Adherent Invasive Escherichia coli Strains from Patients with Crohn's Disease Survive and Replicate within Macrophages without Inducing Host Cell Death (original) (raw)
Abstract
Escherichia coli strains recovered from Crohn's disease (CD) lesions are able to adhere to and invade cultured intestinal epithelial cells. We analyzed the behavior within macrophages of adherent invasive E. coli (AIEC) strains isolated from patients with CD. All the 15 AIEC strains tested were able to replicate extensively within J774-A1 cells: the numbers of intracellular bacteria increased 2.2- to 74.2-fold at 48 h over that at 1 h postinfection. By use of murine peritoneal macrophages and human monocyte-derived-macrophages, the reference AIEC strain LF82 was confirmed to be able to survive intracellularly. Transmission electron micrographs of AIEC LF82-infected macrophages showed that at 24 h postinfection, infected cells harbored large vacuoles containing numerous bacteria, as a result of the fusion of several vacuoles occurring after 8 h postinfection. No lactate dehydrogenase (LDH) release, no sign of DNA fragmentation or degradation, and no binding to fluorescein isothlocyanate-labeled annexin V were observed with LF82-infected J774-A1 cells, even after 24 h postinfection. LF82-infected J774-A1 cells secreted 2.7-fold more tumor necrosis factor alpha (TNF-α) than cells stimulated with 1 μg of lipopolysaccharide (LPS)/ml. No release of interleukin-1β was observed with LPS-prestimulated J774-A1 cells infected with AIEC LF82. These findings showed that (i) AIEC strains are able to survive and to replicate within macrophages, (ii) AIEC LF82 replication does not induce any cell death of the infected cells, and (iii) LF82-infected J774-A1 cells release high levels of TNF-α. These properties could be related to some features of CD and particularly to granuloma formation, one of the hallmarks of CD lesions.
Crohn's disease (CD) is an inflammatory bowel disease characterized by a chronic transmural, segmental, and typically granulomatous inflammation of the intestine in humans. CD lesions develop from an accumulation of lymphocytes and plasma cells, followed by an influx of macrophages, which transform into epithelioid cells and generate nascent granulomas (for a review see reference 12).
The etiology of CD is still unknown. The general concept is that CD pathogenesis is immunologically mediated and greatly influenced by genetic, environmental, and other endogenous host factors. Infectious agents as a possible environmental cause of CD have been investigated since the disease was first recognized. Some characteristic pathologic elements of CD, including aphthous ulcers of the mucosa, mural abscesses, and macrophage and epithelioid cell granulomas, occur in well-recognized infectious diseases, like shigellosis, salmonellosis, and yersinial enterocolitis, in which invasiveness is an essential virulence factor of the bacteria involved (for a review see reference 40).
We previously showed that Escherichia coli is abnormally predominant (between 50 and 100% of the total number of aerobes and anaerobes) in early and chronic ileal lesions of CD and that a great majority of the E. coli strains isolated from Crohn's ileal mucosa adhere strongly in vitro to intestinal epithelial cells (11). The latter property could enable the bacteria to colonize the intestinal mucosa. Qualitative and quantitative analysis of E. coli strain LF82 isolated from a chronic ileal lesion of a patient with CD revealed that it is a true invasive pathogen (6). It efficiently invades a wide range of human epithelial cell lines in vitro, including HEp-2 cells and the intestinal cells Intestine-407, Caco-2, and HCT-8. In contrast with most invasive bacteria belonging to the Enterobactericeae family, its uptake is dependent upon both functioning host cell actin microfilaments and microtubules. It survives and replicates in the host cell cytoplasm after lysis of the endocytic vacuole. Electron microscope examination of LF82-infected epithelial cells revealed a macropinocytosis-like process of entry, characterized by the elongation of membrane extensions which surrounded the bacteria at the sites of contact between the entering bacteria and the epithelial cells (5). The invasive process was found to be original in that LF82 possessed none of the known genetic invasive determinants described for enteroinvasive, enteropathogenic, or enterotoxigenic E. coli, or Shigella flexneri, i.e., the _ipa_C plasmid gene encoding the invasin of S. flexneri, and enteroinvasive E. coli (EIEC), the eae gene encoding the intimin of enteropathogenic E. coli (EPEC), and the tia gene encoding a 25-kDa outer membrane protein involved in enterotoxigenic E. coli (ETEC) invasiveness (6, 7). Recent data have revealed a high prevalence of invasive strains associated with the ileal mucosa of patients with CD compared to that for controls, supporting a putative role of E. coli invasiveness in the pathogenesis of CD (7, 29). Characterization of these strains revealed that they shared the same mechanism of entry as LF82 and that they lacked any of the invasive genetic determinants described above. They were thus clustered in a new potentially pathogenic group of invasive E. coli, which we designate AIEC for adherent invasive E. coli (6, 7).
In CD mucosa the numbers of macrophages and antigen-presenting dendritic cells are increased (37). Macrophages residing in the intestine or attracted to the site of inflammation might normally serve as a first line of defense by nonspecifically eliminating microorganisms that have penetrated from the intestinal lumen. Invasive bacteria have developed various strategies to counteract these mechanisms, which, when successful, enable them to survive and multiply and sometimes to destroy macrophages (for reviews see references 1, 13, 16, and 31). The behavior of the microorganisms within macrophages and host responses are key components in the replication and perpetuation of these intestinal pathogens. Elicitation of an inflammatory cascade may either eliminate the invading bacteria or facilitate further bacterial invasion (33, 34, 42).
As interactions between bacteria and macrophages dictate the outcome of most of the infectious diseases, the aim of the present work was to analyze the behavior within macrophages of AIEC strains isolated from patients with CD. Three different types of macrophages were used: the universally used J774-A1 murine macrophage-like cell line, which allowed us to compare the behavior of AIEC with other invasive bacteria studied so far, murine peritoneal macrophages, and human monocyte-derived macrophages (HMDM). The survival and replication of the phagocytosed bacteria were studied, as well as the behavior of the infected macrophages, by analyzing the synthesis and release of proinflammatory cytokines and cell integrity.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
A total of 15 invasive E. coli strains (LF strains) isolated from patients with CD were tested, including the prototype AIEC strain LF82 of serotype O83:H1, which was isolated from a chronic lesion of a patient with CD (6). The strains were recovered from ileal biopsy specimens from patients with chronic lesions who had been operated on for CD (n = 7), from patients with early endoscopic recurrent lesions at 3, 6, or 12 months postsurgery (n = 7), and from a patient without recurrence of CD up to 2 years postsurgery (n = 1) (11).
The following invasive strains were also used as controls: S. flexneri strain M90T (32), Salmonella enterica serovar Dublin SL2260 (26), and Salmonella enterica serovar Choleraesuis (clinical isolate). E. coli strain K-12 C600 was used as a noninvasive control. All E. coli strains were stored in Mueller-Hinton broth (Institut Pasteur Production, Marnes-la-Coquette, France) with glycerol (15%, vol/vol) at −80°C and grown in Luria-Bertani (LB) broth without shaking, except S. flexneri M90T, which was cultured in trypto-casein-soybean broth (TCS) with shaking overnight at 37°C.
Cell line and cell culture.
The murine macrophage-like cell line J774-A1 (American Type Culture Collection no. TIB67) was maintained in an atmosphere containing 5% CO2 at 37°C in RPMI 1640 medium (Seromed, Biochrom KG, Berlin, Germany) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (FCS; Seromed), 1% l-glutamine (Life Technologies), 100,000 of penicillin/liter, 100 mg of streptomycin/liter, and 25 μg of amphotericin B/liter. J774-A1 cells were seeded in 24-well tissue culture plates (Polylabo, Strasbourg, France) at a density of 105 cells per cm2 and were grown for 18 h in an atmosphere containing 5% CO2 at 37°C.
Isolation and culture of murine peritoneal macrophages.
Peritoneal cells were isolated by intraperitoneal (i.p.) injection of 5 ml of RPMI 1640 (Seromed) supplemented with 5% (vol/vol) heat-inactivated FCS (Seromed), 1% l-glutamine (Life Technologies), 100,000 U of penicillin/liter, 100 mg of streptomycin/liter, and 25 μg of amphotericin B/liter into OF1 mice (IFFA CREDO, Chatillon sur Chalaronne, France), that had been killed by cervical dislocation. Following i.p. injection, mice were shaken to dislodge peritoneal cells, and the lavage fluids were removed by syringe. The resulting cells, in the above-mentioned medium, were placed in 24-well tissue culture plates (Polylabo) at a density of 5 × 105 cells per well and maintained in an atmosphere containing 5% CO2 at 37°C. Two hours later, the medium was removed, and the plastic adherent cells were washed three times in 0.5 ml of phosphate-buffered saline (PBS; pH 7.2) and then incubated in 0.5 ml of complete RPMI medium as above at 37°C in a 5% CO2 incubator. Eighteen hours later, the culture medium was aspirated, and the cells were washed twice with PBS and then incubated with 0.5 ml of the above culture medium lacking antibiotics.
Isolation and culture of HMDM.
Isolation of HMDM was performed as described by Libby et al. (27). Briefly, blood was collected from normal donors in heparinized syringes and in additional sterile glass tubes for use as autologous serum. Monocytes were purified by density gradient centrifugation using Isoprep and a differential adherence procedure with 2% gelatin-coated flasks. Nonadherent cells were removed by washes with RPMI, and adherent cells were eluted with RPMI plus 10 mM EDTA in PBS. The eluted cells were centrifuged and resuspended in RPMI containing 15% autologous serum. Monocytes were seeded into 24-well culture plates at a density of 5 × 105 cells per well. The monocytes were maintained in an atmosphere containing 5% CO2 at 37°C for 5 to 7 days until they differentiated into cells with characteristic macrophage morphology.
Bacterial survival and replication in macrophages.
Bacterial uptake, survival, and replication were measured by the gentamicin protection assay. The MBC (concentration that reduced the bacterial count by 99.99%) of the antibiotic for all strains included in this study was determined in tissue culture medium, and the drug was used at 15- to 100-fold the MBC (the MBC ranged from 0.5 to 4 μg/ml)
Before infection, the cell monolayers were washed twice with PBS and the medium was replaced with 1 ml of RPMI 1640 supplemented with 10% heat-inactivated FCS. J774-A1 monolayers, murine peritoneal macrophages, and HMDM were infected at a multiplicity of infection (MOI) of 10 bacteria per macrophage. After a 2-h incubation at 37°C with 5% CO2, infected macrophages were washed twice with PBS, and fresh cell culture medium containing 100 μg of gentamicin/ml was added to kill extracellular bacteria. After incubation for an additional hour, the medium was removed and fresh medium containing 20 μg of gentamicin/ml was added for longer postinfection periods. To measure intracellular survival beyond 48 h postinfection, fresh cell culture medium containing gentamicin (20 μg/ml) was added daily to the infected cells. The number of internalized bacteria was determined in attached and detached cells. Attached cells were washed once with PBS, and 0.5 ml of 1% Triton X-100 (Sigma Chemical Company, St Louis, Mo.) in deionized water was placed in each well for 5 min to lyse the eukaryotic cells. This concentration of Triton X-100 had no effect on bacterial viability for at least 30 min. Samples were removed, diluted, and plated onto Mueller-Hinton agar plates to determine the number of CFU recovered from the lysed monolayers. To determine the number of bacteria in the detached cells, the culture medium was collected at each time point, centrifuged, washed in PBS, and treated with 1% Triton X-100 as described above. The number of bacteria surviving the gentamicin killing assay was determined after 1, 4, 8, 24, and 48 h of gentamicin treatment. Survival was expressed either as CFU per well or as the mean percentage of the number of bacteria recovered after 1 h postinfection, defined as 100%. In experiments with S. flexneri M90T or serovar Dublin SL2260, plates were centrifuged at 700 × g for 10 min after infection.
Transmission electron microscopy (TEM).
Cross sections of J774-A1 cells were prepared as follows. After infection, cells were fixed with 3% glutaraldehyde in 0.2 M cacodylate buffer at 4°C for 2 h and postfixed in 1% OsO4 in cacodylate buffer at 4°C for 1 h. After dehydration in a graded series of ethanol, the cultures were embedded in a 2-mm-thick Epon coating in the tissue culture well and polymerized for 3 days at 60°C. Suitable areas were oriented parallel to the cell layer surface on Epon blocks with an Epon mixture. Ultrathin sections were contrasted with uranyl acetate and lead citrate.
LDH activity.
J774-A1 cells were seeded in 24-well tissue culture plates at a density of 2 × 105 cells/cm2 and grown for 18 h. They were then infected at MOIs of 10 and 100, and processed as described above. Supernatants of the infected macrophages were sampled at 1, 4, 8, and 24 h of gentamicin treatment, centrifuged at 2,500 × g for 3 min at 4°C, and assayed for lactate dehydrogenase (LDH) activity. Enzymatic activity was determined in supernatants by using NADH as a substrate (LDH kit; Boehringer, Mannheim, Germany). Release of LDH was expressed as units per liter of supernatant. The percent cytotoxicity was calculated as (experimental release − spontaneous release)/(total release − spontaneous release) × 100, where spontaneous release is the amount of LDH activity in supernatants of cells incubated in medium alone and total release is the LDH activity measured in macrophage lysates.
Annexin V binding and propidium iodide staining of infected macrophages.
J774-A1 cells were seeded in 24-well tissue culture plates at a density of 2 × 105 cells per well. At various time points after infection at an MOI of 100 or after treatment with 5 μM gliotoxin (Sigma), cells and supernatant were harvested and stained with fluorescein isothiocyanate (FITC)-labeled annexin V and propidium iodide (annexin V-FITC apoptosis detection kit; Sigma) according to the supplier's protocol. With this protocol, live cells are not stained by either propidium iodide or annexin V-FITC. Cells which appear early in the apoptotic process are stained with annexin V-FITC alone, and necrotic cells are stained by both propidium iodide and annexin V-FITC. The percent stained cells was scored by analyzing 100 cells of three independent experiments in duplicate using a Zeiss fluorescence microscope.
DNA fragmentation.
J774-A1 cells seeded at a density of 3.5 × 106 were infected for 2 h at an MOI of 100, as described above. After a 24-h gentamicin treatment, total genomic DNA was extracted from adherent and floating cells by a standard DNA extraction method (proteinase K and phenol-chloroform), ethanol precipitated, and incubated for 30 min in the presence of 1 μg of RNase (Boehringer)/ml. The DNA solution was analyzed by electrophoresis for 5 h in a 1.5% agarose–0.1% sodium dodecyl sulfate (SDS) gel and stained with ethidium bromide.
Detection of cytokines IL-1β and TNF-α in the supernatant of infected macrophages.
Macrophages seeded at a density of 2 × 105 cells/cm2 were infected at an MOI of 100, and supernatants were collected, centrifuged, and stored at −20°C. For interleukin-1β (IL-1β) only, cells were prestimulated 18 h before infection with 1 μg of E. coli O111:B4 lipopolysaccharide (LPS; Sigma)/ml. The amounts of murine cytokines IL-1β and tumor necrosis factor alpha (TNF-α) released in the culture supernatant or remaining in cells were determined by enzyme-linked immunosorbent assay (ELISA) (Endogen Inc., Woburn, Mass.). The optical density was determined at a wavelength of 450 nm, and cytokine concentrations in picograms per milliliter were assessed according to the manufacturer's instructions.
RESULTS
Survival and replication of AIEC strains within the murine J774-A1 macrophage-like cell line.
In order to examine the resistance of AIEC strains to the intracellular killing by macrophages, murine J774-A1 macrophages were infected with 15 AIEC strains. Unless otherwise stated, a 2-h infection at an MOI of 10 was performed in all the experiments reported. The number of intracellular bacteria surviving the gentamicin exposure was determined after 1, 4, 8, 24, and 48 h of gentamicin treatment. Bacterial uptake was quantified at 1 h postinfection and expressed as CFU per well. Survival at 4, 8, 24, and 48 h postinfection was expressed as mean percentages of the number of bacteria recovered after 1 h of gentamicin treatment, defined as 100% (Table 1).
TABLE 1.
Uptake and ability of E. coli strains isolated from patients with CD to evade killing by J774-A1 cells after 1, 4, 8, 24, and 48 h of gentamicin treatment
| MOI and strain | Uptake of bacteria (104 CFU)a after 1 h of treatment | % Survival of bacteriab after the indicated treatment period | |||
|---|---|---|---|---|---|
| 4 h | 8 h | 24 h | 48 h | ||
| MOI = 10 | |||||
| K-12 C600 | 2.4 ± 0.2 | 122 ± 5 | 124 ± 11 | 6 ± 1 | 0.6 ± 0.1 |
| LF82 | 12.6 ± 1.1 | 217 ± 31 | 280 ± 40 | 504 ± 63 | 1122 ± 297 |
| LF9 | 32.5 ± 20.3 | 132 ± 22 | 201 ± 85 | 969 ± 261 | 1743 ± 798 |
| LF15 | 17.9 ± 6.7 | 74 ± 7 | 98 ± 6 | 624 ± 212 | 700 ± 57 |
| LF16 | 18.5 ± 4.8 | 162 ± 25 | 205 ± 20 | 3537 ± 418 | 7421 ± 1246 |
| LF28 | 8.5 ± 2.2 | 213 ± 92 | 212 ± 62 | 563 ± 120 | 291 ± 85 |
| LF31 | 8.1 ± 3.6 | 130 ± 6 | 146 ± 21 | 850 ± 137 | 1676 ± 317 |
| LF43 | 15.1 ± 7.5 | 258 ± 71 | 455 ± 124 | 820 ± 164 | 910 ± 319 |
| LF46 | 0.5 ± 0.04 | 364 ± 160 | 670 ± 221 | 2782 ± 582 | 4286 ± 633 |
| LF50 | 7.5 ± 1.0 | 156 ± 19 | 346 ± 13 | 2071 ± 490 | 5506 ± 417 |
| LF65 | 12.1 ± 2.1 | 286 ± 42 | 423 ± 81 | 1239 ± 31 | 3267 ± 44 |
| LF71 | 4.8 ± 0.6 | 259 ± 28 | 600 ± 213 | 646 ± 278 | 782 ± 277 |
| LF100 | 13.8 ± 2.9 | 191 ± 16 | 248 ± 46 | 251 ± 47 | 265 ± 24 |
| LF106 | 33.1 ± 1.0 | 156 ± 24 | 184 ± 18 | 365 ± 169 | 225 ± 106 |
| LF107 | 2.2 ± 0.8 | 147 ± 11 | 420 ± 186 | 572 ± 237 | 591 ± 212 |
| LF120 | 10.5 ± 1.2 | 158 ± 21 | 167 ± 67 | 495 ± 137 | 1451 ± 274 |
| MOI = 10 | |||||
| K-12 C600 | 6.7 ± 1.8 | 145 ± 4 | ND | 2.18 ± 1.10 | 0.8 ± 0.1 |
| S. enterica serovar Chloeraesuis | 253.0 ± 61.2 | 134 ± 29 | ND | 0.04 ± 0.02 | ND |
| S. flexneri M90T | 10.4 ± 1.2 | 57 ± 11 | ND | 0.40 ± 0.30 | ND |
| LF82 | 75.0 ± 26.0 | 237 ± 41 | 268 ± 84 | 450 ± 59 | 851 ± 264 |
The uptake of AIEC strains by the J774-A1 cells was efficient, except for two strains (LF46 and LF107). The numbers of internalized bacteria ranged from 4.8 × 104 to 33.1 × 104 CFU per well. Whatever the bacterial uptake level within macrophages, all the AIEC strains survived and replicated extensively within J774-A1 macrophages, increasing in cell number up to 48 h postinfection. The numbers of intracellular bacteria increased as early as 4 h postinfection (except for strain LF15, for which bacterial increase was observed only at 24 h postinfection). At 48 h postinfection, for to the various AIEC strains included in this study, we observed a 2.2- to 74.2-fold increase in the number of bacteria over the number initially internalized. Interestingly, three AIEC strains (strains LF31, LF46, and LF100) which were previously described as producing alpha-hemolysin (11) exhibited the same behavior as the nonhemolytic AIEC strains included in this study. In contrast, the nonpathogenic K-12 C600 reference strain was slowly but efficiently killed following phagocytosis by J774-A1 cells. No intracellular killing was observed at early times postinfection (4 and 8 h). Only 6% of the bacteria initially internalized were recovered at 24 h postinfection, indicating a bactericidal activity of the J774-A1 macrophages.
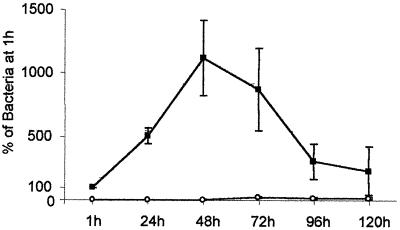
Survival experiments were performed with AIEC strain LF82 until 5 days postinfection. The number of intracellular bacteria relative to that obtained at 1 h of gentamicin treatment, defined as 100%, was determined every day. As shown in Fig. 1, AIEC strain LF82 survived and replicated within macrophages, reaching a maximum level at 48 h postinfection (1,122%). At day 3 postinfection, the percent intracellular bacteria remained very high (869%), and at days 4 and 5 postinfection these percentages were still 305 and 230%, respectively. Very few intracellular bacteria were recovered in detached cells postinfection.
FIG. 1.
Kinetics of intracellular multiplication of AIEC LF82 in J774-AI macrophages. The numbers of viable LF82 bacteria were determined in attached (■) and detached (□) macrophage fractions after different times (hours) of gentamicin treatment. Results are expressed as the number of intracellular bacteria relative to that obtained at 1 h after gentamicin exposure, taken as 100%. Means and standard errors of the means are indicated and correspond to six different experiments.
Survival within murine resident peritoneal macrophages.
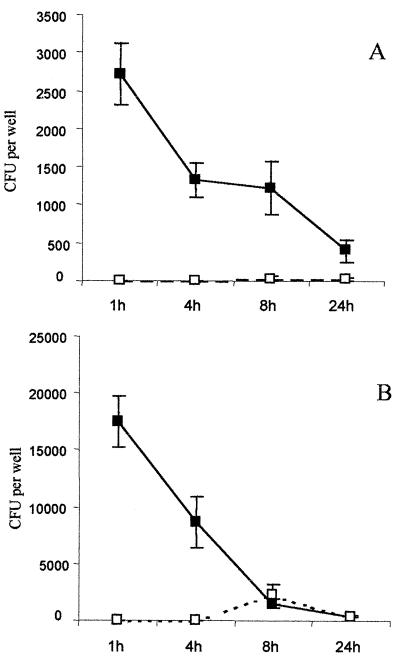
Assays were performed at an MOI of 10 with AIEC strain LF82, serovar Cholereasuis, and nonpathogenic E. coli K-12 C600. Results presented in Fig. 2 show only the intracellular behaviors of AIEC strain LF82 and serovar Choleraesuis. We were unable to count any intracellular bacteria for E. coli K-12 at the postinfection time points. Moreover, experiments performed at an MOI of 100 revealed that E. coli K-12 C600 was efficiently killed as early as 4 h postinfection (data not shown), confirming the very high bactericidal efficiency of these macrophages. The number of serovar Choleraesuis intracellular bacteria recovered at 4 h represented 34.1% of that recovered at 1 h. This dropped to 1.4% at 8 h postinfection in the attached-macrophage fraction. In the detached fraction, 16.0% of the initially phagocytosed bacteria were counted at 8 h postinfection, reflecting the _Salmonella_-induced apoptotic process. In contrast, even though the number of AIEC LF82 bacteria decreased relative to the number initially internalized, 52.0% of the bacteria recovered at 1 h postinfection were also recovered at 4 h postinfection. Hence, the number of live bacteria remained constant over a period of 8 h postinfection and decreased to 14.2% at 24 h postinfection. Only very few bacteria were counted in the detached cells over the postinfection time course.
FIG. 2.
Survival of AIEC LF82 (A) and S. enterica serovar Choleraesuis (B) in murine peritoneal macrophages. Means and standard errors of the mean are indicated and correspond to four different experiments in duplicate wells. Bacterial CFU per well containing 5 × 105 macrophages (y axis) and the time after addition of gentamicin (x axis) are shown. Numbers of viable bacteria were determined in the attached (■) and detached (□) macrophage fractions.
Survival of AIEC strain LF82 within HMDM.
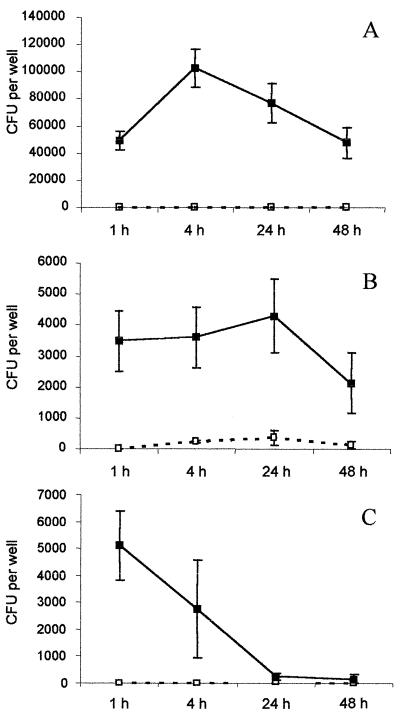
In order to confirm the ability of AIEC to survive in human phagocytic cells, experiments were conducted using the AIEC reference strain LF82 with HMDM. The serovar Dublin strain SL2260, which is well characterized for its behavior in HMDM, was included in our experiments for comparative purposes. Detached and attached macrophage fractions were cultured separately to enumerate intracellular bacteria. The results are shown in Fig. 3. Results with serovar Dublin SL2260 were in accordance with already published data (27). The number of CFU recovered after infection with serovar Dublin SL2260 increased slightly in the attached macrophages over 24 h and reached 122% of the number of bacteria recovered after 1 h postinfection, taken as 100%. Bacteria in detached macrophages were observed as early as 4 h postinfection, and their number increased over the postinfection period. The number of bacteria in the attached fraction of AIEC LF82-infected macrophages increased over 4 h postinfection to reach 208% of the initially internalized bacteria and decreased slightly over 48 h postinfection. However, at 48 h, the percent intracellular bacteria still represented 96% of the bacteria recovered after 1 h of gentamicin treatment. In contrast to serovar Dublin-infected macrophages, no bacteria were recovered in the corresponding detached fractions, confirming the lack of cytotoxicity induced by AIEC LF82 infection of J774-A1 macrophages. With the nonpathogenic strain K-12 C600, a decrease in the number of viable bacteria was observed as early as 4 h postinfection and only 5% of the bacteria initially internalized were still alive after 24 h postinfection.
FIG. 3.
Survival of AIEC LF82 (A) compared to those of serovar Dublin SL2260 (B) and nonpathogenic E. coli K-12 C600 (C) in HMDM. Each time point represents the mean of three independent experiments in triplicate wells. Bacterial CFU per well containing 5 × 105 macrophages (y axis) and the time after addition of gentamicin (x axis) are shown. Numbers of viable bacteria were determined in the attached (■) and detached (□) macrophage fractions.
AIEC strain LF82 did not induce cell death of J774-A1 infected macrophages.
All experiments to analyze the cell integrity of infected macrophages were performed at MOIs of 10 and 100, to check whether a higher number of internalized bacteria was able to induce cell death. The culture supernatants of infected or noninfected macrophages were assayed for the presence of the cytoplasmic enzyme LDH to estimate the membrane integrity of the cells. Samples of the culture medium were tested at 1, 4, 8, and 24 h postinfection (Table 2). S. flexneri M90T and serovar Choleraesuis were included as positive controls, since they are known to induce cell death (30, 42). E. coli K-12 C600 was used as a negative control. The amounts of LDH released were expressed as LDH activity recovered in the supernatant or as a percentage relative to that obtained at 1 h.
TABLE 2.
Absence of LDH release by J774-A1 cells infected with AIEC strain LF82
| Gentamicin treatment period (h) | LDH activitya (% cytotoxicityb) at an MOI of: | |||||||
|---|---|---|---|---|---|---|---|---|
| 10 | 100 | |||||||
| AIEC LF82 | K-12 C600 | S. flexneri M90T | Serovar Choleraesuis | AIEC LF82 | K-12 C600 | S. flexneri M90T | Serovar Choleraesuis | |
| 1 | 20.8 ± 4.7 (2.2) | 16.1 ± 4.2 (0.4) | 43.4 ± 15.6 (10.8) | 27.0 ± 7.0 (4.6) | 19.8 ± 4.8 (1.9) | 21.7 ± 4.4 (2.6) | 82.8 ± 19.2 (25.7) | 29.5 ± 4.5 (5.5) |
| 4 | 22.2 ± 5.2 (2.8) | 23.2 ± 3.1 (3.2) | 83.8 ± 37.1 (26.8) | 31.0 ± 1.0 (6.1) | 19.8 ± 5.2 (1.9) | 23.0 ± 5.7 (3.1) | 131.5 ± 32.0 (44.2) | 56.5 ± 2.5 (15.89) |
| 8 | 29.5 ± 4.5 (5.5) | 24.5 ± 9.5 (3.6) | 177.0 ± 29.5 (61.6) | 35.5 ± 13.5 (7.8) | 26.0 ± 8.0 (4.2) | 27.5 ± 2.5 (4.8) | 202.0 ± 5.0 (70.9) | 125.5 ± 61.5 (41.9) |
| 24 | 41.8 ± 5.3 (10.2) | 43.8 ± 9.6 (10.9) | ND | 148.5 ± 0.4 (50.6) | 42.8 ± 3.8 (10.6) | 42.3 ± 4.4 (10.4) | ND | 208.5 ± 11.5 (73.3) |
Infection of J774-A1 cells at an MOI of 10 with either S. flexneri M90T or serovar Choleraesuis resulted in high LDH release. LDH activity in the cell culture media reached 61.6% at 8 h postinfection for S. flexneri and 50.6% at 24 h postinfection for serovar Choleraesuis. LDH release also increased at these two time points with an MOI of 100, yielding 70.9% for S. flexneri and 73.3% for serovar Choleraresuis. This release reflects a secondary necrosis after apoptosis in vitro.
In contrast, the amounts of LDH released from macrophages infected at an MOI of 10 or 100 with AIEC LF82 (10.2 and 10.6%, respectively) did not differ from those for uninfected cells (14.9%) or cells infected with the avirulent strain K-12 C600 (10.4 and 10.9% at MOIs of 10 and 100, respectively), even after 24 h of gentamicin exposure. We confirmed that the absence of cytotoxicity was not due to a very small number of LF82-infected macrophages compared to the number of, _Shigella_- or _Salmonella_-infected cells. As shown in Table 1, the numbers of CFU per well at 1 h postinfection for the three bacterial strains tested were similar. This suggests that even with a number of internalized bacteria similar to that for S. flexneri or serovar Choleraesuis, AIEC LF82 was not cytotoxic for J774-A1 macrophages.
J774-A1 cells infected at an MOI of 100 were stained with annexin V-FITC and propidium iodide. Apoptotic stimuli, i.e., infection with S. flexneri or serovar Choleraesuis and the NF-κB inhibitor gliotoxin 5 μM, were used as positive controls (30, 39, 42). Results, expressed as percentages of apoptotic and necrotic cells, are presented in Table 3. Infection with S. flexneri, infection with serovar Cholerasuis, and treatment with gliotoxin for 8 h all induced apoptosis, since the percentages of macrophages exhibiting redistribution of phosphatidylserine to the cell surface were 26.6, 25.0, and 36.6%, respectively. At 8 or 24 h after infection with either AIEC LF82 or K-12 C600, the percentage of macrophages labeled with annexin V-FITC was similar to that obtained with untreated cells, and very few necrotic cells were enumerated.
TABLE 3.
Enumeration of apoptotic and necrotic J774-A1 macrophages after bacterial infection or treatment with gliotoxin
| % Apoptotic cellsa | % Necrotic cellsa | |||
|---|---|---|---|---|
| 8 h postinfection | 24 h postinfection | 8 h postinfection | 24 h postinfection | |
| Untreated cells | 10.1 ± 0.9 | 11.2 ± 1.2 | 1.6 ± 1.1 | 2.0 ± 0.7 |
| AIEC LF82 | 7.8 ± 0.2 | 10.0 ± 1.6 | 1.4 ± 0.6 | 4.0 ± 1.4 |
| Gliotoxin (5 μM) | 36.6 ± 1.0 | ND | 4.5 ± 0.4 | ND |
| S. flexneri M90T | 26.6 ± 2.8 | 19.5 ± 4.0 | 5.0 ± 1.2 | 12.4 ± 1.8 |
| Serovar Cholereasuis | 25.0 ± 2.3 | 28.0 ± 0.8 | 2.0 ± 1.2 | 5.5 ± 2.6 |
| K-12 C600 | 10.9 ± 2.5 | 11.7 ± 0.4 | 1.1 ± 1.1 | 0.9 ± 0.0 |
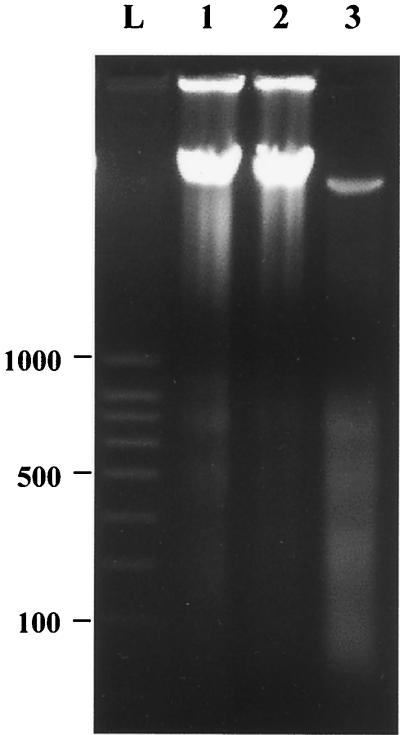
The integrity of J774-A1 cells infected with the AIEC LF82 was confirmed by analyzing total genomic DNA by electrophoresis. Macrophages were infected for 2 h at an MOI of 100 and then underwent gentamicin treatment for 24 h. As shown in Fig. 4, the total DNA from macrophages infected with S. flexneri was cleaved into multimers of 180 to 200 bp, which is a characteristic feature of apoptosis due to specific cleavage between nucleosomes. In contrast, DNA from LF82-infected macrophages showed no evidence of chromatin cleavage or degradation and had a DNA pattern similar to that of uninfected cells. Thus, even at 24 h after infection with AIEC LF82, and despite high levels of intracellular bacterial replication (Table 1), DNA remained intact. This indicates that in contrast to S. flexneri or Salmonella serovar Choleraesuis, AIEC LF82 did not induce any cell death of J774-A1 macrophages.
FIG. 4.
DNA electrophoresis of J774-A1 cells at 24 h postinfection. Lanes: L, ladder of molecular size markers in base pairs (SmartLadder SF; Eurogentec); 1, uninfected cells; 2, cells infected with the AIEC strain LF82; 3, cells infected with S. flexneri M90T. Only M90T-infected cells generated ladders of 180 to 200 bp, characteristic of apoptosis. LF82-infected cells were negative for DNA degradation.
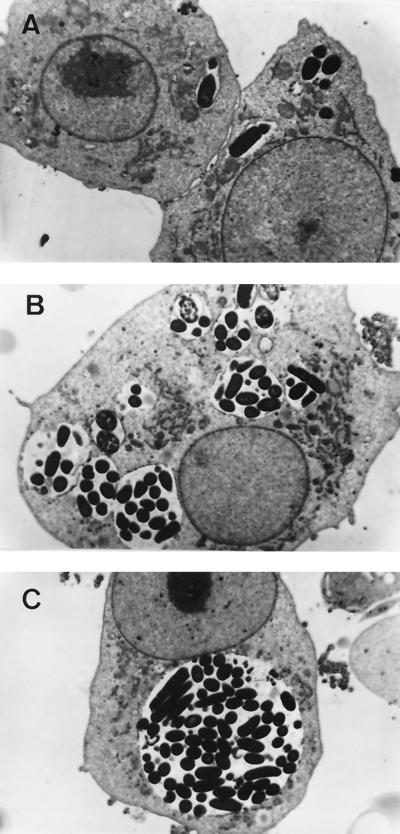
AIEC LF82 induced spacious phagosomes.
Morphological data to confirm the survival and replication abilities of AIEC LF82 within J774-A1 cells were obtained with TEM. One hour after infection, LF82 bacteria were observed in small vacuoles (Fig. 5A). LF82-infected cells exhibited larger phagosomes at 8 h, some of which were observed to fuse, leading to the formation of spacious phagosomes (Fig. 5B). At 24 h postinfection, infected macrophages showed a particularly prominent vacuole containing numerous bacteria. None of the internalized bacteria were free in the cytoplasm. The phagosomes occupied most of the cytoplasmic compartment and did not seem to alter the morphology of the nucleus or that of the cytoplasmic membrane (Fig. 5C). There was no morphological evidence of cytotoxicity, confirming the results of LDH release and DNA analysis. Despite this large number of intracellular bacteria, infected macrophages appeared viable even after 48 h postinfection (data not shown). In contrast, after 24 h, macrophages with internalized E. coli K-12 C600 harbored only a few phagosomes and also had small, round vesicular structures in phagosome vacuoles that appeared to represent fragments of organisms (data not shown). Thus, AIEC LF82 can persist within phagocytic cells for a long period in a large vacuole without inducing any apparent cell death or damage to the infected macrophages.
FIG. 5.
TEMs of J774-A1 macrophages infected at an MOI of 10 bacteria per cell. At 1 h postinfection, AIEC LF82 bacteria were observed in small vacuoles (A). After 8 h of gentamicin treatment, the size of the vacuoles was increased (B). Note phagosome fusion leading to the formation of a large vacuole observed after 24 h of gentamicin treatment (C). Magnification: ×7,200.
AIEC LF82 induced secretion by macrophages of TNF-α but no IL-1β release.
To explore the possible mechanisms in an inflammatory response induced by AIEC LF82, we examined its ability to induce (IL-1β) release and TNF-α secretion by ELISA.
The quantification of IL-1β release presented in Table 4 was obtained with macrophages prestimulated with LPS overnight and then infected with S. flexneri or AIEC LF82 at an MOI of 100. After 1 h of gentamicin treatment, the uninfected cell lysate gave an IL-1β concentration of 97 pg/ml, and the concentration of IL-1β in the supernatant of uninfected cells was less than 10 pg/ml at 1 and 24 h postinfection. As expected, S. flexneri M90T induced a high release of mature IL-1β at 1 h postinfection (40.5 pg/ml recovered in the supernatant). This result was in accordance with previously published findings showing that IL-1β is released when the cells undergo apoptosis (15, 41). In contrast, AIEC LF82 induced no detectable release of IL-1β, even after 24 h of gentamicin treatment.
TABLE 4.
Production of proinflammatory cytokines IL-1β and TNF-α by J774-A1 cells after AIEC LF82 infection
| Gentamicin treatment period (h) | IL-1β concn (pg/ml)a in: | TNF-α concn (pg/ml)b | |||||
|---|---|---|---|---|---|---|---|
| Uninfected cells | Uninfected lysed cells | AIEC LF82- infected cells | S. flexneri M90T infected cells | Uninfected cells | AIEC LF82- infected cells | LPS-stimulated cells | |
| 1 | <10 (0%) | 97.0 ± 2.8 (100%) | <10 (0%) | 40.5 ± 0.7 (41.7%) | 214 ± 48 | 99 ± 6 | 110 ± 5 |
| 24 | <10 (0%) | ND | <10 (0%) | ND | 302 ± 13 | 3,533 ± 111 | 1,300 ± 86 |
ELISA quantification of TNF-α secretion was done with J774-A1 cells infected with AIEC LF82 at an MOI of 100 in comparison with LPS-treated cells (Table 4). Small amounts of TNF-α found in the supernatant of AIEC LF82-infected or LPS-stimulated cells at 1 h postinfection. At 24 h postinfection, a maximum amount of TNF-α, 1,300 pg/ml, was recovered in the supernatant of cells stimulated with 1 μg of LPS/ml. In comparison, AIEC LF82-infected J774-A1 cells secreted a very high level of TNF-α: 3,533 pg/ml was recovered in the culture medium at 24 h postinfection.
DISCUSSION
The search for infectious agents as a possible cause of CD has mainly focused on intracellular pathogens which have evolved to resist phagocytosis and often persist for long periods within macrophages leading to chronic antigenic stimulation and T-cell and macrophage activation. This results in the formation of granulomas surrounding the microbes. Granulomatous inflammation is not only a histologic hallmark of infections with some intracellular bacteria and fungi but also a characteristic feature of CD, with granulomas found in the bowel wall and in regional lymph nodes (12). Moreover the presence of specific E. coli antigens was previously found in macrophages distributed within the lamina propria, in granulomas, and in the germinal centers of mesenteric lymph nodes in patients with CD (9, 28). We previously reported that the ileal mucosa of patients with CD is abnormally colonized by pathogenic E. coli strains termed AIEC, which have the ability to adhere to and invade epithelial intestinal cultured cells (6, 11). The aim of the present work was to determine the intramacrophagic fate of AIEC strains isolated from patients with CD and the behavior of the infected macrophages in order to evaluate the ability of such invasive bacteria to participate in granuloma formation.
We showed in the present study that all the AIEC strains that we isolated from patients with CD were able to survive and to replicate extensively within J774-A1 phagocytic cells. After 48 h postinfection, the number of intracellular AIEC bacteria increased 2.2- to 74.2-fold over the number at initial infection, depending on the AIEC strains studied. Moreover, the AIEC strain LF82 was able to persist efficiently until 5 days postinfection within J774-A1 macrophages. No detached cells, no LDH release, no annexin V-FITC binding, and no sign of DNA fragmentation or degradation were observed with J774-A1 infected cells even after 24 h postinfection, indicating that there was no cell death. The behavior of the AIEC strains within macrophages is different from that of other invasive bacteria, since most of them induce cell death of the infected macrophages (for a review see reference 31). Through cell death, infected macrophages attempt to both prevent the spread of intracellular pathogens and elicit a potent antibacterial immune response via the release of proinflammatory cytokines.
Comparative studies of intracellular survival of AIEC LF82 within two cell models indicated that this strain had a survival advantage in HMBM in comparison with murine peritoneal macrophages. However, in comparison to published data concerning the intracellular resistance of various Salmonella serovars or Shigella within murine peritoneal macrophages at early postinfection times before induction of macrophage cell death, the fraction of AIEC LF82 resistant to bactericidal killing was similar or higher, depending on the strain (2, 8, 19, 20, 22, 34). In the same way, the survival of intracellular AIEC LF82 within HMDM was similar to or even higher than those previously reported for human-pathogenic Salmonella serovars or Shigella (2, 15, 22, 27, 35, 36, 38). However, a great difference was observed between _Salmonella_- or _Shigella_- and AIEC LF82-infected macrophages. No detached cells corresponding to dead cells were observed with AIEC LF82-infected cells, even after 24 or 48 h postinfection.
Strain LF82 does not induce any cell death of the infected macrophages. To our knowledge, this is the first report describing the ability of E. coli strains to replicate extensively within macrophages without inducing cell membrane damage or cell death within a 24-h period. Indeed, the ability to induce macrophage cell death is a common feature of most of the diarrheagenic E. coli strains. Members of all the diarrheagenic pathovars of human E. coli studied so far (enteroaggregative E. coli, enterohemorrhagic E. coli, EIEC, EPEC, ETEC, and diffusely adhering E. coli) were cytotoxic to J774-A1 after 24 h of infection at an MOI of 10 or 100 (24, 25). Except for the ETEC strain H10407, for which the cell death induced has not been elucidated, all the different E. coli pathovars induce cell death via an apoptotic mechanism (25). Infection of HMDM and J774-A1 murine macrophages with several enteroaggregative and cytodetaching E. coli strains demonstrated that these strains were not able to replicate intracellularly and induced macrophage cell death accompanied by LDH and IL-1β release into the culture supernatant (14). Moreover, assays performed with a few invasive E. coli strains that we isolated from stools of healthy controls indicated that these strains were not resistant to macrophage killing within the J774-A1 cells (data not shown). Thus, invasive E. coli strains isolated from CD lesions, which possess none of the known genetic invasive determinants of E. coli, Shigella, or Salmonella, share a virulence factor(s) which confers on the bacteria the ability to evade the bactericidal pathways to which intracellular bacteria are normally exposed within macrophages.
The mechanism by which AIEC strains resist bacterial killing by macrophages is still under investigation. Pathogens that survive within host phagocytes have various mechanisms of survival: (i) by avoiding phagocytosis (13, 17), (ii) by inhibiting fusion of bacteria-containing phagosomes with lysosomes and endosomes (3), (iii) by remodeling their phagosome (10), (iv) by moving out of the phagosome (21, 23), or (v) by resisting the antimicrobial environment of the mature phagolysosome (4).
LF82 bacterial replication does not require bacterial escape into the cytoplasmic compartment, in contrast to its behavior within intestinal epithelial cells (6). Within J774-A1 macrophages, the bacteria induced the formation of a single spacious vacuole by fusion of initial phagosomes. Such large vacuoles containing numerous bacteria have also been observed with J774-A1 cells infected with Salmonella or Yersinia enterocolitica clinical isolates (2, 3, 18). The formation of this specific compartment induced by AIEC LF82 within the infected macrophages is probably the key to its ability to resist macrophage killing. Spacious-phagosome formation may promote LF82 survival by dilution of toxic lysosomal compounds or attenuation of antimicrobial factors, including decreased phagosomal acidification.
It is noteworthy that AIEC strains are able to invade intestinal epithelial cells by a macropinocytosis-like process requiring actin polymerization and recruitment of microtubules and by inducing host cell membrane elongations at the sites of contact with epithelial cells. Type 1 pili were shown to play an essential role in promoting AIEC LF82 internalization within intestinal epithelial cells (5). We thought that they were also involved in stimulating the entry of bacteria into macrophages, and possibly subsequent resistance. Preliminary experiments already performed showed that all type 1 pilus-negative mutants, and the AIEC LF82 wild-type strain in the presence of 2% d-mannose, were able to survive and replicate intracellularly as efficiently as the wild-type LF82. The mechanism of entry, which may involve a specific phagosome formation with an unusual endocytic trafficking as described for _Salmonella_-infected macrophages (3, 30, 34), is currently under investigation.
We addressed the question of whether LF82-infected macrophages released IL-1β or TNF-α, both capable of mediating an inflammatory process. No IL-1β was released even after 24 h post-AIEC LF82 infection. This result was not surprising, since it has been shown that this proinflammatory cytokine was only released in the culture medium during apoptosis of _S. flexneri_-infected macrophages (41). Compared to LPS-stimulated macrophages, a high level of TNF-α was secreted after 24 h of infection with AIEC LF82. As TNF-α is transcribed and translated de novo after macrophage stimulation, the synthesis of this cytokine demonstrated that macrophages were still active even with numerous intracellular bacteria. Moreover, proinflammatory TNF-α secretion reflects an activation of infected macrophages. We can speculate that such AIEC-infected macrophages are continuously activated by the sustained presence of intracellular bacteria resistant for a long period to the bacterial killing.
In conclusion, AIEC strains, which are able to invade intestinal epithelial cells, are also able to trigger uptake into and survival within macrophages without inducing host cell death. These properties could allow the bacteria to translocate across the human intestinal barrier, to move to deep tissues, to continuously activate macrophages, and to potentially induce the formation of granulomas, one of the hallmarks of CD lesions.
ACKNOWLEDGMENTS
This study was supported by grants from Association F. Aupetit, Institut de Recherche des Maladies de l'Appareil Digestif (IRMAD, Laboratories Astra France), and the Ministère de la Recherche et de la Technologie (EA2148). N. Barnich was supported by a grant from Association F. Aupetit.
We are grateful to Christel Neut of the Laboratoire de Bactériologie, Faculté de Pharmacie, Lille, France for providing E. coli strains isolated from patients with CD and for helpful discussions. We also thank Annie Fraisse, Monique Orion, and Josiane Payen of the Electron Microscopy Department of Michel Bourges for technical assistance. We thank Philippe Sansonetti, Institut Pasteur, Paris, France, for providing S. flexneri M90T. We gratefully thank Donald Guiney, La Jolla School of Medicine at the University of California—San Diego, for providing Salmonella serovar Dublin strain SL2260 and for valuable help in preparing HMDM. We also thank Herbert J. Van Kruiningen and Cecilia Berin for helpful discussions and critical reading of the manuscript.
REFERENCES
- 1.Aderem A, Underhill D M. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 2.Alpuche-Aranda C M, Berthiaume E P, Mock B, Swanson J A, Miller S I. Spacious phagosome formation within mouse macrophages correlates with Salmonella serotype pathogenicity and host susceptibility. Infect Immun. 1995;63:4456–4462. doi: 10.1128/iai.63.11.4456-4462.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alpuche-Aranda C M, Racoosin E L, Swanson J A, Miller S I. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J Exp Med. 1994;179:601–608. doi: 10.1084/jem.179.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baca O G, Li Y P, Kumar H. Survival of the Q fever agent Coxiella burnetii in the phagolysosome. Trends Microbiol. 1994;2:476–480. doi: 10.1016/0966-842x(94)90651-3. [DOI] [PubMed] [Google Scholar]
- 5.Boudeau J, Barnich N, Darfeuille-Michaud A. Type 1 pili-mediated adherence of Escherichia coli strain LF82 isolated from Crohn's disease is involved in bacterial invasion of intestinal epithelial cells. Mol Microbiol. 2001;39:1272–1284. doi: 10.1111/j.1365-2958.2001.02315.x. [DOI] [PubMed] [Google Scholar]
- 6.Boudeau J, Glasser A L, Masseret E, Joly B, Darfeuille-Michaud A. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect Immun. 1999;67:4499–4509. doi: 10.1128/iai.67.9.4499-4509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boudeau J, Glasser A L, Neut C, Desreumaux P, Cortot A, Rich C, Joly B, Colombel J F, Darfeuille-Michaud A. Invasive ability of Escherichia coli strains isolated from ileal mucosa in Crohn's disease. Gastroenterology. 2000;118:A1847. [Google Scholar]
- 8.Buchmeier N A, Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989;57:1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cartun R W, Van Kruiningen H J, Pedersen C A, Berman M M. An immunocytochemical search for infectious agents in Crohn's disease. Mod Pathol. 1993;6:212–219. [PubMed] [Google Scholar]
- 10.Clemens D L. Characterization of the Mycobacterium tuberculosis phagosome. Trends Microbiol. 1996;4:113–118. doi: 10.1016/0966-842X(96)81528-9. [DOI] [PubMed] [Google Scholar]
- 11.Darfeuille-Michaud A, Neut C, Barnich N, Lederman E, Di Martino P, Desreumaux P, Gambiez L, Joly B, Cortot A, Colombel J F. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology. 1998;115:1405–1413. doi: 10.1016/s0016-5085(98)70019-8. [DOI] [PubMed] [Google Scholar]
- 12.Duchmann R, Zeitz M. Crohn's disease. In: Ogra P L, et al., editors. Mucosal immunology. 2nd ed. San Diego, Calif: Academic Press; 1999. pp. 1055–1080. [Google Scholar]
- 13.Ernst J D. Bacterial inhibition of phagocytosis. Cell Microbiol. 2000;2:379–386. doi: 10.1046/j.1462-5822.2000.00075.x. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Prada C, Tall B D, Elliott S E, Hoover D L, Nataro J P, Venkatesan M M. Hemolysin-positive enteroaggregative and cell-detaching Escherichia coli strains cause oncosis of human monocyte-derived macrophages and apoptosis of murine J774 cells. Infect Immun. 1998;66:3918–3924. doi: 10.1128/iai.66.8.3918-3924.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-Prada C M, Hoover D L, Tall B D, Venkatesan M M. Human monocyte-derived macrophages infected with virulent Shigella flexneri in vitro undergo a rapid cytolytic event similar to oncosis but not apoptosis. Infect Immun. 1997;65:1486–1496. doi: 10.1128/iai.65.4.1486-1496.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 17.Goosney D L, Celli J, Kenny B, Finlay B B. Enteropathogenic Escherichia coli inhibits phagocytosis. Infect Immun. 1999;67:490–495. doi: 10.1128/iai.67.2.490-495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant T, Bennett-Wood V, Robins-Browne R M. Characterization of the interaction between Yersinia enterocolitica biotype 1A and phagocytes and epithelial cells in vitro. Infect Immun. 1999;67:4367–4375. doi: 10.1128/iai.67.9.4367-4375.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guilloteau L A, Wallis T S, Gautier A V, MacIntyre S, Platt D J, Lax A J. The Salmonella virulence plasmid enhances Salmonella-induced lysis of macrophages and influences inflammatory responses. Infect Immun. 1996;64:3385–3393. doi: 10.1128/iai.64.8.3385-3393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haque M A, Yoshino S, Ohki K, Inada S, Kohashi O. Effect of growth condition on in-vitro susceptibility of Shigella dysenteriae type one killing by murine peritoneal macrophages. J Med Microbiol. 1996;44:99–104. doi: 10.1099/00222615-44-2-99. [DOI] [PubMed] [Google Scholar]
- 21.High N, Mounier J, Prevost M C, Sansonetti P J. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992;11:1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishibashi Y, Arai T. A possible mechanism for host-specific pathogenesis of Salmonella serovars. Microb Pathog. 1996;21:435–446. doi: 10.1006/mpat.1996.0074. [DOI] [PubMed] [Google Scholar]
- 23.Jones S, Preiter K, Portnoy D A. Conversion of an extracellular cytolysin into a phagosome-specific lysin which supports the growth of an intracellular pathogen. Mol Microbiol. 1996;21:1219–1225. doi: 10.1046/j.1365-2958.1996.00074.x. [DOI] [PubMed] [Google Scholar]
- 24.Lai X H, Wang S Y, Uhlin B E. Expression of cytotoxicity by potential pathogens in the standard Escherichia coli collection of reference (ECOR) strains. Microbiology. 1999;145:3295–3303. doi: 10.1099/00221287-145-11-3295. [DOI] [PubMed] [Google Scholar]
- 25.Lai X H, Xu J G, Melgar S, Uhlin B E. An apoptotic response by J774 macrophage cells is common upon infection with diarrheagenic Escherichia coli. FEMS Microbiol Lett. 1999;172:29–34. doi: 10.1111/j.1574-6968.1999.tb13445.x. [DOI] [PubMed] [Google Scholar]
- 26.Libby S J, Adams L G, Ficht T A, Allen C, Whitford H A, Buchmeier N A, Bossie S, Guiney D G. The spv genes on the Salmonella dublin virulence plasmid are required for severe enteritis and systemic infection in the natural host. Infect Immun. 1997;65:1786–1792. doi: 10.1128/iai.65.5.1786-1792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Libby S J, Lesnick M, Hasegawa P, Weidenhammer E, Guiney D G. The Salmonella virulence plasmid spv genes are required for cytopathology in human monocyte-derived macrophages. Cell Microbiol. 2000;2:49–58. doi: 10.1046/j.1462-5822.2000.00030.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Van Kruiningen H J, West A B, Cartun R W, Cortot A, Colombel J F. Immunocytochemical evidence of Listeria, Escherichia coli, and Streptococcus antigens in Crohn's disease. Gastroenterology. 1995;108:1396–1404. doi: 10.1016/0016-5085(95)90687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masseret E, Boudeau J, Colombel J F, Neut C, Desreumaux P, Joly B, Cortot A, Darfeuille-Michaud A. Genetically related Escherichia coli strains associated with Crohn's disease. Gut. 2001;48:320–325. doi: 10.1136/gut.48.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9388. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navarre W W, Zychlinsky A. Pathogen-induced apoptosis of macrophages: a common end for different pathogenic strategies. Cell Microbiol. 2000;2:265–273. doi: 10.1046/j.1462-5822.2000.00056.x. [DOI] [PubMed] [Google Scholar]
- 32.Sansonetti P J, Kopecko D J, Formal S B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982;35:852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sansonetti P J, Tran Van Nhieu G, Egile C. Rupture of the intestinal epithelial barrier and mucosal invasion by Shigella flexneri. Clin Infect Dis. 1999;28:466–475. doi: 10.1086/515150. [DOI] [PubMed] [Google Scholar]
- 34.Schwan W R, Huang X Z, Hu L, Kopecko D J. Differential bacterial survival, replication, and apoptosis-inducing ability of Salmonella serovars within human and murine macrophages. Infect Immun. 2000;68:1005–1013. doi: 10.1128/iai.68.3.1005-1013.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwan W R, Kopecko D J. Serovar-specific differences in Salmonella survival within macrophage cells. Adv Exp Med Biol. 1997;412:277–278. doi: 10.1007/978-1-4899-1828-4_46. [DOI] [PubMed] [Google Scholar]
- 36.Sizemore D R, Elsinghorst E A, Eck L C, Branstrom A A, Hoover D L, Warren R L, Rubin F A. Interaction of Salmonella typhi strains with cultured human monocyte-derived macrophages. Infect Immun. 1997;65:309–312. doi: 10.1128/iai.65.1.309-312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanner A R, Arthur M J, Wright R. Macrophage activation, chronic inflammation and gastrointestinal disease. Gut. 1984;25:760–783. doi: 10.1136/gut.25.7.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vladoianu I R, Chang H R, Pechere J C. Expression of host resistance to Salmonella typhi and Salmonella typhimurium: bacterial survival within macrophages of murine and human origin. Microb Pathog. 1990;8:83–90. doi: 10.1016/0882-4010(90)90072-x. [DOI] [PubMed] [Google Scholar]
- 39.Waring P, Eichner R D, Mullbacher A, Sjaarda A. Gliotoxin induces apoptosis in macrophages unrelated to its antiphagocytic properties. J Biol Chem. 1988;263:18493–18499. [PubMed] [Google Scholar]
- 40.Zumla A, James D G. Granulomatous infections: etiology and classification. Clin Infect Dis. 1996;23:146–158. doi: 10.1093/clinids/23.1.146. [DOI] [PubMed] [Google Scholar]
- 41.Zychlinsky A, Fitting C, Cavaillon J M, Sansonetti P J. Interleukin 1 is released by murine macrophages during apoptosis induced by Shigella flexneri. J Clin Investig. 1994;94:1328–1332. doi: 10.1172/JCI117452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zychlinsky A, Prevost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]