Native Plasmids of Fusobacterium nucleatum: Characterization and Use in Development of Genetic Systems (original) (raw)
Abstract
Three native plasmids of Fusobacterium nucleatum were characterized, including DNA sequence analysis of one plasmid, pFN1. A shuttle plasmid, pHS17, capable of transforming Escherichia coli and F. nucleatum ATCC 10953 was constructed with pFN1. pHS17 was stably maintained in the F. nucleatum transformants, and differences in the transformation efficiencies suggested the presence of a restriction-modification system in F. nucleatum.
Fusobacterium nucleatum is a gram-negative anaerobe of interest due to its central role in the ecology of dental plaque, a complex microbial biofilm that forms on teeth (6, 17), and its association with human infections (5, 12, 21, 22). A significant hindrance to the study of F. nucleatum is the lack of genetic and molecular systems for the construction of trait-specific isogenic mutants which are essential for delineation of gene function in the native cell background. A homologous family of native cryptic plasmids has been reported to occur in 18% of the F. nucleatum strains examined (20). This study was initiated to investigate the utility of native plasmids in the development of gene transfer systems for F. nucleatum. Data are presented on the characterization of three native F. nucleatum plasmids isolated in our laboratory, including determination and analysis of the complete DNA sequence of one plasmid, pFN1. Also described are the use of pFN1 in the construction of an intergeneric shuttle plasmid in Escherichia coli, transformation of F. nucleatum with the shuttle plasmid, and analysis of its stability in the F. nucleatum host cell background. To our knowledge, this is the first report of transformation of F. nucleatum by electroporation.
Isolation and characterization of F. nucleatum plasmids.
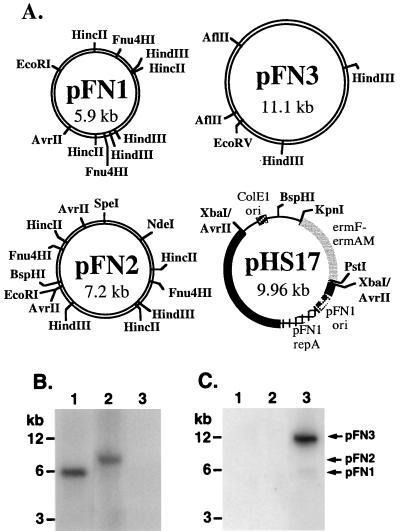
Three native plasmids (pFN1, pFN2, and pFN3) (Table 1; Fig. 1A) were isolated from strains of F. nucleatum by routine techniques (Wizard Plus Minipreps [Promega, Madison, Wis.]; Midi Preps [Qiagen, Inc., Valencia, Calif.]) and visualized on ethidium-stained 0.8% agarose gels. Restriction endonuclease mapping (16) demonstrated that the plasmids varied in size and in the occurrence of several restriction endonuclease sites (Fig. 1A), suggesting that the plasmids were unrelated. However, Southern hybridization studies (15) indicated that pFN1 and pFN2 share homology with each other but not with pFN3 (Fig. 1B and C). Nitrocellulose blots of plasmid and chromosomal DNA preparations from the plasmid-containing host strains were probed with pFN1 and pFN3 DNA. The pFN1 probe hybridized to pFN1 and pFN2 DNA but not pFN3 DNA (Fig. 1B), whereas the pFN3 probe hybridized only to pFN3 DNA (Fig. 1C). No hybridization to chromosomal DNA from any of the host strains was evident (data not shown). The strain harboring pFN3, ATCC 10953, was previously reported to lack plasmid DNA (20). Due to this discrepancy, we obtained a new culture from the American Type Culture Collection (Rockville, Md.) and confirmed the presence of pFN3 in this strain. These data reveal the existence of two nonhomologous groups of plasmids indigenous to F. nucleatum, the first represented by pFN1 and pFN2 and the second represented by pFN3.
TABLE 1.
Relevant characteristics of the bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Relevant characteristicsa | Source and/or reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | Erys | Life Technologies |
| F. nucleatum 12230 | Clns; transtracheal isolate and source of pFN1 | S. Finegold, Wadsworth Anaerobe Laboratory, West Los Angeles VA Medical Center, Los Angeles, Calif. |
| F. nucleatum 10113 | Clns; clinical isolate and source of pFN2 | S. Finegold |
| F. nucleatum ATCC 10953 | Clns; gingival isolate and source of pFN3 | American Type Culture Collection |
| Plasmids | ||
| pBluescript SK(−) | Ampr; 3.0-kb E. coli cloning vector | Stratagene Cloning Systems |
| pVA2198 | Eryr; 9.2-kb plasmid with the ermF-ermAM cassette | F. Macrina (8) |
| pFN1 | 5.9-kb native F. nucleatum plasmid | This study |
| pFN2 | 7.2-kb native F. nucleatum plasmid | This study |
| pFN3 | 11.1-kb native F. nucleatum plasmid | This study |
| pHS17 | Amps Eryr; 10.0-kb plasmid consisting of pFN1, ermF-ermAM, and pBluescript minus its ampicillin resistance determinant | This study |
| pHS19 | Amps Eryr; 4.1-kb plasmid consisting of ermF-ermAM and pBluescript minus its ampicillin resistance determinant | This study |
FIG. 1.
Restriction maps and Southern blots of the F. nucleatum plasmids. (A) Partial restriction map of F. nucleatum plasmids pFN1, pFN2 and pFN3 and shuttle plasmid pHS17. Selected restriction endonuclease sites in the native plasmids are presented. Restriction endonuclease sites indicated for pHS17 relate to the plasmid construction. The pFN1 portion of pHS17 is indicated by the thick solid bar, with the position of the repA homologue (ORF5) and putative ori indicated. (B and C) Plasmids from F. nucleatum strains 12230 (pFN1, lanes 1), 10113 (pFN2, lanes 2), and ATCC 10953 (pFN3, lanes 3) were probed with pFN1 DNA with _Eco_RI digests (B) or pFN3 DNA with _Eco_RV digests (C). The _Hin_cII-digested pFN1 and _Ase_I-digested pFN3 probes were 32P labeled (specific activity of 25 × 108 and 4.5 × 108 dpm/μg of DNA, respectively). The positions of molecular size markers are indicated on the left, and the linear forms of pFN1, pFN2, and pFN3 are indicated on the right.
Determination and analysis of pFN1 DNA sequence.
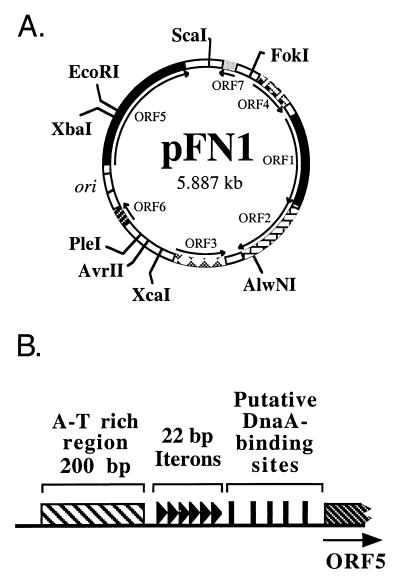
Due to its small size and superior plasmid yields, pFN1 was chosen for further analysis. The DNA sequence was determined for both strands. Analysis of the compiled sequence revealed a circular structure of 5,887 bp with 23% G+C content and seven putative open reading frames (ORFs) (defined as ≥150 bp [Fig. 2A]). Similarity searches were performed using the National Center for Biotechnology Information BLAST server (1, 2). The sequence of pFN1 was highly homologous to the sequence of a 6,281-bp F. nucleatum plasmid (pAD52; GenBank accession no. AF022647). No similarity was found to any gene encoding antibiotic resistance or other selectable phenotypic marker. Antibiotic susceptibility testing indicated that the pFN1 host strain F. nucleatum 12230 was susceptible to penicillin G, tetracycline, chloramphenicol, clindamycin, cefoxitin, ampicillin-sulbactam, imipenem, metronidazole, and streptomycin and resistant to erythromycin at a concentration of 25 μg/ml, as is common in other strains of F. nucleatum (7). These data suggested that pFN1 is a cryptic plasmid with respect to antibiotic resistance, comparable to previous findings with this group of plasmids (20).
FIG. 2.
Physical characteristics of pFN1 based on DNA sequence analysis. (A) ORFs, the putative origin of replication (ori), and selected restriction endonuclease sites are indicated. (B) Structural elements of putative origin of replication found upstream of ORF5, the repA homologue. The putative origin contains an A-T-rich region (crosshatched bar), six perfect 22-bp direct repeats (▸) termed iterons, and several putative DnaA-binding sites (█).
ORF1 is related to DNA relaxase (mobilization) proteins which mediate the initiation of conjugal transfer of plasmid DNA (13). Alignment of the complete predicted amino acid sequences using Clustal W (11, 28) of ORF1 with Staphylococcus plasmid relaxases demonstrated 23 to 29% identity and 30 to 34% similarity. Homology to the four regions of the consensus sequence defined for relaxase proteins (13) was evident (Table 2). The putative active tyrosine was the only residue of N-terminal motif 3 of the consensus sequence identical to a residue of the pFN1 ORF1, but this motif demonstrated a weak consensus sequence in the other proteins analyzed (13). Additional studies are needed to clarify the functional properties of this putative relaxase in F. nucleatum.
TABLE 2.
Amino acid alignment of fusobacterial and staphylococcal plasmid relaxase proteins within the relaxase consensus sequence motifs
| Plasmid and protein | Amino acid alignment in motif: | |||
|---|---|---|---|---|
| 3 | z | 2a | 2 | |
| Consensusa | -----n-Y-- | ----HuUU-Sf--ge | u-ua--uH-d- | --pHuHuuu--u----- |
| Alternate | w t | u | ||
| * | ***** | ***** | ****** | |
| pFN1 ORF1b | LYNILK-YV- 41 | GRQYRHHIQSFKPGE 20 | FDVFISTHIDK | GHIHNHIIINTVNIDTG |
| :* | ******:* | : * :***: | *****:**:: ::** | |
| pS194 R1x | SRA-IN-YA- 33 | VQA-HTVIQSFKPGE 20 | YQVAVYTHTDK | DHYHNHIIINSVNLETG |
| pC223 Orf1 | SRA-IN-YA- 33 | NEG-HVVIQSFKPNE 20 | HQVQVYTHNDT | DHVHNHIVINSIDLETG |
| pC221 OrfA | SRA-IN-YA- 33 | IQA-HTVIQSFKPGE 20 | HWVAVYTHTDK | DHYHNHIVINSVDLETG |
| pSK639 ORF337 | SRA-IN-YA- 32 | GNQGHVIIQSFKPGE 20 | HQVAVYTHNDT | DHVHNHIVINSIDLETG |
| pIP1630 MobA2 | SRA-IN-YA- 32 | GNQGHVIIQSFKPGE 20 | HQVAVYTHNDT | DHVHNHIVINSIDLETG |
| pIP1629 MobA1 | SRA-IN-YA- 32 | GNQGHVIIQSFKPGE 20 | HQVAVYTHADT | DHVHNHIVMNSIDLETG |
ORF5 analyses indicated that it is related to plasmid replication proteins, including those of Lactobacillus acidophilus plasmid pLA103 (14), Staphylococcus aureus plasmid pJE1 (4), and Pediococcus halophilus plasmid pUCL287 (3). Alignment of the complete ORFs of homologues with pFN1 ORF5 demonstrated 10 to 19% identity and 21 to 34% similarity. The association of ORF5 with replication was strongly supported by analyses of the upstream DNA sequence, which demonstrated six perfect 22-bp direct repeats (or iterons) preceded by an approximately 200-bp A-T-rich region (Fig. 2B). Multiple putative DnaA binding sites were also identified, based on matching 8 of the 9 bp comprising the DnaA binding consensus sequence (26). This organization is characteristic of the origin of replication of iteron-regulated theta-replicating plasmids (10). A general model of replication initiation involves the binding of the plasmid replication protein to the iteron sequences, resulting in structural changes (including melting of the adjacent A-T-rich region) to form an open complex. The replication protein, possibly in conjunction with the host DnaA protein, is then responsible for guiding host replication proteins into the open complex (10). It is also significant that the pFN1 replication protein homologue was related to the replication protein of pUCL287, which has been shown to utilize a theta mode of replication (3).
Transformation of F. nucleatum with shuttle plasmid pHS17.
The shuttle plasmid pHS17 (Fig. 1) was constructed sequentially in E. coli DH5α cells (Life Technologies, Gaithersburg, Md.) as follows: _Avr_II-digested pFN1 was cloned into the _Xba_I site of pBluescript; an ermF-ermAM cassette (8) was added by cloning into _Kpn_I-_Pst_I sites; and the pBluescript ampicillin resistance determinant was deleted by digestion with flanking _Bsp_HI sites. The resulting construct included both E. coli and F. nucleatum origins of replication from pBluescript and pFN1, respectively. The junctions of DNA fragments joined by cloning were confirmed by DNA sequencing, and the phenotypic properties of the construct were confirmed on selective medium.
Transformation studies were performed with plasmid DNA isolated by alkaline lysis-column purification techniques (Promega Wizard Plus Minipreps; Qiagen Midi Preps) and further purified by cesium chloride-ethidium bromide density gradient centrifugation (25). Bacterial cells were washed and resuspended in electroporation buffer (8) at a calculated optical density of 6.0, and 100-μl aliquots were electroporated by standard techniques (27). The electroporated cells were immediately diluted in 0.9 ml of Columbia broth (BBL Microbiology Systems, Cockeysville, Md.) with MgCl2, and the number of viable cells were determined by plating a diluted aliquot on nonselective medium. The transformation mix was incubated anaerobically, followed by plating on Columbia agar (BBL Microbiology Systems) with clindamycin. Variables examined included the bacterial cell growth phases (early log, mid-log, and stationary phases), the source of pHS17 DNA (heterologous versus homologous host sources), electroporation parameters (resistance of 50 to 500 Ω; field strength of 24 or 25 kV/cm; capacitance of 25 or 50 μF), the concentration of MgCl2 in the Columbia broth (0.5, 1.0, and 2.0 mM), and the clindamycin concentration used in the selective medium (0.2 or 0.4 μg/ml).
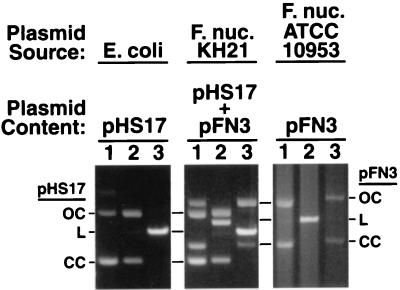
Initial attempts to transform F. nucleatum ATCC 10953 with pHS17 were successful under previously defined conditions (9, 24, 27). Preliminary results indicated optimal recovery of transformants with a field strength of 25 kV/cm, a capacitance of 25 μF, and resistance ranging from 200 to 400 Ω. Analysis of the transformants revealed the presence of pHS17 and the ATCC 10953 native plasmid pFN3. The two plasmids were easily distinguished, based on their sizes and restriction endonuclease digestion patterns (Fig. 3). Electroporation controls included nonelectroporated cells with or without the addition of DNA as well as electroporated cells without DNA added, and all yielded negative results. Electroporation with pHS19 also yielded negative results, suggesting that pFN1 is essential for replication in F. nucleatum. The single parameter found to have a major effect on transformation efficiency was the pHS17 DNA source. Transformation efficiency using 1 μg of plasmid DNA ranged from 1.6 × 102 to 2 × 102 transformants per μg of DNA from the homologous F. nucleatum host, as compared to no transformants with DNA from the heterologous E. coli host (Table 3). Transformation efficiency was optimal at a resistance setting of 200 Ω with the homologous host DNA, although pronounced differences were not evident over the range examined. Transformation with 5 μg of E. coli pHS17 DNA was demonstrated; however, the efficiency was still less than that observed with 1 μg of homologous DNA. The 100-fold or greater increase in transformation efficiency with homologous DNA suggests the presence of a functional restriction-modification system in F. nucleatum ATCC 10953. Restriction-modification systems have been found in F. nucleatum (18, 19). Growth phase also influenced the transformation efficiency, but to a lesser extent than the DNA source. Increased transformation efficiencies were routinely obtained with early-log-phase cells. For example, in one experiment using early-log-phase, mid-log-phase, and stationary-phase recipient cells and an outgrowth period of 5 h (approximately two generations), the transformation efficiencies were 7.2 × 103, 4.8 × 103, and 5.0 × 103, respectively. No significant differences were observed with variations in the concentration of MgCl2 in the outgrowth broth or with 0.2 versus 0.4 μg of clindamycin per ml in the selective agar medium.
FIG. 3.
Plasmid DNA from F. nucleatum ATCC 10953 transformants consists of the shuttle plasmid, pHS17, and the native plasmid, pFN3. Plasmid preparations from E. coli (pHS17), F. nucleatum ATCC 10953 transformant strain KH21 (pHS17 and pFN3), and F. nucleatum ATCC 10953 (pFN3) were analyzed. The preparations were either not digested (lanes 1) or predigested with _Eco_RV (lanes 2) or _Eco_RI (lanes 3), separated on 0.8% agarose gels, stained with ethidium bromide, and visualized under UV illumination. The open circular (OC), linear (L), and covalently closed circular (CC) forms of pHS17 and pFN3 are indicated on the left and right, respectively.
TABLE 3.
Transformation efficiency in F. nucleatum ATCC 10953 with DNA isolated from homologous versus heterologous strainsa
| Resistance (Ω)b | Transformation efficiency | ||
|---|---|---|---|
| Plasmid DNA from heterologous strain (pHS17) | Plasmid DNA (1 μg) from homologous strain (pHS17 + pFN3) | ||
| 1 μg | 5 μg | ||
| 400 | 0 | 1.2 × 101 | 1.6 × 102 |
| 300 | 0 | 0 | 1.9 × 102 |
| 200 | 0 | 1 | 2.0 × 102 |
Stability of shuttle plasmid in F. nucleatum transformants.
The structural stability of pHS17 in representative transformants was evaluated by restriction endonuclease mapping, PCR amplification of pHS17-specific DNA regions, and Southern analysis of the transformant DNA with pFN1 and pHS17 DNA probes (data not shown). In all of the analyses done, no evidence of DNA rearrangement or deletion was detected. The segregational stability of pHS17 was examined in the transformant strain KH21, maintained in liquid cultures without antibiotic selection (23). After 100 generations, the percentage loss of plasmid per generation was 0.02, with an average of 98% of the viable cells demonstrating the clindamycin resistance phenotype. The shuttle plasmid was present in all colonies subcultured at baseline and after 100 generations, with no evidence of DNA rearrangement or deletion. Thus, pHS17 was found to be both structurally and segregationally stable in the F. nucleatum host cell background. Interestingly, both pHS17 and pFN3 were stably maintained in the transformants, indicating that these two plasmids are compatible and that pFN3 may be useful in developing plasmid vectors for use in conjunction with pFN1-derived plasmids.
The data presented here document key findings that provide a foundation for developing F. nucleatum genetic systems. These findings include the following: the occurrence of two distinct groups of plasmids native to F. nucleatum, one of which appears to be a theta-replicating iteron-regulated plasmid; the ability of the ermF-ermAM cassette to confer clindamycin resistance in F. nucleatum; the ability of F. nucleatum to be transformed by electroporation using the pFN1-derived shuttle plasmid; and the stability of the shuttle plasmid in F. nucleatum. Additional studies are in progress to examine the copy number of the native plasmids and to confirm the mode of replication of the pFN1 group of plasmids. Further, we are actively pursuing strategies to identify additional selectable markers and further optimize the structural features of the shuttle plasmid for use in molecular manipulations of fusobacteria.
Nucleotide sequence accession numbers.
The nucleotide sequences of pFN1 and the ermF-ermAM cassette (8) have been deposited in the GenBank database under accession no. AF159249 and AF219231, respectively.
Acknowledgments
Grants from the UCLA School of Dentistry Opportunity Fund and the UCLA Academic Senate and PHS grant DE12639 to S.K.H. supported this work.
The authors thank E. Lee for technical assistance, V. L. Miller and D. A. Haake for helpful discussions and review of this material, and S. Hunt Gerardo for editorial review.
Footnotes
†
We dedicate this work to the memory of Susan E. Valone in recognition of her support and contributions to this research.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benachour A, Frere J, Novel G. pUCL287 plasmid from Tetragenococcus halophila (Pediococcus halophilus) ATCC 33315 represents a new theta-type replicon family of lactic acid bacteria. FEMS Microbiol Lett. 1995;128:167–176. doi: 10.1111/j.1574-6968.1995.tb07518.x. [DOI] [PubMed] [Google Scholar]
- 4.Berg T, Firth N, Apisiridej S, Hettiaratchi A, Leelaporn A, Skurray R A. Complete nucleotide sequence of pSK41: evolution of staphylococcal conjugative multiresistance plasmids. J Bacteriol. 1998;180:4350–4359. doi: 10.1128/jb.180.17.4350-4359.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolstad A I, Jensen H B, Bakken V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin Microbiol Rev. 1996;9:55–71. doi: 10.1128/cmr.9.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradshaw D J, Marsh P D, Watson G K, Allison C. Role of Fusobacterium nucleatum and coaggregation in anaerobe survival in planktonic and biofilm oral microbial communities during aeration. Infect Immun. 1998;66:4729–4732. doi: 10.1128/iai.66.10.4729-4732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brazier J S, Citron D M, Goldstein E J C. A selective medium for Fusobacterium spp. J Appl Bacteriol. 1991;71:343–346. doi: 10.1111/j.1365-2672.1991.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher H M, Schenkein H A, Morgan R M, Bailey K A, Berry C R, Macrina F L. Virulence of a Porphyromonas gingivalis W83 mutant defective in the prtH gene. Infect Immun. 1995;63:1521–1528. doi: 10.1128/iai.63.4.1521-1528.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher M. Measurement of glucose utilization by Pseudomonas fluorescens that are free-living and that are attached to surfaces. Appl Environ Microbiol. 1986;52:672–676. doi: 10.1128/aem.52.4.672-676.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helinski D R, Toukdarian A E, Novick R P. Replication control and other stable maintenance mechanisms of plasmids. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C.: ASM Press; 1996. pp. 2295–2324. [Google Scholar]
- 11.Higgins D G. CLUSTAL V: multiple alignment of DNA and protein sequences. Methods Mol Biol. 1994;25:307–318. doi: 10.1385/0-89603-276-0:307. [DOI] [PubMed] [Google Scholar]
- 12.Holst E, Goffeng A R, Andersch B. Bacterial vaginosis and vaginal microorganisms in idiopathic premature labor and association with pregnancy outcome. J Clin Microbiol. 1994;32:176–186. doi: 10.1128/jcm.32.1.176-186.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilyina T V, Koonin E V. Conserved sequence motifs in the initiator proteins for rolling circle DNA replication encoded by diverse replicons from eubacteria, eukaryotes and archaebacteria. Nucleic Acids Res. 1992;20:3279–3285. doi: 10.1093/nar/20.13.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanatani K, Tahara T, Oshimura M, Sano K, Umezawa C. Identification of the replication region of Lactobacillus acidophilus plasmid pLA103. FEMS Microbiol Lett. 1995;133:127–130. doi: 10.1111/j.1574-6968.1995.tb07872.x. [DOI] [PubMed] [Google Scholar]
- 15.Kinder S A, Badger J L, Bryant G O, Pepe J C, Miller V L. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O8 and construction of a R−M+ mutant. Gene. 1993;136:271–275. doi: 10.1016/0378-1119(93)90478-l. [DOI] [PubMed] [Google Scholar]
- 16.Kinder Haake S, Wang X. Cloning and expression of fomA, the major outer membrane protein gene from Fusobacterium nucleatum T18. Arch Oral Biol. 1997;42:19–24. doi: 10.1016/s0003-9969(96)00105-7. [DOI] [PubMed] [Google Scholar]
- 17.Kolenbrander P E, London J. Adhere today, here tomorrow: oral bacterial adherence. J Bacteriol. 1993;175:3247–3252. doi: 10.1128/jb.175.11.3247-3252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung D W, Lui A C P, Merilees H, McBride B C, Smith M. A restriction enzyme from Fusobacterium nucleatum 4H which recognizes GCNGC. Nucleic Acids Res. 1979;6:17–25. doi: 10.1093/nar/6.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lui A C P, McBride B C, Vovis G F, Smith M. Site specific endonuclease from Fusobacterium nucleatum. Nucleic Acids Res. 1979;6:1–15. doi: 10.1093/nar/6.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKay T L, Ko J, Bilalis Y, DiRienzo J M. Mobile genetic elements of Fusobacterium nucleatum. Plasmid. 1995;33:15–20. doi: 10.1006/plas.1995.1003. [DOI] [PubMed] [Google Scholar]
- 21.Mikamo H, Kawazoe K, Sato Y, Tamaya T. Elastase activity of anaerobes isolated from amniotic fluid with preterm premature rupture of membranes. Am J Obstet Gynecol. 1999;180:378–380. doi: 10.1016/s0002-9378(99)70217-6. [DOI] [PubMed] [Google Scholar]
- 22.Moore W E C, Moore L V H. The bacteria of periodontal diseases. Periodontol 2000. 1994;5:66–77. doi: 10.1111/j.1600-0757.1994.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 23.Roberts R C, Helinski D R. Definition of the minimal plasmid stabilization system from the broad-host-range plasmid RK2. J Bacteriol. 1992;174:8119–8132. doi: 10.1128/jb.174.24.8119-8132.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosey E L, Kennedy M J, Petrella D K, Ulrich R G, Yancey R J., Jr Inactivation of Serpulina hyodysenteriae flaA1 and flaB1 periplasmic flagellar genes by electroporation-mediated allelic exchange. J Bacteriol. 1995;177:5959–5970. doi: 10.1128/jb.177.20.5959-5970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Schaefer C, Messer W. DnaA protein/DNA interaction. Modulation of the recognition sequence. Mol Gen Genet. 1991;226:34–40. doi: 10.1007/BF00273584. [DOI] [PubMed] [Google Scholar]
- 27.Sreenivasan P K, LeBlanc D J, Lee L N, Fives-Taylor P. Transformation of Actinobacillus actinomycetemcomitans by electroporation, utilizing constructed shuttle plasmids. Infect Immun. 1991;59:4621–4627. doi: 10.1128/iai.59.12.4621-4627.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]