Similar Humoral and Cellular Immunological Reactivities to Human Herpesvirus 6 in Patients with Multiple Sclerosis and Controls (original) (raw)
Abstract
Several studies have suggested an association between human herpesvirus 6 (HHV-6) and multiple sclerosis (MS). We have previously studied intrathecal production of antibody to lymphotropic herpesviruses in MS patients and the presence of human herpesvirus 1 to 7 DNAs in cerebrospinal fluid (CSF). In the present study anti-HHV-6 immunoglobulin M (IgM) in serum and anti-HHV-6 IgG subclasses in serum and CSF were examined and the lymphoproliferative response to HHV-6 was analyzed. The PCR examination was refined by purifying DNA from CSF and retesting the samples for HHV-6 DNA. There were no statistically significant differences between the groups concerning IgM positivity, distribution of IgG subclasses, or lymphoproliferative response to HHV-6. The purification of DNA increased the number of PCR-positive samples from 0 of 71 to 4 of 68. The study does not give additional support to the possibility that HHV-6 is a common cause of MS, but a role for the virus in a subset of patients cannot be excluded.
Multiple sclerosis (MS) is a disease of unknown etiology. It is characterized by a relapsing and remitting or a chronic progressive course, and its pathology includes inflammation and destruction of oligodendrocytes which results in plaques of demyelination within the white matter in the central nervous system. Epidemiological studies suggest that an infectious agent may be involved either as an initiating event or as a direct pathogen in plaque formation (6). Earlier studies have pointed to different members of the herpesvirus family as possible agents. Several features make the herpesviruses attractive candidates since the majority of them are neurotropic, they establish latency, they are periodically reactivated, and they have the capacity to induce demyelination. Herpes simplex virus and Epstein-Barr virus (EBV) have been discussed previously (14, 20). Recently, much interest has been focused on human herpesvirus 6 (HHV-6) since HHV-6 has been found in cerebrospinal fluid (CSF) from MS patients (7, 22) and in MS plaques (2). Also, the sera of MS patients have been shown to have increased titers of anti-HHV-6 immunoglobulin G (IgG) antibodies compared to the titers in the sera of healthy controls (18, 22). One recent study reported increased anti-HHV-6 IgM responses in sera from MS patients and reported on the detection of HHV-6 DNA in serum in 30% of the MS patients (19). Fulminant demyelinating disease has also been associated with HHV-6 (15).
In a previous study we examined intrathecal production of antibodies to HHV-6, EBV, cytomegalovirus, and the measles virus in MS patients (5). Elevated titers of antibodies to several herpesviruses were found in CSF from MS patients compared to the titers in the control group, but the results argued more for a nonspecific immunoactivation within the central nervous system than for a specific response to an active intrathecal HHV-6 infection in MS patients. In the present study we have investigated whether an aberrant immunological response indicating an active HHV-6 infection or a defect in immunological control of HHV-6 could be found in MS patients. Anti-HHV-6 IgM antibodies in serum and anti-HHV-6 IgG subclasses in serum and CSF from MS patients were examined. Previous studies have indicated that intrathecal production of virus-specific IgG subclasses other than IgG1 may be a marker of the presence of the antigen (12). Earlier, we analyzed CSF samples from these patients by PCR but did not detect DNAs of human herpesviruses 1 to 7 (11). In the present study we refined the HHV-6 PCR examination by analysis of purified DNA from CSF samples. The T-cell proliferative response to nucleocapsid antigens from the GS strain and the Z29 strain, representing HHV-6 variants A and B, respectively, was examined for evaluation of the cellular immunological response to HHV-6.
MATERIALS AND METHODS
Patients. (i) Serological assays and PCR.
Fifty-five patients (41 females) had clinically definite MS according to the criteria of Poser et al. (17). The median age of the patients was 36 years (age range, 15 to 60 years). Nineteen of the MS patients had early, laboratory-supported definite MS according to the criteria of Poser et al. (17), with laboratory support including oligoclonal bands in CSF and abnormalities on brain magnetic resonance imaging (median duration, 6 weeks). The control group consisted of 20 patients with other neurological diseases: tension headache (5 patients), vertigo (3 patients), cerebrovascular disease (3 patients), Parkinson’s disease (2 patients), migraine (1 patient), borrelia meningitis (1 patient), Alzheimer’s disease (1 patient), communicating hydrocephalus (1 patient), polyneuropathy (1 patient), and mononeuropathy (2 patients). This study was performed retrospectively.
(ii) Lymphoproliferative assays.
Samples from 14 MS patients (10 females) with a median age of 54 years (age range, 36 to 64 years) were examined by lymphoproliferative assays. They had a median duration of disease of 14.5 years (range, 4 to 35 years). For disability and form of disease, see Table 1. In a parallel study, samples from 29 staff members from either the Swedish Institute for Infectious Disease Control or Huddinge Hospital (Stockholm, Sweden) were used for comparison (21). Peripheral blood samples were drawn into tubes containing heparin, and the tubes were stored at room temperature until they were analyzed on the same day of collection.
TABLE 1.
Lymphoproliferative responses to the different antigens in 14 MS patientsa
| Patient no. | Sex | Age (yr) | Duration of disease (yr) | Disability | Form of disease | Lymphoproliferative responses to the different antigens | |||
|---|---|---|---|---|---|---|---|---|---|
| GS | Z29 | VZV | PHA | ||||||
| 1 | F | 54 | 4 | Slight | RR (ex) | + | + | + | + |
| 2 | F | 49 | 5 | Slight | RR (rem) | − | − | + | + |
| 3 | F | 54 | 5 | Moderate | RR (rem) | − | − | + | + |
| 4 | F | 43 | 18 | Moderate | RR (rem) | − | + | + | + |
| 5 | M | 60 | 13 | Moderate | PCP | − | + | + | + |
| 6 | F | 58 | 8 | Moderate | SCP | + | + | − | + |
| 7 | F | 55 | 17 | Moderate | SCP | + | + | + | + |
| 8 | F | 62 | 35 | Moderate | SCP | − | + | + | + |
| 9 | F | 55 | 16 | Moderate | SCP | − | + | + | + |
| 10 | M | 52 | 17 | Severe | SCP | − | − | − | + |
| 11 | M | 48 | 7 | Severe | SCP | − | − | + | + |
| 12 | M | 45 | 16 | Severe | SCP | − | − | + | + |
| 13 | F | 64 | 20 | Severe | SCP | − | + | + | + |
| 14 | F | 36 | 10 | Severe | SCP | + | + | + | + |
PCR with CSF.
DNA was extracted from the CSF samples with the QIAmp Blood Kit (Qiagen GmbH, Hilden, Germany). The instructions from the manufacturer were followed, with the exception that DNA from a 200-μl sample was eluted in 50 μl of water instead of 200 μl of water in order to concentrate the DNA. The PCR was performed as described previously (3) with duplicates of 10-μl sets of the extracted DNA. If only one of the duplicates was positive, the examination was repeated. If one or two of the duplicates were positive the second time, the sample was considered positive. The sensitivity of the PCR system as determined by examination of purified genomic DNA from laboratory strains of HHV-6 (strains GS and Z29) was 2.5 fg of DNA (15 genomes).
Anti-HHV-6 IgM serology.
IgM antibodies to HHV-6 in serum were determined by an indirect immunofluorescence assay by a previously published method (4). Preparations were made from the HSB-2 cell line infected with HHV-6 GS (the cell line and virus were kindly donated by R. Gallo). All samples tested were previously absorbed with rheumatoid factor absorbent (Behring Diagnostics GmbH, Marburg, Germany) according to the instructions of the manufacturer. The serum samples were examined at a 1:20 dilution. Incubation of serum was overnight at 37°C, and after washing, a fluorescein isothiocyanate-labelled rabbit anti-human conjugate (Dakopatts, Copenhagen, Denmark) was added for 30 min at 37°C. The slides were examined in a fluorescence microscope at a magnification of ×400.
IgG subclasses.
IgG subclasses to HHV-6 and EBV viral capsid antigen (VCA) were detected by immunofluorescence assays by previously published methods (8). The same type of HHV-6 preparations used for the HHV-6 IgM assay was used. EBV VCA preparations were made from the EBV-expressing P3HR-1 cell line. The CSF samples were diluted 1:2, and the serum samples were diluted 1:5 to 1:20. The slides were incubated with CSF or serum for 1 h at 37°C. After washing off the serum or CSF, mouse ascitic fluids containing monoclonal antibodies to human IgG1 to IgG4 (clones NL16, GOM1, ZG4, and RJ4 for IgG1 to IgG4, respectively; Oxoid, Hampshire, England) were added at a 1:100 dilution for 1 h at 37°C. Finally, a fluorescein isothiocyanate-labelled rabbit anti-mouse conjugate (Dakopatts, Copenhagen, Denmark) was added, and incubation was continued for another hour at 37°C. The slides were examined in a fluorescence microscope at a magnification of ×400.
Lymphocyte proliferation assay.
The methods for antigen preparation and the lymphocyte proliferation assay have been described previously (9, 21). Briefly, HHV-6 antigens, predominantly containing nucleocapsids, were prepared from HSB-2 cells infected with strain GS (variant A) and MOLT-3 cells infected with strain Z29 (variant B). Virus-infected cells were collected by centrifugation at 420 × g for 15 min, resuspended in 1/20th of the original culture volume of 0.1 M glycine buffer (pH 9.5), sonicated on ice, and centrifuged at 4,700 × g for 60 min at 4°C. The supernatants were collected and used as antigens. Control antigens were prepared from uninfected HSB-2 and MOLT-3 cells. Nuclear antigens from a local varicella-zoster virus (VZV) strain (strain 9/84) grown in fetal fibroblast cells were prepared in the same way and were used as a control antigen for the specific lymphoproliferative assay. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (Pharmacia Biotech AB, Uppsala, Sweden), washed twice with RPMI 1640 medium, and resuspended in culture medium which consisted of RPMI 1640 medium supplemented with glutamine, penicillin, streptomycin, 2-mercaptoethanol, and 10% human type AB-positive serum. The PBMCs were adjusted to 1.5 × 106 cells/ml, and 100 μl of the cell suspension was added to each well of a 96-well flat-bottom plate. One hundred microliters of antigen diluted in RPMI 1640 medium was added to each well. Proliferation was measured by [3H]thymidine incorporation after 6 days of incubation at 37°C and was expressed as counts per minute. Results were expressed as stimulation indices, which were derived by division of the counts per minute obtained after antigen stimulation by the counts per minute obtained after stimulation with the corresponding control antigen. A response was considered positive if the stimulation index was greater than 2 and the net counts per minute (the counts per minute of the antigen reduced by that of the control antigen) was greater than 1,000.
RESULTS
PCR.
Samples from four of the MS patients were excluded from the PCR examination due to a lack of CSF, which left 51 patients in this group. Three (5.9%) of them had detectable HHV-6 DNA in their CSF. In the control group three patients were excluded due to a lack of CSF. One (5.9%) of the 17 CSF samples examined had detectable HHV-6 DNA.
IgM.
The serum of 1 (1.8%) of the 55 MS patients was positive for anti-HHV-6 IgM. Sera from the control group were not examined for IgM.
IgG subclasses.
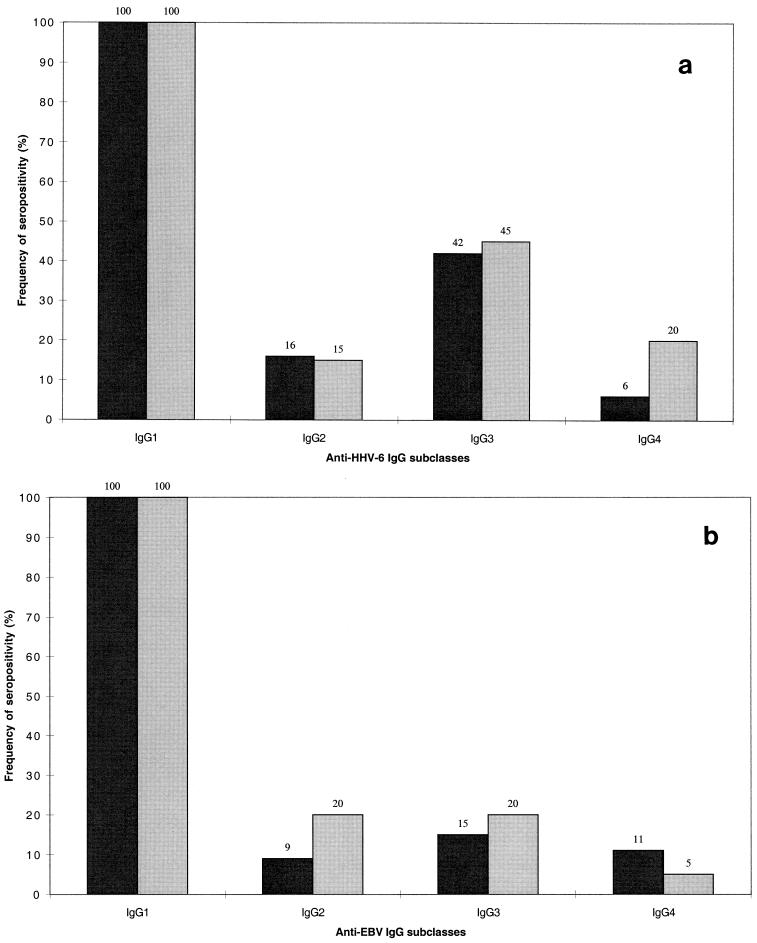
Among the patients in the control group, one patient lacked detectable anti-HHV-6 IgG1, but the sera of all the other patients in the two groups were positive for anti-HHV-6 and anti-EBV IgG1. Anti-HHV-6 IgG2 was found in 9 of 55 (16%) of the MS patients and 3 of 20 (15%) of the controls, IgG3 was found in 23 of 55 (42%) of the MS patients and 9 of 20 (45%) of the controls, and IgG4 was found in 3 of 55 (5.5%) of the MS patients and 4 of 20 (20%) of the controls (Fig. 1a). Anti-EBV IgG2 was found in 5 of 55 (9.1%) of the MS patients and 4 of 20 (20%) of the controls, IgG3 was found in 8 of 55 (15%) of the MS patients and 4 of 20 (20%) of the controls, and IgG4 was found in 6 of 55 (11%) of the MS patients and 1 of 20 (5%) of the controls (Fig. 1b). There was no significant difference between the groups in the distribution of the IgG subclass for antibodies to HHV-6 or EBV in serum (Fisher’s exact test).
FIG. 1.
Distribution in serum of detectable subclasses of IgG to HHV-6 (a) and EBV (b) in patients with MS (heavy shading) and controls (light shading). Percentages are at the top of each column.
The only virus-specific IgG subclass that was found in CSF was IgG1. Anti-HHV-6 IgG1 was found in 15 of 55 (27%) of the MS patients and 0 of 20 of the controls. Anti-EBV IgG1 was found in 33 of 55 (60%) of the MS patients and 2 of 20 (10%) of the controls.
Relation between results of different assays.
The HHV-6 IgM-positive MS patient did not have detectable HHV-6 DNA in CSF or any HHV-6 IgG subclass other than IgG1 in serum. All three patients who were PCR positive for HHV-6 DNA in CSF had HHV-6 IgG1 in their sera, and one also had detectable IgG2 and IgG3. Three of the 23 MS patients with detectable IgG3 antibodies to HHV-6 in their sera also had IgG3 to EBV, but other than these patients, no patients with MS or controls had IgG subclasses other than IgG1 to both HHV-6 and EBV in serum. None of the PCR-positive patients had any detectable virus-specific IgG subclass in CSF.
Lymphocyte proliferation assay.
In the MS group, 5 of 14 (36%) patients had a detectable lymphoproliferative response to HHV-6 variant A and 8 of 14 (57%) patients had a detectable lymphoproliferative response to variant B. In the control group of healthy Swedes (21), a lymphoproliferative response to HHV-6 variant A was demonstrated in 6 of 29 (20%) subjects and a lymphoproliferative response to variant B was demonstrated in 14 of 29 (48%) subjects. There was no significant difference between the MS group and the control group regarding the frequency of positivity for HHV-6 (Fisher’s exact test). The median net counts per minute among the positive patients in the MS group was 2,410 cpm for the HHV-6 variant-A antigen and 7,280 cpm for the variant-B antigen. In the control group the median net counts per minute was 6,000 cpm for the variant-A antigen and 3,000 cpm for the variant-B antigen for the positive patients. Twelve of 14 (86%) patients in the MS group and 20 of 20 (100%) subjects in the control group responded to the VZV antigen. All patients in both groups responded to phytohemagglutinin.
DISCUSSION
A normal IgG subclass response to a viral infection is dominated by IgG1 and IgG3. IgG4 is more infrequently detectable. IgG3 may be a marker for ongoing infection, and IgG4 may be a marker for repeated antigen exposure (10). Increases in the titers of antigen-specific IgG subclasses are found in patients with some autoimmune diseases, such as rheumatoid arthritis, diabetes mellitus type 1, and myasthenia gravis (10). Isotype restriction may suggest an abnormal immune response. A difference in the distribution of anti-HHV-6 IgG subclasses in the MS patients compared to that in the control group would therefore strengthen the hypothesis that HHV-6 is involved in the pathogenesis of MS. In this study we found the same prevalence of IgG1 to IgG4 in the sera of MS patients as in the sera of controls. IgG1 was the dominant specific subclass of IgG antibodies to both EBV VCA and HHV-6. All patients who were previously positive for total IgG (5) were also positive for IgG1. The distributions of the different IgG subclasses were similar both when the MS patients were compared to the control group and when the pattern for HHV-6 was compared to the one for EBV. The only IgG isotype that was found in CSF was IgG1. Exactly the same patients who were previously positive for total IgG to HHV-6 or EBV were positive for anti-HHV-6 or anti-EBV IgG1, respectively, in CSF (5). Additional evidence supporting a role for HHV-6 in MS was thus not obtained by analysis of specific IgG subclasses.
The anti-HHV-6 IgM positivity rate for the sera of the MS patients was 1.8%. That is a frequency similar to what we found when we examined 162 healthy Swedish blood donors (unpublished data). In that group four subjects (2.5%) were positive. Similar rates have also been published in reports of other studies (16, 19). When preparing slides for HHV-6 immunofluorescence assays for both IgM and IgG subclasses, we have used cells infected only with the GS strain of the virus. However, these samples have earlier been examined for total anti-HHV-6 IgG by an immunofluorescence assay with both GS- and Z-29-infected cells. There was very little difference when the titers obtained with the two strains were compared, and we believe that the results of this study would not have been different if the Z-29 strain had been used. In another study in which an IgM response was detected, HHV-6 early antigen (p41/38) was used (19). An antigenic difference may explain the lack of IgM in our study, but with the use of a monoclonal antibody (1) we have demonstrated that p41/38 is indeed expressed in our preparations.
There were no significant differences in lymphoproliferative responses when the MS patients were compared to the control group of healthy Swedes (Fisher’s exact test). This argues against a different T-cell response to HHV-6 in MS patients. It is interesting, however, that the patient with exacerbated relapsing and remitting disease had a positive lymphoproliferative response to both HHV-6 variant A and HHV-6 variant B, while two of the patients with relapsing and remitting disease in remission were negative for responses to both variants, and one was positive for a response only to variant B. A follow-up study with subsequently obtained samples could therefore be of interest.
The purification of DNA gave a slightly higher frequency of patients whose CSF was positive for HHV-6 DNA than that obtained in our previous study, in which we failed to detect any HHV-6 DNA-positive samples (11). This is probably due both to the increased concentration of DNA obtained by the DNA purification process and to a possible decreased inhibition of the PCR when purified DNA instead of complete CSF is used. Still, the frequency of HHV-6 DNA in CSF was quite low, 5.9% both in the MS group and in the control group, and does not indicate that HHV-6 is a major cause of MS. In a previous study in which extracted DNA from 50 μl of CSF was used, the CSF of 14% of MS patients was found to be positive for HHV-6 DNA, whereas the CSF of none of the patients with other neurological diseases was found to be positive for HHV-6 DNA (22). It is possible that a more sensitive assay was used and that an increased sensitivity would further increase the number of positive samples in our study. We have, however, no reason to believe that this would lead to any major differences between the two groups since with the increase in sensitivity in this study, HHV-6 DNA was detected in CSF equally frequently in MS patients and controls.
In a subanalysis of the 19 MS patients with an early form of the disease, we found nothing that indicated a different response to HHV-6 in this group compared to that in the other MS patients. There were no statistically significant differences between the results for two subgroups of MS patients by any of the assays.
Several studies have suggested that HHV-6 is involved in the pathogenesis of MS (2, 7, 18, 19, 22). An aberrant immunological activity to HHV-6 in these patients would strengthen this hypothesis. In this study, however, we found no indications of a different immunological response to HHV-6 or a more active HHV-6 infection in the MS patients compared to the response to HHV-6 and the level of activity of the infection in the control group. The CSF of the MS patients also did not have a high prevalence of detectable HHV-6 DNA. These findings agree with those of a recently published study in which HHV-6 DNA was found only infrequently in PBMCs from MS patients (13). If there is an association between MS and HHV-6 it is probably limited to relatively few patients. Thus, our study and those of other investigators do not support the idea that HHV-6 is a major cause of MS but it may be of importance for individual patients. Antiviral treatment may be an option if HHV-6 and other herpesviruses are involved in the pathogenesis of MS in a subset of patients. If ongoing studies verify that antiviral agents are beneficial for patients with MS, it will be extremely important to find suitable methods for the identification of patients who should be treated, and further studies should probably focus on that issue.
REFERENCES
- 1.Balachandran N, Amelse R E, Zhou W W, Chang C K. Identification of proteins specific for human herpesvirus 6-infected human T cells. J Virol. 1989;63:2835–2840. doi: 10.1128/jvi.63.6.2835-2840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Challoner P B, Smith K T, Parker J D, MacLeod D L, Coulter S N, Rose T M, Schultz E R, Bennett J L, Garber R L, Chang M, et al. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci USA. 1995;92:7440–7444. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cinque P, Vago L, Dahl H, Brytting M, Terreni M R, Fornara C, Racca S, Castagna A, Monforte A D, Wahren B, Lazzarin A, Linde A. Polymerase chain reaction on cerebrospinal fluid for diagnosis of virus-associated opportunistic diseases of the central nervous system in HIV-infected patients. AIDS. 1996;10:951–958. doi: 10.1097/00002030-199610090-00004. [DOI] [PubMed] [Google Scholar]
- 4.Dahl H, Linde A, Sundqvist V A, Wahren B. An enzyme-linked immunosorbent assay for IgG antibodies to human herpes virus 6. J Virol Methods. 1990;29:313–323. doi: 10.1016/0166-0934(90)90058-n. [DOI] [PubMed] [Google Scholar]
- 5.Enbom M, Martin C, Fredrikson S, Jägdahl L, Dahl H, Linde A. Intrathecal antibody production to lymphotropic herpesviruses in patients with multiple sclerosis. Neurol Infect Epidemiol. 1997;2:107–111. [Google Scholar]
- 6.Kurtzke J F. Epidemiologic evidence for multiple sclerosis as an infection. Clin Microbiol Rev. 1993;6:382–427. doi: 10.1128/cmr.6.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liedtke W, Malessa R, Faustmann P M, Eis-Hubinger A M. Human herpesvirus 6 polymerase chain reaction findings in human immunodeficiency virus associated neurological disease and multiple sclerosis. J Neurovirol. 1995;1:253–258. doi: 10.3109/13550289509114021. [DOI] [PubMed] [Google Scholar]
- 8.Linde A, Andersson J, Lundgren G, Wahren B. Subclass reactivity to Epstein-Barr virus capsid antigen in primary and reactivated EBV infections. J Med Virol. 1987;21:109–121. doi: 10.1002/jmv.1890210203. [DOI] [PubMed] [Google Scholar]
- 9.Ljungman P, Wahren B, Sundqvist V-A. Lymphocyte proliferation and IgG production with herpesvirus antigens in solid phase. J Virol Methods. 1985;12:199–208. doi: 10.1016/0166-0934(85)90130-2. [DOI] [PubMed] [Google Scholar]
- 10.Maran R, Dueymes M, Corre R L, Renaudineau Y, Shoenfeld Y, Youinou P. IgG subclasses of human autoantibodies. Ann Med Int. 1997;148:29–38. [PubMed] [Google Scholar]
- 11.Martin C, Enbom M, Soderstrom M, Fredrikson S, Dahl H, Lycke J, Bergstrom T, Linde A. Absence of seven human herpesviruses, including HHV-6, by polymerase chain reaction in CSF and blood from patients with multiple sclerosis and optic neuritis. Acta Neurol Scand. 1997;95:280–283. doi: 10.1111/j.1600-0404.1997.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 12.Mathiesen T, Linde A, Olding-Stenkvist E, Wahren B. Antiviral IgM and IgG subclasses in varicella zoster associated neurological syndromes. J Neurol Neurosurg Psychiatry. 1989;52:578–582. doi: 10.1136/jnnp.52.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayne M, Krishnan J, Metz L, Nath A, Auty A, Sahai B M, Power C. Infrequent detection of human herpesvirus 6 DNA in peripheral blood mononuclear cells from multiple sclerosis patients. Ann Neurol. 1998;44:391–394. doi: 10.1002/ana.410440317. [DOI] [PubMed] [Google Scholar]
- 14.Munch M, Riisom K, Christensen T, Moller-Larsen A, Haahr S. The significance of Epstein-Barr virus seropositivity in multiple sclerosis patients? Acta Neurol Scand. 1998;97:171–174. doi: 10.1111/j.1600-0404.1998.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 15.Novoa L J, Nagra R M, Nakawatase T, Edwards-Lee T, Tourtellotte W W, Cornford M E. Fulminant demyelinating encephalomyelitis associated with productive HHV-6 infection in an immunocompetent adult. J Med Virol. 1997;52:301–308. doi: 10.1002/(sici)1096-9071(199707)52:3<301::aid-jmv11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 16.Patnaik M, Komaroff A L, Conley E, Ojo-Amaize E A, Peter J B. Prevalence of IgM antibodies to human herpesvirus 6 early antigen (p41/38) in patients with chronic fatigue syndrome. J Infect Dis. 1995;172:1364–1367. doi: 10.1093/infdis/172.5.1364. [DOI] [PubMed] [Google Scholar]
- 17.Poser C M, Paty D W, Scheinberg L, McDonald W I, Davis F A, Ebers G C, Johnson K P, Sibley W A, Silberberg D H, Tourtellotte W W. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 18.Sola P, Merelli E, Marasca R, Poggi M, Luppi M, Montorsi M, Torelli G. Human herpesvirus 6 and multiple sclerosis: survey of anti-HHV-6 antibodies by immunofluorescence analysis and of viral sequences by polymerase chain reaction. J Neurol Neurosurg Psychiatry. 1993;56:917–919. doi: 10.1136/jnnp.56.8.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soldan S S, Berti R, Salem N, Secchiero P, Flamand L, Calabresi P A, Brennan M B, Maloni H W, McFarland H F, Lin H C, Patnaik M, Jacobson S. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat Med. 1997;3:1394–1397. doi: 10.1038/nm1297-1394. [DOI] [PubMed] [Google Scholar]
- 20.Vahlne A, Edstrom S, Hanner P, Andersen O, Svennerholm B, Lycke E. Possible association of herpes simplex virus infection with demyelinating disease. Scand J Infect Dis Suppl. 1985;47:16–21. [PubMed] [Google Scholar]
- 21.Wang F-Z, Dahl H, Ljungman P, Linde A. Lymphoproliferative responses to human herpesvirus-6 variant A and B in healthy adults. J Med Virol. 1999;57:134–139. [PubMed] [Google Scholar]
- 22.Wilborn F, Schmidt C A, Brinkmann V, Jendroska K, Oettle H, Siegert W. A potential role for human herpesvirus type 6 in nervous system disease. J Neuroimmunol. 1994;49:213–214. doi: 10.1016/0165-5728(94)90198-8. [DOI] [PubMed] [Google Scholar]