Transformation of Acinetobacter sp. Strain BD413(pFG4ΔnptII) with Transgenic Plant DNA in Soil Microcosms and Effects of Kanamycin on Selection of Transformants (original) (raw)
Abstract
Here we show that horizontal transfer of DNA, extracted from transgenic sugar beets, to bacteria, based on homologous recombination, can occur in soil. Restoration of a 317-bp-deleted nptII gene in Acinetobacter sp. strain BD413(pFG4) cells incubated in sterile soil microcosms was detected after addition of nutrients and transgenic plant DNA encoding a functional nptII gene conferring bacterial kanamycin resistance. Selective effects of the addition of kanamycin on the population dynamics of Acinetobacter sp. cells in soil were found, and high concentrations of kanamycin reduced the CFU of Acinetobacter sp. cells from 109 CFU/g of soil to below detection. In contrast to a chromosomal _nptII_-encoded kanamycin resistance, the pFG4-generated resistance was found to be unstable over a 31-day incubation period in vitro.
Bacterial antibiotic resistance markers are the most frequently inserted genes in transgenic plants. However, the resistance genes do not encode desirable traits in commercially used plant varieties. Of the 15 different resistance genes incorporated into plants (17, 30), several encode resistance to clinically used antibiotics. Since plant DNA has been shown to persist in soil over extended periods of time (8, 25, 38, 39), concerns that these transgenes may spread horizontally to bacteria have been raised (17, 18, 22, 29). Sequence comparisons of genes isolated from wild plants and bacteria have indicated that horizontal gene transfer has occurred naturally between them (13, 32). Moreover, whole-genome analyses of bacteria suggest horizontal transfer of genetic material to be common and a major force in bacterial evolution (14, 40).
One mechanism of gene transfer that allows uptake of genetic material from diverged species in bacteria is natural transformation, which facilitates uptake of naked DNA in competent bacteria (15). Based on this mechanism, several laboratory studies have been conducted to elucidate the potential for plant-harbored resistance determinants to be taken up by naturally occurring bacterial recipients (2, 3, 21, 28). These studies have, however, not been able to demonstrate uptake of such determinants, nor have studies of bacteria obtained from soil samples from field trials with transgenic plants (8, 25). Detection of horizontal transfer in these studies relied upon the uptake of expressed and selectable genes in the bacterial recipients grown under optimized conditions or a positive DNA hybridization signal or PCR amplification of plant transgenes in the bacterial fraction of soil. However, direct analyses of DNA from soil samples often fail to demonstrate integration of plant transgenes into bacterial genomes. Transfer of smaller DNA fragments or nonexpressed or nonselected genes would rarely be detected in these studies.
Recently, uptake of transgenic plant-harbored DNA fragments by bacteria based on restoration of a partially deleted (10- or 317-bp internal deletion) bacterial kanamycin (KM) resistance gene (nptII) after recombination with transgenic plant-inserted homologues was demonstrated (5, 7). By exposing the naturally transformable bacterium Acinetobacter sp. strain BD413 to DNA isolated from transgenic sugar beet plants, these groups showed that the bacterium can access plant DNA under optimized in vitro conditions if homologous stretches of DNA are present. Studies done under optimized in vitro conditions often have little relevance to natural systems such as soil. For instance, agricultural soils are continually exposed to DNA from decaying plant material. In this study, we demonstrate that horizontal transfer of DNA isolated from transgenic sugar beet (Beta vulgaris) plants to bacteria, based on homologous recombination, can occur in sterile soil microcosms. Since the numbers of transformants generated in soil is expected to be very low, a selective advantage for their enrichment and, hence, environmental significance is needed. In this study we have therefore also determined the effects of increasing kanamycin concentrations on the population dynamics of transformant and recipient Acinetobacter sp. strain BD413 cells in soil and liquid soil suspension. In addition, the in vitro stability of the kanamycin-resistant bacterial phenotypes was determined over a 31-day period.
Recipient preparation and isolation of donor DNA.
The gram-negative soil bacterium Acinetobacter sp. strain BD413(pFG4) with a 317-bp deletion in the plasmid-harbored nptII gene was used as the recipient in all transformations (7, 11). The recipient inoculum was prepared as described by Nielsen et al. (19, 23). Portions of 100 μl (108 CFU per ml of water) were used for each filter and soil microcosm. For enumeration of CFU, aliquots were spread on solidified LB medium (19) supplemented with the antibiotics (all at 50 μg ml−1) rifampin (chromosomally encoded resistance), ampicillin (pFG4 encoded resistance), and KM (pFG4-encoded resistance) for selection of transformants and incubated at 30°C for 3 days. Each experiment was repeated and done in triplicate or more on three to eight agar plates. Transgenic sugar beets containing a functional nptII gene (16) were used for purification of donor DNA. The transgenic sugar beet insert contained, from the left border of the T-DNA, the bar gene, the bidirectional promoter Tr1/Tr2, the nptII gene, the 3′ ocs terminator, the cAMV35S promoter, and the cpBNYVV gene (see references 7 and 21 for a further description of the transgenic plant material). DNA from conventionally grown sugar beets from adjacent field sites was used as a control. The plant DNA, isolated according to Trinker et al. (34), was reextracted with phenol-chloroform and chloroform and precipitated with isopropanol and ethanol before quantification on a UV spectrophotometer and DNA fluorimeter.
Transformations on filters and in soil microcosms.
Filter and soil transformations were done as described in Nielsen et al. (19, 20). For filter transformation with purified transgenic plant DNA, 100 μl of a DNA solution (at concentrations of 10, 40, 80, and 160 μg DNA per 160-μl solution) was mixed with the bacterial inoculum. As controls, we used DNA from nontransgenic plants mixed with the inoculum and transgenic plant DNA only to check for sterility. A Flevo silt loam soil, sampled from microplots in Wageningen, The Netherlands, sterilized by gamma irradiation (4 megarads) with a 60Co source (Gammaster BV, Ede, The Netherlands) was used for all microcosm studies (35). The sterile soil microcosms consisted of polypropylene cylinders of 1-cm3 volume and 7 mm tall filled with 1.2 g (dry weight) of Flevo silt loam soil (see references 19 and 20 for a detailed description of the soil microcosms). After addition of the inoculum by careful distribution of the solution on top of the columns, the microcosms with the adsorbed bacterial suspension were incubated for 24 h before water or nutrients (100 μl) were administered similarly; purified DNA was added after a further 1-h incubation. In addition to water, two different nutrient solutions were used. Nutrient solution A has previously been described (20) and contained 2% lactic acid in addition to 5× M9 minimal medium salts with a 25× concentration of P salts. Solution B has been modified from A to include half-strength salt solution, different organic acids (0.2% each of acetate, lactate, citrate, tartrate, and succinate), and in addition the amino acids glutamic acid, proline, alanine, glycine, leucine, serine, arginine, glutamine, and valine at an amount corresponding to 25 times the concentration found per gram of maize rhizosphere soil (see reference 12). Following a 24-h transformation period at 20 or 30°C, the microcosms were sampled as described previously (20), and CFU were enumerated after a 72-h incubation period at 30°C. As controls, those described for the filter transformations were used and, in addition, transgenic sugar beet DNA was added to soil microcosms containing bacterial inoculum, nutrient-stimulated for 24 h at the time of plating, to check for the occurrence of plate transformants; no Kmr colonies were found in these experiments. Since sampling of bacterial cells was normally done at least 24 h after addition of DNA to the soil microcosms, our experimental procedures did not encourage transformants to occur during the plating procedure. Previous studies have shown that chromosomal DNA incubated in sterile soil for over 6 h was not available as a source of transforming DNA to competent Acinetobacter sp. strain BD413 cells (19, 23). PCR amplification spanning the partially deleted or repaired nptII gene on plasmid pFG4 was used to confirm the restoration of the nptII gene in restreaked transformants of Acinetobacter sp. cells. The sequences were amplified by the method described by Hofmann and Brian, (10) with primer set P1 and P2: P1 (1236), 5′ TGC TAA AGG AAG CGG AAC 3′; P2 (2929), 5′ AGG TCA ACA GGC GGT AAC 3′ (7). The primers were designed to amplify the Tn_5_ region 1236 to 2929, which includes the nptII promoter, the complete nptII gene, and the bleo gene present on pFG4 in the Acinetobacter sp. cells. As described above, the transgenic sugar beet insert contained the nptII gene expressed with a different promoter and terminator region. PCR signals were not obtained from the transgenic plant material with these primers (data not shown). PCR conditions were applied as described previously (7, 23).
Selective effects of KM in soil and soil suspensions.
For studies on the selective effects of KM in sterile soil, microcosms with inoculated Kms (recipients) or Kmr (transformants) Acinetobacter cells (inoculated as described for the soil transformation studies) were treated with 100 μl of KM at concentrations of 0, 5, 12, 20, 50, and 100 mg ml of water−1. After a further 24 h of incubation at room temperature, the microcosms were sampled as described for the soil transformation studies. To monitor the selective effects of KM in liquid soil suspensions, inoculated microcosms (see above) were suspended in 2 ml of LB medium with 1 g of gravel added (“aquarium sand,” 2 to 4 mm in diameter, to aid in the suspension of soil particles) and the following antibiotics: rifampin, 100 μg ml−1; ampicillin, 100 μg ml−1; and 0, 10, 15, 20, 25, 50, and 100 mg of KM dissolved in 1 ml of water. The soil suspension was incubated at 27°C with shaking (180 rpm) for 24 h before sampling and enumeration of CFU. Numbers of replicates and repeats were as described for the soil transformations.
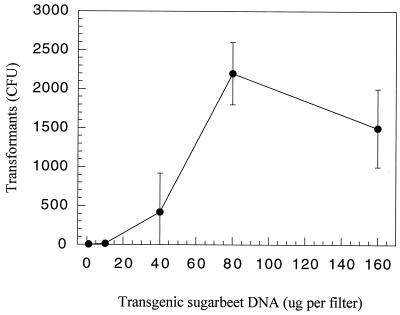
To determine the saturation level of transgenic plant DNA for transformation of Acinetobacter sp. strain BD413(pFG4) cells, increasing amounts of DNA were mixed with the recipient bacteria and incubated for 24 h on nitrocellulose filters (Millipore, Bedford, Mass.). As shown in Fig. 1, saturation for transformation was reached at a concentration of approximately 100 μg of DNA per 4 × 109 recipient CFU per filter. This DNA concentration, corresponding to the amount found in approximately 6.4 g of fresh plant leaves (producing 1 transformant per 2.9 mg of plant material or 11,600 copies of the nptII gene) (1, 5), was used for all the transformation studies in soil microcosms. de Vries and Wackernagel (5) obtained roughly the same numbers of transformants per microgram of plant DNA added when transforming Acinetobacter sp. strain BD413 cells in liquid cultures. However, they reported saturation of transformation to occur at a DNA concentration of 5 μg per 20 ml of liquid bacterial suspension. Since the number of recipient CFU was similar in both studies (4 × 109 to 5 × 109), our 20-fold-higher number of transformants was also reflected in the correspondingly higher transformation frequency of 5.7 × 10−7 on solidified medium compared to that of 2.0 × 10−8 obtained in liquid culture (5).
FIG. 1.
Natural transformation and restoration of a 317-bp internal deletion in the nptII gene in Acinetobacter sp. strain BD413(pFG4) cells incubated on nitrocellulose filters with increasing concentrations of transgenic sugar beet DNA. T bars, standard deviations.
As shown in Table 1, noncompetent (19, 20) Acinetobacter sp. strain BD413 cells residing in sterile soil microcosms for 24 h could, after addition of nutrients, be induced to integrate a bacterial marker gene inserted in transgenic sugar beets by homologous recombination. Both of the nutrient solutions used to stimulate competence development in Acinetobacter sp. strain BD413 populations contained inorganic salts and, in addition, simple organic compounds corresponding to those which have been frequently detected in the rhizosphere of various plants (4, 6, 12, 27). As seen from Table 1, solution B was efficient at inducing Acinetobacter sp. cells to higher transformation frequencies in soil microcosms. At 20°C, water or nutrient solution A was not able to promote transformation of the recipient with the transgenic sugar beet DNA. However, when solution A was provided to bacteria incubated in sterile soil for only 1 h, transformants were seen at a frequency of 2.2 × 10−8. After 24 h of incubation in soil microcosms at a temperature of 30°C, both nutrient solutions A and B were able to generate transformants of Acinetobacter sp. cells (Table 1), producing up to 1.4 × 10−8 transformants per recipient, which corresponds to 1.2 × 10−7 transformants per plant-harbored copy of the nptII gene. Water was not able to induce any transformants in our studies, nor was nontransgenic plant DNA, soil microcosms with only nutrients and inoculum added, or soil microcosms with nutrient-stimulated bacteria and transgenic sugar beet DNA added at the time of sampling. The latter control indicated that transformation did not occur on the selective plates. The restoration of the nptII gene in some of these randomly picked colonies was revealed by PCR amplification (Fig. 2). Inoculation of GN-Biolog plates, quantified on a Biolog MicroLog station (Biolog Microlog3; Biolog, Inc, Hayward, Calif.), with transformants also revealed that their metabolic pattern was indistinguishable from the one obtained from the inoculant strain.
TABLE 1.
Natural transformation and restoration of a 317-bp-deleted nptII gene in Acinetobacter sp. strain BD413(pFG4) cells residing in a sterile silt loam soil microcosm for 24 h, with added purified DNA from transgenic sugar beet (B. vulgaris) with a functional nptII gene
| Additionb | 20°C soil temperaturea | 30°C soil temperaturea | ||||||
|---|---|---|---|---|---|---|---|---|
| Transformants (CFU) | Recipients (CFU) | Transformation frequencyc | Transformants/nptII copy | Transformants (CFU) | Recipients (CFU) | Transformation frequencyc | Transformants/nptII copy | |
| Water | ndd | 9.5 (1.2)e × 107 | <1.1 × 10−8 | nd | 1.2 (0.1) × 108 | <8.3 × 10−9 | ||
| Nutrients A | nd | 1.8 (0.1) × 108 | <5.6 × 10−9 | 2.4 (3.8) | 2.1 (0.2) × 108 | 1.2 × 10−8 | 7.6 (11.9) × 10−8 | |
| Nutrients B | 3.1 (3.4) | 2.1 (0.1) × 108 | 1.4 × 10−8 | 9.8 (10.7) × 10−8 | 3.7 (3.8) | 2.7 (0.2) × 108 | 1.4 × 10−8 | 1.2 (11.9) × 10−7 |
FIG. 2.
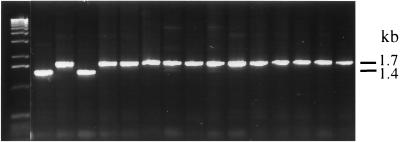
PCR amplification of the Tn_5_ region 1236 to 2929, which includes the nptII promoter, the complete nptII gene, and the bleo gene of pFG4, demonstrating the presence of the restored nptII gene construct in the transformant colonies and the 317-bp partially deleted gene in the recipient bacterium. Lane 1 from the left side, 1-kb ladder (Gibco-BRL); lane 2, Acinetobacter sp. strain BD413(pFG4) Kms; lane 3, Acinetobacter sp. strain BD413(pFG4) Kmr; lane 4, Acinetobacter sp. strain BD413(pFG4) Kms recipient; lane 5 to 16, Acinetobacter sp. strain BD413(pFG4) Kmr transformants obtained with transgenic _nptII_-containing sugar beet DNA.
Transformation of Acinetobacter sp. strain BD413(pFG4) cells with transgenic sugar beet DNA was not detected in nonsterile soil microcosms (data not shown). In previous gene transfer studies with homologous bacterial DNA in sterile and nonsterile soil microcosms, a 10- to above 100-fold drop in the transformation frequencies has been observed with the change from sterile to nonsterile soil conditions (19). Thus, we infer that the transformation frequencies of Acinetobacter sp. cells in nonsterile soil microcosms, given similar purified transgenic plant DNA and nutrient conditions, would be at a level of 10−10 to 10−11 transformants per recipient. Transformations occurring with this efficiency were below our limit of detection (Table 1), and the identification of such events in nonsterile soil, using the same experimental setup as for the sterile soil, would require screening of approximately 20,000 agar plates. We emphasize that the estimates proposed here are based upon integration of the transgenic plant-harbored marker genes into the bacterial chromosome after homologous recombination, using purified transgenic plant DNA. Experimental studies by our groups (21) and others (2, 3, 28) have confirmed the low probability of integration of transgenes in recipient bacteria if DNA homology is not present. Bacterial genes and vector sequences are however present in most transgenic plants (see Table 3 in reference 22). Strictly homology-based recombination of plant transgenes in competent bacteria is unlikely to cause any environmental effect due to the already existing homologues in the bacterial chromosomes; however, studies of gene transfer by natural transformation in competent bacteria have revealed that additive integration of nonhomologous genetic material can occur at high frequencies when flanking homology is present. For instance, in Acinetobacter sp. cells, uptake and integration, by natural transformation, of three nonhomologous foreign genes (nptII, cryIVb, and aadB) occurs, when flanking homologous bacterial DNA is present, at frequencies (transformants per recipient) up to 1% in vitro and 10−5 in nonsterile soil (19, 20). Thus, the presence in transgenic plants of various prokaryotic markers and vector sequences may facilitate additive insertion of foreign genetic material into bacterial hosts after homology-based heteroduplex formation.
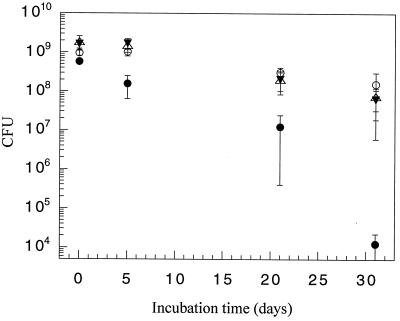
Due to the expected low level of formation of transformants in soil, random genetic drift and selection (see below) may contribute to their possible amplification and hence environmental significance. Stability of the acquired resistance trait would be a prerequisite for random genetic drift to fixation. Stability in vitro of the Kmr phenotype in Acinetobacter sp. strain BD413(pFG4) bearing restored KM resistance was compared to the stability of the Kmr phenotype of Acinetobacter sp. strain BD413 with a chromosomally inserted nptII gene (19). Both resistant strains were inoculated (six replicates) in separate aliquots of 5 ml of nonselective LB medium and incubated at 27°C with agitation (180 rpm). The aliquots were sampled after 0, 5, 21, and 31 days on LB plates with or without KM (50 mg/liter), and CFU were enumerated after 72 h. As shown in Fig. 3, the Kmr phenotype in the strain harboring a chromosomally inserted nptII gene was stable, without selection, once the resistance trait had been acquired. The Kmr phenotype in the strain harboring the restored pFG4 plasmid was in contrast unstable and resistance was partially lost during the long-term incubation. The loss of KM resistance could be shown to be accompanied by a loss of the plasmid-encoded ampicillin resistance, suggesting that the altered Kms phenotype seen was caused by plasmid instability.
FIG. 3.
Stability of KM-resistant phenotypes of Acinetobacter sp. strain BD413(pFG4::nptII) (circles) and Acinetobacter sp. strain BD413 chr::nptII (triangles) in liquid LB medium measured after plating on KM-free (open symbols) and KM-containing (filled symbols) LB medium. T bars, standard deviations. CFU are per 5 ml.
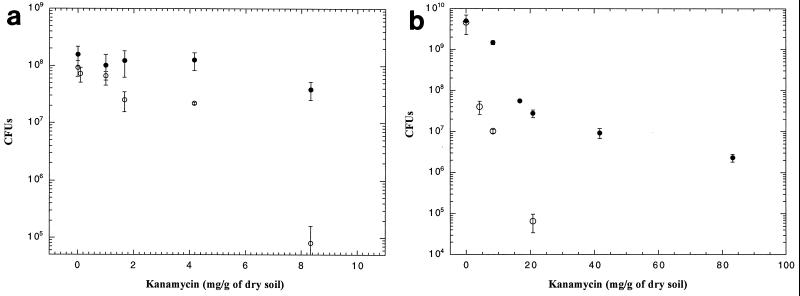
KM-resistant bacteria, but not nptII_-encoded phenotypes (31), are abundant in natural soils (found at levels of 105 CFU/g of soil), suggesting a possible selection of this phenotype in the soil environment. Despite this observation, the natural occurrence of antibiotics, like KM, in soil has been difficult to quantify, and the selective effects and role of antibiotics in soil remain unclear (24, 33, 36, 37). To our knowledge, no studies have detected KM in natural soil, and the antibiotic has been suggested to be inactivated in soil due to binding to clay minerals (9). Putative selective effects of KM on Kms (recipients) or Kmr (transformants) Acinetobacter sp. cells in sterile soil microcosms were investigated in this study by administering increasing amounts of the antibiotic to the inoculated microcosms. As seen from Fig. 4a, the addition of up to 8.3 mg of KM (per g [dry weight] of soil) did not substantially influence the survival of Kmr Acinetobacter sp. cells in soil. In contrast, the Kms cells were susceptible to the selective effects of the KM. However, only high amounts of KM reduced the number of recipients substantially over a 24-h period. The addition of up to 4.2 mg of KM reduced the CFU numbers less than fivefold, whereas a 1,100-fold drop of the recipient CFU was seen when 8.3 mg of KM was added. The latter addition is close to the upper limit of administering liquid KM in soil and equals 100 μl of a 100-mg ml−1 KM stock solution per 1.2 g (dry weight) of soil. As seen from Fig. 4a, 8 × 104 CFU of Kms bacteria were still present in soil after this KM amendment and a 24-h selection period. To reveal if it was possible to obtain stronger selective effects of KM on soil bacteria, the microcosms were suspended in a total of 3 ml of liquid medium. This facilitated the addition of 10-fold-higher concentrations of KM to the bacterial suspension. Although negative selective effects of this high KM amendment were seen for the Kmr transformants, with a 540-fold reduction in CFU from 0 to 42 mg of KM added per g of dry soil (Fig. 4b), the same 42-mg addition of KM to the soil suspension with Kms bacteria reduced their CFU from 4.6 × 109 to below detection over a 24-h period of selection. Reactivation of the selective effects of KM, inactivated after distribution and incubation in soil microcosms, after suspension of the soil in liquid culture was not seen (data not presented). Oliveira et al. (24) investigated the effects of KM amendment in soil microcosms on detection of Tn_5 (nptII)-carrying Pseudomonas fluorescens. Addition of 0.018 or 0.18 mg of KM g of dry soil−1 had no noticeable effect on the survival of the inoculated bacteria. Thus, both studies confirm that the addition of high levels of KM does not substantially influence the population dynamics of resistance-carrying bacteria in different soil microcosms. In contrast to Oliveira et al. (24) we were able to see effects of the added antibiotic on KM-sensitive bacteria after a 24-h incubation period in sterile soil, but only after high levels of antibiotic amendment: 8 to 42 mg of KM g of soil−1, corresponding to a 1,000-fold-higher concentration than normal amendment in bacterial media.
FIG. 4.
CFU of KM-sensitive recipients (open symbols) and KM-resistant transformants (filled symbols) of Acinetobacter sp. strain BD413(pFG4) isolated from soil after addition of increasing amounts of kanamycin to soil microcosms (a) or liquid soil suspensions (b). T bars, standard deviations. CFU are per microcosm of 1.2 g (dry weight) of soil.
Based on the above studies with sterile soil microcosms regarding the effects of KM amendment on the population dynamics of Acinetobacter sp. cells, the earlier reported rapid KM inactivation by clays, and the apparent low presence of KM in soil, we suggest that natural soil conditions rarely would produce the selective pressure required for fixation of possible transfers of the nptII gene from transgenic plants into the recipient bacterium studied. However, we note that KM is able to select for different phenotypes in sterile soil microcosms. Furthermore, based on studies using purified DNA and sterile soil conditions, we indicate that homologous recombination, and possible additive integration, of bacterial marker genes harbored in transgenic plants into competent soil bacteria like Acinetobacter spp. may take place in soil and that the environmental significance of such rare events depends upon selection of the acquired character.
Acknowledgments
K.M.N. was supported by the Norwegian Research Council (MU:121733).
We thank F. Gebhard for providing the pFG4 plasmid and E. Torsetnes and L. Lankwarden for excellent technical assistance.
REFERENCES
- 1.Arumuganathan K, Earle E D. Nuclear DNA content of some important plant species. Plant Mol Biol Rep. 1991;9:208–218. [Google Scholar]
- 2.Becker J, Siegert H, Logemann J, Schell J. Biologische Sicherheit. Vol. 3. 1994. Begleitende sicherheitsforschung zur freisetzung gentechnisch veränderter petunien; pp. 563–578. Bundesministerium für Forschung und Technologie, Bonn, Germany. [Google Scholar]
- 3.Broer I, Dröge-Laser W, Gerke M. Examination of the putative horizontal gene transfer from transgenic plants to agrobacteria. In: Schmidt E R, Hankeln T, editors. Transgenic organisms and biosafety, horizontal gene transfer, stability of DNA and expression of transgenes. Heidelberg, Germany: Springer Verlag; 1996. pp. 67–70. [Google Scholar]
- 4.Cieslinski G, van Rees K C J, Szmigielska A M, Huang P M. Low molecular weight organic acids released from roots of durum wheat and flax into sterile nutrient solutions. J Plant Nutr. 1997;20:753–764. [Google Scholar]
- 5.de Vries J, Wackernagel W. Detection of nptII (kanamycin resistance) genes in genomes of transgenic plants by marker-rescue transformation. Mol Gen Genet. 1998;257:606–613. doi: 10.1007/s004380050688. [DOI] [PubMed] [Google Scholar]
- 6.Futamata H, Sakai M, Ozawa H, Urashima Y, Sueguchi T, Matsuguchi T. Chemotactic response to amino acids of fluorescent pseudomonads isolated from spinach roots grown in soil with different salinity levels. Soil Sci Plant Nutr. 1998;44:1–7. [Google Scholar]
- 7.Gebhard F, Smalla K. Transformation of Acinetobacter sp. strain BD413 by transgenic sugar beet DNA. Appl Environ Microbiol. 1998;64:1550–1554. doi: 10.1128/aem.64.4.1550-1554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gebhard F, Smalla K. Monitoring field releases of genetically modified sugar beets for persistence of transgenic plant DNA and horizontal gene transfer. FEMS Microbiol Ecol. 1999;28:261–272. [Google Scholar]
- 9.Gottlieb D. The production and role of antibiotics in soil. J Antibiot. 1976;29:987–1000. doi: 10.7164/antibiotics.29.987. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann M A, Brian D A. Sequencing PCR DNA amplified directly from a bacterial colony. BioTechniques. 1991;11:30–31. [PubMed] [Google Scholar]
- 11.Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972;112:917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraffczyk G, Trolldenier G, Beringer H. Soluble root exudates of maize: influence of potassium supply and rhizosphere microorganisms. Soil Biol Biochem. 1984;16:315–322. [Google Scholar]
- 13.Lamour V, Quevillon S, Diriong S, N'Guyen V C, Lipinski M, Mirande M. Evolution of the Glx-tRNA synthetase family: the glutaminyl enzyme as a case of horizontal gene transfer. Proc Natl Acad Sci USA. 1994;91:8670–8674. doi: 10.1073/pnas.91.18.8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrence J G, Ochman H. Molecular archeology of the Escherichia coli genome. Proc Natl Acad Sci USA. 1998;95:9413–9417. doi: 10.1073/pnas.95.16.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorenz M G, Wackernagel W. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol Rev. 1994;58:563–602. doi: 10.1128/mr.58.3.563-602.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannerlöf M, Lennersfors B L, Tenning P. Reduced titer of BNYVV in transgenic sugar beet expressing the BNYVV coat protein. Euphytica. 1996;90:293–299. [Google Scholar]
- 17.Metz P L J, Nap J P. A transgene-centered approach to the biosafety of transgenic plants: overview of selection and reporter genes. Acta Bot Neerl. 1997;46:25–50. [Google Scholar]
- 18.Nap J-P, Bijvoet J, Willem J. Biosafety of kanamycin-resistant transgenic plants. Transgenic Res. 1992;1:239–249. doi: 10.1007/BF02525165. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen K M, van Weerelt M D M, Berg T N, Bones A M, Hagler A N, van Elsas J D. Natural transformation and availability of transforming DNA to Acinetobacter calcoaceticus in soil microcosms. Appl Environ Microbiol. 1997;63:1945–1952. doi: 10.1128/aem.63.5.1945-1952.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen K M, Bones A M, van Elsas J D. Induced natural transformation of Acinetobacter calcoaceticus in soil microcosms. Appl Environ Microbiol. 1997;63:3972–3977. doi: 10.1128/aem.63.10.3972-3977.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen K M, Gebhard F, Smalla K, Bones A M, van Elsas J D. Evaluation of possible horizontal gene transfer from transgenic plants to the soil bacterium Acinetobacter calcoaceticus BD413. Theor Appl Genet. 1997;95:815–821. [Google Scholar]
- 22.Nielsen K M, Bones A M, Smalla K, van Elsas J D. Horizontal gene transfer from transgenic plants to terrestrial bacteria—a rare event? FEMS Microbiol Rev. 1998;22:79–103. doi: 10.1111/j.1574-6976.1998.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen K M, Smalla K, van Elsas J D. Natural transformation of Acinetobacter sp. strain BD413 with cell lysates of Acinetobacter sp., Pseudomonas fluorescens, and Burkholderia cepacia in soil microcosms. Appl Environ Microbiol. 2000;66:206–212. doi: 10.1128/aem.66.1.206-212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliveira R D G B, Wolters A C, van Elsas J D. Effects of antibiotics in soil on the population dynamics of transposon Tn5 carrying Pseudomonas fluorescens. Plant Soil. 1995;175:323–333. [Google Scholar]
- 25.Paget E, Lebrun M, Freyssinet G, Simonet P. The fate of recombinant plant DNA in soil. Eur J Soil Biol. 1998;34:81–88. [Google Scholar]
- 26.Recorbet G, Givaudan A, Steinberg C, Bally R, Normand P, Faurie G. Tn5 to assess soil fate of genetically marked bacteria: screening for aminoglycoside-resistance advantage and labelling specificity. FEMS Microbiol Ecol. 1992;86:187–194. [Google Scholar]
- 27.Schilling G, Gransee A, Deubel A, Lezovic G, Ruppel S. Phosphorus availability, root exudates, and microbial activity in the rhizosphere. Z Pflanzenernähr Bodenk. 1998;161:464–478. [Google Scholar]
- 28.Schlüter K, Fütterer J, Potrykus I. “Horizontal” gene transfer from a transgenic potato line to a bacterial pathogen (Erwinia chrysanthemi) occurs—if at all—at an extremely low frequency. Bio/Technology. 1995;13:1094–1098. doi: 10.1038/nbt1095-1094. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt, E. R., and T. Hankeln (ed.). 1996. Transgenic organisms and biosafety, horizontal gene transfer, stability of DNA and expression of transgenes. Springer Verlag, Heidelberg, Germany.
- 30.Schrott M. Selectable marker and reporter genes. In: Potrykus I, Spangenberg G, editors. Gene transfer to plants—a laboratory manual. Berlin, Germany: Springer Verlag; 1995. pp. 325–336. [Google Scholar]
- 31.Smalla K, van Overbeek L S, Pukall R, van Elsas J D. Prevalence of nptII and Tn5 in kanamycin resistant bacteria from different environments. FEMS Microbiol Ecol. 1993;13:47–58. [Google Scholar]
- 32.Smith M W, Feng D-F, Doolittle R F. Evolution by acquisition: the case for horizontal gene transfer. Trends Biochem Sci. 1992;17:489–493. doi: 10.1016/0968-0004(92)90335-7. [DOI] [PubMed] [Google Scholar]
- 33.Thomashow L S, Weller D M, Bonsall R F, Pierson L S., III Production of the antibiotic phenazine-1-carboxylic acid by fluorescent Pseudomonas species in the rhizosphere of wheat. Appl Environ Microbiol. 1990;56:908–912. doi: 10.1128/aem.56.4.908-912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trinker N A, Fortin M G, Mather D E. Random amplified polymorphic DNA and pedigree relationship in spring barley. Theor Appl Genet. 1993;85:976–984. doi: 10.1007/BF00215037. [DOI] [PubMed] [Google Scholar]
- 35.van Elsas J D, Dijkstra A F, Govaert J M, van Veen J A. Survival of Pseudomonas fluorescens and Bacillus subtilis introduced into two soils of different texture in field microplots. FEMS Microbiol Ecol. 1986;38:151–160. [Google Scholar]
- 36.van Elsas J D, Smalla K. Antibiotic (kanamycin and streptomycin) resistance traits in the environment. Mitt Biol Bundesanstalt Land- Forstwirtsch Berlin-Dahlem. 1995;309:61–69. [Google Scholar]
- 37.Wellington E M H, Marsch P, Toth I, Cresswell N, Huddleston L, Schilhabel M B. The selective effects of antibiotics in soil. In: Guerrero R, Pedros-Alio C, editors. Trends in microbial ecology. Barcelona, Spain: Spanish Society for Microbiology; 1993. pp. 331–336. [Google Scholar]
- 38.Widmer F, Seidler R J, Watrud L S. Sensitive detection of transgenic plant marker gene persistence in soil microcosms. Mol Ecol. 1996;5:603–613. [Google Scholar]
- 39.Widmer F, Seidler R J, Donegan K K, Reed G L. Quantification of transgenic marker gene persistence in the field. Mol Ecol. 1997;6:1–7. [Google Scholar]
- 40.Wolf Y I, Aravind L, Koonin E V. Rickettsiae and Chlamydiae: evidence of horizontal gene transfer and gene exchange. Trends Genet. 1999;15:173–175. doi: 10.1016/s0168-9525(99)01704-7. [DOI] [PubMed] [Google Scholar]