Viability and Recovery of Peripheral Blood Mononuclear Cells Cryopreserved for up to 12 Years in a Multicenter Study (original) (raw)
Abstract
The Multicenter AIDS Cohort Study (MACS), an ongoing prospective study of the natural history of human immunodeficiency virus (HIV), has stored biologic specimens, including peripheral blood mononuclear cells (PBMC), from 5,622 participants for up to 12 years. The purpose of the present analysis was to evaluate the quality of the PBMC in the MACS repository in order to test the validity and feasibility of nested retrospective studies and to guide the planning of future repositories. PBMC were collected from MACS participants at four centers at 6-month intervals from 1984 to 1995, cryopreserved, and transported to a central repository for storage. A total of 596 of these specimens were subsequently tested for viability and used to evaluate cell function, to conduct immunophenotype analysis, or to isolate HIV. Simple linear regression models were applied to evaluate trends in recovery and viability over time and by center. Results indicated that from a nominal 107 cells cryopreserved per vial at all four centers, the median number of viable cells recovered was at least 5 × 106 (50% of the number stored) and the median viability was at least 90%. Results suggested that cryopreserved cells can be stored for at least 12 years with no general tendency toward cell loss over time. Furthermore, there were no statistically significant changes in the percent cell viability according to the length of time frozen, regardless of HIV serostatus or the level of CD4+ lymphocytes. Storing 107 PBMC per vial yields sufficient viable cells for phenotypic and/or functional analysis. Results from the MACS provide the basis for the planning of future repositories for use by investigators with similar research goals.
Repositories of biologic specimens from cohort studies are valuable resources for medical research. Newly developed assays can take advantage of the specimens in the repository to measure markers that might have been available at the time the samples were obtained. Although many long-term cohort studies invest substantial resources in establishing repositories of specimens that are key for intensive laboratory pathogenesis studies, seldom have reports documenting the quality and utility of those repositories been prepared. The preservation of viable peripheral blood mononuclear cells (PBMC) for virologic and immunologic investigations has been undertaken by several multicenter studies of human immunodeficiency virus (HIV) disease, but few data are available on how long materials can be stored, on typical numbers of cells recovered after different intervals, or on the uses for which such samples are appropriate. Every sample frozen in a cohort study is potentially valuable, and care must be taken to determine the best application for these specimens. Therefore, the random testing of such samples simply to validate the quality of materials is avoided. However, if investigators plan to use samples from repositories for important issues such as genetic research or the investigation of other surrogate markers of disease, measures of the quality and validity of the samples are imperative.
In studying the natural history of HIV, properly preserved PBMC specimens have proven to be invaluable to elucidate HIV pathogenesis. The Multicenter AIDS Cohort Study (MACS) is an ongoing prospective study of the natural history of HIV infection in 5,622 homosexual or bisexual men, conducted since 1984 in four centers located in Baltimore, Md.; Chicago, Ill.; Pittsburgh, Pa.; and Los Angeles, Calif. Details about the recruitment and characteristics of the MACS cohort and the study protocol have been reported previously (2, 10). Applications for the study have included culturing of virus, studies of immune function, immunophenotyping by flow cytometry, and assays of immune function. The purpose of this analysis was to evaluate the viability and recovery of stored PBMC specimens from the MACS that were frozen and thawed over a 12-year follow-up period in conjunction with specific scientific research initiatives utilizing these specimens. This information may be helpful in planning future repositories or as a comparison for other studies.
MATERIALS AND METHODS
Cryopreservation, shipment, and storage of lymphocytes.
Ninety-milliliter blood samples were collected at 6-month intervals in heparinized tubes (Becton Dickinson Inc., Rutherford, N.J.). The method used was essentially that described previously in detail (8). Whole blood was centrifuged for 15 min at 300 × g. Next, the buffy coats for each sample were placed in a 50-ml polypropylene tube, and an equal volume of isotonic saline was added. This was overlaid onto Ficoll-Hypaque density gradients. Mononuclear cell suspensions were isolated by centrifugation at 700 × g for 10 min. The cell pellets were washed twice at room temperature with Hanks buffered salt solution by centrifugation at 300 × g for 10 min. The final pellets were resuspended in 2 ml of RPMI 1640 containing 20% human AB serum or fetal calf serum, and the suspensions were gently mixed and chilled on ice. Cells were counted on a Coulter Counter in the centers in Baltimore and Los Angeles by using 20 μl of cell suspension in 10 ml of isotonic buffered saline plus 3 drops of Zap-oglobin2 lysing solution or on a hemocytometer in the centers in Chicago and Pittsburgh. Cells were diluted to 2 × 107 cells per ml in cold RPMI 1640 plus 20% serum. Then, an equal volume of RPMI 1640 plus 20% dimethyl sulfoxide (DMSO) as a cryoprotectant was added. Next, the cells were quickly aliquoted into freezing vials (1 ml per vial). Usually six vials were saved for each blood specimen, and each vial contained 107 PBMC. The final freezing solution consisted of RPMI 1640 containing 10% serum and 10% DMSO. All blood samples were processed and frozen by sterile techniques, within 24 h (range, 5 to 24 h) of collection, at the center where they were obtained. For the vast majority of samples, freezing occurred in less than 10 h after collection.
For the initial part of the cryopreservation procedure, cells were placed overnight either in controlled-rate freezers (Gordinier Cryogenics Electronics, Roseville, Mich.), used in the center in Los Angeles from the initiation of the study and in the center in Pittsburgh after August 1988, or in insulated containers (Sigma, St. Louis, Mo.) filled with isopropanol, used in the centers in Baltimore, Chicago, and Pittsburgh before August 1988. Cryovials from the controlled-rate freezers were transferred directly to −135°C freezers (Queue Systems Pacific Sciences, Inc., El Segundo, Calif.) or into the vapor phase of liquid nitrogen for long-term storage. Cryovials within the insulated containers were placed in a −70°C freezer overnight and transferred to the long-term, colder storage the next morning. Frozen tubes were shipped in a customized TA-60 dry-nitrogen shipper (MVE, New Prague, Minn.) to Biomedical Research Incorporated (BRI) (Rockville, Md.), which, under contract to the National Institutes of Health Division of AIDS, received, catalogued, stored, and dispersed clinical specimens from MACS participants in Division of AIDS studies. MACS samples have now been stored at BRI in vapor-phase freezers for up to 12 years. Data in this report include those for samples collected through 1995.
Thawing cryopreserved cells and determining viability.
Samples for this study were shipped from BRI in dry-nitrogen shippers (see above) and were then stored continuously in liquid nitrogen or at −135°C until immediately prior to their use. The method used was essentially that described previously (8). Cells were removed from storage and rapidly thawed by immersion in a 37°C water bath with gentle agitation, by trained technical staff in two MACS pathogenesis laboratories. Each tube was thawed individually until only a small amount of ice crystals remained, a procedure that took about 40 s. Samples were immediately transferred to round-bottom tissue culture tubes. Then, 10 to 14 ml of RPMI 1640 containing 10% human AB serum or fetal calf serum was added over a period of 4 min with agitation, and the tubes were centrifuged immediately at 100 to 400 × g for 10 min. After the DMSO-containing liquid had been removed by aspiration, the cells were washed once in medium containing at least 10% serum or an alternate protein source. Next, the cells were resuspended in about 1 ml of buffer appropriate to the assay to be done, and counts and viability were determined with a hemocytometer and the trypan blue dye exclusion technique. With this method, dead cells appear blue and are therefore distinguishable from viable cells. Viable cells for this project were defined as PBMC that had survived the freeze-thaw process. They were used (i) to evaluate cell function, (ii) for immunophenotype analysis by flow cytometry, (iii) to isolate HIV for subsequent analysis, or (iv) for immortalization by Epstein-Barr virus transformation.
Statistical analysis.
Data reported to the Center for the Analysis and Management of the MACS included (i) the date the sample was originally collected, processed, and frozen (freeze date); (ii) the date the sample was thawed for laboratory testing (thaw date); (iii) the total number of cells recovered regardless of viability; and (iv) the percent cell viability. The number of viable cells recovered was calculated by multiplying items iii and iv. We addressed two main questions: first, whether cell recovery and viability differed over time according to the duration of freezing, and second, whether cell viability differed by center, the original processing or freeze date, the HIV serostatus of the donor, and the CD4+-lymphocyte count of the donor.
The number of viable cells and cell viability were summarized via percentiles, overall and across centers. Simple linear regression models were used to evaluate trends in viability over time with regard to the length of time frozen (thaw date − freeze date) and the initial date of sample collection and processing. Nonparametric Kruskal-Wallis tests (4) were applied to assess the significance of observed differences in viability by center, serostatus, and CD4+-cell count.
RESULTS
Viability testing.
A total of 596 PBMC samples from 147 individuals were tested for cell viability. They had initially been stored at one of the four MACS centers (179 samples in Baltimore, 170 in Chicago, 88 in Pittsburgh, and 159 in Los Angeles). The center where the sample was originally collected and stored may or may not coincide with the city or laboratory where the sample was thawed, evaluated for cell viability, and used for a pathogenesis study. A total of 197 specimens were from HIV-seronegative donors, and 399 specimens were from HIV-seropositive donors.
Of the 596 PBMC samples evaluated, the median number of total cells recovered per vial across all centers was 7.75 × 106. The 10th and 90th percentiles were 3.9 × 106 and 14.0 × 106, respectively, and the range was 1 × 106 to 25 × 106 total cells recovered per vial. The viability assessment of these cells by trypan blue dye exclusion resulted in a median of 92% of the cells recovered remaining viable (range, 24 to 100%). The median number of viable cells recovered per vial across all centers was 6.9 × 106 (Table 1). The 10th and 90th percentiles were 3.5 × 106 and 12.9 × 106, respectively, and the range was 0.4 × 106 to 23 × 106 viable cells recovered per vial. The distributions of numbers of total and viable cells recovered and of percent cell viability were significantly different across centers (P < 0.05 [Kruskal-Wallis test]). However, the median number of viable cells was at least 5 × 106 for each center, and the median viability was at least 90%. Recovery was often considerably lower than the expected 6 × 106 cells. At the same time, recovery from some vials, especially those stored in the center in Chicago early in the study, was higher than 107 cells, indicating that more cells than prescribed by the protocol must have been stored.
TABLE 1.
Numbers of viable cells recovered and percent viability of recovered cells overall and by center
| Location of center | No. of samples | 10th, 25th, 50th, 75th, and 90th percentiles | |
|---|---|---|---|
| Viable cells recovered (106) | % Viability of recovered cells | ||
| Total | 596 | 3.5, 4.7, 6.9, 9.6, 12.9 | 82, 89, 92, 96, 98 |
| Baltimore | 179 | 2.4, 3.9, 5.0, 7.2, 10.5 | 82, 88, 92, 95, 98 |
| Chicago | 170 | 4.3, 7.4, 10.0, 12.5, 15.5 | 82, 89, 94, 97, 99 |
| Pittsburgh | 88 | 2.6, 4.0, 6.0, 8.5, 9.2 | 71, 86, 90, 94, 96 |
| Los Angeles | 159 | 4.4, 5.2, 7.0, 8.8, 12.6 | 87, 90, 93, 96, 98 |
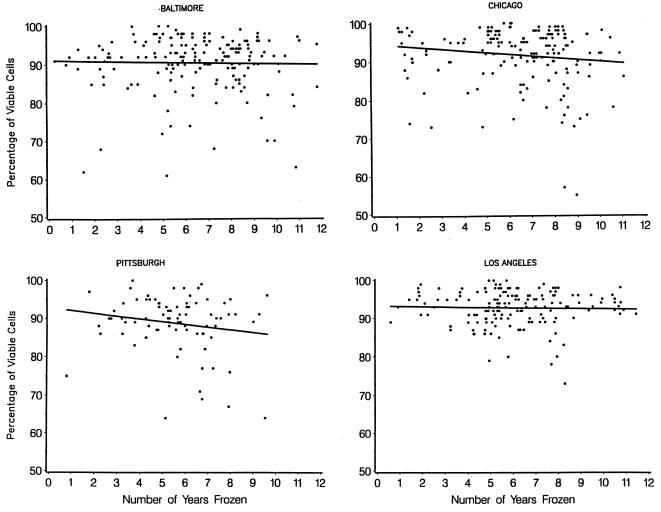
In order to address the center differences and analyze the effect of time on the viability and number of viable cells recovered, the data were evaluated separately by center. Four specimens from the center in Pittsburgh which yielded viabilities of less than 35% occurred early in the study and were excluded from the analysis. Scatter plots by center of the 596 individual cell viabilities (Fig. 1) and counts of viable cells (Fig. 2) over the length of time frozen (from 0 to 12 years), together with a corresponding regression line, were used to illustrate changes over time. There were statistically insignificant decreases in viability in all centers, with estimated losses per year of 0.10% in Baltimore, 0.47% in Chicago, 0.73% in Pittsburgh, and 0.09% in Los Angeles (Fig. 1). Overall, there was an estimated decrease (average slope) in viability of 2.9% over the entire 12 years of the study (0.24% per year), which was of borderline statistical significance (P = 0.06). Viabilities did not vary according to the year the sample was collected at the clinic center (P = 0.36 [data not shown]). Additionally, the median viabilities were 93% for seronegatives and 92% for seropositives, while the median numbers of viable cells were 6.4 × 106 and 7.2 × 106 for seronegatives and seropositives, respectively. These differences were not statistically significant.
FIG. 1.
Percent cell viability over time by center. β̂ (per year) for Baltimore is −0.10%, that for Chicago is −0.47%, that for Pittsburgh is −0.73% (after excluding four specimens with <35% viability, originally stored from 1985 to 1986), and that for Los Angeles is −0.09%.
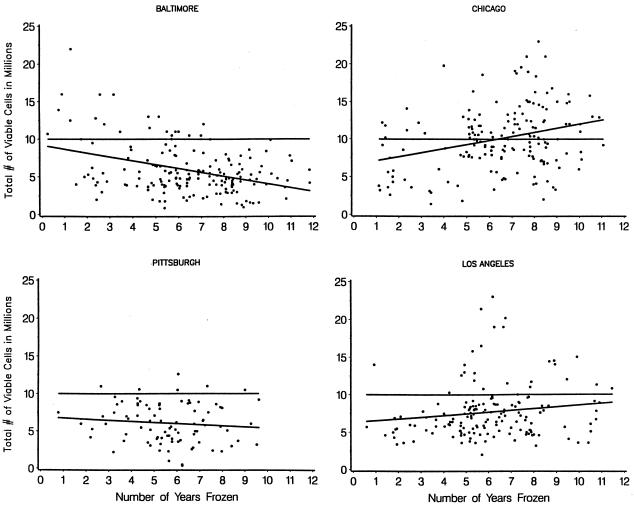
FIG. 2.
Viable-cell recovery over time by center. β̂ (per year) for Baltimore is −5.1 × 105, that for Chicago is 5.4 × 105, that for Pittsburgh is −1.5 × 105, and that for Los Angeles is 2.3 × 105. Reference lines at 107 mark the number of PBMC stored as suggested by the MACS protocol.
The rate of change for the number of viable cells recovered with respect to the number of years frozen differed by center. However, the directionality of the trends was not consistent for all centers (Fig. 2). Data from the centers in Baltimore and Pittsburgh revealed estimated losses of 5.1 × 105 and 1.5 × 105 cells per year, respectively. In contrast, the numbers of cells at the centers in Chicago and Los Angeles increased by an estimated 5.4 × 105 and 2.3 × 105 cells per year, respectively. The rates of change observed in the specimens from the Baltimore and Chicago centers were significantly different from 0 (P < 0.01).
The median number of CD4+ lymphocytes from the 399 samples from HIV-seropositive donors was 607/μl (range, 8 to 1,784/μl). After adjusting for the number of years frozen, there was a relatively strong positive association between the number of viable cells recovered and the CD4+-cell counts in the Chicago and Los Angeles samples (P ≤ 0.001). There was no apparent relationship between CD4+-cell count and total cell viability in the other two centers or between CD4+-cell count and percent viability in any center. Therefore, we conclude that our data do not indicate decreased viability in samples due to patients being immunosuppressed.
Data from the Pittsburgh center were used in order to assess the effect of using a controlled-rate freezer on viability by analyzing the results for two groups selected according to the method of cryopreservation within the same laboratory: (i) an isopropanol-filled insulated container used prior to August 1988 and (ii) a controlled-rate freezer used after August 1988. By using one laboratory for this assessment, we were able to control for differences by center that may be unrelated to the type of freezer container. There was a significant improvement in percent viability over time, with an estimated loss of 2.23% per year when alcohol-filled containers were used for freezing, compared to only a 0.01% loss per year with the controlled-rate freezer (P = 0.04). In order to assess whether the differences that we observed in the Pittsburgh center may be due to a learning curve, we repeated the analysis in the Los Angeles center, because a controlled-rate freezer had been used there consistently since the beginning of the study. We found no suggestion of improvement over time and therefore conclude that the trends seen in the center in Pittsburgh were not entirely due to a technique improved over time.
We also classified all 596 samples into two groups depending on the freezing process, that using a controlled-rate freezer (n = 198) and that using an alcohol-filled container (n = 398). Overall, there were no significant differences in the median viability (93 versus 92%) or the number of cells recovered (6.6 × 106 versus 7.0 × 106) between the two freezing methods. However, using cutoffs of 85, 80, 75, and 50%, there were consistently more samples below the cutoffs when the alcohol-filled container was used. For example, when we applied a cutoff of 80%, there were more samples with less than 80% viability when the alcohol-filled container was used (9.5%) than when the controlled-rate freezer was used (2.5%). Although consistent with the literature, these data alone are not sufficient to conclude that a controlled-rate freezer is better than an alcohol-filled container for the cryopreservation of cells.
In addition to the issues related to cell freezing, it is worth noting that after controlling for the center that cryopreserved the cells, significant differences in the median viable-cell recovery persisted between the two pathogenesis laboratories that thawed the samples (8.4 × 106 [n = 167] versus 6.2 × 106 [n = 429]).
Use of cryopreserved PBMC for pathogenesis studies.
One example of a typical use of the samples in this repository for pathogenesis studies is a study that included a subset of the 596 samples, a total of 112 samples from all time points between seroconversion and T-cell inflection (12), a span of up to 10 years. In all cases, one vial per person visit yielded sufficient cells to complete flow cytometry, analysis of cytotoxic-T-lymphocyte precursors, proviral HIV load measurements, and HIV sequencing (16). In another study, 37 of the 596 samples were from donors who were severely immunosuppressed (CD4+-cell counts, <50 per mm3). Every sample provided enough cells to obtain immunophenotyping results, and culturable virus was recovered from all except one of the samples (6). In another series of studies (1, 14), 118 samples from HIV-seropositive donors were tested for cell surface markers and immune function.
In other studies requiring viable cells where similar protocols for freezing and thawing were used but viability and recovery were not measured, comparably good results were obtained. For example, in a MACS virology laboratory, virus has been isolated from frozen viable cells in more than 90% of attempted isolations (13). In addition, 1,185 of 1,312 (90%) PBMC specimens used were transformed with Epstein-Barr virus for development of B lymphoblastoid cell lines used to extract DNA for genetic typing. Despite being cultured for up to several weeks these samples showed little or no bacterial contamination.
DISCUSSION
The data presented here indicate that 90% of the time, the yield of viable cells from cryopreserved MACS specimens was at least 3.5 × 106, with a viability of 82% or higher. By obtaining cell counts and measuring cell viability for each frozen sample as it was thawed for ongoing research, we established the consistency of the cryopreservation method across time and centers for MACS specimens.
We had anticipated the recovery of a minimum of 6 × 106 viable cells per vial from the initial 107 cells stored with a viability of at least 85%. Our results indicate that 519 of the 596 specimens met our goal for viability, but only slightly more than half of them met the goal for numbers of viable cells recovered. A reduction of recovery from 107 to 6 × 106 cells might be expected due to cell loss during the freeze-thawing and subsequent washing process. The reason why substantially fewer than the expected 6 × 106 cells per vial were recovered in some cases is not known but most likely relates in part to variations in protocol with regard to the number of cells originally placed in the vials. There was substantial variation in this protocol, as shown by the recovery of >107 cells from some vials and by the increased cell recovery in relation to the time frozen in the center in Chicago. Since the directionality of changes in cell recovery was not consistent across centers, cell loss in the MACS is probably not due to the length of time frozen.
Although additional data are needed for confirmation, the use of controlled-rate freezers and sample processing within 6 h of blood collection could have contributed to the low variability of data observed in the Los Angeles center. Furthermore, after switching to a controlled-rate freezer in the Pittsburgh center, there was a significant improvement in the maintenance of viability over time. However, it is important to note that the sample size was small (45 samples without and 39 with a controlled-rate freezer), and other possible confounding factors were not addressable.
Some variability in the numbers of cells recovered could be due to variations among technicians who performed the cell counts, but the MACS does not include data on this. In addition, differences in the methods and instruments for cell counting at the time of freezing could contribute to variation, but this possibility was also not assessed. The MACS team developed a method manual that was provided to all the technicians who stored cells. However, our data suggest that future studies may need to incorporate methods to reduce the contribution of technical variables to cell recovery by using cross-center training and standardization programs. Upon thawing, if the cells are used for assays of lymphocyte function, it may be important to determine the composition of the recovered cells, including the percentages of lymphocytes and monocytes, which was not done in the present study.
The experience with the 596 specimens reported here indicates that cells stored for up to 12 years can be used for immunophenotype analysis and for assays of specific anti-HIV immunity, as well as for the characterization of viral phenotype. Cryopreserved lymphocytes from the MACS have been used for the assay of specific HIV-directed memory cytotoxic-T-lymphocyte activity (15, 17), the recovery of HIV by cell culture (7), lymphocyte immunophenotyping by flow cytometry (3, 7), human lymphocyte antigen genetic typing (11), and the detection of inherited resistance mutations (5, 9, 18). An additional 1,562 cell samples, not reported on in detail here, were dispensed in 1996 and 1997 for other ongoing pathogenesis projects by 18 investigators throughout the United States and Australia, both internal and external to the MACS, for studies of a variety of facets of HIV pathogenesis. Although detailed data on recovery are not available for these specimens, their utility for scientific initiatives is documented by publications on the findings from these samples (5, 9, 18).
Our experience suggests several steps to follow and quality control measures to undertake in setting up a cell repository. Some of these have been discussed elsewhere (8). It is important to have enough personnel to allow the separation and storage of blood within 6 h after the blood is drawn. Use of a controlled-rate freezer may be desirable. Nitrogen storage tanks should be fitted with alarms which will alert technicians if the level of nitrogen is not acceptable or the temperature is too warm. Keeping vials in the nitrogen vapor phase is recommended for maintaining a high cell viability during long-term storage, and the use of vials internally threaded with a gasket is generally thought to be best for liquid-nitrogen storage. On-site training by a central technical coordinator to standardize methods for cell counting, freezing, and thawing across sites would be expected to reduce variation in cell recovery. An external quality control program would also be valuable in enabling the optimization of viability and cell recoveries across sites. More predictable yields with high viabilities will maximize the value of PBMC collected and stored for research studies.
This approach to the quality assessment of cryopreserved PBMC may be useful for other studies involving stored specimens, particularly in planning for the recovery of sufficient cells to accomplish the desired research. Successful repositories also require consistent attention to detail by skilled technical staff, including attention to the numbers of cells stored. The quality of specimens should be examined early during the course of a research study, so that any problems in sample storage are recognized and corrected before valuable resources are lost.
ACKNOWLEDGMENTS
Data presented in this paper were collected by the MACS, with centers (and principal investigators) at the Johns Hopkins School of Public Health (Joseph Margolick and Alvaro Muñoz); Howard Brown Health Center and Northwestern University Medical School (John Phair); University of California, Los Angeles (UCLA) (Roger Detels and Janis V. Giorgi); and the University of Pittsburgh (Charles Rinaldo). The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute (grants U01-AI-35042, 5-M01-RR-00052 [GCRC], U01-AI-35043, U01-AI-37984, U01-AI-35039, U01-AI-35040, U01-AI-37613, and U01-AI-35041).
We thank the MACS participants for their dedication, BRI for storage and shipment of specimens, Alvaro Muñoz for insightful comments, John Fahey for his participation in initially establishing the repository and for managing the storage of specimens at UCLA, Marcella Guccione for assistance with data management, and Xiao-Li Huang, Mary Ann Hausner, and the many personnel at the local and national repositories who have provided laboratory assistance.
REFERENCES
- 1.Bass H Z, Fahey J L, Nishanian P, Detels R, Cumberland W, Kemeny M, Plaeger S. Relation of impaired lymphocyte proliferative function to other major human immunodeficiency virus type 1-induced immunological changes. Clin Diagn Lab Immunol. 1997;4:64–69. doi: 10.1128/cdli.4.1.64-69.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chmiel J S, Detels R, Kaslow R, VanRaden M, Kingsley L, Brookmeyer R. Factors associated with prevalent human immunodeficiency virus (HIV) infection in the Multicenter AIDS Cohort Study. Am J Epidemiol. 1987;126:568–577. doi: 10.1093/oxfordjournals.aje.a114696. [DOI] [PubMed] [Google Scholar]
- 3.Chou C C, Gudeman V, O’Rourke S, Isacescu V, Detels R, Williams G, Mitsuyasu R, Giorgi J. Phenotypically defined memory CD4+ cells are not selectively decreased in chronic HIV disease. J Acquired Immune Defic Syndr. 1994;7:665–675. [PubMed] [Google Scholar]
- 4.Conover W J. Practical nonparametric statistics. New York, N.Y: John Wiley and Sons; 1980. [Google Scholar]
- 5.Dean M, Carrington M, Winkler C, Huttley G, Smith M, Allikmets R, Goedert J, Buchbinder S, Vittinghoff E, Gonperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, O’Brien S Hemophilia Growth and Development Study; Multicenter AIDS Cohort Study; Multicenter Hemophilia Cohort Study; San Francisco City Cohort; Alive Study. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 6.Giorgi, J. V., L. E. Hultin, J. A. McKeating, T. Johnson, R. Shih, D. Wiley, B. Owens, J. Lewis, L. Jacobson, J. Phair, S. Wolinsky, and R. Detels. Survival in advanced HIV disease is more stringently related to lymphocyte activation as reflected by expression of CD38 on T cells than to plasma HIV viral burden. Submitted for publication.
- 7.Giorgi J V, Ho H N, Hirji K, Chou C, Hultin L, O’Rourke S, Park L, Margolick J, Ferbas J, Phair J. CD8+ lymphocyte activation at human immunodeficiency virus type 1 seroconversion; development of HLA-DR+ CD38− CD8+ cells is associated with subsequent stable CD4+ cell levels. J Infect Dis. 1994;170:775–781. doi: 10.1093/infdis/170.4.775. [DOI] [PubMed] [Google Scholar]
- 8.Gjerset G, Nelson K A, Strong D M. Methods for cryopreserving cells. In: Rose N R, de Macario E C, Fahey J L, Friedman H, Penn G M, editors. Manual of clinical laboratory immunology. 4th ed. Washington, D.C: American Society for Microbiology; 1992. pp. 61–67. [Google Scholar]
- 9.Huang Y, Paxton W A, Wolinsky S M, Neumann A, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, Kunstman K, Erickson D, Dragon E, Landau N, Phair J, Ho D, Koup R. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 10.Kaslow R A, Ostrow D G, Detels R, Phair J, Polk B, Rinaldo C. The Multicenter AIDS Cohort Study: rationale, organization and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 11.Kaslow R A, Carrington M, Apple R, Park L, Muñoz A, Saah A, Goedert J, Winkler C, O’Brien S, Rinaldo C, Detels R, Blattner W, Phair J, Erlich H, Mann D. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- 12.Margolick J B, Muñoz A, Donnenberg A, Park L, Galai N, Giorgi J, O’Gorman M, Ferbas J. Failure of T-cell homeostasis preceding AIDS in HIV-1 infection. Nat Med. 1995;1:674–680. doi: 10.1038/nm0795-674. [DOI] [PubMed] [Google Scholar]
- 13.Masters, B., and H. Farzadegan. Personal communication.
- 14.Plaeger, S., H. Bass, P. Nishanian, J. Thomas, N. Aziz, R. Detels, J. King, W. Cumberland, M. Kemeny, and J. Fahey. The prognostic significance in HIV infection of immune activation represented by cell surface antigen and plasma activation marker changes. Clin. Immunol. Immunopathol., in press. [DOI] [PubMed]
- 15.Rinaldo C, Huang X-L, Fan Z, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, Gupta P. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinaldo, C. R., P. Gupta, Z. Huang, Z. Fan, J. Mullins, S. Gange, H. Farzadegan, R. Shankarappa, A. Muñoz, and J. Margolick. Anti-HIV-1 memory cytotoxic T lymphocyte responses correlate with changes in CD4+ cell numbers in progression of HIV-1 infection. AIDS Res. Hum. Retroviruses, in press. [DOI] [PubMed]
- 17.Rinaldo C R, Beltz L A, Huang X, Gupta P, Fan Z, Torpey D. Anti-HIV type 1 cytotoxic T lymphocyte effector activity and disease progression in the first 8 years of HIV type 1 infection of homosexual men. AIDS Res Hum Retroviruses. 1995;11:481–489. doi: 10.1089/aid.1995.11.481. [DOI] [PubMed] [Google Scholar]
- 18.Zimmerman P A, Buckler-White A, Alkhatib G, Spalding T, Kubofcik J, Combadiere C, Weissman D, Cohen O, Rubbert A, Lam G, Vaccarezza M, Kennedy P, Kumaraswami V, Giorgi J, Detels R, Hunter J, Chopek M, Berger E, Fauci A, Nutman T, Murphy P. Inherited resistance to HIV-1 conferred by an inactivating mutation in CC chemokine receptor 5: studies in populations with contrasting clinical phenotypes, defined racial background and quantified risk. Mol Med. 1997;3:23–36. [PMC free article] [PubMed] [Google Scholar]