Expression of the Nitric Oxide Synthase 2 Gene Is Not Essential for Early Control of Mycobacterium tuberculosis in the Murine Lung (original) (raw)
Abstract
The interleukin-12 and gamma interferon (IFN-γ) pathway of macrophage activation plays a pivotal role in controlling tuberculosis. In the murine model, the generation of supplementary nitric oxide by the induction of the nitric oxide synthase 2 (NOS2) gene product is considered the principal antimicrobial mechanism of IFN-γ-activated macrophages. Using a low-dose aerosol-mediated infection model in the mouse, we have investigated the role of nitric oxide in controlling Mycobacterium tuberculosis in the lung. In contrast to the consequences of a systemic infection, a low dose of bacteria introduced directly into the lungs of mice lacking the NOS2 gene is controlled almost as well as in intact animals. This is in contrast to the rapid progression of disease in mice lacking IFN-γ or a key member of the IFN signaling pathway, interferon regulatory factor 1. Thus while IFN-γ is pivotal in early control of bacterial growth in the lung, this control does not completely depend upon the expression of the NOS2 gene. The absence of inducible nitric oxide in the lung does, however, result in increased polymorphonuclear cell involvement and eventual necrosis in the pulmonary granulomas of the infected mice lacking the NOS2 gene.
Tuberculosis is principally a disease of the lung and is transmitted by aerosol droplets containing only a few bacteria. These infected droplets must be deposited in the alveoli for infection to be established, and thus the exposure of the bacteria to the immune response is limited during the early stages of infection. This discreet entry into the lung means that innate responses must bear the burden of controlling the infection until the acquired response recognizes that infection has occurred. Currently, only two products of the immune response, gamma interferon (IFN-γ) (7) and tumor necrosis factor alpha (2), have been shown to be crucial to the early control of low-dose aerosol infection with virulent Mycobacterium tuberculosis. These two cytokines are known to activate macrophages to a microbistatic and/or microbicidal state.
The exact molecular mechanisms of macrophage activation that limit mycobacteria have not yet been determined. One of the likely mediators of mycobacterial control is the expression of high levels of nitric oxide (28) following induction of the inducible nitric oxide synthase (iNOS) gene (NOS2). This molecule is thought to act in concert with superoxide radicals within acidic phagosomes to generate toxic products capable of limiting the survival and growth of M. tuberculosis. Indeed, studies performed using systemic models of mycobacterial infection confirmed that this molecule is crucial to the containment of systemic M. tuberculosis infection (4, 5, 16). A potential problem with the relevance of these studies to tuberculosis is that the bacteria are introduced intravenously and become lodged within the liver, spleen, and interstitium of the lung, resulting in an extensive, systemic bacterial infection. This level of infection requires the maximum protective responses of the host and results in a very rapid induction of the acquired immune response (3); neither situation occurs during a natural aerosol infection. In addition, not all strains of M. tuberculosis appear to be susceptible to nitric oxide-dependent toxic products (24).
In order to confirm a principal role for nitric oxide in controlling mycobacterial growth within the lung, we infected NOS2 gene-disrupted (NOS2-KO) mice with low numbers of M. tuberculosis and followed bacterial growth and granuloma development. Surprisingly, the phenotype for NOS2-KO was one of prolonged bacterial control, which is in stark contrast to the highly susceptible phenotype expressed by mice lacking IFN-γ (7) or the IFN-γ-stimulated inducer of NOS2 expression, interferon regulatory factor 1 (IRF-1). While mice lacking the NOS2 gene eventually succumbed to infection, they were clearly capable of mediating control of bacterial growth within the lung. Intriguingly, the quality of the granulomas produced by these mice was altered, with increased cellularity and central caseating necrosis.
MATERIALS AND METHODS
Mice and infections.
Mice lacking the NOS2 (15) or IRF-1 (18) gene and C57BL/6 mice were purchased from the Jackson Laboratories (Bar Harbor, Maine). Mice lacking the IFN-αβ receptor (IFN-αβ R-KO) and their B6/129 controls (19) were bred in-house at Colorado State University from breeders kindly provided by P. Marrack (National Jewish Hospital, Denver, Colo.). Mice were housed in the Biohazard Level 3 facility and given mouse chow and water ad libitum. Infected knockout mice were monitored for failure to thrive and were euthanized when they exhibited signs of weight loss.
A virulent strain of M. tuberculosis (Erdman) was grown from a low-passage-number seed in Proskauer-Beck liquid media to mid-log phase, aliquoted, and frozen at −70°C. Mice were infected using a Glas-Col aerosol generator (Glas-Col, Terre Haute, Ind.) such that 100 bacteria were deposited in the lungs of each animal. The numbers of viable bacteria in target organs were determined at various time points by plating serial dilutions of organ homogenates on nutrient Middlebrook 7H11 agar and counting colonies after 20 days of incubation at 37°C. A determination of the infecting dose was performed on lungs of mice infected for 1 day. Infection experiments were performed three times, and the statistical difference between the mean bacterial numbers in C57BL/6 and knockout tissues was determined by Student's t test.
Histological analysis.
The lower right lobe of each mouse was inflated with 10% neutral buffered saline and processed routinely for light microscopy. Sections were then stained with hematoxylin and eosin. Slides were examined without knowledge of the experimental group and were subjectively graded for both quantity and quality of cellular accumulations. Repeat evaluations were performed to confirm that grading was reproducible. In addition, sections from formalin-fixed tissue were prepared as previously described (11) to determine the expression of the NOS2 gene product in situ. Specific staining was confirmed using preimmune rabbit serum.
RESULTS
NOS2-KO mice can control aerosol infection with M. tuberculosis to a greater degree than IRF-1-KO mice.
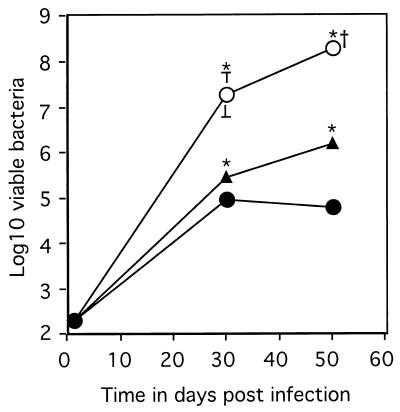
In order to determine the role NOS2 plays in the control of mycobacterial growth in the lung, mice lacking either the NOS2 or the IRF-1 gene (required for IFN-γ induction of NOS2 [14]) were infected via the low-dose aerogenic route. The lack of IRF-1 resulted in the rapid growth of bacteria, with a 580-fold increase over levels in control mice by day 30 and death by day 50 (Fig. 1). In contrast, control mice and mice lacking the NOS2 gene had similar numbers of bacteria (within two- to threefold) in the lung at day 30 (Fig. 1). By day 50, however, the absence of the NOS2 gene resulted in a 22-fold increase from the bacterial burden in control mice (Fig. 1). This increase did not, however, resemble the increase in either the IRF-1-KO mice observed by us or in the IFN-γ-KO mice reported previously (7).
FIG. 1.
The course of M. tuberculosis infection in mice lacking components of the IFN-γ-mediated macrophage activation pathway. Mice were infected aerogenically with 100 virulent bacteria, and the numbers of bacteria detected in the lungs of C57BL/6 (solid circles), IRF-1-KO (empty circles) and NOS2-KO mice (triangles) were determined. Data represent the mean log10 number of viable bacteria per organ (n = 4) ± the standard error. ∗, means which were statistically different from the C57BL/6 values as determined by Student's t test (P ≤ 0.05). †, mice were euthanized due to morbidity. The graph reflects one experiment representative of two similar experiments.
As in the lung, the growth of bacteria in the spleen was rapid in the IRF-1-KO mice (log10 of 7.12 ± 0.31 by day 30) (P ≤ 0.05), whereas the growth of bacteria within the NOS2-KO mice was more similar to that seen in the spleens of the C57BL/6 mice (C57BL/6, log10 of 4.2 ± 0.16; NOS2, log10 of 4.73 ± 0.21) (P ≤ 0.05). The difference between C57BL/6 and NOS2-KO mice increased at day 50, when the log10 numbers of bacteria in the spleen were 4.22 ± 0.09 and 5.89 ± 0.17, respectively (P ≤ 0.05).
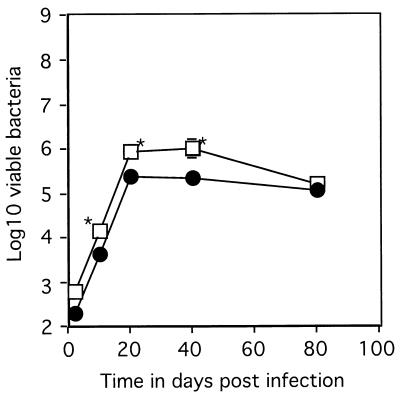
To confirm that the defect in the IRF-1-KO mice was not due to the loss of the protective effects of IFN-α or -β, mice lacking the receptor for these cytokines were also infected. These mice did show a slightly reduced ability to limit early bacterial growth but did not demonstrate the profound susceptibility of the IRF-1-KO mice (Fig. 2).
FIG. 2.
The course of M. tuberculosis infection in mice lacking the IFN-αβ signal transduction receptor. Mice were infected aerogenically with 100 virulent bacteria, and the numbers of bacteria detected in the lungs of B6/129 control mice (circles) and IFN-αβ R-KO mice (squares) were determined. Data represent the mean log10 number of viable bacteria per organ (n = 4) ± the standard error. ∗, means which were statistically different from the B6/129 values as determined by Student's t test (P ≤ 0.05). The graph reflects one experiment representative of two similar experiments.
In an attempt to determine long-term survival of the NOS2-KO mice when infected with M. tuberculosis, mice infected with a low dose of bacteria were monitored for 180 days. As reported above, the NOS2-KO mice were slightly more susceptible with moderately increased bacterial numbers in the lung at day 50 (Table 1). All mice survived to day 180; however, by this time the bacterial load in the NOS2-KO mice was considerably higher than in the C57BL/6 mice (Table 1). Perhaps the most profound difference between the C57BL/6 and NOS2-KO mice was the high number of bacteria in the livers of the NOS2-KO mice. (IRF-1-KO mice were not included in this study, as by day 50 of the previous study most were failing to thrive and had to be euthanized).
TABLE 1.
NOS2 mice gradually fail to contain bacterial growth following a pulmonary infection with M. tuberculosis a,b
| Organ processed | Results (mean ± SEM)ab | |||
|---|---|---|---|---|
| Day 50 | Day 180 | |||
| C57BL/6 | NOS2d | C57BL/6 | NOS2d | |
| Lung | 4.31 ± 0.09 | 5.36 ± 0.12 | 4.87 ± 0.13 | 7.76 ± 0.39 |
| Spleen | 3.73 ± 0.19 | 5.36 ± 0.12 | 4.43 ± 0.24 | 8.59 ± 0.15 |
| Liver | NDc | 3.93 ± 0.25 | ND | 7.51 ± 0.42 |
NOS2-KO mice generate large, diffuse granulomas in the lung.
One acknowledged function of nitric oxide (the product of the NOS2 gene) is as a down regulator of immune function. Once we had determined that the NOS2-KO mouse was not profoundly susceptible to M. tuberculosis infection, we investigated the role of nitric oxide in limiting inflammation within the mycobacterially infected lung. Confirmation of the inability of the NOS2-KO mice to generate the iNOS enzyme was seen in the absence of staining for this enzyme following immunohistochemical analysis (data not shown). C57BL/6 mice expressed the iNOS enzyme strongly throughout infection, as reported previously (11) (data not shown).
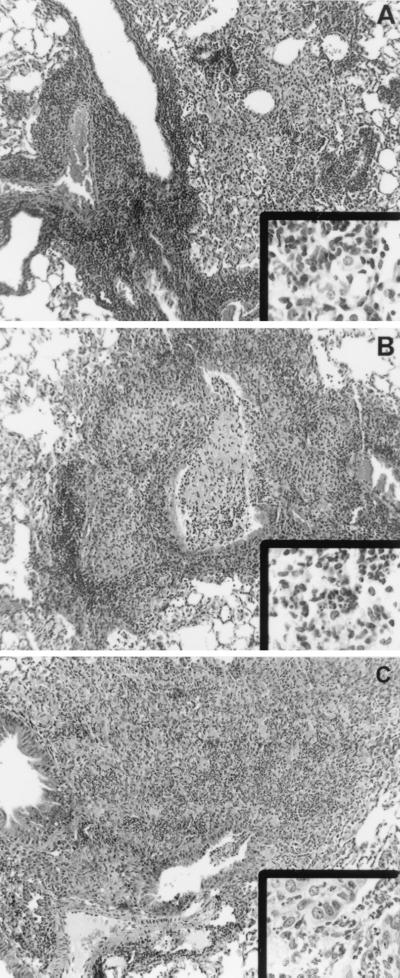
Histological analysis revealed that the characters of the IRF-1-KO, NOS2-KO, and C57BL/6 granulomas were different. Thus, while bacterial numbers in the lung were 2.5-fold higher in NOS2-KO and 580-fold higher in IRF-1-KO than in C57BL/6 mice, the granulomatous response in the NOS2-KO mice was more akin to the IRF-1-KO response than to the C57BL/6 response (Fig. 3). The cellular components of the granulomas were also different, with both the NOS2-KO and IRF-1 lesions containing polymorphonuclear cells (Fig. 3, insets). Importantly, this increased purulence correlated with the breakdown of the lung architecture and the release of pus in the airways (Fig. 3).
FIG. 3.
Representative photomicrographs (from two experiments) of formalin-fixed sections from the lungs of C57BL/6 (A), NOS2-KO (B), and IRF-1-KO (C) mice aerogenically infected 30 days previously. The granuloma is primarily mononuclear in nature in the lungs of C57BL/6 mice, compared to the neutrophilic accumulation in the lungs of NOS2-KO (B, inset) and IRF-1-KO (C, inset) mice. In both the NOS2-KO and the IRF-1-KO mice, purulent debris can be seen breaking into the airways (B and C, main images). Magnification for main panels, ×200; for insets, ×1,000.
DISCUSSION
The observations reported here confirm the critical role of the IFN-γ-initiated pathway of macrophage activation in controlling pulmonary tuberculosis. Our data also demonstrate that the principal mediator of this control in the lung is not IFN-γ-induced nitric oxide. Clearly, IFN-γ serves to activate macrophages to a bacteriostatic state via IRF-1; however, the induction of the NOS2 gene (a known consequence of IFN-γ induction of IRF-1 [14]) is not required for this purpose. Although early bacterial control was not totally dependent upon NOS2 gene expression, a clear consequence of the lack of inducible nitric oxide was the altered quality of the granulomatous response in the NOS2-KO mice.
The much-reduced susceptibility of the NOS2-KO mouse compared to that of the IFN-γ-KO mouse following low-dose aerosol infection was surprising, considering the highly susceptible phenotype of the NOS2-KO mouse following systemic infection (16). The likely explanation for this difference may lie in the differences between the liver (the primary organ in a systemic challenge) and the lung. These two organs have very different responses to mycobacterial infection; in particular, the liver is much more able to control mycobacterial growth than the lung (3, 6, 23). That the protective response in the liver is dependent upon nitric oxide is seen in the susceptibility of the NOS2-KO mouse to systemic infection (16) and the increased susceptibility of this organ following aerosol infection.
In contrast to the liver, the lung, while able to limit bacterial growth, is unable to reduce bacterial load even with a full complement of immune responses at its disposal (25). The mechanisms mediating control in the lung are dependent upon IFN-γ (7) and tumor necrosis factor alpha (2), but the protective mechanism induced by these cytokines is not known. The obvious candidate is the NOS2 gene product, and although NOS2 is expressed in the lung during pulmonary infection (11), it would appear that this gene product is not the primary protective element induced by IFN-γ via the IRF-1 pathway.
There are no other IFN-γ-mediated antimycobacterial mechanisms as potent as the production of excess nitric oxide; however, other mechanisms do exist and may play a more dominant role in the lung. It has been shown that while nonactivated macrophages house mycobacteria within nontoxic early endosomes (29, 30), activation by IFN-γ results in the maturation of the phagosome (27). The consequences of this maturation are reduced transferrin trafficking and lower pH, which result in reduced viability of the bacteria (27). While these toxic effects are not dramatic in vitro, they may serve to restrict bacterial growth within the lung. A second mechanism, which may also limit bacterial growth, is the generation of superoxide, as in vivo studies have identified a limited protective role for this molecule in the lung (1, 8).
The absence of an acutely susceptible phenotype in the NOS2-KO mouse allowed examination of the role of excess nitric oxide in limiting inflammatory damage in the lung. An important observation reported here is that even when bacterial numbers are similar (day 30 of infection), the granulomas in the NOS2-KO mice are altered in character. This defect is of particular importance in a chronic disease of the lung, such as tuberculosis. Control of the granulomatous response in murine tuberculosis is important not only in limiting bacterial growth and dissemination but also in limiting damage to the delicate lung tissue (21, 22, 26). In this regard nitric oxide has been identified as a mediator of hyporesponsiveness during infection (17, 20). Indeed, in its absence the granulomas induced by Mycobacterium avium are increased in size, with no concomitant increase in bacterial numbers (10, 12, 13). This role of nitric oxide in controlling cellular responses to mycobacteria has recently been confirmed in systemic Mycobacterium bovis BCG infection, wherein T-cell activation is controlled by IFN-γ- and nitric oxide-dependent apoptosis (9). These reports and the data reported here strongly support the hypothesis that nitric oxide is instrumental in regulating the cellular response to mycobacterial infection.
It is not the aim of this paper to deny the role of nitric oxide in the antibacterial response of the host. Indeed, this molecule is certainly required for the control of mycobacterial growth. What nitric oxide does not do is mediate all the IFN-γ- and IRF-1-induced antimycobacterial control seen in the mouse lung. Other mechanisms are clearly involved, and improvement in vaccines or immunotherapy will depend upon the identification of these mechanisms.
ACKNOWLEDGMENTS
We thank the staff of the Laboratory Animal Resources Center, Colorado State University, for excellent animal care.
This work was supported by National Institutes of Health grants AI-40488 and AI-44072 and a grant from the Deutsche Forschungsgemeinschaft (SFB 367-C9).
REFERENCES
- 1.Adams L B, Dinauer M C, Morgenstern D E, Krahenbuhl J L. Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tuber Lung Dis. 1997;78:237–246. doi: 10.1016/s0962-8479(97)90004-6. [DOI] [PubMed] [Google Scholar]
- 2.Bean A G D, Roach D R, Briscoe H, France M P, Korner H, Sedgewick J D, Britton W J. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- 3.Cardona P-J, Cooper A M, Luquin M, Ariza A, Filipo F, Orme I M, Ausina V. The intravenous model of murine tuberculosis is less pathogenic than the aerosol model owing to a more rapid induction of systemic immunity. Scand J Immunol. 1999;49:362–366. doi: 10.1046/j.1365-3083.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- 4.Chan J, Tanaka K, Carroll D, Flynn J L, Bloom B R. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan J, Xing Y, Magliozzo R, Bloom B R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper A M, Callahan J E, Keen M, Belisle J T, Orme I M. Expression of memory immunity in the lung following re-exposure to Mycobacterium tuberculosis. Tuber Lung Dis. 1997;78:67–73. doi: 10.1016/s0962-8479(97)90017-4. [DOI] [PubMed] [Google Scholar]
- 7.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper A M, Segal B H, Frank A A, Holland S M, Orme I M. Transient loss of resistance to pulmonary tuberculosis in p47phox−/− mice. Infect Immun. 2000;68:1231–1234. doi: 10.1128/iai.68.3.1231-1234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalton D K, Haynes L, Chu C Q, Swain S L, Wittmer S. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J Exp Med. 2000;192:117–122. doi: 10.1084/jem.192.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty T M, Sher A. Defects in cell-mediated immunity affect chronic, but not innate, resistance of mice to Mycobacterium avium infection. J Immunol. 1997;158:4822–4831. [PubMed] [Google Scholar]
- 11.D'Souza C D, Cooper A M, Frank A A, Ehlers S, Turner J, Bendelac A, Orme I M. A novel nonclassic β2-microglobulin-restricted mechanism influencing early lymphocyte accumulation and subsequent resistance to tuberculosis in the lung. Am J Respir Cell Mol Biol. 2000;23:188–193. doi: 10.1165/ajrcmb.23.2.4063. [DOI] [PubMed] [Google Scholar]
- 12.Ehlers S, Kutsch S, Benini J, Cooper A M, Hahn C, Gerdes J, Orme I M, Martin C, Rietschel E T. NOS2-derived nitric oxide regulates the size, quantity and quality of granuloma formation in Mycobacterium avium-infected mice without affecting bacterial loads. Immunology. 1999;98:313–323. doi: 10.1046/j.1365-2567.1999.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes M S, Florido M, Pais T F, Appelberg R. Improved clearance of Mycobacterium avium upon disruption of the inducible nitric oxide synthase gene. J Immunol. 1999;162:6734–6739. [PubMed] [Google Scholar]
- 14.Kamijo R, Harada H, Matsuyama T, Bosland J, Gerecitano J, Shapiro J, Le J, Koh S I, Kimura T, Green S J, Mak T W, Taniguchi T, Vicek J. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science. 1994;263:1612–1615. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- 15.Laubach V E, Shesley E G, Smithies O, Sherman P A. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide death. Proc Natl Acad Sci USA. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacMicking J D, North R J, LaCourse R, Mudgett J, Shah S K, Nathan C F. Identification of NOS2 as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martins G A, Vieira L Q, Cunha F Q, Silva J S. Gamma interferon modulates CD95 (fas) and CD95 ligand (Fas-L) expression and nitric oxide-induced apoptosis during the acute phase of Trypanosoma cruzi infection: a possible role in immune response control. Infect Immun. 1999;67:3864–3871. doi: 10.1128/iai.67.8.3864-3871.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuyama T, Kimura T, Kitagawa M, Pfeffer K, Kawakami T, Watanabe N, Kundig T M, Amakawa R, Kishihara K, Wakeham A, Potter A, Furlonger C L, Narendran A, Suzuki H, Ohashi P S, Paige C J, Taniguchi T, Mak T W. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell. 1993;75:83–97. [PubMed] [Google Scholar]
- 19.Muller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aquet M. Functional role of type I and type II interferons in anti-viral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 20.Nabeshima S, Nomoto M, Matsuzaki G, Kishihara K, Taniguchi H, Yoshida S, Nomoto K. T-cell hyporesponsiveness induced by activated macrophages through nitric oxide production in mice infected with Mycobacterium tuberculosis. Infect Immun. 1999;67:3221–3226. doi: 10.1128/iai.67.7.3221-3226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orme I M. The immunopathogenesis of tuberculosis: a new working hypothesis. Trends Microbiol. 1998;6:94–97. doi: 10.1016/s0966-842x(98)01209-8. [DOI] [PubMed] [Google Scholar]
- 22.Orme I M, Cooper A M. Cytokine/chemokine cascades in immunity to tuberculosis. Immunol Today. 1999;20:307–312. doi: 10.1016/s0167-5699(98)01438-8. [DOI] [PubMed] [Google Scholar]
- 23.Orme I M, Miller E S, Roberts A D, Furney S K, Griffin J P, Dobos K M, Chi D, Rivoire B, Brennan P J. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992;148:189–196. [PubMed] [Google Scholar]
- 24.Rhoades E, Orme I. Susceptibility of a panel of virulent strains of Mycobacterium tuberculosis to reactive nitrogen intermediates. Infect Immun. 1997;65:1189–1195. doi: 10.1128/iai.65.4.1189-1195.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhoades E R, Frank A A, Orme I M. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber Lung Dis. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 26.Saunders B M, Frank A A, Orme I M. Granuloma formation is required to contain bacilli growth and delay mortality in mice chronically infected with Mycobacterium tuberculosis. Immunology. 1999;98:324–328. doi: 10.1046/j.1365-2567.1999.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaible U E, Sturgill-Koszycki S, Schlesinger P H, Russell D G. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J Immunol. 1998;160:1290–1296. [PubMed] [Google Scholar]
- 28.Stuer D J, Gross S S, Sukuma I, Levi R, Nathan C F. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med. 1989;169:1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 30.Xu S, Cooper A M, Sturgill-Koszycki S, van Heyningen T, Chatterjee D, Orme I, Allen P, Russell D G. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J Immunol. 1994;153:2568–2578. [PubMed] [Google Scholar]