Interleukin-7 or Interleukin-15 Enhances Survival of Mycobacterium tuberculosis-Infected Mice (original) (raw)
Abstract
Both antigen-presenting cells and immune effector cells are required to effectively eradicate or contain _Mycobacterium tuberculosis_-infected cells. A variety of cytokines are involved to ensure productive “cross talk” between macrophages and T lymphocytes. For instance, infection of macrophages with mycobacteria leads to effective interleukin-7 (IL-7) and IL-15 secretion, and both cytokines are able to maintain strong cellular immune responses of α/β and γ/δ T cells. Here we show that either cytokine is able to enhance survival of _M. tuberculosis_-infected BALB/c mice significantly compared to application of IL-2, IL-4, or phosphate-buffered saline (as a control). Enhanced survival could be achieved only when IL-7 or IL-15 was delivered as a treatment (i.e., 3 weeks postinfection), not when it was administered at the time of infection. Increased survival of _M. tuberculosis_-infected animals was observed following passive transfer of spleen cells harvested from _M. tuberculosis_-infected, IL-7- or IL-15-treated animals, but not after transfer of spleen cells obtained from mice which received either cytokine alone. Histological examination revealed that IL-7 and IL-15 failed to significantly impact on the number and composition of granulomas formed or the bacterial load. Our data indicated that administration of IL-7 or IL-15 to _M. tuberculosis_-treated animals resulted in a qualitatively different cellular immune response in spleen cells as reflected by increased tumor necrosis factor alpha and decreased gamma interferon secretion in response to _M. tuberculosis_-infected antigen-presenting cells.
A third of the world's population is infected with the intracellular pathogen Mycobacterium tuberculosis, which contributes to substantial mortality (2). Control of the infection by the host immune system relies on the eradication and/or containment of viable bacilli. A combination of immune cells is involved in immune recognition of _M. tuberculosis_-infected cells, including CD8+ α/β T-cell receptor-positive (TCR+) lymphocytes (15, 44; C. D. D'Souza, A. M. Cooper, A. A. Frunk, and L. M. Orme, p. 47, Keystone Symp. Mol. Methods Immunol. Aspects, 1998). CD4+ α/β TCR+ lymphocytes (23), CD8− CD4− α/β TCR+ T cells (4, 32), γ/δ TCR+ cells (38, 40), and NK cells (21). Potential restricting elements for _M. tuberculosis_-associated antigens presented to these immune cells are the classical major histocompatibility complex (MHC) class I and MHC class II antigens (23, 44), monomorphic β-2 microglobulin-associated CD1b and CD1c antigens capable of presenting nonpeptide ligands derived from mycolic acids or glycolipids (4, 5, 32, 33, 38), and as-yet ill-defined nonpolymorphic MHC class Ib antigens other than CD1 (24). Both macrophages and T cells are required to elicit strong, effective, and long-lasting cellular immune responses. The quality and quantity of an effective antimycobacterial immune response appear to be influenced by T-cell-elaborated cytokines capable of enhancing the bactericidal activity of macrophages. Candidates for such cytokines are tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ).
Studies using IFN-γ or TNF receptor gene-deleted mice (13, 14) have provided evidence that the absence of these immune mediators is associated with enhanced susceptibility to M. tuberculosis infection. Conversely, infected-macrophage-secreted cytokines which activate and expand T lymphocytes may also be crucial in controlling M. tuberculosis infection. Previous experiments have indicated that infection of macrophages with mycobacteria will lead to effective secretion of interleukin-7 (IL-7) (34) and IL-15 (11). Both IL-7 and IL-15 act on particular T-lymphocyte subsets. IL-7 represents an exceptional cytokine since, unlike IL-2, IL-4, or IL-10, it mediates lymphopoiesis in mice in a nonredundant fashion (42). It is secreted by both immune and nonimmune (e.g., epithelial) cells (26), and it is able to maintain strong cellular immune responses (α/β and γ/δ T cells) for several months (25). Like IL-7, IL-15 is not secreted by T cells (19). Infected macrophages or dendritic cells appear to represent the major source of biologically meaningful IL-15 protein secretion (3, 11). Based on data obtained from animal studies, IL-15 is able to preferentially activate individual T-cell subsets: effective and selective stimulation of memory-phenotype (CD44hi and CD62Llow) CD8+, but not CD4+ T lymphocytes, has been shown to occur in vivo upon IL-15 application in mice (43). Here we explore the role of IL-7 and IL-15 in a murine model of M. tuberculosis infection and show that each of these cytokines is able to enhance survival of infected animals.
MATERIALS AND METHODS
Animals.
Female BALB/c mice (6 to 10 weeks of age) were obtained from the local breeding facility at the University of Mainz. Mice were found to be free of infectious agents prior to experiments.
Infections and cytokines.
A freshly isolated M. tuberculosis strain, designated as M.tub.MZ#610, from the sputum of a patient was grown in Middlebrook 7H10 medium once and frozen in aliquots. Before infection, an aliquot was thawed in phosphate-buffered saline (PBS) containing 0.05% Tween 80, sonicated, and plated onto Middlebrook 7H10 agar plates (Difco Laboratories, Augsburg, Germany). Mice were infected by intravenous (i.v.) injection, via the tail vein, of 3 × 106 live bacilli in 0.1 ml of PBS.
Recombinant IL-15 (rIL-15; 4.45 × 108 U/mg) was kindly provided by Tony Troutt, Immunex Corporation, Seattle, Wash. rIL-7 (2 × 106 U/mg, as determined by a cell proliferation assay using phytohemagglutinin-activated peripheral blood leukocytes) was supplied by Natalio Vita, Sanofi Corporation, Labege, France. rIL-2 (3.43 × 105 U/mg) was provided by Chiron, Ratingen, Germany. rIL-4 (108 U/mg) was supplied by Satwant Narula, Schering Plough, Kenilworth, N.J. Cytokines were diluted in PBS for injection into animals.
Histology.
Tissues were fixed in 10% PBS-buffered formalin and embedded in paraffin blocks. Sections were prepared and stained either with hematoxylin-eosin (HE) or with Ziehl-Neelsen stain for detecting acid-fast bacilli.
Determination of CFU.
Organs from infected mice were homogenized in PBS–0.05% Tween 80, and dilutions were plated onto Middlebrook 7H10 agar plates. Colony counts were determined after a 20-day incubation at 37°C.
Flow cytometry.
BALB/c mice with or without M. tuberculosis infection were injected with PBS, IL-2, IL-4, IL-7, or IL-15 for 1 week as indicated in Fig. 1. Spleens were harvested, and single-cell suspensions were prepared and analyzed by flow cytometry using a Coulter Epics XL flow cytometer equipped with the XL system software, version 2.1 (Beckman/Coulter, Krefeld, Germany). The following monoclonal antibodies, purchased from Beckman/Coulter, were directly labeled with either fluorescein isothiocyanate or phycoerythrin: rat anti-murine CD3 (immunoglobulin G2A [IgG2a]; clone KT3), rat anti-murine CD4 (IgG2a; clone KT6), rat anti-murine CD8 (IgG2a; clone KT15), rat anti-murine 62L (IgG2a; clone MEL-141), hamster anti-murine α/β TCR (IgG; clone H57-597), and hamster anti-murine γ/δ TCR (IgG; clone GL3). Rat anti-murine CD44 (IgG2b; clone IM7), rat anti-murine CD19 (IgG2a; clone ID3), and rat anti-NK cells (IgM; clone DX5) were obtained from PharMingen, Hamburg, Germany. Appropriate phycoerythrin- or fluorescein isothiocyanate-coupled isotype control antibodies were obtained from Beckman/Coulter.
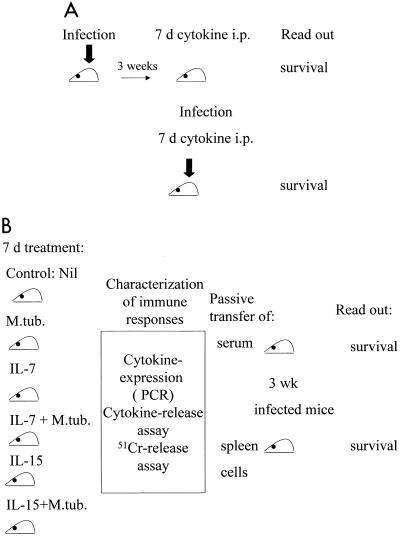
FIG. 1.
Treatment schedule. (A) Mice (n = 15/group) were i.v. infected with viable bacilli. After 3 weeks, they were treated i.p. for 7 consecutive days with three 100-ng doses of IL-2, IL-4, IL-7, or IL-15 or with PBS as a control. Alternatively, mice were infected and treated concomitantly for 7 days with cytokines. The bold arrows indicate infection with M. tuberculosis. (B) Animals were either injected with PBS or infected with M. tuberculosis (M.tub.). Each group (infected or noninfected [nil]) was treated i.p. for 7 days with either IL-7 or IL-15 (three 100-ng doses/day). After 7 days, spleen cells were harvested and tested for cytokine mRNA expression, cytotoxicity, and cytokine release. Spleen cells (3 × 107) or serum (0.3 ml) from individual animals were transferred by tail vein injection to individual animals which had been preinfected 3 weeks earlier with M. tuberculosis.
Cytotoxicity and cytokine release assays.
The NK and/or LAK-sensitive target cell lines YAC and RMA-S and the H-2d) mastocytoma cell line P815 served as controls. Peritoneal macrophages were obtained from BALB/c mice and selected by adherence to plastic for 2 h followed by three consecutive washing steps in order to ensure minimal contamination with splenic lymphocytes. Macrophages were infected with mycobacteria 24 h prior to assay, and infection was evaluated by Ziehl-Neelson staining. Cells were cultured in RPMI 1640 supplemented with fetal calf serum, l-glutamine, and penicillin (GIBCO BRL, Heidelberg, Germany). A standard 4-h chromium release assay was used to assess cytolytic recognition of target cells by freshly harvested spleen cells from individual animals. Unless otherwise indicated, an effector-to-target cell ratio of 30:1 was used. 51Cr-labeled target cell suspensions were adjusted to a density of 105 cells/ml, and 100 μl of this cell suspension was added to individual assay wells in triplicate determinations. Spleen cell suspensions (100-μl volumes) were added to the experimental wells, and the plates were incubated for 4 h at 37°C. Spontaneous-release wells received 100 μl of RPMI medium supplemented with 10% fetal calf serum, and maximum-release wells received 100 μl of Triton X-100 (10% [vol/vol] in water). Aliquots (100 μl) were harvested from each well, and radioactivity was determined in a gamma counter (Pharmacia LKB, Uppsala, Sweden). In cytokine release assays, cells were prepared as for cytotoxicity assays except that stimulator cells were infected with viable mycobacteria 24 h prior to testing, fixed with 1% formaldehyde, and incubated for 16 h with freshly harvested spleen cells. Supernatants were harvested and assayed for IFN-γ, TNF-α, IL-4, and IL-10 by enzyme-linked immunosorbent assay (R&D Systems, Wiesbaden, Germany) in accordance with the manufacturer's instructions.
Template cDNA preparation and RT-PCR.
Total RNA from 5 × 106 to 1 × 107 spleen cells was extracted by using RNAzol in accordance with the method of Chomczynski and Sacchi (9). First-strand cDNA synthesis was performed by heating the reaction mixture at 37°C for 1 h; this was followed by a 5-min denaturation step at 95°C with a Perkin-Elmer PCR thermal cycler. The 40-μl reaction volume contained 8 μg of RNA in 16 μl of H2O, 8 μl of 5× reaction buffer (ABI, Weiterstadt, Germany), 4 μl of dithiothreitol (final concentration, 10 mM), 2 μl of deoxynucleoside triphosphate solution (dATP, dCTP, dGTP, and dTTP; final concentration of each, 1 mM), 3 μl of RNase inhibitor (120 U), 1 μl of actinomycin D (2 μg), 4 μl of oligo(dT) random primers (0.8 μg), and 2 μl of Moloney murine leukemia virus reverse transcriptase (ABI; final concentration, 400 IU/ml). Individual reverse transcription (RT)-PCR conditions and primer pairs for β-actin, murine IL-2 (mIL-2), mIL-4, mIFN-γ, mTNF-α, and mIL-10 have already been described in detail elsewhere (30).
Transfer of spleen cells or serum.
Mice were sacrificed, and serum was obtained from each animal. Similarly, spleens from mice of each experimental group were harvested, and single-cell suspensions were prepared by careful repetitive aspiration with a 21-gauge needle and a 5-ml syringe. Spleen cells from the individual animals were counted and transferred (3 × 107 cells in 0.3 ml of PBS, i.v.) to a different individual animal as indicated in Fig. 1B. Control mice received either 0.3 ml of PBS i.v. or 0.3 ml of serum i.v.
Treatment schedule.
Mice (n = 15/group) were i.v. infected with viable bacilli. After 3 weeks, they were treated intraperitoneally (i.p.) for 7 consecutive days with three 100-ng of doses of IL-2, IL-4, IL-7, or IL-15 or with PBS as a control (Fig. 1A). Alternatively, mice were infected and treated concomitantly for 7 days with cytokines as indicated in Fig. 1A. In passive-transfer experiments, animals were either injected with PBS or infected with M. tuberculosis. Each group (infected or noninfected) was treated i.p. for 7 days with either IL-7 or IL-15 (three 100-ng doses/day). The dosages of cytokines were based on their ability to induce maximal proliferation of single-cell suspensions obtained from spleens of BALB/c mice (data not shown). After 7 days, spleen cells were harvested from individual animals and tested for cytokine mRNA expression, cytotoxicity, and cytokine release. Spleen cells (3 × 107) or serum (0.3 ml) from individual animals was transferred to individual animals which had been preinfected 3 weeks earlier with M. tuberculosis, as summarized in Fig. 1B.
Statistical analysis.
Survival data for mice were tested for statistical significance by the log rank test. Survival rates within each treatment schedule were compared to application with the cytokine diluent (PBS). In addition, survival rates with the various cytokine therapies (e.g., IL-7 application) were compared to those for different starting points of the treatment (treatment concomitant with infection or 3 weeks after infection). In passive-transfer experiments (with either serum or cells), survival of _M. tuberculosis_-infected animals was compared to that of mice to which no cells or serum was transferred. Counts of viable bacilli in organs as well as cytokine release data obtained from _M. tuberculosis_-infected animals treated with different cytokines were tested for statistical differences by the exact two-sided Wilcoxon test. Differences were considered significant if the P value was <0.01.
RESULTS
IL-7 and IL-15 increase survival of _M. tuberculosis_-infected mice.
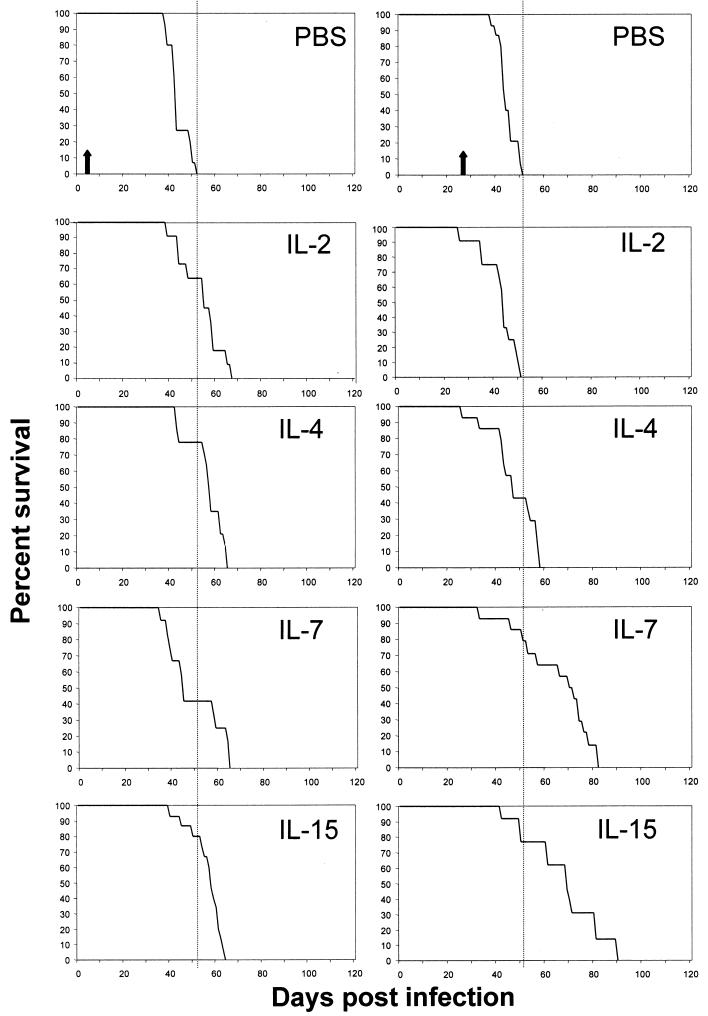
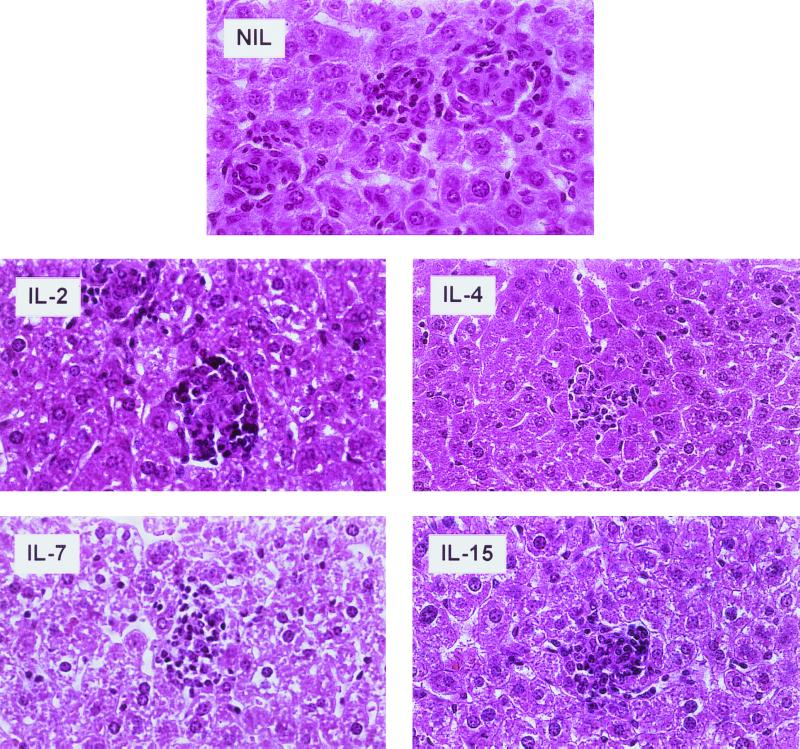
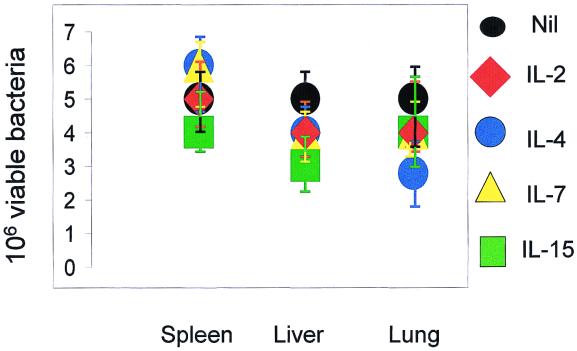
BALB/c mice were individually infected i.v. with 3 × 106 bacilli and received concomitantly, for 7 days, three 100-ng doses of IL-2, IL-4, IL-7, or IL-15 daily i.p. (Fig. 1A). The control group received PBS. Most of the animals, regardless of the particular cytokine treatment, had succumbed to the disease by day 63 postinfection (Fig. 2 and Table 1). Mice which had been preinfected with M. tuberculosis 3 weeks prior to treatment showed marked differences. Application of three 100-ng doses of IL-7 or IL-15 daily resulted in up to a 30-day prolongation of survival compared to animals which received IL-2, IL-4, or PBS alone (Fig. 2 and Table 1). Application of IL-7 3 weeks after infection was beneficial compared to IL-7 application at the time of infection (P = 0.0008). The same was found to be true for IL-15 (IL-15 application 3 weeks after infection versus IL-15 injection concomitantly with administration of bacilli; P = 0.0113). Thus, cytokines showed beneficial effects if given as a therapy, but not if injected concomitantly with M. tuberculosis. In a parallel experiment, the spleens, livers, and lungs of animals preinfected (3 weeks) with mycobacteria and treated with individual cytokines as indicated in Fig. 1 were harvested following 7 days of treatment and examined for granuloma formation by HE and for the presence of acid-fast bacilli by Ziehl-Neelsen staining. No significant differences in overall numbers and compositions of granulomas between treatment groups were observed in any of the animals (Fig. 3). Additionally, we did not observe differences in the numbers of viable bacilli (Fig. 4 and Table 2). Flow-cytometric analysis (Table 3) of spleen cells from these animals indicated no major differences pertaining to NK cells or γ/δ TCR+ cells. In general, animals infected with M. tuberculosis exhibited smaller numbers of α/β TCR+ cells than mice which had been injected with cytokines. Notably, exclusively _M. tuberculosis_-infected animals treated with either IL-7 or IL-15 exhibited a lower CD4/CD8 cell ratio (1) than mice given cytokine treatment alone or animals infected with bacilli and receiving either no treatment (CD4/CD8 cell ratio of 3) or treatment with IL-2 or IL-4 (CD4/CD8 cell ratios of 3 and 2, respectively).
FIG. 2.
Effects of IL-2, IL-4, IL-7, IL-15, and PBS on survival of _M. tuberculosis_-infected BALB/c mice. Mice were treated either at the time of infection (left panel) or 3 weeks after infection with viable bacilli (right panel). Data are from one representative experiment (n = 15 mice/group) which was performed two times. Note that IL-7 or IL-15 enhanced survival if provided as a treatment (right panel). The arrows indicate completion of the 7-day cytokine treatment (left panel, days 1 to 7; right panel, days 21 to 27). See Table 1 for associated statistical analysis data.
TABLE 1.
Statistical analysis of data from Fig. 2
| Cytokine used in treatment | P value for treatment startinga | |
|---|---|---|
| Concomitantly with infection | 3 weeks postinfection | |
| IL-2 | 0.0542 | 0.3826 |
| IL-4 | 0.0001 | 0.0654 |
| IL-7 | 0.4198 | 0.0001 |
| IL-15 | 0.0001 | 0.0003 |
FIG. 3.
Lack of a significant effect of IL-2, IL-4, IL-7, IL-15, or PBS on granuloma formation or liver pathology in animals killed at day 8 after infection and concomitant cytokine treatment. Formalin-fixed organs were stained with HE. Magnifications: NIL (PBS), ×680; IL-2, ×620, IL-4, ×516; IL-7, ×360; IL-15, ×564.
FIG. 4.
M. tuberculosis CFU in organs of infected BALB/c mice. Mice either were not infected (Nil) or infected with M. tuberculosis and after 3 weeks were treated with cytokines for 7 days as indicated in Materials and Methods. Organs were retrieved and CFU were determined. There were 5 mice per group. Experiments were performed twice. See Table 2 for associated statistical analysis of data from one experiment.
TABLE 2.
Statistical analysis of data from Fig. 4
| Cytokine used in treatment | P value for CFU in cells froma: | ||
|---|---|---|---|
| Liver | Lung | Spleen | |
| IL-2 | 1 | 1 | 0.1270 |
| IL-4 | 1 | 1 | 0.4048 |
| IL-7 | 0.8810 | 0.3968 | 0.6349 |
| IL-15 | 0.6429 | 0.6825 | 0.3175 |
TABLE 3.
Immunophenotyping of spleen cellsa
| Immune marker analyzed | % Positive-staining cells, with and without mycobacterial infection, for treatment group: | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PBS | IL-2 | IL-4 | IL-7 | IL-15 | ||||||
| − | + | − | + | − | + | − | + | − | + | |
| αβ TCR | 25 | 12 | 23 | 9 | 22 | 18 | 30 | 25 | 40 | 26 |
| γδ TCR | 11 | 2 | 10 | 3 | 15 | 2 | 12 | 1.5 | 8 | 2 |
| CD4/CD8 | 1.6 | 3 | 2.2 | 2 | 2.1 | 3 | 3.4 | 1 | 1.6 | 1 |
| CD19 | 58 | ND | 54 | ND | 44 | ND | 56 | ND | 38 | ND |
| NK | 11 | 4 | 10 | 6 | 14 | 5 | 12 | 6 | 7 | 4 |
Differential cytokine release in response to _M. tuberculosis_-infected cells as a result of IL-7 or IL-15 treatment.
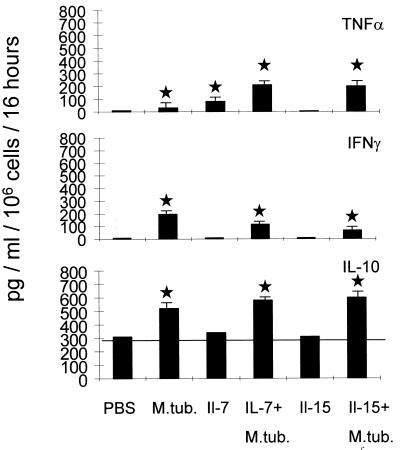
Animals which had been infected with M. tuberculosis received either PBS, IL-7, or IL-15 i.p., the cytokines being administered in three 100-ng doses daily. Spleens from individual mice were harvested after 7 days of daily cytokine application and tested for (i) cytokine mRNA expression and (ii) T-cell responses directed against _M. tuberculosis_-infected cells as determined by cytokine release and cytotoxicity assays. No gross differences in cytokine mRNA expression between treatment groups could be observed in these groups (Fig. 5). In general, in comparison to control cells, spleen cells obtained from _M. tuberculosis_-infected animals exhibited decreased IL-2 and IL-4 and enhanced IL-10 mRNA expression, irrespective of cytokine treatment. Functional assays were performed with freshly harvested spleen cells as effector cells and with murine peritoneal macrophages which had been preinfected with M. tuberculosis 24 h prior to experiments as antigen-presenting cells. Appropriate controls included macrophages without bacilli, or macrophages pulsed with bovine serum albumin, and the LAK/NK-sensitive target cell lines RMA-S and YAK. We were unable to observe any significant differences with regard to cytotoxic T-cell responses directed against any of the targets tested. In contrast, examination of cytokines released in response to _M. tuberculosis_-infected macrophages revealed that IL-7 and IL-15 enhanced TNF-α secretion in spleen cells (Fig. 6 and Table 4). Spleen cells from _M. tuberculosis_-infected animals secreted up to 83 pg of TNF-α/ml of medium. In contrast, spleen cells obtained from IL-7- or IL-15-treated, _M. tuberculosis_-infected animals secreted up to 200 or 180 pg of TNF-α, respectively. This has not been found to be true for IFN-γ secretion. Spleen cells from _M. tuberculosis_-infected animals secreted up to 200 pg of IFN-γ/ml, cells from IL-7-treated infected mice secreted up to 110 pg/ml, and IL-15-treated animals secreted 80 pg/ml of medium in response to _M. tuberculosis_-infected macrophages. Spleen cells obtained from mice treated with IL-7 or IL-15 but not exposed to M. tuberculosis infection failed to secrete detectable amounts of IFN-γ in response to _M. tuberculosis_-infected stimulator cells.
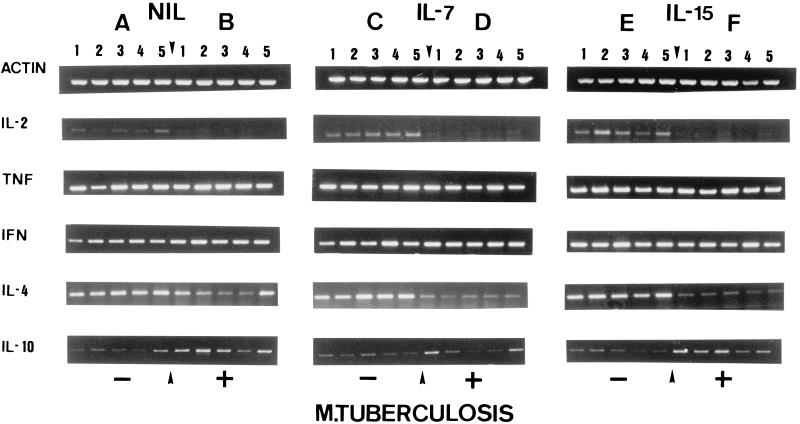
FIG. 5.
Cytokine gene expression in spleens. Mice were either noninfected (−) or infected with M. tuberculosis (+) and treated with IL-7 or IL-15. Five representative samples from individual animals are shown for each group. RNA was extracted, reverse transcribed into cDNA, and analyzed for cytokine expression by RT-PCR. Amplification of β-actin served as a positive control. Groups: A, M. tuberculosis negative, no treatment (NIL); B, M. tuberculosis positive, no treatment; C, M. tuberculosis negative, IL-7 treatment alone; D, M. tuberculosis infection plus IL-7 application; E, M. tuberculosis negative, IL-15 treatment alone; F, M. tuberculosis infection plus IL-15 treatment. No significant differences in cytokine expression could be observed within different treatment groups. In general, M. tuberculosis infection leads to decreased IL-2 and IL-4 and increased IL-10 mRNA expression.
FIG. 6.
Cytokines released in response to M. tuberculosis (M.tub)-infected macrophages. Peritoneal macrophages were harvested and infected with viable M. tuberculosis bacilli and served as antigen-presenting cells. Spleen cells were obtained either from noninfected animals, or from _M. tuberculosis_-infected mice which received either PBS, IL-7, or IL-15. Spleen cells from animals which received either IL-7 or IL-15 (without M. tuberculosis infection) served as controls. Note that antigen-presenting cells alone, i.e., without responder cells, secreted up to 310 pg of IL-10/ml (horizontal bar). However, IL-10 secretion as a response to _M. tuberculosis_-positive antigen-presenting cells was observed in spleen cells harvested from animals which had been infected with M. tuberculosis, irrespective of cytokine application. IL-7 and IL-15 treatment of _M. tuberculosis_-infected animals enhanced TNF-α secretion in spleen cells. Spleen cells from _M. tuberculosis_-infected animals secreted up to 83 pg of TNF-α/ml. In contrast, spleen cells obtained from IL-7- or IL-15-treated, _M. tuberculosis_-infected animals secreted up to 200 or 180 pg TNF-α/ml, respectively. In contrast, IL-7 or IL-15 treatment appears to decrease IFN-γ secretion as a response to _M. tuberculosis_-infected stimulator cells. Spleen cells from IL-7-treated infected mice secreted up to 110 pg and those from IL-15-treated animals secreted 80 pg of IFN-γ/ml in response to _M. tuberculosis_-infected macrophages, whereas spleen cells from _M. tuberculosis_-infected animals secreted up to 200 pg of IFN-γ/ml. No IL-4 secretion could be observed (data not shown). Data for each cytokine represent mean values for five individual animals as determined by ELISA. Error bars indicate standard deviations. P values of <0.01 are indicated with stars. Levels of cytokine secretion by spleen cells obtained from different treatment groups were compared to that by cells obtained from PBS (diluent)-injected mice. Exact P values (Wilcoxon two-sample test) are given in Table 4.
TABLE 4.
Statistical analysis of data from Fig. 6
| Cytokine | M. tuberculosis infection | P value for treatment group: | ||
|---|---|---|---|---|
| INF-γ | TNF-α | IL-10 | ||
| None | + | 0.0079 | 0.0079 | 0.0079 |
| IL-7 | − | 0.1667 | 0.0079 | 0.1111 |
| IL-7 | + | 0.0079 | 0.0079 | 0.0079 |
| IL-15 | − | 1.0000 | 0.5556 | 0.6111 |
| IL-15 | + | 0.0079 | 0.0079 | 0.0079 |
No great differences could be observed with regard to IL-10 secretion in the different groups of _M. tuberculosis_-infected animals. Of note, _M. tuberculosis_-infected stimulator cells alone secreted up to 310 pg of IL-10/ml. Secretion of up to 580 pg of IL-10/ml could be detected in animals infected with M. tuberculosis and administered PBS, IL-7, or IL-15. Within the detection limits of the assay, we were unable to observe IL-4 secretion. In summary, freshly isolated spleen cells obtained from _M. tuberculosis_-infected, IL-7- or IL-15-treated animals revealed no gross differences in IL-10 secretion but rather exhibited decreased IFN-γ secretion and increased TNF-α secretion as a response to _M. tuberculosis_-infected target cells.
Passive transfer of spleen cells obtained from _M. tuberculosis_-infected, IL-7- or IL-15-treated mice confers prolonged survival in _M. tuberculosis_-infected animals.
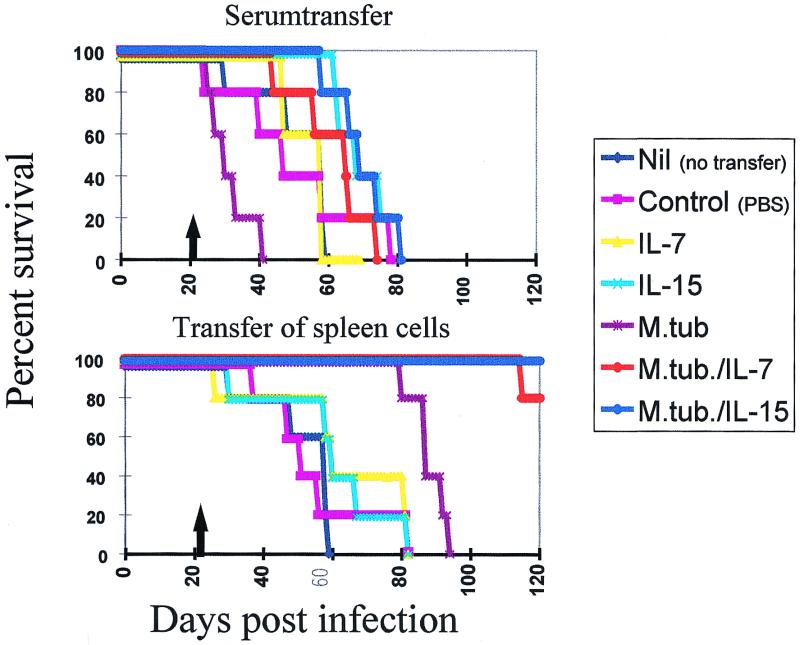
Spleen cells (3 × 107) from individual mice were resuspended in 300 μl of PBS and passively transferred into mice preinfected with M. tuberculosis (Fig. 7 and Table 5). In summary, only the cells obtained from animals which were infected with M. tuberculosis and treated with IL-7 or IL-15 exhibited prolonged survival. Fifty percent of animals which had received either PBS, spleen cells from normal control mice, or spleen cells harvested from IL-7- or IL-15-treated animals without M. tuberculosis infection succumbed within 60 days after infection (Fig. 7 and Table 5). In contrast, animals which had received spleen cells from _M. tuberculosis_-infected animals that did not undergo cytokine treatment succumbed to the infection within 93 days. Significantly, mice which received spleen cells from either IL-7- or IL-15-treated animals lived up to 120 days after infection (P values are given in the Table 5). Serum transfer did not yield enhanced survival of _M. tuberculosis_-infected mice (Fig. 7 and Table 5).
FIG. 7.
Passive transfer of spleen cells from M. tuberculosis (M.tub.)-infected animals treated with IL-7 or IL-15 enhances survival of animals which had been preinfected with M. tuberculosis. Animals were infected with viable bacilli and treated for 7 consecutive days with either IL-7, IL-15, or PBS. As a control, noninfected animals were treated with IL-7, IL-15, or PBS (see Fig. 1B). Spleen cells were harvested, and 3 × 107 cells from an individual animal were passively transferred via tail vein injection into an animal which had been preinfected (3 weeks earlier) with M. tuberculosis. As a control, serum from these individual treatment groups was passively transferred in _M. tuberculosis_-preinfected mice. Exclusively spleen cells harvested from animals which had been infected and treated with IL-7 or IL-15 could confer enhanced survival in preinfected mice (n = 15 mice/group). The arrows mark the points of passive transfer of serum or cells, respectively. See Table 5 for associated statistical analysis.
TABLE 5.
Statistical analysis of data from Fig. 7a
| Treatment group | P value obtained with passive transfer of: | ||
|---|---|---|---|
| Treatment | M. tuberculosis infection | Serum | Cells |
| PBS | − | 0.0011 | 0.4200 |
| IL-7 | − | 0.1793 | 0.0001 |
| IL-7 | + | 0.0035 | 0.0001 |
| IL-15 | − | 0.0001 | 0.0014 |
| IL-15 | + | 0.0001 | 0.0001 |
| Nil | + | 0.0001 | 0.0001 |
DISCUSSION
The three salient findings of this study are (i) that IL-7 and IL-15 are capable of enhancing survival of _M. tuberculosis_-infected animals, (ii) that these beneficial effects are exclusively observed when cytokines are applied in a treatment setting (3 weeks after the infection) but not when they are administered at the time of infection, and (iii) that enhanced survival is mediated by transfer of spleen cells obtained from animals which were treated with either IL-7 or IL-15 and infected with M. tuberculosis but not by cells from animals which had been treated with cytokines alone.
Several immune effector mechanisms may account for enhanced survival in _M. tuberculosis_-infected animals treated with IL-7 or IL-15, e.g., (i) activation of distinct subsets of T lymphocytes, (ii) activation of monocytes/macrophages, (iii) modulation of the cytokine milieu, or (iv) augmentation of antigen-specific immune responses.
(i) Activation of immune effector cells.
CD8+ α/β+ TCR T cells, NK cells, and γ/δ+ T cells have been shown to be activated by IL-7 or IL-15 in animal models with intracellular infections: Female A/J mice infected with Toxoplasma gondii and treated daily with 2 μg of IL-7 for 2 weeks survived, while control animals succumbed to the infection (22). NK cells, CD8+ T cells, and endogenously produced IFN-γ appear to be responsible for these effects (22). The relative increase in number of CD8+ T cells (Table 3) in _M. tuberculosis_-infected animals treated with IL-7 or IL-15 suggested that this T-cell subset may indeed play a role in enhanced survival of animals. However, we have not been able to determine whether α/β+ CD4+ and α/β+ CD8+ T cells in these animals exhibit a naive or memory phenotype. Double staining for CD8 and CD4 T cells with CD44 in order to demonstrate an activated and/or memory phenotype (CD44high CD62Llow) did not provide a clear pattern, which is consistent with previous reports, namely that the distinction between low- and high-level CD44 expression is prominent in C57BL/6 but not in BALB/c mice (6).
Yet, a different model of infection with intracellular bacteria has indicated that IL-7, in concert with IL-1β, enhances survival of _Listeria monocytogenes_-infected mice. IL-7-facilitated expansion of γ/δ T cells during the early phase of infection may be responsible in part for the favored outcome in IL-7-treated animals (36). Based on flow-cytometric data obtained from animals which had been infected with M. tuberculosis for 3 weeks and then subjected to a 7-day cytokine treatment schedule, no significant expansion of NK cells or γ/δ TCR+ cells was observed (Table 3). However, it may be possible that γ/δ TCR+ cells are expanded later in _M. tuberculosis_-infected animals than in those infected with L. monocytogenes. Additionally, IL-15 has been shown to protect mice from i.p. infection with Escherichia coli, presumably also by augmenting γ/δ T-cell responses (37). A number of other immune cells, which are particularly responsive to IL-15- but not to IL-2-mediated activation, may able to mediate these effects, e.g., “natural” T cells in mice (CD4− CD8− α/β TCR+ NK1.1+ cells) which express the canonical TCR alpha variable chain VA14 (31).
(ii) Enhanced antimicrobial activity of macrophages.
Alternatively, IL-7 or IL-15 elaborated in situ may directly activate macrophages, which may contribute to the effective containment of M. tuberculosis. Examination of skin biopsy specimens from patients with Hansen's disease indicated that IL-7 may indeed play a role in effective immune reactivity directed against Mycobacterium leprae. Enhanced IL-7 mRNA expression correlated with the tuberculoid form of the disease, contributing to the containment of viable bacilli (34). These observations have been substantiated by in vitro experiments showing that IL-7 appears to inhibit the intracellular growth of Mycobacterium avium complex (39). The beneficial role of IL-7 in the immune response to infections with intracellular bacteria may be mediated by several mechanisms. For instance, IL-7 potentiates the secretion of proinflammatory cytokines, e.g., IL-1β, IL-6, and TNF-α (1). Alternatively, antimycobacterial effects may be mediated by the induction of nitric oxide or superoxide radicals induced by IL-7 in susceptible cells, e.g., monocytes or macrophages (1, 18). Similarly, IL-15 has also been shown to enhance the antimicrobial activity of macrophages, leading to enhanced killing of Candida albicans (41).
(iii) Different cytokine milieu.
IL-7 or IL-15 may also contribute to a qualitatively or quantitatively different cytokine milieu during the infection with M. tuberculosis. This hypothesis is supported by the notion that IL-7 may indeed exert not only beneficial but also deleterious effects in infectious diseases. Despite the observation that IL-7 enhances in vitro anti-leishmanicidal effects of macrophages infected with Leishmania major, the application of IL-7 to BALB/c mice at the onset of infection results in a 40-fold increase in parasite burden and leads to accelerated death (17). A more-detailed analysis revealed that lymphocytes obtained from IL-7-treated mice produced levels of the Th2-associated cytokines IL-4 and IL-10 that were similar to those of nontreated animals but produced less IFN-γ in response to antigen (17). Our data may support this observation, in that IL-7 or IL-15 treatment leads to decreased IFN-γ secretion by spleen cells in response to peritoneal macrophages infected with M. tuberculosis (Fig. 6). In the initial phase of mycobacterial infection, TNF-α and IFN-γ appear to be crucial for the control of the intracellular infection (12). However, if a Th1-polarized cellular immune response ensues, it may also be able to exert deleterious effects and be responsible for the immunopathology of the disease (8, 20). This may be ameliorated by enhanced Th2 cytokine production or be reduced by the presence of Th1-associated cytokines, e.g., IFN-γ.
(iv) Augmentation of antigen-specific T-cell responses.
Of note, granuloma formation in IL-7- or IL-15-treated animals was not grossly different from that evident in animals treated with IL-2, IL-4, or PBS. The effects appear to be negligible as determined by studies of HE-stained (Fig. 3) and Ziehl Neelson-stained (data not shown) tissue sections or by measuring the CFU obtained from the lungs, spleen, and liver of each individual animal (Fig. 4). This is in contrast to IL-12-mediated effects in animals infected with M. tuberculosis; IL-12-treated animals exhibited less granuloma formation, and fewer viable bacilli could be obtained from organs of IL-12-treated mice (16). However, IL-12 may exert its beneficial effects in the induction phase of an immune response, while IL-7 and IL-15 may exert theirs in the effector phase (10, 16). The observation that IL-15 causes selective expansion of CD8+ CD44low CD62Lhigh memory cells in a murine model (43) supports the hypothesis that IL-15 is beneficial in maintaining an ongoing immune response as opposed to facilitating the expansion of naive T cells which have not yet encountered their specific target antigens (27, 28). Similarly, IL-7 is able to effectively maintain antigen-specific T cells, while IL-2 is not (25). Of note, the effects of IL-7 and IL-15 appear to be antigen specific, since the passive transfer of spleen cells in _M. tuberculosis_-infected mice from animals which received IL-7 or IL-15 alone did not result in enhanced survival (Fig. 7). Either IL-7 or IL-15 may also contribute to sustaining an effective cellular immune response via potent chemoattractant activities and by preventing apoptosis in activated immune cells (7, 29). In summary, IL-7 and IL-15 may be candidate molecules for implementing therapeutic intervention in established infections, but not necessarily for augmenting primary immune responses directed against intracellular pathogens.
REFERENCES
- 1.Alderson M R, Tough T W, Ziegler S F, Grabstein K H. Interleukin 7 induces cytokine secretion and tumoricidal activity by human peripheral blood monocytes. J Exp Med. 1991;173:923–930. doi: 10.1084/jem.173.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arachi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 3.Bamford R N, Battiata A P, Waldmann T A. IL-15: the role of translational regulation in their expression. J Leukoc Biol. 1996;59:476–480. doi: 10.1002/jlb.59.4.476. [DOI] [PubMed] [Google Scholar]
- 4.Beckman E M, Melian A, Behar S M, Sieling P A, Chatterjee D, Furlong S T, Matsumoto R, Rosat J P, Modlin R L, Porcelli S A. CD1c restricts responses of mycobacteria-specific T cells. Evidence for antigen presentation by a second member of the human CD1 family. J Immunol. 1996;157:2795–2803. [PubMed] [Google Scholar]
- 5.Beckman E M, Porcelli S A, Morita C T, Behar S M, Furlong S T, Brenner M B. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 6.Budd R, Cerottini J, Harvath C, Bron C, Pedrazzini T, Howe R, MacDonald H. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pyp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987;138:3120–3129. [PubMed] [Google Scholar]
- 7.Bulfone-Paus S, Ungureanu D, Pohl T, Lindner G, Paus R, Ruckert R, Krause H, Kunzendorf U. Interleukin-15 protects from lethal apoptosis in vivo. Nat Med. 1997;3:1124–1128. doi: 10.1038/nm1097-1124. [DOI] [PubMed] [Google Scholar]
- 8.Cadranel J, Philippe C, Perez J, Milleron B, Akoun G, Ardaillou R, Baud L. In vitro production of tumor necrosis factor and prostaglandin E2 by peripheral blood mononuclear cells from tuberculosis patients. Clin Exp Immunol. 1990;81:319–324. doi: 10.1111/j.1365-2249.1990.tb03338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 10.Cooper A M, Magram J, Ferrante J, Orme L M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doherty T M, Seder R A, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immunol. 1996;156:735–741. [PubMed] [Google Scholar]
- 12.Flesch I E A, Kaufmann S H E. Activation of tuberculostatic macrophage functions by gamma interferon, interleukin-4, and tumor necrosis factor. Infect Immun. 1990;58:2675–2677. doi: 10.1128/iai.58.8.2675-2677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 15.Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn J L, Goldstein M M, Triebold K J, Sypek J, Wolf S, Bloom B R. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995;155:2515–2524. [PubMed] [Google Scholar]
- 17.Gessner A, Will A, Vieth M, Schroppel K, Rollinghoff M. Stimulation of B-cell lymphopoiesis by interleukin-7 leads to aggravation of murine leishmaniasis. Immunology. 1995;84:416–422. [PMC free article] [PubMed] [Google Scholar]
- 18.Gessner A, Vieth M, Will A, Schröppel K, Rollinghoff M. Interleukin-7 enhances antimicrobial activity against Leishmania major in murine macrophages. Infect Immun. 1993;61:4008–4012. doi: 10.1128/iai.61.9.4008-4012.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grabstein K H, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn M A, Ahdieh M, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Pando R, Rook G A. The role of TNF-α in T-cell-mediated inflammation depends on the Th1/Th2 cytokine balance. Immunology. 1994;82:591–595. [PMC free article] [PubMed] [Google Scholar]
- 21.Jullien D, Sieling P A, Uyemura K, Mar N D, Rea T H, Modlin R L. IL-15, an immunomodulator of T cell responses in intracellular infection. J Immunol. 1997;158:800–806. [PubMed] [Google Scholar]
- 22.Kasper L H, Matsuura T, Khan L A. IL-7 stimulates protective immunity in mice against the intracellular pathogen, Toxoplasma gondii. J Immunol. 1995;155:4798–4804. [PubMed] [Google Scholar]
- 23.Kaufmann S H, Ladel C H. Role of T cell subsets in immunity against intracellular bacteria: experimental infections of knock-out mice with Listeria monocytogenes and Mycobacterium bovis BCG. Immunobiology. 1994;191:509–519. doi: 10.1016/S0171-2985(11)80457-2. [DOI] [PubMed] [Google Scholar]
- 24.Lewinsohn D M, Alderson M R, Briden A L, Riddell S R, Reed S G, Grabstein K H. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen-presenting cells. J Exp Med. 1998;187:1633–1640. doi: 10.1084/jem.187.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch D H, Miller R E. Interleukin-7 promotes long-term in vitro growth of anti-tumor cytotoxic T-lymphocytes with immunotherapeutic efficacy in vivo. J Exp Med. 1994;179:31–42. doi: 10.1084/jem.179.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsue H, Bergstresser P R, Takashima A. Keratinocyte-derived IL-7 serves as a growth factor for dendritic epidermal T cells in mice. J Immunol. 1993;151:6012–6019. [PubMed] [Google Scholar]
- 27.McInnes I B, Al-Muhales J, Field M, Leung B P, Huang F P, Dixon R, Sturrock R D, Wilkinson P C, Liew F Y. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2:175–182. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 28.McInnes I B, Leung B P, Sturrock R D, Field M, Liew F Y. Interleukin-15 mediates T cell dependent regulation of tumor necrosis factor-α production in rheumatoid arthritis. Nat Med. 1997;3:189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki T, Liu Z J, Kawahara A, Minami Y, Yamada K, Tsujimoto Y, Barsoumian E L, Permutter R M, Taniguchi T. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell. 1995;81:223–231. doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 30.Murray L J, Lee R, Martens C. In vivo cytokine gene expression in T cell subsets of the autoimmune MRL/Mp-lpr/lpr mouse. Eur J Immunol. 1990;20:163–170. doi: 10.1002/eji.1830200124. [DOI] [PubMed] [Google Scholar]
- 31.Nishizawa K, Koyasu S. IL-2 and IL-7 differentially induce CD4− CD8− αβ TCR+ NK1.1+ large granular lymphocytes and IL-4 producing cells from CD4− CD8− αβ TCR+ NK1.1− cells: implications for the regulation of Th1- and Th2-type responses. Int Immunol. 1997;9:1123–1129. doi: 10.1093/intimm/9.8.1123. [DOI] [PubMed] [Google Scholar]
- 32.Porcelli S, Morita C T, Brenner M B. CD1b restricts the response of human CD4−8− T lymphocytes to a microbial antigen. Nature. 1992;260:593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- 33.Sieling P A, Chatterjee D, Porcelli S A, Prigozy T I, Mazzaccaro R J, Soriano T, Bloom B R, Brenner M B, Kronenberg M, Brennan P J, et al. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 34.Sieling P A, Sakimura L, Uyemura K, Yamamura M, Oliveros J, Nickolof B J, Rea T H, Modlin R L. IL-7 in the cell-mediated immune response to a human pathogen. J Immunol. 1995;154:2775–2783. [PubMed] [Google Scholar]
- 35.Skeen M J, Ziegler H K. Intercellular interaction and cytokine responsiveness of peritoneal α/β and γ/δ T cells from Listeria-infected mice: synergistic effects of interleukin 1 and 7 on T cells. J Exp Med. 1993;178:985–996. doi: 10.1084/jem.178.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skeen M J, Ziegler H K. Induction of murine peritoneal gamma/delta T cells and their role in resistance to bacterial infection. J Exp Med. 1993;178:971–984. doi: 10.1084/jem.178.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takano M, Nishimura H, Kimura Y, Mokuno Y, Washizu J, Itohara S, Nimura Y, Yoshikai Y. Protective role of γδ T cells and interleukin-15 in Escherichia coli infection in mice. Infect Immun. 1998;66:3270–3278. doi: 10.1128/iai.66.7.3270-3278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka Y, Morita T, Tanaka Y, Nieves E, Brenner M B, Bloom B R. Natural and synthetic nonpeptide antigens recognized by human γδ T-cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 39.Tantawichien T, Young L S, Bermudez L E. Interleukin-7 induces anti-Mycobacterium avium activity in human monocyte-derived macrophages. J Infect Dis. 1996;174:574–582. doi: 10.1093/infdis/174.3.574. [DOI] [PubMed] [Google Scholar]
- 40.Tsukaguchi K, Balaji K N, Boom W H. CD4+ αβ T-cell and γδ T-cell responses to Mycobacterium tuberculosis. Similarities and differences in antigen recognition, cytotoxic effector function, and cytokine production. J Immunol. 1995;154:1786–1796. [PubMed] [Google Scholar]
- 41.Vázquez N, Walsh T J, Friedman D, Chanock S J, Lyman C A. Interleukin-15 augments superoxide production and microbicidal activity of human monocytes against Candida albicans. Infect Immun. 1998;66:145–150. doi: 10.1128/iai.66.1.145-150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Freeden Jeffry U, Vieira P, Lucian L A, McNeil T, Burdach S E, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Sun S, Hwang I, Tough D, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 44.Zhu X, Venkataprasad N, Thangeraj H S, Hill M, Singh M, Ivanyi J, Vordermeier H M. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 1997;158:5921–5926. [PubMed] [Google Scholar]