Carcinoembryonic Antigen Family Receptor Specificity of Neisseria meningitidis Opa Variants Influences Adherence to and Invasion of Proinflammatory Cytokine-Activated Endothelial Cells (original) (raw)
Abstract
The carcinoembryonic antigen (CEA) family member CEACAM1 (previously called biliary glycoprotein or CD66a) was previously shown to function as a receptor that can mediate the binding of Opa protein-expressing Neisseria meningitidis to both neutrophils and epithelial cells. Since neutrophils and polarized epithelia have both been shown to coexpress multiple CEACAM receptors, we have now extended this work to characterize the binding specificity of meningococcal Opa proteins with other CEA family members. To do so, we used recombinant Escherichia coli expressing nine different Opa variants from three meningococcal strains and stably transfected cell lines expressing single members of the CEACAM family. These infection studies demonstrated that seven of the nine Opa variants bound to at least one CEACAM receptor and that binding to each of these receptors is sufficient to trigger the Opa-dependent bacterial uptake by these cell lines. The other two Opa variants do not appear to bind to either CEACAM receptors or heparan sulfate proteoglycan receptors, which are bound by some gonococcal Opa variants, thus implying a novel class of Opa proteins. We have also extended previous studies by demonstrating induction of CEACAM1 expression after stimulation of human umbilical vein endothelial cells with the proinflammatory cytokine tumor necrosis factor alpha, which is present in high concentrations during meningococcal disease. This induced expression of CEACAM1 leads to an increased Opa-dependent bacterial binding and invasion into the primary endothelia, implying that these interactions may play an important role in the pathogenesis of invasive meningococcal disease.
Neisseria meningitidis is a common resident of the human nasopharyngeal mucosa of humans, yet meningococcal disease is a relatively infrequent occurrence. Meningococci can, therefore, be considered commensal organisms when isolated from the throat but are a major concern when isolated from deeper tissues. Annual rates of disease due to N. meningitidis may range between 0.001 and 1% of the population per year, depending on geographic region. Serogroup B and C strains predominate as the cause of endemic disease in Europe and North America, while strains of serogroup A are the cause of large meningococcal epidemics. China and the Sahel region of West Africa have been particularly prone to meningococcal outbreaks of meningitis and meningococcemia, with epidemics recurring each decade (32, 33). The most recent pandemic of N. meningitidis disease began in China and Nepal in the early 1980s and then spread to Mecca, Saudi Arabia, during the annual Haj pilgrimage of 1987. Healthy pilgrims carried the serogroup A epidemic strain back to their countries of origin, ultimately resulting in isolated cases of meningococcal disease in these countries, including the United States, the United Kingdom, and France, and the outbreak of epidemics in eastern and central Africa.
Primary contact with the nasopharyngeal mucosa is likely mediated by the neisserial type 4 pili (21, 30, 37). Subsequent to this, the colony opacity-associated Opa proteins can mediate a tight secondary binding that may lead to bacterial engulfment by polarized epithelial cells (49). A single strain of N. meningitidis may contain up to 4 different opa alleles (36), while a single gonococcal strain may possess as many as 11 (5, 23). Opa protein expression from each of these alleles is turned on and off at a frequency of around 10−3 due to RecA-independent genetic rearrangements within the leader peptide sequence that result in the addition or deletion of pentanucleotide complementary repeat sequences (28, 35). This phase variation constantly generates a mixed population of phenotypic variants within a single culture, since the combination of Opa variants expressed can influence the outcome of bacterial interactions with epithelial, endothelial, and phagocytic cells (11). The horizontal transfer of opa genes or gene fragments can also occur (20, 27), facilitated by the naturally high competence of pathogenic Neisseria to genetic transformation (14). Together, these rearrangements present a constantly changing assortment of antigenic and phenotypic characteristics to the human host.
Recently, the differential tropism of opaque variants was ascribed to the fact that there are two distinct classes of Opa protein based on their differential binding specificity for cellular receptors. One class, best exemplified by the Neisseria gonorrhoeae Opa50 protein, binds to the heparan sulfate proteoglycan-containing syndecan receptors (8, 42) and to the extracellular matrix proteins vitronectin (12, 13, 16) and fibronectin (41). The other class of Opa proteins has been shown to bind to host cellular receptors of the carcinoembryonic antigen (CEA) gene family. For N. gonorrhoeae, different Opa protein variants have been shown to bind one or more of the CEACAM (previously called CD66 [2]) subset of the CEA family, with some Opa variants binding CEACAM1 (CD66a; biliary glycoprotein), CEACAM3 (CD66d; CEA gene family member 1 [CGM1]), CEACAM5 (CD66e; CEA), and CEACAM6 (CD66c; nonspecific cross-reacting antigen), while the binding of others is restricted to either CEACAM1 and CEACAM5 or to CEACAM5 alone (6, 10, 18, 19). No Opa variants characterized to date have been seen to bind to CEACAM4 (CGM7), CEACAM7 (CGM2), or CEACAM8 (CD66b; CGM6) despite their strong homology at the sequence and predicted structural levels with the other CEACAM receptors (34, 38). In each case, gonococcal binding to CEACAM receptors correlates with bacterial engulfment into stably transfected epithelial cells expressing single CEACAM receptors. Virji et al. (43, 44) have also shown that each of the Opa variants from meningococcal strain C751 bound to CEACAM1, CEACAM3, CEACAM5, and CEACAM6 with various affinities, but they did not see invasion into the CEACAM6-expressing cell line. The CEACAM receptor sequences that are responsible for Opa binding are protein-protein interactions and have now been well characterized (7, 34, 43). Together, these studies have shown that the sequences recognized by Opa proteins lie within the nonglycosylated β-strands C, F, and G face of the N-terminal domain and that a heterologous triplet of amino acid residues is responsible for the differential binding of some Opa variants to CEACAM1 and CEACAM5 versus CEACAM6.
Detailed analyses of an extensive collection of meningococcal strains demonstrated that the Opa repertoire of isolates collected before the Mecca outbreak was different from that of isolates recovered during or subsequent to this event (27). This resulted from a point mutation in opaD and the acquisition of a novel opaB allele by transformation with DNA from an unrelated strain. Whether these genotypic changes contribute to the apparent differences in virulence of these strains is not known. In this study, we have used stably transfected HeLa cell lines expressing individual CEACAM family members to determine the binding specificity of the Opa variants cloned from the N. meningitidis serogroup A strains which predominated before and after the Mecca outbreak. The Opa variants encoded by a representative serogroup C isolate were also characterized in order to compare the binding specificity of the Opa repertoire expressed by an independent group of bacteria. We then assessed the ability of these Opa variants to mediate attachment and invasion into proinflammatory cytokine-stimulated primary human umbilical vein endothelial cells (HUVECs).
MATERIALS AND METHODS
Bacterial strains and cell lines.
The construction and maintenance of transfected HeLa cell lines stably expressing individual CEACAM receptor proteins and the isolation and propagation of HUVECs have been described previously (19).
Recombinant Escherichia coli DH5 strains expressing isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible Opa variants cloned from N. meningitidis isolates recovered before and after the Mecca outbreak were also previously described (27). The genes encoding Opa variants from N. meningitidis strain C1938 (36) were cloned using the approach of Kupsch et al. (23). Briefly, the four chromosomal opa genes were amplified by PCR using oligonucleotide primers TM46 (5′-TCTTTGTTATTTAGCAGCTTACTGTTCAGCTCATTACTGTTTTCTTCCGCAGCGCAGGCGGCA-3′) and EMK04 (5′-GGTACAAAGCTTTCAGAAGCGGTAGCGCACGCCC-3′), and the resulting product was then reamplified using TM45 (5′-GGCGCGGATCCAAGGAGCCGAAAATCAACCCAGCCCCCAAAAAACCTTCTTTGTTATTTAGCAGCTTA-3′) and EMK04. This resulted in (i) the deletion of the coding repeat sequence within the Opa leader peptides in order to prevent phase variation of the cloned genes and (ii) the addition of _Bam_HI and _Hin_dIII restriction enzyme sites at the 5′ and 3′ ends, respectively, of the amplified products. The resulting products were then cloned into the IPTG-inducible E. coli-N. gonorrhoeae shuttle vector Hermes-10, or into Hermes-6a behind the constitutively expressed opa promoter (22). To assess Opa function in a heterologous neisserial host, the Hermes-10 constructs were then transformed into N. gonorrhoeae by using the shuttle system of Kupsch et al. (22, 23). In each case, appropriate Opa protein expression was verified by immunoblot analysis of total bacterial extracts (5 μg/ml), using the cross-reactive anti-Opa monoclonal antibody (MAb) clone 4B12/C11 (1). See Table 1 for designations of recombinant strains.
TABLE 1.
Meningococcal Opa variants used in this studya
| opa allele | Opa protein | Parent Nme | Recombinant | GenBank accession no. | Reference | |
|---|---|---|---|---|---|---|
| Eco | Ngo | |||||
| A132 | 132 | 00170/F6124 | H2984 | AF001180 | 27 | |
| B92 | 92 | 00170 | H2985 | AF001199 | 27 | |
| B94 | 94 | F6124 | H2986 | AF001201 | 27 | |
| D100 | 100 | 00170/F6124 | H2987 | AF001195 | 27 | |
| J101 | 101 | 00170/F6124 | H2988 | AF001179 | 27 | |
| ND | C1938-1 | C1938 | H2936 | N377 | NA | This study |
| ND | 41 | C1938 | H2937 | N378 | X06445 | 36 |
| ND | 40 | C1938 | H2938 | N379 | X06446 | 36 |
| ND | C1938-4 | C1938 | H2939 | N380 | NA | This study |
Bacterial infection assays.
The quantification of bacterial binding and entry into stably transfected HeLa cell lines was done using standard gentamicin-based assays with dilution plating to recover associated viable bacteria as outlined before (18, 19). The infection of HUVECs to quantify adherent and intracellular bacteria was performed as described above for the HeLa infection assays except that the cells were grown in the presence or absence of tumor necrosis factor alpha (TNF-α; 10 ng/ml) prior to infections, as indicated, and these infection assays were done in M199 growth medium (Life Technologies, Paisley, United Kingdom) supplemented with 10% fetal calf serum. To confirm the intracellular localization of Opa-expressing E. coli, immunofluorescent staining and confocal laser scanning microscopic analysis of fixed infected samples were also performed using the protocols outlined previously (18, 19). Bacterial association was determined by differentially counting intracellular and extracellular bacteria associated with at least three samples of 20 cells each. Data presented are representative of the results obtained with at least three independent experiments.
Analysis of CEACAM expression in HUVECs.
To determine which CEACAM receptors were induced by the proinflammatory cytokine TNF-α, cells were grown in the presence or absence of TNF-α (10 ng/ml) and then harvested into phosphate-buffered saline containing 10 mM EDTA. The cells recovered were then pelleted by centrifugation, resuspended in phosphate-buffered saline containing 1 mM MgCl2, 0.5 mM CaCl2, 10 mM EDTA, 1% Triton X-100, 10 mM phenylmethylsulfonylfluoride, pepstatin A (1 μg/ml), leupeptin (2.5 μg/ml), and aprotinin (2 μg/ml), diluted into an equal volume of 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and then boiled for 15 min. Component proteins were then resolved by SDS-PAGE and immunoblot analysis using the CEACAM1, CEACAM3, CEACAM5, and CEACAM6 cross-specific MAb D14HD11, the CEACAM6-specific antibody 9A6, the CEACAM8-specific MAb 80H3 (Immunotech, Marseille, France), and the CEACAM3 and CEACAM5 cross-specific Col1 antibody (Zymed, Munich, Germany). D14HD11 and 9A6 were both generously provided by Fritz Grunert, University of Freiburg, Freiburg, Germany. Bound antibody was detected using a peroxidase-conjugated goat anti-mouse secondary antibody and the ECL chemiluminescent detection system (Amersham Life Sciences, Little Chalfont, Buckinghamshire, United Kingdom).
CEACAM1-encoding transcript levels were determined by a semiquantitative reverse transcriptase-mediated PCR (RT-PCR) method based on that outlined previously for the assessment of CEACAM1 levels in T84 epithelial cell lines (49).
RESULTS
Meningococcal Opa protein expression in E. coli.
The high-level variability of neisserial surface antigens, including the expression of pili, Opa proteins, and the Opc adhesin and capsule, makes the assignment of Opa binding function in clinical isolates difficult. We thus sought to assess the CEACAM binding characteristics of each Opa variant in isolation. Our recent success with the nonadherent laboratory strain E. coli DH5 to characterize the receptor specificities of Opa proteins cloned from N. gonorrhoeae MS11 (19) prompted us to use a similar approach to characterize the binding specificity of meningococcal variants. Meningococcal isolates collected before the Mecca outbreak differed from those that were isolated afterwards. Associated with this clonal replacement was the exchange of the allele opaB92 with the unrelated allele opaB94 (27). To determine whether this genotypic difference corresponded to a functional difference that may have contributed to the apparently heightened virulence of the latter strains, we chose to determine the binding specificity of the Opa proteins encoded by the pre- and post-Mecca outbreak strains. These alleles had previously been cloned and expressed in E. coli (27), supporting the premise that meningococcal Opa proteins would be functionally expressed in a manner similar to what we had previously found for the gonococcal proteins.
N. meningitidis from serogroup A tend to be associated with epidemic outbreaks, while the those of serogroup C are associated with endemic disease. The opa alleles carried by the serogroup C1938 strain were also cloned and expressed in E. coli DH5 in order to determine whether Opa binding functions would differ between individual strains from these two serogroups. This provided us with a set of recombinant E. coli strains expressing nine different alleles isolated from three different strains. By cloning the serogroup C1938 opa variants into the Hermes-6a shuttle vectors (22), we were also able to transfer these expression constructs into an N. gonorrhoeae MS11 derivative (see below).
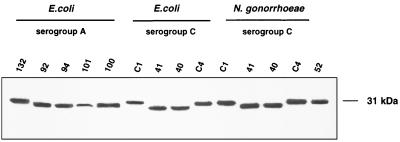
Opa expression by the recombinant E. coli and N. gonorrhoeae strains was assessed by immunoblot analysis of total bacterial lysates using a MAb, 4B12/C11, that recognizes all Opa proteins. As shown in Fig. 1, minor electrophoretic differences were observed between the individual Opa variants. The proteins were expressed at similar levels in all of the E. coli strains, with the exception that the level of Opa101 expression was consistently less than for the other variants. The N. meningitidis C1938 Opa proteins were indistinguishable by this analysis whether expressed by N. gonorrhoeae or E. coli.
FIG. 1.
Recombinant Opa protein expression. Opa protein variants cloned from serogroup A or C strains of N. meningiditis were cloned and expressed in E. coli DH5 or a recombinant N. gonorrhoeae MS11 derivative that has deletions in both pilin (pilE) loci and in the opaC30 locus that encodes the heparan sulfate proteoglycan-specific Opa30. Component proteins from bacterial lysate (5 μg/lane) were separated by SDS-PAGE, and immunoblots were probed with the Opa-specific MAb 4B12/C11 (1).
CEACAM receptor binding specificity of meningococcal Opa variants.
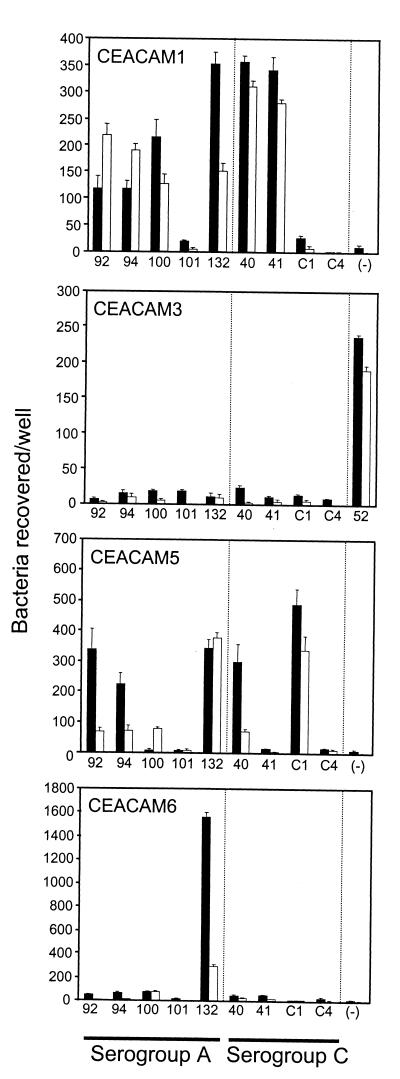
To characterize Opa binding interactions with each member of the CEACAM receptor family, we took advantage of a series of stably transfected HeLa cell lines expressing each of the CEACAM proteins (18). Each cell line was infected with the recombinant E. coli DH5 strains expressing meningococcal Opa proteins. Bacterial association with these cell lines was determined by quantifying CFU recovered after saponin lysis of washed infected cells. Virji et al. (44) had previously demonstrated that all three of the Opa variants, Opa132, Opa135, and Opa137, encoded by N. meningitidis serogroup C strain C751 could bind to CEACAM1. We obtained the same results with the cloned Opa132 protein and for three other Opa proteins encoded from the serogroup A strains (Opa92, Opa94, and Opa100) and for two of the four variants encoded by strain C1938 (Opa40 and Opa41): all these proteins mediated binding to HeLa-CEACAM1 (Fig. 2). However, Opa101 (serogroup A) and OpaC1938-4 (serogroup C) did not bind any CEACAM receptor tested, while OpaC1938-1 bound to HeLa-CEACAM5 but not HeLa-CEACAM1. Opa100 and Opa41 binding was restricted to HeLa-CEACAM1, while strains expressing Opa132 adhered to HeLa-CEACAM1, HeLa-CEACAM5, and HeLa-CEACAM6. Opa40, Opa92, and Opa94 appear to be functionally conserved, since each mediates binding to HeLa-CEACAM1 and HeLa-CEACAM5 but not to HeLa-CEACAM3 or HeLa-CEACAM6 (Fig. 2). None of the cloned meningococcal Opa proteins mediated binding to the parental HeLa cell line or to HeLa cells expressing CEACAM8 (data not shown).
FIG. 2.
Opa-mediated interactions with stably transfected CEACAM-expressing cell lines. HeLa cell lines expressing either CEACAM1, CEACAM3, CEACAM5, or CEACAM6 were infected for 3 h with E. coli DH5 strains expressing the indicated Opa variants. After washing, bacteria which associated with the transfected cells were quantified by lysing the HeLa cell membranes with 1% saponin and then dilution plating (black bars; 10−4 CFU). To determine the relative levels of bacteria which were intracellular, the washed samples were instead incubated in the presence of gentamicin prior to cellular lysis (grey bars; 10−2 CFU) and quantified. None of the Opa variants tested here bound to HeLa cells transfected with the empty expression vector or to stably transfected HeLa cells expressing CEACAM8 (data not shown). Assays were performed in triplicate on at least three different occasions, and the data illustrate the mean ± standard deviation of one representative experiment.
Unlike some gonococcal proteins (e.g., Opa52), none of the meningococcal Opa variants bound to CEACAM3, and some did not bind to any of the CEACAM receptor family. It seemed possible that the latter Opa variants also did not mediate binding to heparan sulfate proteoglycan receptors (8, 42) because E. coli expressing these Opa proteins was no more adhesive to any cell line tested than was the parental strain. These attributes might potentially be explained by improper processing of these Opa variants, because one gonococcal Opa variant (Opa60) mediated binding to HeLa-CEACAM3 cells when expressed in E. coli JM103 (18) but not when expressed in E. coli DH5 (19). We therefore transferred the expression constructs encoding the N. meningitidis C1938 Opa variants into a recombinant N. gonorrhoeae strain that has deletions in both pilin loci (pilE1 and pilE2) and in the opaC30 locus that encodes the heparan sulfate proteoglycan-specific Opa30. This allowed us to test the function of the Opa variants in a neisserial background that had been previously used to characterize the binding specificities of the gonococcal Opa proteins (19, 23). All of the Opa proteins conferred similar binding patterns whether expressed in E. coli DH5 (Fig. 2) or in N. gonorrhoeae (data not shown), thus supporting the use of recombinant E. coli as a model of meningococcal Opa binding characteristics.
To determine whether any of the recombinant E. coli strains were being internalized by the transfected HeLa cell lines, viable bacteria counts were measured after 2 h of treatment of the infected cells with bactericidal concentrations of gentamicin. In each case, the ability to adhere to transfected epithelial cell lines correlated with bacterial entry into these cells (Fig. 2). This is consistent with previous studies showing that gonococcal Opa expression is sufficient to mediate cellular invasion in the absence of other neisserial factors (9, 10, 18, 19).
CEACAM1 expression by HUVECs following stimulation with the proinflammatory cytokine TNF-α.
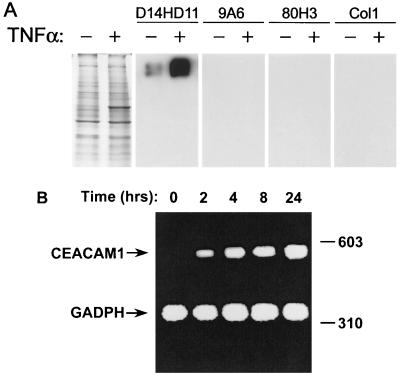
TNF-α is one of the principal mediators of endotoxic shock (4, 26, 39, 40). High levels of this proinflammatory cytokine are present in the bloodstream during invasive meningococcal infections, and the severity of disease correlates with serum TNF-α concentrations present (15, 47, 48). Previously, we have shown that TNF-α stimulates CEACAM receptor expression in primary HUVECs (19). The results with fluorescence-activated cell sorting (FACS) analysis using anti-CEACAM MAbs with two different binding specificities indicated that CEACAM1 expression was induced by TNF-α. However, the results did not exclude the concurrent stimulation of other CEACAM receptors. Therefore, we have now investigated CEACAM expression pattern of activated HUVECs by immunoblot analysis using a spectrum of MAbs with more restricted CEACAM binding specificities. As shown in Fig. 3A, MAb D14HD11, which is cross-reactive with CEACAM1, CEACAM3, CEACAM5, and CEACAM6, revealed the strong induction of a high-molecular-weight CEACAM protein following TNF-α treatment. MAb 9A6 (specific for CEACAM6), MAb 80H3 (specific for CEACAM8), and MAb Col1 (reacts with CEACAM3 and CEACAM5) did not react with any protein in the HUVEC cell lysates, thus confirming that CEACAM1 is the only Opa receptor expressed by these cells. FACS analysis using these MAbs also confirmed these results (data not shown).
FIG. 3.
Induced pattern of CEACAM receptor expression in TNF-α-stimulated HUVECs. (A) Component proteins of lysates prepared from primary HUVECs grown in the presence (+; final concentration, 10 ng/ml) or absence (−) of TNF-α were separated by SDS-PAGE, and immunoblots were probed with either the CEACAM1-, CEACAM3-, CEACAM5-, and CEACAM6-specific MAb D14HD11, the CEACAM6-specific MAb 9A6, the CEACAM8-specific MAb 80H3, or the CEACAM3- and CEACAM5-specific MAb Col1. Coomassie blue-stained samples run in parallel to the immunoblotted samples are shown in the first panel in order to confirm that protein loading was equal in the stimulated and nonstimulated cells. (B) CEACAM1 transcript expression by TNF-α-stimulated HUVECs. Total RNA was isolated after the indicated time intervals following the addition of TNF-α to the growth media. Semiquantitative duplex RT-PCR was then performed using a combination of primer pairs for the specific amplification of CEACAM and GADPH (glyceraldehyde-3-phosphate dehydrogenase) transcript fragments from total mRNA. Resulting DNA fragments were separated by electrophoresis, and amplified fragments are labeled, with the constitutively expressed GADPH standard being used to verify consistent sample amplification and loading. Sizes are indicated in base pairs.
Since the progression of invasive meningococcal disease can be rapid, the time course of CEACAM1 expression following exposure to TNF-α must be quick if it is to be biologically relevant. We therefore studied the time course of CEACAM1 expression using semiquantitative RT-PCR. CEACAM1 transcripts were induced within 2 h of stimulation by TNF-α and persisted for at least 24 h (Fig. 3B). This stimulation at the transcriptional level also corresponded with the expression of new receptor protein, since CEACAM1 continued to accumulate in HUVECs for at least 48 h (data not shown). Together, these data indicate that the TNF-α-mediated induction of CEACAM1 occurs on a time scale that is relevant for neisserial disease.
Opa-mediated association with HUVECs.
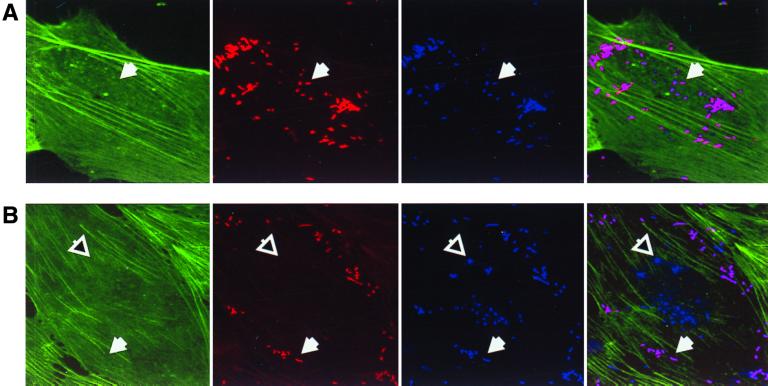
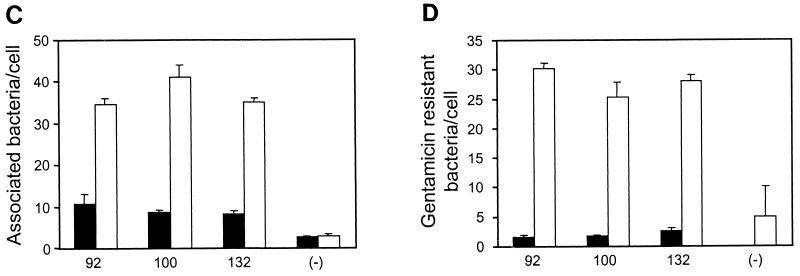
We next determined whether the binding of Opa-expressing E. coli strains to HUVECs increased in association with the increased CEACAM1 receptor expression following TNF-α stimulation. HUVECs cells that had been stimulated with TNF-α for 48 h were infected in parallel with control cells that were grown in the absence of this cytokine. After 3 h of infection, the infected samples were washed, fixed, and immunofluorescently labeled to allow the differential counting of intracellular and extracellular bacteria associated per cell by confocal laser scanning microscopy (Fig. 4A and B). Alternatively, viable bacteria that remained bound to the infected HUVECs after washing were quantified by dilution plating following saponin lysis of the eukaryotic membranes, and intracellular bacteria were recovered following gentamicin treatment of the infected cells (data not shown). The two approaches yielded consistent results, showing a clear increase in bacterial association following TNF-α treatment. As shown in Fig. 4C, approximately fourfold more bacteria bound to the TNF-α-treated cells than to the untreated controls. The effect of TNF-α treatment on cellular invasion by Opa-expressing bacteria was even more pronounced, with 10 to 20 times more bacteria being found in the stimulated cells than in the untreated controls (Fig. 4D).
FIG. 4.
Opa-mediated association with primary endothelial cells. HUVECs seeded on glass coverslips and grown in the presence or absence of TNF-α (10 ng/ml) were infected with recombinant E. coli DH5 strains expressing the indicated meningococcal Opa variants. After 3 h, samples were fixed and then immunofluorescently labeled for analysis by confocal laser scanning microscopy. (A and B) Fixed samples of untreated (A) and TNF-α-stimulated (B) cells that have been infected with E. coli DH5 expressing meningococcal Opa94 were stained to display actin cytoskeleton (green), total bacteria (blue), and extracellular bacteria (red). Representative intracellular and extracellular bacteria are indicated by open and closed arrows, respectively. (C) Total bacteria associated per cell. Black bars, adherence to untreated HUVECs; grey bars, adherence to TNF-α-activated HUVECs. (D) Intracellular bacteria per cell. Black bars, intracellular bacteria associated with untreated HUVECs; grey bars, intracellular bacteria associated with TNF-α-activated HUVECs.
DISCUSSION
Opa proteins are expressed by both mucosal and disease isolates of N. meningitidis, and 87.5% of meningococcal strains tested bind to CEACAM1 (46). In this study, we have used recombinant E. coli strains expressing Opa proteins together with stably transfected HeLa epithelial cell lines expressing each member of the CEACAM receptor family in isolation in order to characterize the binding specificity of Opa proteins from representative meningococcal strains. Based upon this, the serogroup A strains were found to express Opa variants that bind CEACAM1 (Opa100), CEACAM1 and CEACAM5 (Opa92/Opa94), CEACAM1, CEACAM5, and CEACAM6 (Opa132), or no CEACAM receptor (Opa101), while the serogroup C1938 strain was found to express variants that bound either CEACAM1 (Opa41), CEACAM5 (OpaC1938-1), both of these receptors (Opa40), or no CEACAM proteins (OpaC1938-4 [Fig. 2]). Whether the serogroup A strain's ability to bind to CEACAM6 (i.e., via Opa132) and/or the serogroup C strain's possession of an Opa protein that binds only to CEACAM5 (Opa1938-1) has functional significance with respect to virulence properties remains to be determined. It is also interesting that Opa92 and Opa94 are not different with respect to receptor binding, since this implies that the clonal replacement of opaB92 with the opaB94 allele did not result in a functional change that could contribute to the apparently different virulence of the isolates which predominated before and after the Mecca epidemic (27).
All gonococcal Opa variants tested to date bind to either CEACAM or heparan sulfate proteoglycans (6, 19). The data presented here are the first indication that Opa variants exist (Opa101 and OpaC1938-4) which did not bind to either of these receptors. Since these variants also did not mediate any bacterial binding to primary endothelial cells (Fig. 4), it remains to be determined what function they confer upon the meningococci. It was also unexpected that none of the meningococcal Opa proteins tested were able to bind to CEACAM3, since several gonococcal variants efficiently bind and mediate bacterial entry into CEACAM3-expressing cell lines (6, 9, 19). It seems unlikely that this could reflect improper processing in E. coli because most Opa proteins bound to CEACAM1, CEACAM6, and/or CEACAM5, and the binding specificities of Opa proteins expressed in recombinant N. gonorrhoeae (data not shown) and E. coli (Fig. 2) were indistinguishable. The inability to bind to CEACAM3 might be related to the different manifestations of disease caused by N. meningitidis and N. gonorrhoeae. CEACAM3 is expressed exclusively on neutrophils and contains a sequence reminiscent of the immunoreceptor tyrosine-based activating motif of various immunoglobulin family receptors (29). Engagement of such receptors, which include components of B-cell, T-cell, and Fc receptors, typically triggers immune cellular activation unless their inhibitory coreceptor is also stimulated. The absence of an activation signal upon meningococcal contact with neutrophils might alter the immune response to these bacteria, potentially explaining why gonococcal infections often provoke an intense inflammatory immune response whereas meningococcal mucosal colonization is generally asymptomatic. Such speculation warrants further study and is the focus of our ongoing work.
The TNF-α-induced increase in CEACAM1 expression by HUVECs (Fig. 3) correlates with their increased binding and uptake of Opa-expressing bacteria (Fig. 4), and anti-CEACAM antibody does block meningococcal binding to activated HUVECs (43). These results provide an important insight into our understanding of meningococcal disease. During invasive N. meningitidis infections, TNF-α levels in the blood correlate with the severity of septicemia (15, 47, 48), and patients having concentrations of more than 440 U/ml invariably die (48). Nassif et al. (31) previously demonstrated that passive immunization with polyclonal antiserum against TNF-α protected infant rats against the mortality associated with meningococcemia despite the fact that the bacterial growth kinetics in vivo were unchanged. The authors suggested that the pathophysiological events induced by these elevated levels of TNF-α might be required for meningococcal pathogenicity. We have recently shown that neisserial immunoglobulin A1 protease is a potent inducer of TNF-α in peripheral blood mononuclear cells (25). Likewise, meningococcal lipopolysaccharide is known to stimulate proinflammatory responses. Based on the results presented here, the TNF-α-induced CEACAM1 expression by endothelial cells would allow meningococcal adherence to vascular walls and, potentially, its exit from the bloodstream. Such a model parallels that which has been proposed for Plasmodium falciparum, since TNF-α induces the expression of this parasite's receptors on the surface of cerebral blood vessels (3, 17), and elevated TNF-α is associated with cerebral symptoms and other organ impairment in infected patients (24). The TNF-α-mediated induction of CEACAM1 could also help to explain the correlation between invasive meningococcal disease and recent viral infection, if these infections have triggered the expression of CEACAM1 on tissues where they are not normally present.
It is likely that various combinations of CEACAM receptors are expressed on most tissues that meningococci encounter during infection (11), and the Opa variant(s) expressed should determine which of these will be engaged. Although CEACAM proteins are extremely conserved in their extracellular domains, the cellular response to binding each protein likely differs (38). In addition, the N. meningitidis arsenal also possesses adhesins other than Opa. For example, the meningococcal Opc adhesin has been shown to bind arginine-glycine-aspartate (RGD)-containing serum proteins such as vitronectin, a function which can lead to cellular invasion through subsequent binding of the RGD protein to its cognate integrin receptors (45). How each of these binding functions may benefit the bacteria's ability to persist remains an important mystery that must be further unraveled if we are to understand the pathogenic mechanism of this medically important microbe.
ACKNOWLEDGMENTS
We are grateful to Andreas Popp for insightful discussion and technical assistance throughout this study.
This work was supported in part by the Deutsche Forschungsgemeinschaft (Me705/5-1) and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Achtman M, Neibert M, Crowe B A, Strittmatter W, Kusecek B, Weyse E, Walsh M J, Slawig B, Morelli G, Moll A, Blake M. Purification and characterization of eight class 5 outer membrane protein variants from a clone of Neisseria meningitidis serogroup A. J Exp Med. 1988;168:507–525. doi: 10.1084/jem.168.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beauchemin N, Draber P, Dveksler G, Gold P, Gray-Owen S, Grunert F, Hammarstrom S, Holmes K V, Karlsson A, Kuroki M, Lin S-H, Lucka L, Najjar S M, Neumaier M, Obrink B, Shively J E, Skubitz K M, Stanners C P, Thomas P, Thompson J A, Virji M, von Kleist S, Wagener C, Watt S, Zimmermann W. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res. 1999;252:243–249. doi: 10.1006/excr.1999.4610. [DOI] [PubMed] [Google Scholar]
- 3.Berendt A R, Simmons D L, Tansey J, Newbold C I, Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989;341:57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- 4.Beutler B, Milsark I W, Cerami A. Passive immunization against cachectin/tumor necrosis factor protects mice against lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 5.Bhat K S, Gibbs C P, Barrera O, Morrison S G, Jahnig F, Stern A, Kupsch E M, Meyer T F, Swanson J. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol Microbiol. 1991;5:1889–1901. doi: 10.1111/j.1365-2958.1991.tb00813.x. . (Erratum, 6:1073–1076, 1992.) [DOI] [PubMed] [Google Scholar]
- 6.Bos M P, Grunert F, Belland R J. Differential recognition of members of the carcinoembryonic antigen family by Opa variants of Neisseria gonorrhoeae. Infect Immun. 1997;65:2353–2361. doi: 10.1128/iai.65.6.2353-2361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bos M P, Hogan D, Belland R J. Homologue scanning mutagenesis reveals CD66 receptor residues required for neisserial Opa protein binding. J Exp Med. 1999;190:331–340. doi: 10.1084/jem.190.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen T, Belland R, Wilson J, Swanson J. Adherence of pilus− Opa+ gonococci to epithelial cells in vitro involves heparan sulfate. J Exp Med. 1995;182:511–517. doi: 10.1084/jem.182.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen T, Gotschlich E C. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc Natl Acad Sci USA. 1996;93:14851–14856. doi: 10.1073/pnas.93.25.14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T, Grunert F, Medina-Marino A, Gotschlich E C. Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J Exp Med. 1997;185:1557–1564. doi: 10.1084/jem.185.9.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dehio C, Gray-Owen S D, Meyer T F. The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol. 1998;6:489–495. doi: 10.1016/s0966-842x(98)01365-1. [DOI] [PubMed] [Google Scholar]
- 12.Dehio M, Gomez-Duarte O G, Dehio C, Meyer T F. Vitronectin-dependent invasion of epithelial cells by Neisseria gonorrhoeae involves αv integrin receptors. FEBS Lett. 1998;424:84–88. doi: 10.1016/s0014-5793(98)00144-6. [DOI] [PubMed] [Google Scholar]
- 13.Duensing T D, van Putten J P. Vitronectin mediates internalization of Neisseria gonorrhoeae by Chinese hamster ovary cells. Infect Immun. 1997;65:964–970. doi: 10.1128/iai.65.3.964-970.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frosch M, Meyer T F. Transformation-mediated exchange of virulence determinants by co-cultivation of pathogenic Neisseriae. FEMS Microbiol Lett. 1992;79:345–349. doi: 10.1111/j.1574-6968.1992.tb14062.x. [DOI] [PubMed] [Google Scholar]
- 15.Girardin E, Grau G E, Dayer J-M, Roux-Lombard P, Lambert P-H. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1988;319:397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Duarte O G, Dehio M, Guzman C A, Chhatwal G S, Dehio C, Meyer T F. Binding of vitronectin to opa-expressing Neisseria gonorrhoeae mediates invasion of HeLa cells. Infect Immun. 1997;65:3857–3866. doi: 10.1128/iai.65.9.3857-3866.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grau G, Fajardo L, Piguet P, Allet B, Lambert P, Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987;237:1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- 18.Gray-Owen S D, Dehio C, Haude A, Grunert F, Meyer T F. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J. 1997;16:3435–3445. doi: 10.1093/emboj/16.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray-Owen S D, Lorenzen D R, Haude A, Meyer T F, Dehio C. Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol Microbiol. 1997;26:971–980. doi: 10.1046/j.1365-2958.1997.6342006.x. [DOI] [PubMed] [Google Scholar]
- 20.Hobbs M M, Malorny B, Prasad P, Morelli G, Kusecek B, Heckels J E, Cannon J G, Achtman M. Recombinational reassortment among opa genes from ET-37 complex Neisseria meningitidis isolates of diverse geographical origins. Microbiology. 1998;144:157–166. doi: 10.1099/00221287-144-1-157. [DOI] [PubMed] [Google Scholar]
- 21.Kellogg D S, Jr, Peacock W L, Deacon W E, Brown L, Pirkle C I. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J Bacteriol. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kupsch E-M, Aubel D, Gibbs C P, Kahrs A F, Rudel T, Meyer T F. Construction of Hermes shuttle vectors: a versatile system useful for genetic complementation of transformable and non-transformable Neisseria mutants. Mol Gen Genet. 1996;250:558–569. doi: 10.1007/BF02174444. [DOI] [PubMed] [Google Scholar]
- 23.Kupsch E-M, Knepper B, Kuroki T, Heuer I, Meyer T F. Variable opacity (Opa) outer membrane proteins account for the cell tropisms displayed by Neisseria gonorrhoeae for human leukocytes and epithelial cells. EMBO J. 1993;12:641–650. doi: 10.1002/j.1460-2075.1993.tb05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwiatkowski D, Hill A V S, Sambou I, Twumasi P, Castracane J, Manogue K R, Cerami A, Brewster D R, Greenwood B M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;ii:1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzen D R, Dux F, Wolk U, Tsirpouchtsidis A, Haas G, Meyer T F. Immunoglobulin A1 protease, an exoenzyme of pathogenic Neisseriae, is a potent inducer of proinflammatory cytokines. J Exp Med. 1999;190:1–10. doi: 10.1084/jem.190.8.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathison J C, Wolfson E, Ulevitch R J. Participation of tumor necrosis factor in the mediation of Gram negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Investig. 1988;81:1925–1937. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morelli G, Malorny B, Muller K, Seiler A, Wang J F, del Valle J, Achtman M. Clonal descent and microevolution of Neisseria meningitidis during 30 years of epidemic spread. Mol Microbiol. 1997;25:1047–1064. doi: 10.1046/j.1365-2958.1997.5211882.x. [DOI] [PubMed] [Google Scholar]
- 28.Murphy G L, Connell T D, Barritt D S, Koomey M, Cannon J G. Phase variation of gonococcal protein II: regulation of gene expression by slipped-strand mispairing of a repetitive DNA sequence. Cell. 1989;56:539–547. doi: 10.1016/0092-8674(89)90577-1. [DOI] [PubMed] [Google Scholar]
- 29.Nagel G, Grunert F, Kuijpers T W, Watt S M, Thompson J, Zimmermann W. Genomic organization, splice variants and expression of CGM1, a CD66-related member of the carcinoembryonic antigen gene family. Eur J Biochem. 1993;214:27–35. doi: 10.1111/j.1432-1033.1993.tb17892.x. [DOI] [PubMed] [Google Scholar]
- 30.Nassif X, Marceau M, Pujol C, Pron B, Beretti J L, Taha M K. Type-4 pili and meningococcal adhesiveness. Gene. 1997;192:149–153. doi: 10.1016/s0378-1119(96)00802-5. [DOI] [PubMed] [Google Scholar]
- 31.Nassif X, Mathison J C, Wolfson E, Koziol J A, Ulevitch R J, So M. Tumour necrosis factor alpha antibody protects against lethal meningococcaemia. Mol Microbiol. 1992;6:591–597. doi: 10.1111/j.1365-2958.1992.tb01505.x. [DOI] [PubMed] [Google Scholar]
- 32.Olyhoek T, Crowe B A, Achtman M. Clonal population structure of Neisseria meningitidis serogroup A isolated from epidemics and pandemics between 1915 and 1983. Rev Infect Dis. 1987;9:665–692. doi: 10.1093/clinids/9.4.665. [DOI] [PubMed] [Google Scholar]
- 33.Peltola H. Meningococcal disease: still with us. Rev Infect Dis. 1983;5:71–91. doi: 10.1093/clinids/5.1.71. [DOI] [PubMed] [Google Scholar]
- 34.Popp A, Dehio C, Grunert F, Meyer T F, Gray-Owen S D. Molecular analysis of neisserial Opa protein interactions with the CEA family of receptors: identification of determinants contributing to the differential specificities of binding. Cell Microbiol. 1998;1:169–181. doi: 10.1046/j.1462-5822.1999.00017.x. [DOI] [PubMed] [Google Scholar]
- 35.Stern A, Brown M, Nickel P, Meyer T F. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986;47:61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- 36.Stern A, Meyer T F. Common mechanism controlling phase and antigenic variation in pathogenic neisseriae. Mol Microbiol. 1987;1:5–12. doi: 10.1111/j.1365-2958.1987.tb00520.x. [DOI] [PubMed] [Google Scholar]
- 37.Swanson J, Robbins K, Barrera O, Corwin D, Boslego J, Ciak J, Blake M, Koomey J M. Gonococcal pilin variants in experimental gonorrhea. J Exp Med. 1987;165:1344–1357. doi: 10.1084/jem.165.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson J A, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991;5:344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- 39.Tracey K J, Beutler B, Lowry S F, Merryweather J, Wolpe S, Milsark I W, Hariri R J, Fahey III T J, Zentella A, Alberta J D, Shires G T, Cerami A. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–473. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 40.Tracey K J, Fong Y, Hesse D G, Manogue K R, Lee A T, Kuo G C, Lowry S F, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteremia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 41.van Putten J, Duensing T D, Cole R L. Entry of Opa+ gonococci into HEp-2 cells requires concerted action of glycosaminoglycans, fibronectin and integrin receptors. Mol Microbiol. 1998;29:369–379. doi: 10.1046/j.1365-2958.1998.00951.x. [DOI] [PubMed] [Google Scholar]
- 42.van Putten J P, Paul S M. Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrhoeae entry into human mucosal cells. EMBO J. 1995;14:2144–2154. doi: 10.1002/j.1460-2075.1995.tb07208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Virji M, Evans D, Hadfield A, Grunert F, Teixeira A M, Watt S M. Critical determinants of host receptor targeting by Neisseria meningitidis and Neisseria gonorrhoeae: identification of Opa adhesiotopes on the N-domain of CD66 molecules. Mol Microbiol. 1999;34:538–551. doi: 10.1046/j.1365-2958.1999.01620.x. [DOI] [PubMed] [Google Scholar]
- 44.Virji M, Makepeace K, Ferguson D J P, Watt S. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol Microbiol. 1996;22:941–950. doi: 10.1046/j.1365-2958.1996.01551.x. [DOI] [PubMed] [Google Scholar]
- 45.Virji M, Makepeace K, Moxon E R. Distinct mechanisms of interactions of Opc-expressing meningococci at apical and basolateral surfaces of human endothelial cells; the role of integrins in apical interactions. Mol Microbiol. 1994;14:173–174. doi: 10.1111/j.1365-2958.1994.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 46.Virji M, Watt S, Barker K, Makepeace K, Doyonnas R. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol Microbiol. 1996;22:929–939. doi: 10.1046/j.1365-2958.1996.01548.x. [DOI] [PubMed] [Google Scholar]
- 47.Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. J Exp Med. 1989;170:1859–1867. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waage A, Halstensen A, Espevik T. Association between tumor necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987;i:355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Gray-Owen S D, Knorre A, Meyer T F, Dehio C. Opa binding to cellular CD66 receptors mediates the transcellular traversal of Neisseria gonorrhoeae across polarised T84 epithelial cell monolayers. Mol Microbiol. 1998;30:657–671. doi: 10.1046/j.1365-2958.1998.01102.x. [DOI] [PubMed] [Google Scholar]