Reduction in Exopolysaccharide Viscosity as an Aid to Bacteriophage Penetration through Pseudomonas aeruginosa Biofilms (original) (raw)
Abstract
To cause an infection, bacteriophages must penetrate the alginate exopolysaccharide of Pseudomonas aeruginosa to reach the bacterial surface. Despite a lack of intrinsic motility, phage were shown to diffuse through alginate gels at alginate concentrations up to 8% (wt/vol) and to bring about a 2-log reduction in the cell numbers in 20-day-old biofilms of P. aeruginosa. The inability of alginate to act as a more effective diffusional barrier suggests that phage may cause a reduction in the viscosity of the exopolysaccharide. Samples (n = 5) of commercial alginate and purified cystic fibrosis (CF) alginate were incubated with 2 × 108 purified phage per ml for 24 h at 37°C. After incubation the samples and controls were subjected to rheological analysis with a Carrimed controlled stress rheometer. The viscosities of phage-treated samples were reduced by up to 40% compared to those of controls incubated in the absence of phage. The experiment was repeated by using phage concentrations of 1010 and 1012 phage per ml and samples taken for analysis at intervals up to 4 h. The results indicated that there was a time- and concentration-dependent reduction in viscosity of up to 40% compared to the viscosities of the controls. Commercial and purified CF alginate samples, both phage treated and untreated, were subjected to gel filtration chromatography by using Sephacryl High Resolution S-400 medium in order to obtain evidence of degradation. The results demonstrated that alginate treated with phage had a lower molecular weight than untreated alginate. The data suggest that bacteriophage migration through P. aeruginosa biofilms may be facilitated by a reduction in alginate viscosity brought about by enzymic degradation and that the source of the enzyme may be the bacterial host itself.
Pseudomonas aeruginosa is an opportunistic human pathogen that causes major problems in a number of clinical settings, particularly in patients with burns or other wounds, patients who have indwelling medical devices, and cystic fibrosis (CF) sufferers. Patients with CF are prone to respiratory infections caused by mucoid strains of P. aeruginosa, and these strains are recalcitrant to treatment with antibiotics. This bacterium can grow as a biofilm of considerable thickness (>200 μm) and can secrete large quantities of alginate exopolysaccharide (14). The exopolysaccharide surrounds the cells, forming a glycocalyx that can act as a significant barrier to the penetration of antibiotics (2, 24). When grown in the biofilm mode, P. aeruginosa may exhibit up to 100 times greater resistance to biocides than the corresponding planktonic cells (3, 4, 27, 37).
In addition, the resistance of biofilm cells to β-lactams, tobramycin, quaternary ammonium compounds, and the quinolones has been attributed to a slow growth rate (10, 20, 23), a condition common in biofilms (15, 25). Changes in growth rate and nutrient limitation can also give rise to altered cell wall and membrane structure (3, 14, 19).
As an alternative to the use of antibiotics, some workers have attempted to use bacteriophages as a means of combating bacterial infections, and this strategy has had some success (1, 6).
The concept of using phages as antimicrobial agents to treat infections was initiated in 1915 when Twort and d'Herelle independently observed the phenomenon of transmissible lysis of bacteria. Bacteriophages were administered to cholera patients in an attempt to cure the disease, and the initial results were encouraging. Bruynoghe and Maisin (11) later had some success in treating staphylococcal skin infections with phage. However, apart from a few isolated successes, the use of bacteriophages as treatment for bacterial infections did not progress due in part to exaggerated claims based on a lack of understanding but due principally to the advent of antibiotics in the 1940s.
More recently, there has been renewed interest in the use of bacteriophages as antimicrobial agents now that the spectre of antibiotic resistance has become a reality. Smith and Huggins (32) compared the efficacy of phage therapy with that of conventional antibiotics by using Escherichia coli O18:K1 as a model pathogen for mice. A single intramuscular dose of anti-K1 phage was found to be more effective than multiple intramuscular doses of antibiotics, and the bacteria did not proliferate in the brains of the mice that had been inoculated with phage. Smith and Huggins (33) also attempted to treat diarrhea caused by enteropathogenic E. coli in calves, piglets, and lambs. They found that the E. coli cells isolated after treatment with phage were much less virulent than the parent bacteria. Later, Smith et al. (31, 34) performed additional studies on the control of diarrhea in calves with bacteriophage and found that severe enteropathogenic E. coli diarrhea could be cured with a single oral dose of 10,000 phage particles or by feeding on litter sprayed with phage.
Cislo et al. (12) used phage successfully to treat patients with postoperative skin infections caused by a variety of pathogens, including P. aeruginosa, staphylococci, and Klebsiella sp. Soothill (35) tested the efficacy of bacteriophage for treating experimental infections caused by Acinetobacter, Pseudomonas, and Staphylococcus species. While the results of Soothill exposed some of the limitations of phage therapy, they did demonstrate that phage can be used to control bacterial infections in a clinical setting. In a later study, Soothill (36) examined skin grafts in guinea pigs artificially infected with P. aeruginosa with and without bacteriophage treatment. After 5 days the grafts took in six of seven guinea pigs treated with phage and failed in all untreated animals.
There are a number of potential advantages of using bacteriophages to control infections; in particular, phages which are highly specific for the infecting bacterium and thus are harmless to the host can be chosen. The initial dose of the phage can be low since the virus multiplies in the bacterial cells, releasing new phage particles on lysis. This process of enhancement of the number of phage should continue until all the bacteria have been destroyed. The problem of development of resistance can be reduced by concurrent administration of a number of different phages, each of which acts on the same type of cells. The phages are likely to be effective even against the bacteria that are multiply resistant to antibiotics. Using bacteriophages to treat biofilm-associated infections, however, is likely to be more problematic due to the presence of a glycocalyx that acts as a diffusional barrier to the virus (16, 17).
In this paper we describe an investigation into the ability of bacteriophage to diffuse through the alginate matrix secreted by P. aeruginosa in order to lyse the bacteria in a biofilm.
MATERIALS AND METHODS
Microorganisms, media, and maintenance of cultures.
Bacteriophage F116, together with its host, P. aeruginosa NCIMB 10548, was obtained from the Welsh School of Pharmacy, University of Wales, Cardiff, United Kingdom. A second P. aeruginosa bacteriophage, designated GL1, together with its host bacterial strain, was obtained from the School of Pharmaceutical Sciences, University of Nottingham, Nottingham, United Kingdom. A mucoid strain of P. aeruginosa was a respiratory clinical isolate obtained from a patient at Brighton General Hospital (Brighton, United Kingdom) suffering from CF. The other bacteriophage suspensions used were suspensions of E. coli lambda phage and a Staphylococcus aureus bacteriophage, which was originally obtained as an environmental isolate. Both of the latter bacteriophages were obtained from the culture collection at the University of Brighton, Brighton, United Kingdom. All bacterial cultures were stored frozen at −75°C in 10% glycerol. Every 2 to 3 weeks a vial of frozen culture was thawed and propagated overnight in tryptone soya broth (TSB) (Oxoid, Basingstoke, United Kingdom) at 37°C. The liquid cultures were used to inoculate tryptone soya agar (TSA) (Oxoid), which was incubated at 37°C for 18 to 24 h. The plates were checked for purity, and individual colonies were identified by using the API 20E method.
Propagation and purification of bacteriophage.
One milliliter of an overnight culture of host bacteria was added to 100 ml of TSB and incubated in a shaking incubator at 37°C. The optical density of the culture was monitored at 600 nm. When the culture reached an optical density of 0.5, we added 1 ml of bacteriophage stock diluted in lambda buffer (containing [per liter of water] 0.73 g of Trizma base, 0.5 g of gelatin, and 2.5 g of MgSO4 · 7H2O) to a concentration of 1 × 1010 PFU/ml. After mixing, the flask was kept stationary for 15 min, and then shaking was resumed. Incubation was continued until bacterial lysis was evident by visual inspection. When lysis had occurred, 10 ml of chloroform was added to the flask, which was then shaken for a further 10 min at 37°C. The lysed culture was cooled to room temperature, and sodium chloride was added to a final concentration of 1 M. The NaCl was dissolved by swirling, and the preparation was stored on ice for 1 h. Bacterial debris was removed by centrifugation at 11,000 × g for 10 min at 4°C. Polyethylene glycol 10,000 was added to the supernatant liquid to a final concentration of 10% (wt/vol) and was slowly dissolved by stirring at room temperature. The mixture was left overnight at 4°C to allow the bacteriophage particles to form a precipitate, which was recovered by centrifugation at 11,000 × g for 10 min at 4°C. The pellet was resuspended in 2 ml of lambda buffer, and 1 ml of chloroform was added; this was followed by vortexing for 30 s. The organic and aqueous phases were separated by centrifugation at 3,000 × g for 15 min at 4°C, and the aqueous phase containing purified bacteriophage was stored at 4°C until it was required.
Bacteriophage plaque assay.
Bacteriophage samples were serially diluted 10- and 100-fold in lambda buffer, and 0.1-ml portions were added to 5 ml of molten overlay agar at 50°C. An overnight culture (0.1 ml) of the host bacterium was then added to each preparation, and the molten agar was gently mixed before it was poured over the surface of a TSA plate. The agar was allowed to set, and the plate was incubated at 37°C for 18 to 24 h. The number of plaques that arose was then counted, and the concentration of phage particles was expressed as the number of PFU per milliliter. The assay was sensitive down to a concentration of 100 PFU/ml.
Preparation of bacterial biofilms.
P. aeruginosa NCIMB 10548 biofilms were grown on 12-mm-diameter discs of poly(methyl)methacrylate (PMMA). The discs were prepared as follows. PMMA was dissolved in dichloromethane to a final concentration of 10% (wt/vol). The solution was poured into a clean glass tray and left under laminar airflow until the solvent evaporated. Discs were then cut with a 12-mm-diameter circular cutter. The discs were placed individually in the wells of a microtiter tray, an overnight culture of P. aeruginosa NCIMB 10548 was added, and the preparations were incubated for 2 h at 37°C. The discs were then washed in three changes of sterile phosphate-buffered saline (PBS) to remove nonadherent cells before being placed in fresh TSB. The discs were then incubated at 37°C for up to 20 days with frequent changes of broth. Biofilms grown under static conditions are exposed to increased environmental stress due to stagnation and starvation compared to biofilms grown under flow conditions (14). It has been suggested that this may lead to more confluent biofilms with increased extracellular polysaccharide production and may also influence the susceptibility of biofilms to biocides (8, 14). At intervals discs were removed, washed twice in sterile PBS, and placed in 10 ml of sterile PBS. The biofilm cells on each disc were removed by scraping with a sterile glass scraper, and the liquid and disc were subjected to sonication for 4 min in a sonic bath. The resulting cell suspension was serially diluted and plated onto replicate TSA plates for enumeration of viable cells.
Exposure of biofilms to bacteriophage.
Discs containing 5-, 10-, 15-, and 20-day-old biofilms were washed twice in sterile PBS to remove nonadherent cells and placed in individual wells of a microtiter tray. Control experiments were carried out in which the adherent cells were removed and quantified to obtain estimates of viable counts in the biofilms at different times. Based on these estimates, a bacteriophage suspension was added to give final phage/cell ratios of 100:1 and 1,000:1, and the mixtures were incubated at 37°C for 24 h. At the end of the exposure period the viable bacteria remaining in the biofilms were quantified as described above. The experiment was repeated with cells removed from biofilms of similar ages, and the planktonic cells were exposed at the same phage/cell ratios in PBS.
Preparation and purification of P. aeruginosa extracellular polysaccharide.
Isolated colonies of a mucoid clinical isolate of P. aeruginosa from a CF patient were suspended in 10 ml of sterile water. The resulting suspension was poured over the surface of overdried TSA in an assay plate (12 by 12 in.). The plate was sealed and incubated at 37°C for 3 days. After incubation the surface growth was removed from the plate and transferred into sterile distilled water by using a sterile glass spreader. The suspension was vortex mixed and centrifuged at 13,000 × g for 1 h at 4°C. The supernatant liquid containing extracellular polysaccharide was removed, and the pellet was discarded. Three volumes of absolute alcohol was added to the supernatant liquid to precipitate the exopolysaccharide. The precipitate was finally redissolved in sterile distilled water to produce a viscous solution, which was stored at −20°C prior to use.
Diffusion of bacteriophage through alginate gels.
We used a glass, two-chamber diffusion cell to investigate the ability of bacteriophage to penetrate alginate gels containing different concentrations of alginate. The diffusion cell was prepared by using 0.45-μm-pore-size membrane filters to enclose either pH 2 buffer, solutions of sodium alginate (BDH Laboratory Supplies, Dorset, United Kingdom) at concentrations of 4, 6, 8, and 12% (wt/vol), or purified P. aeruginosa extracellular polysaccharide. The donor chamber contained bacteriophage suspended at a concentration of 1010 PFU/ml, while the receptor chamber contained buffer alone. The entire unit was placed in a water bath at 37°C. Samples were removed from the receptor chamber at intervals and assayed to determine the phage concentration by the overlay plaque assay, using P. aeruginosa NCIMB 10548 as the indicator organism. Plaque counts of zero were recorded as 100 PFU/ml as this concentration was the limit of sensitivity of the assay.
Rheological analysis of alginate gels.
Different concentrations of commercial alginate (4, 6, 8, and 12% [wt/vol]) were prepared in sterile water. Five-milliliter samples of each preparation were incubated with 0.1 ml of bacteriophage at a concentration of 1010 PFU/ml. Controls were also prepared with 0.1 ml of lambda buffer in place of the bacteriophage and 0.1-ml samples of an autoclaved bacteriophage suspension. All tubes were incubated at 37°C for 24 h. After incubation 1-ml samples were subjected to a rheological analysis at 10 points by using a Carrimed controlled stress rheometer operating at 25°C and frequencies ranging from 1 to 10 Hz. The instrument was used in the oscillatory mode with cone and plate geometry. Purified bacterial exopolysaccharide was inoculated with bacteriophage as described above, and samples were removed for rheological analysis at intervals over a 4-h period and after overnight incubation. The experiment was repeated by using an 8% commercial alginate gel incubated with bacteriophage at concentrations of 1010 and 1012 PFU/ml, and the rheological characteristics were determined over a 4-h period. Bacteriophage were mixed with 8% commercial alginate at a concentration of 1010 PFU/ml, and the mixtures were incubated at 4 instead of 37°C. An uninfected culture of P. aeruginosa NCTC 10548 was grown in TSB at 37°C, and the whole culture was subjected to the procedure used for bacteriophage purification. The purified bacterial extract, devoid of bacteriophage, was added to 8% commercial alginate, and the mixture was incubated at 37°C for 4 h. Samples were removed at intervals to assess the reduction in viscosity. A variety of other bacteriophages were used in place of F116 (P. aeruginosa phage GL1, E. coli lambda phage, and a staphylococcal bacteriophage). These phages were incubated with 8% commercial alginate at 37°C, and samples were removed for analysis over a 4-h period.
Gel filtration chromatography of alginate samples.
Commercial alginate and purified P. aeruginosa exopolysaccharide, both freshly prepared and bacteriophage treated, were subjected to gel filtration chromatography to look for evidence of polymer degradation. Sephacryl High Resolution S-400 medium was used in a column (2.5 by 19 cm). The column was equilibrated with 200 ml of lambda buffer (pH 7.2); the flow rate was 0.9 ml/min initially and was reduced to 0.65 ml/min when 2-ml samples were added to the reservoir. The outlet passed through a flowthrough cuvette, and two detection wavelengths were used: 260 nm for the commercial alginate samples and 249 nm for the bacterial exopolysaccharide. These wavelengths were chosen on the basis of preliminary UV scans conducted with each of the alginate gels.
RESULTS
Treatment of biofilms with bacteriophage.
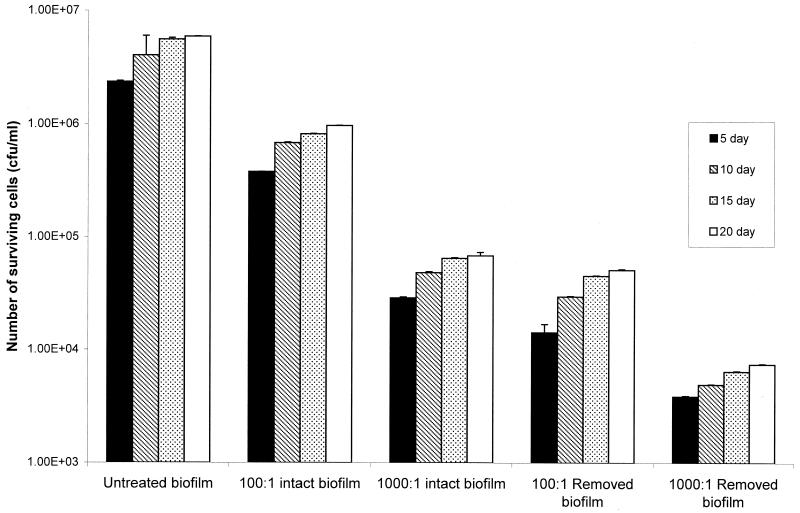
Biofilms of P. aeruginosa NCIMB 10548 were prepared on sheets of PMMA as preliminary experiments had shown that this was a substrate to which the bacteria readily adhered and on which they grew to higher densities than they grew on silicone rubber, glass, or polystyrene. Anwar et al. (4) showed that a P. aeruginosa biofilm grown for 7 days exhibited significantly greater resistance to tobramycin than a biofilm grown for 2 days exhibited and that the resistance of both of biofilms was much greater than that of planktonic cells. Therefore, in this study biofilm growth was allowed to proceed for up to 20 days. Exposure of biofilm cells to bacteriophage for 24 h showed that there was a reduction in viable counts that was dependent on the initial ratio of phage to cells and on whether the cells were attached to the substrate (Fig. 1). A phage/cell ratio of 100:1 resulted in a 1-log reduction in the viable cell number in an intact biofilm, while the number of detached cells resuspended before bacteriophage exposure declined by just over 2 logs. When the ratio was increased to 1,000:1, the log reduction factor for an intact biofilm increased to 2, whereas the log reduction factor for resuspended cells was 3. Younger biofilms did not seem to be any more sensitive to bacteriophage than 20-day-old biofilms; biofilms of all ages resulted in the same log reduction factors for all treatments. For comparison, cells grown planktonically in liquid cultures exhibited log reduction factors greater than of 5 (data not shown), suggesting that the resuspended biofilm-derived cells still had increased resistance to treatment.
FIG. 1.
Effect of bacteriophage on biofilms of P. aeruginosa. P. aeruginosa NCIMB 10548 biofilms were grown for 5, 10, 15, and 20 days. They were treated with bacteriophage, and the numbers of surviving bacteria were determined. Bacteriophage/cell ratios of 100:1 and 1,000:1 were used with both intact biofilms and resuspended biofilm cells. For all experiments n = 6. The error bars indicate standard deviations.
Diffusion of bacteriophage through alginate gels and exopolysaccharide.
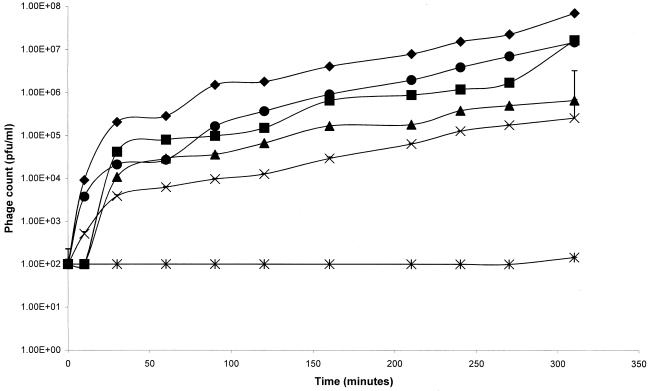
The composition of bacterial exopolysaccharides is complex and incompletely understood. Many bacteria are known to produce extracellular polysaccharides during planktonic growth, and it is not known whether the molecules produced in the biofilm mode of growth are chemically different polymers or whether they have different physical properties. It is generally agreed, however, that the exopolysaccharide produced by P. aeruginosa is a polyanionic alginate that is a block copolymer of mannuronic and guluronic acids. In order to investigate the diffusion of bacteriophage through this exopolysaccharide system and to analyze the effects of different viscosities, commercial alginate gels were prepared and compared to exopolysaccharide purified from a mucoid strain of P. aeruginosa. The rate of diffusion of bacteriophage through the intermediate concentrations of commercial alginate (4, 6, and 8% [wt/vol]) was only slightly retarded compared to the rate of diffusion through buffer, while diffusion through exopolysaccharide was similar to diffusion through 4% alginate (Fig. 2). In each case, however, the number of bacteriophage found in the receptor compartment decreased as the viscosity of the gels increased. Increasing the alginate concentration to 12% (wt/vol) greatly inhibited bacteriophage diffusion over the 5-h experiment. When incubated overnight, however, the bacteriophage was found to have penetrated into the receptor compartment, albeit at a concentration 5 logs less than the concentration in the buffer control.
FIG. 2.
Diffusion of bacteriophage through alginate gels. The rate of diffusion of bacteriophage was determined with commercial alginate gels containing 4% (■), 6% (▴), 8% (×), and 12% (∗) alginate. The data were compared to the data for diffusion through a buffer control (⧫). Bacteriophages were also assessed to determine their rate of diffusion through purified P. aeruginosa exopolysaccharide (●). For all experiments n = 6. The error bars indicate standard deviations. Phage counts of zero were recorded as 100 PFU/ml, which was the limit of detection of the assay.
Rheological analysis of alginate gels.
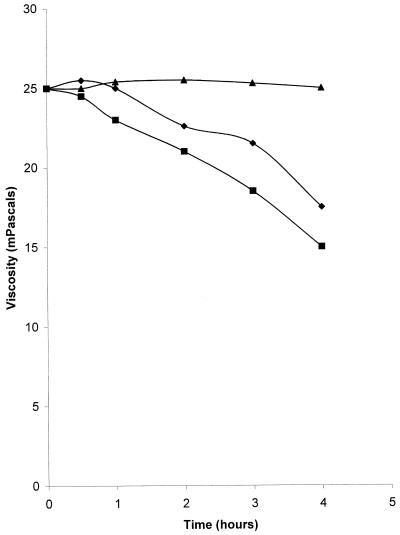
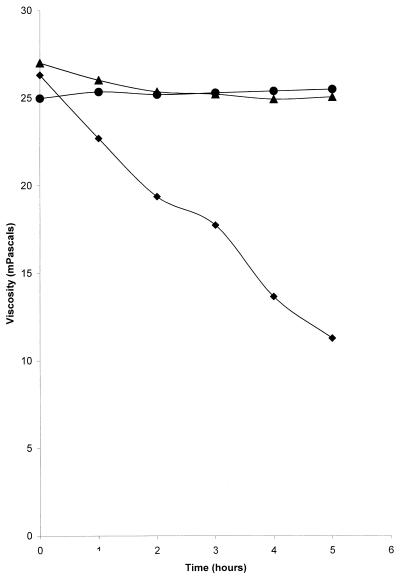
The viscosities of commercial alginate treated with bacteriophage were up to 40% less than the viscosities of controls incubated for the same time in the absence of phage (Table 1). The viscosities in the control tubes, including those containing heat-killed bacteriophage, were unchanged after overnight incubation. The experiment was repeated with bacteriophage concentrations of 1010 and 1012 PFU/ml, and samples were taken for rheological analysis at intervals for up to 4 h. The results indicate that there were time- and concentration-dependent reductions in viscosity of up to 40% compared to the viscosities of the controls (Fig. 3). Phage-alginate mixtures incubated at 4°C did not exhibit any reduction in viscosity over the same time periods (data not shown). Purified exopolysaccharide from P. aeruginosa showed a similar reduction in viscosity with time when it was treated with bacteriophage (Table 2). In order to ensure that the reductions in viscosity observed were not due to bacterial enzymes carried over in the purified bacteriophage suspensions, a culture of uninfected P. aeruginosa was subjected to the procedure used for bacteriophage purification. When samples of the purified extract (diluted appropriately to reflect the extent to which the phage stock was diluted) were added to alginate solutions, the reduction in viscosity observed was significantly less than that seen in the presence of bacteriophage (P = 0.022). A second suspension of purified P. aeruginosa bacteriophage was tested in order to determine the specificity of the interaction. The host bacterium of GL1 was a different strain of P. aeruginosa, and GL1 was propagated from this strain, although it was also lytic for NCIMB 10548. Figure 4 shows that GL1 was able to reduce the viscosities of the alginate samples to the same extent as F116. The ability to reduce the viscosity of alginate was restricted to Pseudomonas bacteriophages, however, since E. coli lambda phage and staphylococcal phage did not reduce the viscosity compared to the viscosity of the controls (Fig. 4).
TABLE 1.
Effect of bacteriophage addition on the viscosities of different concentrations of commercial alginate
| Alginate concn (%, wt/vol) | Viscosity of untreated control (mPa) | Viscosity after phage addition (mPa) |
|---|---|---|
| 4 | 2.76 ± 0.0621a | 2.077 ± 0.03 |
| 6 | 17.95 ± 0.011 | 12.95 ± 0.09 |
| 8 | 25.39 ± 0.339 | 15.21 ± 0.043 |
| 12 | 112.78 ± 2.752 | 88.85 ± 0.183 |
FIG. 3.
Effect of bacteriophage concentration on the viscosities of commercial alginate gels. Gels containing 8% alginate were treated with bacteriophage at concentrations of 1010 PFU/ml (⧫) and 1012 PFU/ml (■), and the viscosities were measured with a Carrimed controlled stress rheometer at different times. Controls consisted of gels which were not treated with bacteriophage (▴).
TABLE 2.
Effect of addition of bacteriophage on the viscosity of purified P. aeruginosa exopolysaccharide
| Time (h) | Viscosity of untreated control (mPa) | Viscosity after phage addition (mPa) |
|---|---|---|
| 0.5 | 2.33 | 2.33 ± 0.031a |
| 1 | 2.35 | 2.22 ± 0.019 |
| 2 | 2.32 | 2.18 ± 0.023 |
| 3 | 2.35 | 2.11 ± 0.011 |
| 4 | 2.34 | 2.06 ± 0.015 |
| 18 | 2.33 | 1.76 ± 0.037 |
FIG. 4.
Effect of bacteriophage type on viscosities of commercial alginate gels. Gels containing 8% alginate were treated with GL1 (⧫), E. coli lambda phage (▴), and a staphylococcal bacteriophage (●). Viscosities were measured with a Carrimed controlled stress rheometer at different times.
Gel filtration chromatography of alginate samples.
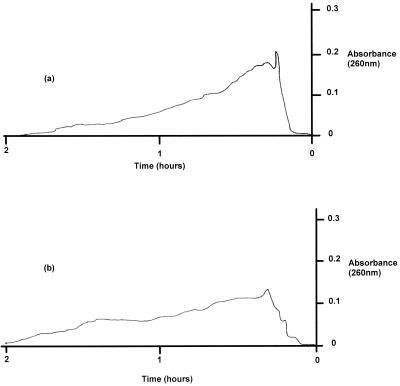
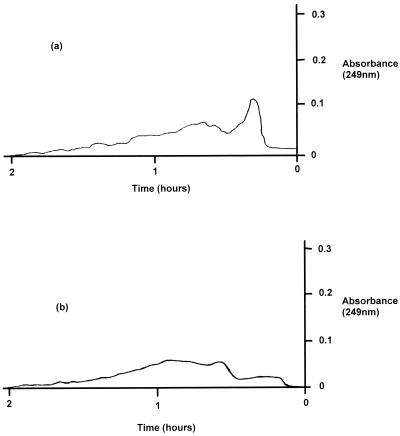
Commercial alginate samples and purified exopolysaccharide before and after treatment with bacteriophage were analyzed by gel filtration chromatography to assess the chain length profiles of the gel systems. The absorbance readings for the commercial alginate are shown in Fig 5, and the peak area of the control sample represented 48.8% of the total area under the curve. When a preparation was treated with bacteriophage, the peak height for the major component was reduced by approximately 60% and the peak area decreased to 25.6% of the total area under the curve. Similar results were obtained with the purified exopolysaccharide (Fig. 6); the peak height for the major component of similar molecular weight was reduced by approximately 75%, and the peak area expressed as a percentage of the total area decreased from 48 to 21% following phage treatment. In both cases an obvious shift from larger molecules to smaller molecules after bacteriophage treatment was evident, indicating that degradation of the polymer to shorter-chain-length units occurred. The apparently reduced void volumes observed for the phage-treated samples were attributed to the emergence of phage particles from the column prior to the alginate peaks.
FIG. 5.
Gel chromatography of commercial alginate gels before treatment (a) and after treatment (b) with P. aeruginosa bacteriophage.
FIG. 6.
Gel chromatography of purified P. aeruginosa exopolysaccharide before treatment (a) and after treatment (b) with P. aeruginosa bacteriophage.
DISCUSSION
Growth of bacteria in planktonic form is relatively rare in nature and tends, therefore, to be confined to unrepresentative laboratory cultures. The more common mode of growth in natural environments involves adhesion to a substrate in the form of a biofilm, usually in a mixed consortium of cell types (9, 14, 29, 39, 40). Under these conditions the bacteria frequently express phenotypic characteristics different from those seen in planktonic cultures, and the rate of growth can be very low (3, 14, 19). Biofilm growth has several advantages for adherent cells, including better access to nutrients, possibilities for genetic exchange, and protection against desiccation and predation (22). Secretion of exopolysaccharide, which not only impedes phagocytic cells but also imposes diffusional constraints on materials entering and leaving the system (13), enhances the protective function of the biofilm. Furthermore, the cells embedded in a biofilm are protected by the architecture of the biofilm itself. In a clinical setting the presence of exopolysaccharide has been shown to have a dramatic effect on the ability of antibiotics to control biofilm-associated infections. Patients with CF who acquire mucoid P. aeruginosa in their lungs very rarely eradicate it, while some infected medical implants can be treated successfully only if the device is removed from the body.
In this study P. aeruginosa was chosen not only because it is of clinical significance when it is growing as a biofilm but also because the nature of the exopolysaccharide is well understood. It should be noted, however, that the highly charged, polyanionic character of the alginate exopolymer is not typical of most bacterial exopolysaccharides. There is much diversity in the exopolysaccharides secreted by different bacterial species, and for this reason care should be taken when attempts to extrapolate results from one system to another are made. Indeed, secretion of alginate by different strains of P. aeruginosa is highly variable; the CF clinical isolate used in this study produces copious quantities, while NCIMB 10548 secretes much less.
Bacteriophages occur in large numbers in natural ecosystems and undoubtedly have a significant influence on the bacterial populations present (28, 30). However, very little work has been done on the effects of bacteriophages on bacterial cells embedded in biofilms (5, 16, 17). Recently Hughes et al. (26) examined a lytic bacteriophage active against biofilms of Enterobacter agglomerans and found that the bacteriophage stimulated production of polysaccharide depolymerases by the bacteria. These enzymes were capable of degrading the exopolysaccharide produced by E. agglomerans biofilms, which clearly assisted bacteriophage dispersal.
If bacteriophages are to exert control over biofilm bacteria, they must first be able to reach the embedded cells by migrating through the exopolysaccharide. Then they must locate the specific adhesion sites on the cell wall before entering a cell by utilizing its own metabolism to propagate new viruses. Each of these stages places considerable burdens on a bacteriophage, not least because phages are not intrinsically motile. However, Drury et al. (18) have shown that 1-μm-diameter latex particles are capable of diffusion through P. aeruginosa biofilms that are approximately 34 μm thick within 24 h. A similar problem confronts mammalian viruses that must penetrate a viscous covering of mucus in order to attack underlying epithelial cells. Very little information has been published in this area, but Bisaillon et al. (7) have shown that reovirus ς1 protein possesses glycosyl hydrolase activity which degrades mucus to aid diffusion. In addition, insect baculovirus possesses an enhancin in its outer coat which is a metalloprotease enzyme capable of degrading insect intestinal mucins. The insect intestinal mucins are composed of glycoproteins and have structural similarities to mammalian mucins. Degradation of the insect intestinal mucins by the enhancin leads to higher viral infection rates (38).
The results reported here clearly demonstrate that bacteriophage can reduce the number of viable bacteria in a biofilm by up to 99% despite the presence of exopolysaccharide. Biofilm cells that were detached from the substrate surface and resuspended before exposure, however, had increased susceptibility, showing that biofilm architecture does play an important role in limiting access to bacteriophage. A similar picture has been reported for biocide treatment of biofilms. Surface-adhered Listeria monocytogenes cells were shown to exhibit much greater resistance to benzalkonium chloride. Removal of the adherent cells from the surface increased their biocide sensitivity, but they were still not as sensitive as planktonically grown cells (21). The data suggest, therefore, that bacteriophages are capable of penetration through a gel network by diffusion. The failure to obtain levels of killing comparable to those seen with planktonic cells may be a reflection of other differences, such as the cellular metabolic rate.
Experiments were performed with purified bacteriophage suspensions in the absence of host bacteria in order to test the ability of the phage to diffuse through gel systems having differing viscosities. The results showed that even at the highest concentration of alginate used (12%, wt/vol), bacteriophage could penetrate during prolonged incubation. At the lower concentrations of commercial alginate and in the presence of purified P. aeruginosa exopolysaccharide, phage diffusion was rapid. The viscosity of alginate gels is affected significantly by the cation concentration, and high concentrations of Ca2+, for example, result in significant cross-linking. However, in natural environments the amount of calcium is expected to be low; for example, the concentration of calcium found in lung secretions is approximately 6 mM, which would not cause significant cross-linking. The purpose of these experiments was to determine whether the bacteriophage was able to influence polymer chain length rather than the degree of cross-linking, and so no additional calcium was included in the buffer solutions.
The flow characteristics of polymeric gel systems are complex and can be described in terms of viscoelastic behavior. The Carrimed controlled stress rheometer enables accurate, nondestructive analysis of rheological characteristics over a wide range of sheer stresses. The results presented here show that addition of bacteriophage to solutions of alginate reduced the viscosities of the gel systems in a manner that was concentration and time dependent. The viscosities of alginate solutions may be altered by changing the degree of cross-linking or decreasing the polymer chain length. The polyanionic nature of alginate gels means that the cation cross-linking can be easily reversed. It is possible, therefore, that the reduction in viscosity was merely due to the phage particles neutralizing the Ca2+ ions which were cross-linking the polymers. The data presented above for heat-treated bacteriophage suspensions suggest that this was not the case and are reinforced by the observation that the kinetics of viscosity reduction, particularly the temperature dependence, resembled an enzymatic process that resulted in polymer degradation. The reduction in viscosity also appeared to be highly specific, as other bacteriophages (E. coli lambda phage and staphylococcal phage) did not have any effect compared to controls.
Enzymatic degradation of the alginate polymer was confirmed by analyzing the gels by column chromatography. Samples that had been exposed to bacteriophage clearly showed that there was a reduction in polymer size compared to the size in the controls.
The bacteriophage suspensions used in this study were highly purified from the original culture lysate, but it is possible that the depolymerase enzyme responsible for alginate degradation may have been present as a contaminant. When the extract from a whole-cell culture of P. aeruginosa was subjected to the procedure used in the phage purification process, the resulting solution did possess some depolymerase activity, but it was significantly less than that shown by the bacteriophage suspension (P = 0.022). This suggests that low levels of alginate-degrading enzyme are present in the host bacterium itself. Bacteriophage may, therefore, upregulate production of the alginate depolymerase enzyme by the bacterium during lysis and may subsequently utilize this enzyme (possibly by adsorbing it to its coat proteins) to assist penetration of biofilm exopolysaccharide.
REFERENCES
- 1.Alisky J, Iczkowski K, Rapoport A, Troitsky N. Bacteriophages show promise as antimicrobial agents. J Infect. 1998;36:5–15. doi: 10.1016/s0163-4453(98)92874-2. [DOI] [PubMed] [Google Scholar]
- 2.Anwar H, Costerton J W. Effective use of antibiotics in the treatment of biofilm-associated infections. ASM News. 1992;58:665–668. [Google Scholar]
- 3.Anwar H, Strap J L, Costerton J W. Establishment of aging biofilms: possible mechanism of bacterial resistance to antimicrobial therapy. Antimicrob Agents Chemother. 1992;36:1347–1351. doi: 10.1128/aac.36.7.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anwar H, van Biesen T, Dasgupta M, Lam K, Costerton J W. Interaction of biofilm bacteria with antibiotics in a novel in vitro chemostat system. Antimicrob Agents Chemother. 1989;33:1824–1826. doi: 10.1128/aac.33.10.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araki M, Hosomi M. Using bacteriophage for slime control in the paper mill. Tappi J. 1990;73:155–158. [Google Scholar]
- 6.Barrow P A, Soothill J S. Bacteriophage therapy and prophylaxis: rediscovery and renewed assessment of potential. Trends Microbiol. 1997;5:268–271. doi: 10.1016/S0966-842X(97)01054-8. [DOI] [PubMed] [Google Scholar]
- 7.Bisaillon M, Senechal S, Bernier L, Lemay G. A glycosyl hydrolase activity of ς1 protein can contribute to viral infection through a mucus layer. J Mol Biol. 1999;286:759–773. doi: 10.1006/jmbi.1998.2495. [DOI] [PubMed] [Google Scholar]
- 8.Bourion F, Cerf O. Disinfection efficacy against pure culture and mixed population biofilms of Listeria innocua and Ps. aeruginosa on stainless steel, Teflon and rubber. Sci Aliments. 1996;16:151–166. [Google Scholar]
- 9.Bronzel V S. Biofilms: a neglected bacterial habitat. S Afr J Sci. 1994;90:200. [Google Scholar]
- 10.Brown M R W, Allison D G, Gilbert P. Resistance of bacterial biofilms to antibiotics: a growth related effect. J Antimicrob Chemother. 1988;22:777–780. doi: 10.1093/jac/22.6.777. [DOI] [PubMed] [Google Scholar]
- 11.Bruynoghe R, Maisin J. Essais de therapeutique an moyen du bacteriophage. C R Soc Biol. 1921;85:1120–1121. [Google Scholar]
- 12.Cislo M, Dabrowska M, Woyton A. Bacteriophage treatment of supperative skin infection. Arch Immunol Ther Exp. 1987;35:175–183. [PubMed] [Google Scholar]
- 13.Cooksey K E. Extracellular polymers in biofilms. In: Melo L F, Bott T R, Fletcher M, Capdeville B, editors. Biofilms: science and technology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 137–148. [Google Scholar]
- 14.Costerton J W, Cheng K-J, Geesey G G, Ladd T I, Nickel J C, Dasgupta M, Marrie T J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 15.Costerton J W, Lappin-Scott H M. Behavior of bacteria in biofilms. ASM News. 1989;55:650–654. [Google Scholar]
- 16.Doolittle M M, Cooney J J, Caldwell D E. Lytic infections of Escherichia coli biofilms by bacteriophage T4. Can J Microbiol. 1995;41:12–18. doi: 10.1139/m95-002. [DOI] [PubMed] [Google Scholar]
- 17.Doolittle M M, Cooney J J, Caldwell D E. Tracing the interaction of bacteriophage with bacterial biofilms using fluorescent and chromogenic probes. J Ind Microbiol. 1996;16:331–341. doi: 10.1007/BF01570111. [DOI] [PubMed] [Google Scholar]
- 18.Drury W J, Stewart P S, Characklis W G. Transport of 1 mm latex particles in Pseudomonas aeruginosa biofilms. Biotechnol Bioeng. 1993;42:111–117. doi: 10.1002/bit.260420115. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher M. The physiological activity of bacteria attached to solid surfaces. Adv Microb Physiol. 1991;32:53–85. doi: 10.1016/s0065-2911(08)60005-3. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher M. Bacterial metabolism in biofilms. In: Melo L F, Bott T R, Fletcher M, Capdeville B, editors. Biofilms: science and technology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 113–124. [Google Scholar]
- 21.Frank J F, Koffi R A. Surface-adherent growth of Listeria monocytogenes is associated with increased resistance to sanitizers and heat. J Food Prot. 1990;53:550–554. doi: 10.4315/0362-028X-53.7.550. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert P. Attachment and biofilm formation: the critical event in microbial pathogenesis. J Pharm Pharmacol. 1997;49(Suppl. 4):8. [Google Scholar]
- 23.Gilbert P, Collier P J, Brown M R W. Influence of growth rate on susceptibility to antimicrobial agents: biofilms, cell cycle, dormancy, and stringent response. Antimicrob Agents Chemother. 1990;34:1865–1868. doi: 10.1128/aac.34.10.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Govan J R W, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoyle B D, Jass J, Costerton J W. The biofilm glycocalyx as a resistance factor. J Antimicrob Chemother. 1990;26:1–6. doi: 10.1093/jac/26.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Hughes K A, Sutherland I W, Clark J, Jones M V. Bacteriophage and associated polysaccharide depolymerases—novel tools for study of bacterial biofilms. J Appl Microbiol. 1998;85:583–590. doi: 10.1046/j.1365-2672.1998.853541.x. [DOI] [PubMed] [Google Scholar]
- 27.Keevil C W, Mackerness C W, Colbourne J S. Biocide treatment of biofilms. Int Bioremed. 1990;26:169–179. [Google Scholar]
- 28.Kokjohn T A, Sayler G S, Miller R V. Attachment and replication of Pseudomonas aeruginosa bacteriophages under conditions simulating aquatic environments. J Gen Microbiol. 1991;137:661–666. [Google Scholar]
- 29.Lappin-Scott H M, Costerton J W, Marrie T J. Biofilms and biofouling. Encycl Microbiol. 1992;1:277–282. [Google Scholar]
- 30.Ogunseitan O A, Sayler G S, Miller R V. Dynamic interactions of Pseudomonas aeruginosa and bacteriophages in lake water. Microb Ecol. 1990;19:171–185. doi: 10.1007/BF02012098. [DOI] [PubMed] [Google Scholar]
- 31.Smith H W, Huggins M B, Shaw K M. The control of experimental E. coli diarrhoea in calves by means of bacteriophages. J Gen Microbiol. 1987;133:1111–1126. doi: 10.1099/00221287-133-5-1111. [DOI] [PubMed] [Google Scholar]
- 32.Smith H W, Huggins M B. Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. J Gen Microbiol. 1982;128:307–318. doi: 10.1099/00221287-128-2-307. [DOI] [PubMed] [Google Scholar]
- 33.Smith H W, Huggins M B. Effectiveness of phages in treating experimental E. coli diarrhoea in calves, piglets and lambs. J Gen Microbiol. 1983;129:2659–2675. doi: 10.1099/00221287-129-8-2659. [DOI] [PubMed] [Google Scholar]
- 34.Smith H W, Huggins M B, Shaw K M. Factors influencing the survival and multiplication of bacteriophages in calves and their environment. J Gen Microbiol. 1987;133:1127–1135. doi: 10.1099/00221287-133-5-1127. [DOI] [PubMed] [Google Scholar]
- 35.Soothill J S. Treatment of experimental infections of mice with bacteriophages. J Med Microbiol. 1992;37:258–261. doi: 10.1099/00222615-37-4-258. [DOI] [PubMed] [Google Scholar]
- 36.Soothill J S. Bacteriophage prevents destruction of skin grafts by Pseudomonas aeruginosa. Burns. 1994;20:209–211. doi: 10.1016/0305-4179(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 37.Stickler D, Hewitt P. Activity of antiseptics against biofilms of mixed bacterial species growing on silicone surfaces. Eur J Clin Microbiol Infect Dis. 1991;10:416–421. doi: 10.1007/BF01968021. [DOI] [PubMed] [Google Scholar]
- 38.Wang P, Granados R R. An intestinal mucin is the target substrate for a baculovirus enhancin. J Microbiol. 1999;13:6977–6982. doi: 10.1073/pnas.94.13.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wimpenny J W T. The spatial organisation of biofilm. In: Wimpenny J W T, Nichols W W, Stickler D J, Lappin-Scott H M, editors. Bacterial biofilms and their control in medicine and industry. Cardiff, United Kingdom: Bioline Publications; 1993. pp. 1–6. [Google Scholar]
- 40.Zottola E A. Microbial attachment and biofilm formation: a new problem for the food industry? Food Technol. 1994;48:107–113. [Google Scholar]