB-12 | Pseudomonas lemoignei (94.0) | White, smooth | 3 | nt | nt | nt | nt |
| MC-2 | Pseudomonas lemoignei (92.5) | White, hard, rough, swollen | nt | nt | 3 | nt | nt |
| MC-10 | Pseudomonas lemoignei (93.9) | White, smooth | nt | nt | 2 | nt | nt |
| ML-6 | Pseudomonas lemoignei (92.1) | Yellowish clear, with swollen center | nt | 2 | nt | nt | nt |
| MB-6 | Ralstonia eutropha (98.3) | Yellowish brown | 2 | — | — | — | — |
| MC-5 | Ralstonia pickettii (96.5) | Yellow, motile, with swollen center | — | 2 | 2 | — | — |
| ML-7 | Ralstonia pickettii (99.2) | Cream colored, motile, with swollen center | — | 2 | 1 | — | — |
| ML-10 | Ralstonia pickettii (99.1) | Dark yellow, motile, with swollen center | — | 2 | 2 | — | — |
| YL-13 | Ralstonia pickettii (97.9) | Clear, motile, flat | 1 | 2 | 2 | — | — |
| Comamonas group | |||||||
| MB-7 | Matsuebacter chitosanotabidus (96.9) | Opaque yellow | 3 | — | — | — | — |
| YB-11 | Matsuebacter chitosanotabidus (97.4) | Opaque, large, soft, viscous | 3 | 1 | — | — | — |
| MC-11 | Roseateles depolymerans (99.5) | Clear, faintly pink, motile, with swollen center | — | 3 | 2 | 3 | — |
| MC-12 | Roseateles depolymerans (99.5) | Clear, faintly pink, large, smooth, viscous | — | 3 | 2 | 3 | — |
| 61A | Roseateles depolymeransd | Pink, small, smooth, soft | — | 3 | 2 | 3 | — |
| 61B2 | Roseateles depolymerans | Pink, smooth, hard | — | 3 | 2 | 3 | — |
| MB-18 | Rhodoferax fermentans (92.0) | White, small, hard, eroded agar surface | 2 | — | — | — | — |
| MB-1 | Type 0803 filamentous bacterium Ben05B (97.8) | Clear, small, rough, with swollen center | 3 | 2 | 3 | — | — |
| MB-2 | Type 0803 filamentous bacterium Ben05B (95.9) | Opaque, small | 3 | 1 | 2 | — | — |
| MB-4 | Type 0803 filamentous bacterium Ben05B (96.4) | Yellowish clear | 3 | 1 | 2 | — | — |
| MC-3 | Type 0803 filamentous bacterium Ben05B (96.0) | Yellow, small | 3 | 2 | 3 | — | — |
| MB-16 | Variovorax paradoxus (97.5) | Yellow, motile, with swollen center | 3 | — | — | — | — |
| WFF52 | Variovorax paradoxus (99.0) | Yellow, motile, with swollen center | 3 | 1 | 3 | — | — |
| Gamma subclass of the Proteobacteria | |||||||
| Enterobacter group | |||||||
| KL-13 | Serratia marcescens (100) | Produced red diffusible pigment | — | 1 | — | — | — |
| Pseudomonas group | |||||||
| ML-11 | Acinetobacter calcoaceticus (96.2) | Yellowish clear, smooth, large | 1 | 3 | 2 | — | — |
| ML-13 | Acinetobacter calcoaceticus (97.4) | Yellowish white, smooth, large | — | 2 | 2 | — | — |
| ML-21 | Acinetobacter calcoaceticus (98.1) | White, small | — | 3 | 2 | — | — |
| ML-12 | Acinetobacter junii (100) | White, small, with swollen center | — | 2 | 2 | 1 | — |
| KL-14 | Pseudomonas pavonaceae (99.3) | Clear, small | — | 2 | 1 | — | — |
| KL-15 | Pseudomonas rhodesiae (99.8) | White, large viscous | nt | 2 | nt | nt | nt |
| ML-1 | Pseudomonas amygdali (99.6) | White, smooth, large | — | 1 | — | — | — |
| 35L | Pseudomonas veronii (98.9) | White, smooth | — | 3 | 2 | — | — |
| Xanthomonas group | |||||||
| YB-12 | Iron-oxidizing lithotroph ES-1 (99.4) | White | 3 | — | — | — | — |
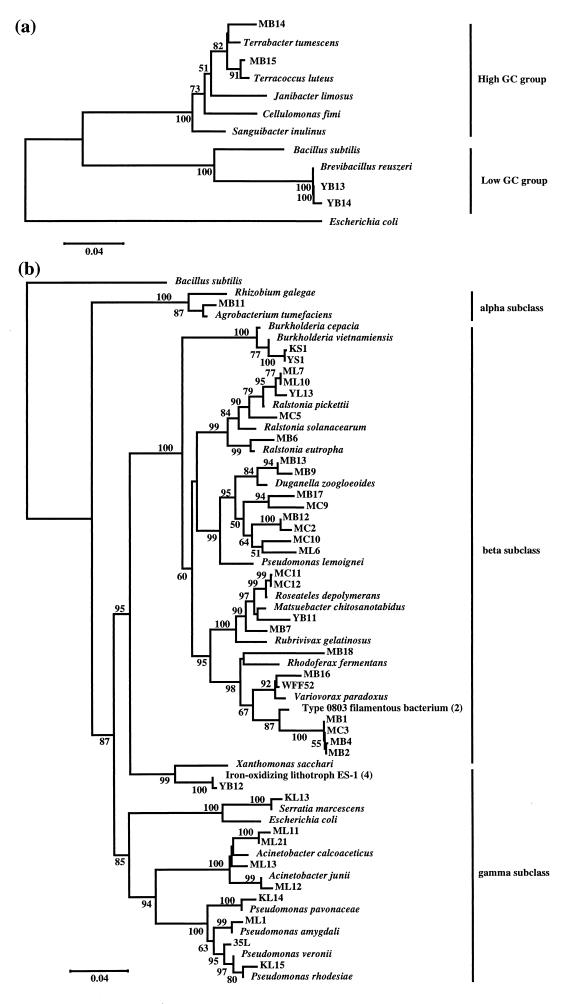
For phylogenetic analysis, the single colony was washed and frozen-thawed twice by liquid nitrogen in 100 μl of Tris-EDTA buffer (pH 8.0). Proteinase K solution (10 μg in 10 μl) (Sigma) and 50 μl of buffer containing 1% (wt/wt) Tween 20, 0.2% (wt/wt) Nonidet P-40, and 0.2 mM EDTA (pH 8.0) were mixed with the samples. The mixture was incubated at 60°C for 20 min to allow the proteinase K to digest proteins and then at 95°C for 10 min to inactivate the enzyme. The supernatant was collected for gene amplification. Genes of 16S rRNA were amplified as described previously (14), and approximately 500 bp were sequenced with an ABI 377 DNA sequencing system (Applied Biosystems) by using the primer complementary to Escherichia coli positions 536 to 518 (3, 17). The sequences were compared with sequences in GenBank-EMBL DNA database by using the BLAST algorithm (1). Phylogenetic analysis was performed with the CLUSTAL W1.6 (16) and MEGA (5) programs. Four PHC-degrading strains, 35L, WFF52, 61A, and 61B2, which we had previously isolated from aquatic environments (14), were included in the analysis. The phylogenetic positions of the strains are shown in Fig. 2. All of the strains were phylogenetically classified into the alpha, beta, and gamma subclasses of the Proteobacteria, and the high and low GC groups of the Firmicutes (gram-positive bacteria). PHA-degrading isolates were distributed in all of the taxa described above. All of the PCL-, PTS-, and PHC-degrading isolates were classified into the beta and gamma subclasses of the Proteobacteria. Most of the strains that were classified into the gamma subclass of the Proteobacteria were isolates degrading PCL (Fig. 2). The sequence similarities to the known closest relatives of the strains are listed in Table 1.
FIG. 2.
Phylogenetic positions of new isolates of the Firmicutes (a) and the Proteobacteria (b) among neighboring species. The phylogenetic tree was constructed by the method of neighbor joining (13), based on pairwise comparisons of 16S ribosomal DNA sequences. The percentages (50% or higher) of 1,000 bootstrap trials that support each topological element are indicated beside the nodes.
The degrading abilities of each strain for the five polymers are also listed in Table 1. Of the five polymers tested, PHA, PCL, and PHC were much more easily degraded than the others. Of eight PTS-degrading strains, only four strains, phylogenetically related to the genus Roseateles, showed very high PTS-degrading activity. None of the isolates degraded PLA. Pranamuda et al. reported that PLA-degrading bacteria were rarely isolated, and they obtained only one PLA-degrading strain from only 1 of 45 soil samples they collected (12), suggesting that PLA is not an easy substrate, at least for soil bacteria. With regard to PCL, PHC, and PTS degradation, there were distinctive patterns of substrate specificity: PCL-degrading bacteria include bacteria which can degrade PHC. PHC-degrading bacteria include bacteria which can degrade PTS. The utilization patterns of PCL, PHC, and PTS by the strains indicate that there are three groups of bacteria capable of degrading these substrates: group 1 is constituted of bacteria degrading PCL, PHC, and PTS; group 2 is constituted of bacteria degrading PCL and PHC; and group 3 is constituted of bacteria degrading PCL. PHA was also a major substrate for our isolates, but PHA-degrading ability and degrading activity for the other three polymers seem to be unrelated.
Our research indicates that bacteria capable of degrading biodegradable polymers are phylogenetically diverse. Very interestingly, there were characteristic patterns in terms of the substrate range. Although we must be careful to avoid overinterpretation because of the limitations of sites and sample size, these findings are very important for understanding the organisms responsible for the degradation of biodegradable plastics in situ.
Nucleotide sequence accession numbers.
The nucleotide sequences determined in the present study have been deposited in the DDBJ/EMBL/GenBank database under accession no. AB013401 through AB013439.
Acknowledgments
We thank Tadao Hamazaki (National Institute of Agro-Environmental Sciences), Satoshi Hanada (National Institute of Bioscience and Human Technology), and Haruo Nishida (Tokuyama Corp.) for advice and precious information, and we thank Aiko Sukegawa and Ying Wu (National Institute of Bioscience and Human Technology) for assistance.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Bradford D, Hugenholtz P, Seviour E M, Cunningham M A, Stratton H, Seviour R J, Blackall L L. 16S rRNA analysis of isolates obtained from Gram-negative filamentous bacteria micromanipulated from activated sludge. Syst Appl Microbiol. 1996;19:334–343. [Google Scholar]
- 3.Brosius J, Palmer J L, Kennedy J P, Noller H F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci USA. 1978;75:4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emerson D, Moyer C. Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl Environ Microbiol. 1997;63:4784–4792. doi: 10.1128/aem.63.12.4784-4792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S, Tamura K, Nei M. MEGA: molecular evolutionary genetics analysis, version 1.0. University Park: Pennsylvania State University; 1993. [Google Scholar]
- 6.Mergaert J, Schirmer A, Hauben L, Mau M, Hoste B, Kersters K, Jendrossek D, Swings J. Isolation and identification of poly(3-hydroxyvalerate)-degrading strains of Pseudomonas lemoignei. Int J Syst Bacteriol. 1996;46:769–773. doi: 10.1099/00207713-46-3-769. [DOI] [PubMed] [Google Scholar]
- 7.Mergaert J, Swings J. Biodiversity of microorganisms that degrade bacterial and synthetic polyesters. J Ind Microbiol. 1996;17:463–469. [Google Scholar]
- 8.Mergaert J, Wouters A, Swings J. Estimation of the intrinsic biodiversity among poly(3-hydroxyalkanoates) degrading streptomycetes using gas chromatographic analysis of fatty acids. Syst Appl Microbiol. 1994;17:601–612. [Google Scholar]
- 9.Nishida H, Tokiwa Y. Distribution of poly(β-hydroxybutyrate) and poly(ɛ-caprolactone) aerobic degrading microorganisms in different environments. J Environ Polym Degrad. 1993;1:227–233. [Google Scholar]
- 10.Nishida H, Tokiwa Y. Confirmation of poly(1,3-dioxolan-2-one) degrading microorganisms in environment. Chem Lett. 1994;1994:421–422. [Google Scholar]
- 11.Pranamuda H, Tokiwa Y, Tanaka H. Microbial degradation of an aliphatic polyester with a high melting point, poly(tetramethylene succinate) Appl Environ Microbiol. 1995;61:1828–1832. doi: 10.1128/aem.61.5.1828-1832.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pranamuda H, Tokiwa Y, Tanaka H. Polylactide degradation by an Amycolatopsis sp. Appl Environ Microbiol. 1997;63:1637–1640. doi: 10.1128/aem.63.4.1637-1640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 14.Suyama T, Hosoya H, Tokiwa Y. Bacterial isolates degrading aliphatic polycarbonates. FEMS Microbiol Lett. 1998;161:255–261. doi: 10.1111/j.1574-6968.1998.tb12956.x. [DOI] [PubMed] [Google Scholar]
- 15.Suyama, T., T. Shigematsu, S. Takaichi, Y. Nodasaka, S. Fujikawa, H. Hosoya, Y. Tokiwa, T. Kanagawa, and S. Hanada. Roseateles depolymerans gen. nov., sp. nov., a new bacteriochlorophyll _a_-containing obligate aerobe belonging to the β subclass of the Proteobacteria. Int. J. Syst. Bacteriol., in press. [DOI] [PubMed]
- 16.Thompson J D, Higgins D G, Gibson T J. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisburg W G, Burns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]