Salmonella enterica Serovars Typhimurium and Dublin Can Lyse Macrophages by a Mechanism Distinct from Apoptosis (original) (raw)
Abstract
Salmonella enterica serovars Typhimurium and Dublin lysed primary bovine alveolar macrophages and immortalized J774.2 macrophage-like cells in the absence of either the morphological changes or DNA fragmentation characteristic of apoptosis. Macrophage lysis was dependent on a subset of caspases and an intact sipB gene.
There is currently great interest in the modulation of eukaryotic cell apoptosis by microbial pathogens. Several viral pathogens can inhibit apoptosis and this may allow viral replication in host cells, which would otherwise be cleared by normal immune mechanisms (reviewed in reference 22). In contrast, several facultative intracellular bacterial pathogens have been reported to induce apoptosis in host cells (16, 34), and it is proposed that this will initiate an inflammatory response by the activation of interleukin-1 by caspase 1 (also known as interleukin-1-converting enzyme) leading to tissue damage and bacterial spread (reviewed in reference 33). However, this proposal is inconsistent with the widely held view that apoptosis limits the inflammatory response potentially associated with eukaryotic cell death (19). In addition, the fate of the intracellular bacteria, which would presumably become trapped within the apoptotic cell, is unclear.
Some of the evidence that bacterially induced cell death is due to apoptosis is controversial. Several investigators have monitored cell death by the uptake of non-membrane-permeative dyes. However, the reported steady uptake over time of such dyes (5, 16) is inconsistent with cell death by apoptosis, in which the integrity of the plasma membrane is maintained until the onset of secondary necrosis, at which point there is a sudden and rapid loss of membrane integrity. In addition, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining and several other assays detecting DNA fragmentation are not specific for apoptosis, since DNA fragmentation may also occur during necrosis (4, 8, 29). Salmonella infection of macrophages induces the formation of TUNEL-positive cells, but it was not determined that the TUNEL-positive cells were apoptotic (5, 12, 16). We have previously reported that Salmonella enterica serovars Typhimurium and Dublin induce a steady and rapid disruption of the plasma membrane of murine peritoneal macrophages and bovine alveolar macrophages (9, 27). In the present study, we further investigated the mechanism of _Salmonella_-induced macrophage lysis.
Preparation of bacterial strains and eukaryotic cells.
Salmonella serovar Typhimurium strains ST4/74, C5, and 14028 and Salmonella serovar Dublin strain SD2229 and its derivative sipB mutant, B1, have been described previously and characterized extensively (1, 3, 6, 7, 9, 10, 13, 18, 25, 27, 28, 31, 32). Alveolar macrophages were isolated from healthy Friesian cattle by bronchoalveolar lavage as described previously (9). J774.2 cells are immortalized, macrophage-like cells. Both cell types were incubated in Dulbecco's modified Eagle's medium–Ham's F-12 nutrient mix without phenol red and containing 5% fetal calf serum and were infected with logarithmic-phase bacteria at a ratio of five bacteria to each eukaryotic cell. Overgrowth of extracellular bacteria in cell monolayers incubated for 20 h was prevented by using gentamicin in a manner similar to that of previous studies (7, 16). Actinomycin–d-mannitol, an inducer of apoptosis, was added to control monolayers at a final concentration of 1 μg ml−1.
Electron microscopy of macrophages.
The ultrastructure of bovine alveolar macrophages was examined by transmission electron microscopy. The majority of macrophages infected with either serovar Typhimurium or serovar Dublin appeared to be necrotic, with a loss of pseudopodia and disrupted nuclear and plasma membranes (Fig. 1). These changes were evident at 3 h after infection and were considerably more severe at 20 h after infection. Incubation with actinomycin–d-mannitol for 3 h had little effect, but by 5 h, marginalization of condensed chromatin and membrane blebbing was apparent, and by 20 h, the majority of macrophages were undergoing secondary necrosis as a consequence of death by apoptosis. Opsonization of bacteria in 10% autologous bovine serum had little or no effect on the appearance of the monolayers compared to that of unopsonized bacteria (data not shown). Infection of J774.2 cells also induced a range of morphological changes which were not characteristic of apoptosis (data not shown).
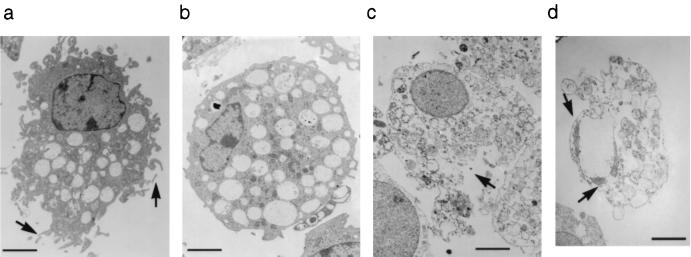
FIG. 1.
Transmission electron microscopy of bovine alveolar macrophages left uninfected (a), infected with Salmonella serovar Dublin SD2229 for 3 h (b), infected with serovar Dublin SD2229 for 20 h (c), or incubated with actinomycin–d-mannitol for 20 h (d). The macrophage in panel a has the typical appearance of a healthy cell, with many pseudopodia (arrows) and a normal nuclear morphology. Note in panel b the absence of pseudopodia and in panel c the disruption to the plasma membrane (arrow). Panel d shows a macrophage undergoing secondary necrosis as a consequence of apoptosis. The remains of the marginalized, condensed chromatin are indicated with arrows, the cell is reduced in size, and although the cytoplasm is disintegrating, it is still relatively well contained by the plasma membrane. Micrograph negatives were scanned using a linotype Saphir flatbed scanner, and the image was converted to positive and the contrast was adjusted using Adobe Photoshop 3.0. Bar = 2 μm.
Characterization of macrophage DNA.
The mechanism of _Salmonella_-induced macrophage lysis was investigated further by examining macrophage DNA by electrophoresis for the characteristic laddering pattern associated with apoptosis. DNA was extracted by the method of Zychlinsky et al. (34). There was no appearance of DNA laddering following infection of bovine alveolar macrophages with any of the Salmonella strains at either 3 h (Fig. 2) or 20 h (data not shown) compared to the uninfected controls. Opsonization of bacteria did not affect the appearance of DNA (data not shown). DNA from macrophages incubated for 20 h with actinomycin–d-mannitol exhibited a laddering pattern. Similar results were observed with J774.2 cells (data not shown).
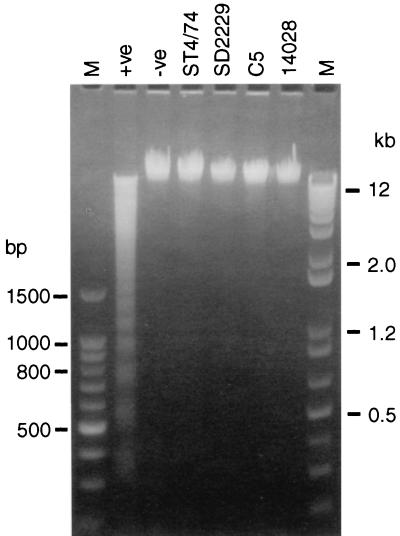
FIG. 2.
Characterization of DNA extracted from bovine alveolar macrophages infected for 3 h with Salmonella serovar Typhimurium ST4/74, C5, or 14028 or with Salmonella serovar Dublin SD2229. Uninfected macrophages were used as the negative control (−ve), and macrophages incubated with actinomycin–d-mannitol for 20 h were used as the positive control (+ve). The original photograph of the gel was scanned using a Kodak DCS420 digital camera, and the contrast of the image was adjusted using Adobe Photoshop 3.0. Lanes M contain molecular size markers.
Effect of caspase inhibitors and mutation of sipB on macrophage lysis.
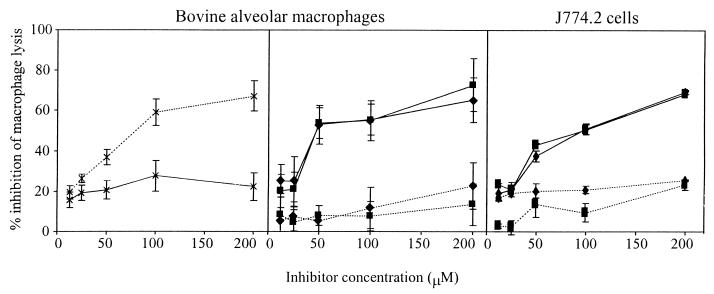
Macrophage lysis was quantified by measuring the release of lactate dehydrogenase (LDH) from cell monolayers as described previously (9). Peptide inhibitors of caspase 1 (Ac-YVAD-aldehyde) and caspase 3 (Ac-DEVD-aldehyde) (Bachem, Saffron Walden, United Kingdom) were added to the monolayers at a range of concentrations 1 h before infection. Macrophage lysis was measured at 3 h after infection with Salmonella and 20 h after the addition of actinomycin–d-mannitol (incubation with actinomycin–d-mannitol for only 3 h does not induce significant release of LDH). Ac-YVAD-aldehyde inhibited _Salmonella_-induced lysis of both bovine macrophages and J774.2 cells by 40 to 50% or more at a concentration of 50 μM or above, but it did not inhibit lysis induced by incubation with actinomycin–d-mannitol (Fig. 3). In contrast, Ac-DEVD-aldehyde inhibited actinomycin–d-mannitol-induced macrophage lysis over 20 h by 40 to 50% or more at concentrations of 100 μM or above, but it did not inhibit _Salmonella_-induced macrophage lysis. Inhibition of actinomycin–d-mannitol-induced apoptosis by caspase 3 but not by caspase 1 inhibitors has been reported previously (15). The present study confirmed that the inhibition of _Salmonella_-induced macrophage lysis was not due to a reduction in bacterial uptake and that it correlated to a reduction in interleukin-1 release by the macrophages (data not shown).
FIG. 3.
Inhibition of lysis of bovine alveolar macrophages and J774.2 cells by specific caspase inhibitors. Cell monolayers were incubated with different concentrations of Ac-YVAD-aldehyde (——) or Ac-DEVD-aldehyde (........) before incubation with actinomycin–d-mannitol (×) or infection with Salmonella serovar Typhimurium ST4/74 (■) or Salmonella serovar Dublin SD2229 (⧫). The error bars represent the standard errors of the means of quadruplicate samples for bovine alveolar macrophages and triplicate samples for J774.2 cells.
Macrophage lysis was significantly reduced (P < 0.001) by mutation of the sipB gene. In bovine alveolar macrophages, the release of LDH after infection with either wild-type serovar Dublin SD2229 or its derivative sipB mutant was 46.29% ± 2.17% and 10.79% ± 0.24%, respectively. In J774.2 cells, the corresponding LDH release was 93.37% ± 0.21% and 12.50% ± 0.06%. The LDH release from uninfected bovine alveolar macrophages and J774.2 cells was 9.69% ± 0.56% and 10.06% ± 0.27%, respectively. These data are the means of triplicate samples and are representative of three separate experiments. These results are in agreement with our previous observation that disruption of the type three secretion system 1 (TTSS-1) following mutation of the invH gene reduced lysis of bovine alveolar macrophages (27). However, due to the pleiotropic nature of mutations affecting TTSS-1, which will affect the translocation of several effector proteins, these results do not implicate the direct involvement of any one TTSS-1-associated gene product in nonapoptotic macrophage lysis.
In summary, _Salmonella_-induced lysis of bovine alveolar macrophages did not have any of the typical characteristics of apoptosis (2, 14). Specifically, there was no double-stranded, internucleosomal DNA cleavage and no morphological changes characteristic of apoptosis. Similar results were obtained during infection of J774.2 cells. Inhibition of caspase 3, one of the key execution molecules in apoptosis (reviewed in reference 20), did not prevent lysis of either bovine alveolar macrophages or J774.2 cells. Taken together, these results unequivocally demonstrate that Salmonella strains are able to kill bovine alveolar macrophages by a mechanism distinct from apoptosis and that other types of macrophages may be killed by a similar, nonapoptotic mechanism. This is in contrast to the conclusions of several other studies (5, 10, 13, 16) and has important implications for future studies of _Salmonella_-induced macrophage lysis, in which it cannot be assumed that lysis is a result of apoptosis without performing the appropriate, controlled experiments.
We have reproduced the reduction in _Salmonella_-induced macrophage lysis using a tetrapeptide inhibitor of caspase 1, as reported previously (10). Although such inhibitors are not entirely specific (24), the involvement of caspase 1 has been further demonstrated by the decrease in _Salmonella_-induced lysis of macrophages isolated from caspase 1 knockout mice (10). However, the specific contribution of caspase 1 to apoptosis is controversial. The current evidence suggests that if it does have any role at all, it is either minor or redundant (reviewed in references 17, 20, and 30)). Macrophages from caspase 1 knockout mice are able to undergo apoptosis normally (11), and therefore the reduction in _Salmonella_-induced lysis in these macrophages (10) is unlikely to be due to their inability to undergo apoptosis. Several recent studies have shown that necrosis may be less “accidental” or more regulated than previously thought, with some involvement of caspases and with regulatory links to apoptosis (23, 26; reviewed in reference 14). Further study of the mechanism of _Salmonella_-induced macrophage lysis will shed light on the regulation and mechanism of cell death other than apoptosis and may also be applicable to cell death induced by other pathogens in which caspase 1, but not apoptosis, is involved (21).
Acknowledgments
This work was supported by Ministry for Agriculture Food and Fisheries grant contract number OZ0352 and Biological and Biotechnological Science Research Council grant CEL04652.
We acknowledge the skilled assistance of P. Monaghan, H. Cook, and T. Smith during the electron microscopic analysis.
REFERENCES
- 1.Ahmer B M M, van Reeuwijk J, Watson P R, Wallis T S, Heffron F. Salmonella SirA is a global regulator of genes mediating enteropathogenesis. Mol Microbiol. 1999;31:971–982. doi: 10.1046/j.1365-2958.1999.01244.x. [DOI] [PubMed] [Google Scholar]
- 2.Allen R T, Hunter III W J, Agrawal D K. Morphological and biochemical characterization and analysis of apoptosis. J Pharmacol Toxicol Methods. 1997;37:215–228. doi: 10.1016/s1056-8719(97)00033-6. [DOI] [PubMed] [Google Scholar]
- 3.Baird G D, Manning E J, Jones P W. Evidence for related virulence sequences in plasmids of Salmonella dublin and Salmonella typhimurium. J Gen Microbiol. 1985;131:1815–1823. doi: 10.1099/00221287-131-7-1815. [DOI] [PubMed] [Google Scholar]
- 4.Bortner C D, Oldenburg N B E, Cidlowski J A. The role of DNA fragmentation in apoptosis. Trends Cell Biol. 1995;5:21–26. doi: 10.1016/s0962-8924(00)88932-1. [DOI] [PubMed] [Google Scholar]
- 5.Chen L M, Kaniga K, Galán J E. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 6.Farrant J L, Sansone A, Canvin J R, Pallen M J, Langford P R, Wallis T S, Dougan G, Kroll J S. Bacterial copper- and zinc-cofactored superoxide dismutase contributes to the pathogenesis of systemic salmonellosis. Mol Microbiol. 1997;25:785–796. doi: 10.1046/j.1365-2958.1997.5151877.x. [DOI] [PubMed] [Google Scholar]
- 7.Fields P I, Swanson R V, Haidaris C G, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Herman R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis and autolytic cell death: a cautionary note. Hepatology. 1995;21:1465–1468. doi: 10.1002/hep.1840210534. [DOI] [PubMed] [Google Scholar]
- 9.Guilloteau L A, Wallis T S, Gautier A V, MacIntyre S, Platt D J, Lax A J. The Salmonella virulence plasmid enhances Salmonella-induced lysis of macrophages and influences inflammatory responses. Infect Immun. 1996;64:3385–3393. doi: 10.1128/iai.64.8.3385-3393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hersh D, Monack D M, Smith M R, Ghori N, Falkow S, Zychlincky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, Towne E, Trasey D, Wardwell S, Wei F-Y, Wong W, Kamen R, Seshardi T. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 12.Lindgren S W, Stojiljkovic I, Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundberg U, Vinatzer U, Berdnik D, von Gabain A, Baccarini M. Growth phase-regulated induction of Salmonella-induced macrophage apoptosis correlates with transient expression of SPI-1 genes. J Bacteriol. 1999;181:3433–3437. doi: 10.1128/jb.181.11.3433-3437.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McConkey D J. Biochemical determinants of apoptosis and necrosis. Toxicol Lett. 1998;99:157–168. doi: 10.1016/s0378-4274(98)00155-6. [DOI] [PubMed] [Google Scholar]
- 15.Mohr S, Zech B, Lapetina E G, Brüne B. Inhibition of caspase-3 by S-nitrosation and oxidation caused by nitric oxide. Biochem Biophys Res Commun. 1997;238:387–391. doi: 10.1006/bbrc.1997.7304. [DOI] [PubMed] [Google Scholar]
- 16.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson D W, Thornberry N A. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 18.Riikonen P, Mäkelä P H, Saarilahti H, Sukupolvi S, Tairi S, Rhen M. The virulence plasmid does not contribute to growth of Salmonella in cultured macrophages. Microb Pathog. 1992;13:281–291. doi: 10.1016/0882-4010(92)90038-p. [DOI] [PubMed] [Google Scholar]
- 19.Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61:375–380. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- 20.Stennicke H R, Salvesen G S. Properties of the caspases. Biochim Biophys Acta. 1998;1387:17–31. doi: 10.1016/s0167-4838(98)00133-2. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi R, Tsutsumi H, Osaki M, Haseyama K, Mizue N, Chiba S. Respiratory syncytial virus infection of human alveolar epithelial cells enhances interferon regulatory factor 1 and interleukin-1β-converting enzyme gene expression but does not cause apoptosis. J Virol. 1998;72:4498–4502. doi: 10.1128/jvi.72.5.4498-4502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tschopp J, Thome M, Hofmann K, Meinl E. The fight of viruses against apoptosis. Curr Opin Genet Dev. 1998;8:82–87. doi: 10.1016/s0959-437x(98)80066-x. [DOI] [PubMed] [Google Scholar]
- 23.Tsujimoto Y, Shimizu S, Eguchi Y, Kamiike W, Matsuda H. Bcl-2 and Bcl-xL block apoptosis as well as necrosis: possible involvement of common mediators in apoptotic and necrotic signal transduction pathways. Leukemia. 1997;11(Suppl. 3):380–382. [PubMed] [Google Scholar]
- 24.Villa P, Kaufmann S H, Earnshaw W C. Caspases and caspase inhibitors. Trends Biochem Sci. 1997;22:388–393. doi: 10.1016/s0968-0004(97)01107-9. [DOI] [PubMed] [Google Scholar]
- 25.Wallis T S, Paulin S M, Plested J S, Watson P R, Jones P W. The Salmonella dublin virulence plasmid mediates systemic but not enteric phases of salmonellosis in cattle. Infect Immun. 1995;63:2755–2761. doi: 10.1128/iai.63.7.2755-2761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warny M, Kelly C P. Monocyte cell necrosis is mediated by potassium depletion and caspase-like proteases. Am J Physiol. 1999;276:C717–C724. doi: 10.1152/ajpcell.1999.276.3.C717. [DOI] [PubMed] [Google Scholar]
- 27.Watson P R, Galyov E E, Paulin S M, Jones P W, Wallis T S. Mutation of invH, but not stn, reduces Salmonella-induced enteritis in cattle. Infect Immun. 1998;66:1432–1438. doi: 10.1128/iai.66.4.1432-1438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson P R, Paulin S M, Bland A P, Jones P W, Wallis T S. Characterization of intestinal invasion by Salmonella typhimurium and Salmonella dublin and effect of a mutation in the invH gene. Infect Immun. 1995;63:2743–2754. doi: 10.1128/iai.63.7.2743-2754.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolvekamp M C J, Darby I A, Fuller P J. Cautionary note on the use of end-labelling DNA fragments for detection of apoptosis. Pathology. 1998;30:267–271. doi: 10.1080/00313029800169426. [DOI] [PubMed] [Google Scholar]
- 30.Wong W W. ICE family proteases in inflammation and apoptosis. Agents Actions Suppl. 1998;49:5–13. doi: 10.1007/978-3-0348-8857-8_2. [DOI] [PubMed] [Google Scholar]
- 31.Wood M W, Jones M A, Watson P R, Hedges S, Wallis T S, Galyov E E. Identification of a pathogenicity island required for Salmonella enteropathogenicity. Mol Microbiol. 1998;29:883–891. doi: 10.1046/j.1365-2958.1998.00984.x. [DOI] [PubMed] [Google Scholar]
- 32.Wood M W, Rosqvist R, Mullan P B, Edwards M H, Galyov E E. SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a sip-dependent mechanism and promotes bacterial entry. Mol Microbiol. 1996;22:327–338. doi: 10.1046/j.1365-2958.1996.00116.x. [DOI] [PubMed] [Google Scholar]
- 33.Zychlinsky A, Sansonetti P J. Apoptosis as a proinflammatory event: what can we learn from bacterial-induced cell death. Trends Microbiol. 1997;5:201–204. doi: 10.1016/S0966-842X(97)01044-5. [DOI] [PubMed] [Google Scholar]
- 34.Zychlinsky A, Prevost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]