Analysis of the structure of human telomerase RNA in vivo (original) (raw)
Abstract
Telomerase is a ribonucleoprotein reverse transcriptase that synthesises telomeric DNA. The RNA component of telomerase acts as a template for telomere synthesis and binds the reverse transcriptase. In this study, we have performed in vivo and in vitro structural analyses of human telomerase RNA (hTR). In vivo mapping experiments showed that the 5′-terminal template domain of hTR folds into a long hairpin structure, in which the template sequence occupies a readily accessible position. Intriguingly, neither in vivo nor in vitro mapping of hTR confirmed formation of a stable ‘pseudoknot’ helix, suggesting that this functionally essential long range interaction is formed only temporarily. In vitro control mappings demonstrated that the 5′-terminal template domain of hTR cannot fold correctly in the absence of cellular protein factors. The 3′-terminal domain of hTR, both in vivo and in vitro, folds into the previously predicted box H/ACA snoRNA-like ‘hairpin–hinge–hairpin–tail’ structure. Finally, comparison of the in vivo and in vitro modification patterns of hTR revealed several regions that might be directly involved in binding of telomerase reverse transcriptase or other telomerase proteins.
INTRODUCTION
Telomerase is a ribonucleoprotein (RNP) reverse transcriptase that adds telomeric DNA repeats to the ends of eukaryotic chromosomes (1–3). All telomerase enzymes consist of at least two essential components, the telomerase reverse transcriptase (TERT) and telomerase RNA. The telomerase RNA has at least two functions, it provides a scaffold for binding of RNP proteins and carries the template sequence that is copied into telomeric DNA by the associated TERT.
Telomerase RNAs have been identified in many phylogenetically distant species, including 24 ciliated protozoa, 2 yeasts and 35 vertebrates. Although they differ considerably in their size and primary structure, each telomerase RNA seems to feature a pseudoknot structure located close to the template sequence (4–8). Ciliate telomerase RNAs are relatively small (148–209 nt) and are composed of a pseudoknot domain and a short 3′-terminal hairpin (4–7). The 3′-half of vertebrate telomerase RNAs carries a box H/ACA small nucleolar RNA-like (snoRNA-like) domain (8,9), therefore they are longer (382–559 nt) than their ciliate counterparts. Phylogenetic comparison of 35 vertebrate telomerase RNAs revealed eight conserved regions (CR1–CR8) which build up four conserved secondary structure elements, namely the 5′-terminal pseudoknot domain, the CR4–CR5 domain, the box H/ACA snoRNA-like domain and the CR7 domain (8). The latter three elements are not found in ciliate RNAs, but play important roles in the function, stability, processing and intracellular trafficking of vertebrate telomerase RNAs. The conserved structural elements of telomerase RNAs likely function through binding of the protein components of the telomerase holoenzyme (10–14).
In the current work, to obtain a more detailed insight into the structure of human telomerase RNA (hTR), we performed in vivo and in vitro conformational analyses. Previous structure probing experiments showed that protozoan telomerase RNAs fold differently under in vivo and in vitro conditions (15,16). Since the in vivo modification pattern showed better agreement with the structure proposed by phylogenetic comparative analyses (4–7), the authors concluded that ciliate telomerase RNAs are misfolded in vitro (15,16). In vivo probing of hTR revealed that the 3′-terminal box H/ACA snoRNA-like domain of the RNA folds into a structure very similar to that found in living human cells. However, the 5′ template domain of hTR, similarly to ciliate telomerase RNAs, showed largely different architectures in vivo and in vitro. Our experiments defined the secondary structure of hTR in more detail and got closer to functional implications, especially of the 3′-domain of the RNA.
MATERIALS AND METHODS
In vivo chemical modification and purification of HeLa nuclear RNAs
For each modification reaction, ∼5–10 × 107 exponentially growing human HeLa cells (S3 line) were washed with ice-cold phosphate-buffered saline and swelled in buffer A (100 mM HEPES–KOH pH 7.9, 1.5 mM MgCl2, 10 mM KCl) for 10 min. Cells were homogenised in a Dounce homogeniser by 15 strokes with pestle B and nuclei were collected by centrifugation at 1000 g for 10 min. For dimethyl sulfate (DMS) treatment (15), nuclei were resuspended in 1 ml of buffer B (10 mM HEPES–KOH pH 7.9, 10 mM MgCl2, 3 mM CaCl2, 250 mM glucose). DMS (Aldrich) was added to 0.05, 0.25, 1.0, 1.5 or 2% final concentration and the suspension was incubated with gentle agitation at room temperature for 2 min. The reaction was terminated by addition of β-mercaptoethanol at 0.7 M final concentration. For kethoxal (α-keto-β-ethoxybutyraldehyde) treatment (17), nuclei were suspended in 200 µl of buffer C (10 mM Tris–HCl pH 6.8, 10 mM MgCl2, 3 mM CaCl2, 250 mM glucose, 20 mM Na borate) and disrupted by sonication for 4 × 15 s with a Branson Sonifier equipped with a microtip (setting 1). Kethoxal (ICN) was added at 0.05, 0.1 and 0.2% final concentration and gently agitated for 10 min at room temperature. For RNA isolation, treated and control nuclei were lysed in 10 vol of 50 mM Na acetate pH 5.1, 140 mM NaCl, 0.3% SDS and extracted twice with water-saturated phenol by vigorous shaking at 65°C for 10 min. RNAs were ethanol precipitated and dissolved in sterile water (DMS treatment) or 25 mM K borate, pH 7 (kethoxal treatment).
Structure probing of in vitro synthesised human telomerase RNA and U19 snoRNA
Full-length U19 and telomerase RNAs were synthesised by T7 RNA polymerase (18) using linearised pU19cod/T7 (19) and phTR1 (20) pasmids as templates. About 10–15 pmol gel-purified RNA transcripts were 3′-end-labelled with [α-32P]pCp and T4 RNA ligase (NEB) (21) or 5′-end-labelled with [γ-32P]ATP and T4 polynucleotide kinase (NEB) after dephosphorylation with calf intestine phosphatase (22). The end-labelled and gel-purified RNAs (∼5 × 104 c.p.m.) were partially digested at 20°C for 10 min with 0.001 or 0.04 U T1, T2 or V1 RNase in 50 mM Tris–HCl pH 7.5, 10 mM MgCl2, 50 mM KCl in the presence of 2.5 µg tRNA. Limited digestion with 0.4 U S1 nuclease was performed at 20°C for 12 and 25 min in 25 mM Na acetate pH 4.5, 10 mM MgCl2, 50 mM KCl, 1 mM ZnCl2 in the presence of 2.5 µg tRNA. Partial alkaline hydrolysis and RNase T1 sequencing was performed according to Kiss et al. (23). The samples were loaded directly onto a 7, 10 or 15% polyacrylamide–urea gel.
For structural mapping of the internal regions of the human telomerase and U19 RNA ∼40–80 ng in vitro synthesised RNA were dissolved in the appropriate reaction buffer lacking magnesium. RNAs were denatured at 90°C for 30 s and incubated at 65°C for 1 min. After cooling to room temperature, MgCl2 was added to 10 mM final concentration. DMS modification was carried out in 150 µl of DMS buffer (50 mM sodium cacodylate pH 7.5, 10 mM MgCl2, 50 mM KCl) in the presence of 10 µg Escherichia coli tRNA (Boehringer) and 0.15 or 0.3% DMS at 20°C for 15 min. The reaction was terminated by adding 60 µl of DMS stop (0.5 M 2-β-mercaptoethanol, 0.75 M Na acetate, pH 5.5). Kethoxal modification was performed in 80 µl of DMS buffer supplemented with 1.2 µg tRNA and 0.05, 0.2 or 0.5% kethoxal at 20°C for 6 min. The reaction was stopped by adding 32 µl of 0.5 M K borate pH 7, containing 10 µg tRNA. _N_-cyclohexyl-_N_′-[b-(_N_-methylmorpholino)-ethyl]-carbodiimide-_p_-toluenesulfonate (CMCT) modification was achieved in 300 µl of 50 mM Na borate pH 8, 10 mM MgCl2, 50 mM KCl containing 15 µg tRNA and 25 mM CMCT (Merck) at 20°C for 30 min. For lead acetate probing, ∼0.1 µg test RNA was dissolved in 20 µl of 50 mM HEPES–KOH pH 7.5, 10 mM Mg acetate, 50 mM K acetate containing 10 µg tRNA. Lead acetate was added to 5 or 20 mM final concentration and the mixture incubated at 25°C for 15 min. The reaction was terminated by adding EDTA at 33 mM final concentration (24). Modified RNAs were collected by ethanol precipitation and dissolved in sterile water or, in the case of kethoxal modification, in 25 mM K borate pH 7. Partial hydrolyses with RNase V1 (cobra venom RNase V1; Pharmacia) was carried out at 20°C for 10 min in 50 µl of 50 mM Tris–HCl pH 7.5, 10 mM MgCl2, 50 mM KCl in the presence of 2.5 µg tRNA and 0.5 or 1.5 U RNase V1. To terminate the reaction, 3 µl of 100 mM EDTA and 10 µg tRNA were added. After phenol extraction, RNAs were ethanol precipitated.
Positions of modified nucleotides and cleavage sites were determined by primer extension analyses using terminally labelled sequence-specific oligonucleotides as primers (25,26). For each elongation reaction, 10–20 µg nuclear RNA and 50 ng in vitro transcribed RNA were used as template. The following oligodeoxynucleotides were used for mapping of telomerase (CR) and U19 RNAs: CR1, CGGCGCCTACGCCCTTC; CR2, CTGACATTTTTTGTTTGCTC; CR3, CCGCCGCAGGTCCCCGGG; CR4, CCCAACTCTTCGCGGTGGCAG; CR5, CCTCTTCCTGCGGCCTG; CR6, GCATGTGTGAGCCGAGTCCTG; U19H, AGTGCTGGAGCCAAACCTCAATAA.
RESULTS AND DISCUSSION
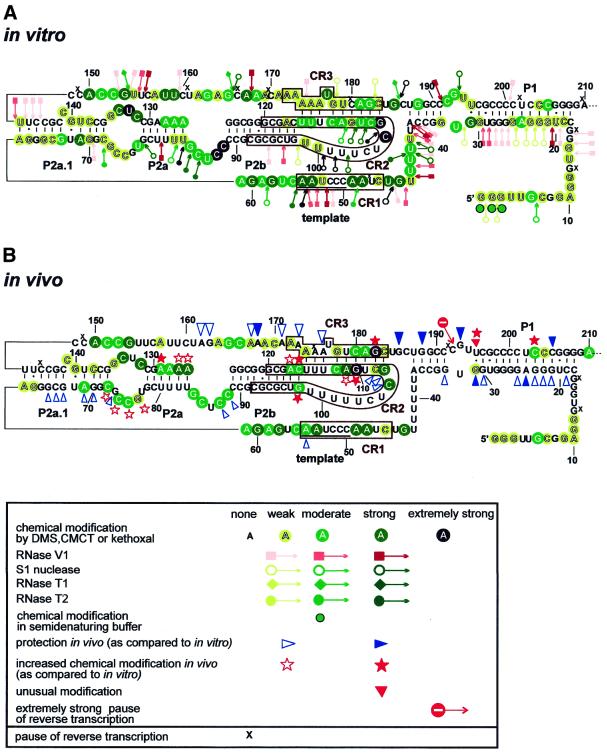
In vivo structural analysis of human telomerase RNA (hTR) is largely hampered by the fact that hTR accumulates at fairly low concentrations (∼1000 copy/cell) in most human tissues and cell lines. To increase the relative amount of hTR, intact or sonicated human HeLa cell nuclei (considered to represent in vivo conditions and termed as such), rather than whole cells, were treated with DMS or kethoxal. DMS methylates adenine at the N1 position and cytosine at the N3 position and kethoxal modifies guanine at the N1 and N2 positions. These base modifications, however, only occur if the target groups are not engaged in hydrogen bonding interactions forming RNA helices, tertiary structures or RNA–protein interactions. The covalently modified nucleotides were detected by primer extension analysis using terminally labelled sequence-specific primers. As a control, in vitro synthesised hTR was also probed with chemical reagents as well as double (RNase V1) and single strand-specific (RNase T1, RNase T2 and nuclease S1) endonucleases. A summary of our in vitro and in vivo structure probing experiments is represented by taking the previously proposed structures of the 5′-terminal pseudoknot (Fig. 1) and 3′-terminal box H/ACA snoRNA-like (see Fig. 3) domains of hTR (8,9). The assignment of week, moderate, strong and extremely strong modification sites was based on visual assessment of autoradiograms obtained in at least three independent experiments.
Figure 1.
Schematic representation of the results of in vitro (A) and in vivo (B) structure probing of the 5′-terminal domain of hTR. The structure of hTR has been adopted from Chen et al. (8) and Mitchell et al. (9). The phylogenetically conserved regions (CR1, CR2 and CR3) are boxed and the template sequence is underlined. Nucleotides modified by DMS, CMCT or kethoxal are circled in green. Positions of nuclease cleavage are indicated by arrows linked to pink squares (RNase V1, double strand-specific) and green diamonds (RNase T1, G-specific) and open (S1 nuclease, single strand-specific) and filled (RNase T2, single strand-specific) circles. The intensity of the colour indicates the intensity of chemical modification and nucleolytic cleavage. Extremely strong modifications are shown in black. Green circles with black outlines indicate altered modifications under semi-denaturing conditions. Unaffected nucleotides are not marked. Nucleotides with decreased in vivo accessibility are indicated by open (moderately protected) and filled (fully protected) blue triangles. Open and filled red stars indicate nucleotides with moderately or highly increased in vivo reactivity, respectively. Red triangles indicate unusual uridine modifications. Pauses (x) and strong stops (stop signal) of reverse transcriptase are also indicated.
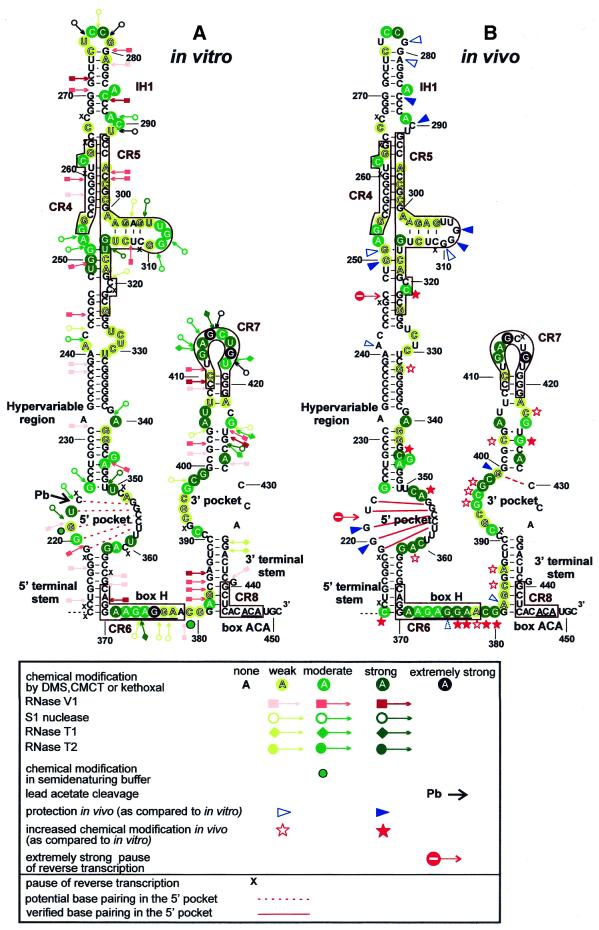
Figure 3.
Schematic representation of the results of in vitro (A) and in vivo (B) structure probing of the box H/ACA snoRNA-like domain of hTR. The phylogenically conserved CR4, CR5, CR6, CR7 and CR8 regions are boxed. The box H and ACA sequences are underlined. Extremely strong cleavage induced by lead acetate (Pb) is marked by a black arrow. Potential and verified base pairings in the 5′ pocket are marked by red dotted and continuous lines, respectively. For other details, see the legend to Figure 1.
The template region of hTR is accessible in vivo
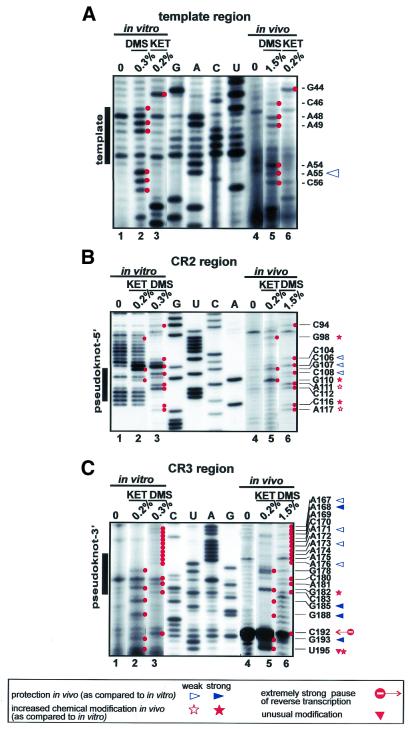
Vertebrate telomerase RNAs contain eight evolutionarily conserved sequence regions (CR1–CR8) (8). Three of these regions (CR1–CR3) are located in the 5′-terminal pseudoknot domain of the RNA (Fig. 1). The CR1 region of hTR (46CUAACCCUAA55) encompasses the template sequence (underlined) that directs faithful synthesis of human telomeric DNA. Our in vivo structure probing experiments showed that nucleotides in the CR1 region and its immediate flanking areas (from A54 to A61, and G44) are generally accessible to single strand-specific chemical agents (Fig. 1B; for mappings see Fig. 2A, lanes 4–6). This indicates that the template region of hTR is not involved in RNA–RNA or RNA–protein interactions in general. However, three C residues in the template sequence (C50–C52) were found to be highly protected under in vivo conditions. It remains unclear whether these residues are involved in base pairing interactions, tertiary RNA structures, protein binding or are highly stacked in the three-dimensional structure of the RNA. Under in vitro conditions, nucleotides in the CR1 region of hTR reacted with both single and double strand-specific agents (Figs 1A and 2A, lanes 1–3, and data not shown), strongly suggesting that this region of the naked hTR is misfolded in the test tube. It is noteworthy that a similar conclusion was obtained upon in vitro structural mapping of Tetrahymena telomerase RNA (15).
Figure 2.
Secondary structure analyses of the CR1 template (A), and the CR2 (B) and CR3 (C) pseudoknot regions of hTR. In vitro (lanes 1–3) and in vivo (lanes 4–6) modified hTR was analysed by primer extension using terminally labelled sequence-specific oligonucleotide primers. The extended products were fractionated on a 6% sequencing gel. Modified nucleotides are marked by red spots and listed on the right. Concentrations of chemical reagents (DMS and kethoxal) are indicated at the top. Control lanes (0) show primer extension reactions performed on non-treated RNAs. Lanes G, A, C and U represent dideoxy sequencing reactions performed with the same primer. Nucleotides with decreased in vivo accessibility are indicated by open (moderately protected) and filled (fully protected) blue triangles. Open and filled red stars indicate nucleotides with moderately or highly increased in vivo reactivity, respectively. Red triangles indicate unusual uridine modifications. Strong stops (stop signal) of reverse transcriptase are also indicated.
Structure probing of hTR fails to detect a stable pseudoknot structure
Phylogenetic comparisons revealed the presence of a conserved pseudoknot structure in both ciliate and vertebrate telomerase RNAs (4–6,8). Although this putative pseudoknot was dispensable for the activity of in vitro reconstituted Tetrahymena telomerase RNP (27,28), it was critical for in vivo assembly of functional telomerase enzyme (29). Likewise, deletion and mutation analyses of mammalian telomerase RNAs strongly supported the idea that pseudoknot formation is essential for efficient telomere synthesis both in vivo and in vitro (30–34).
In the 5′-terminal domain of hTR, the CR2 (from C92 to G120) and CR3 (from A172 to C183) regions were proposed to partially interact with each other and to form a pseudoknot structure (8). To assess the architecture of the proposed pseudoknot region of hTR, the CR2 and CR3 regions were mapped both in vitro and in vivo (Fig. 2B and C). The in vitro modification pattern of the CR2 region and its flanking regions (from C89 to G125) revealed a classical terminal stem–loop conformation that consists of a 10 bp stable helix and a 17 nt long free loop (Figs 1A and 2B, lanes 1–3). In vivo, however, new modifications appeared in the helix structure at the conserved residues G98, C116 and A117 (Figs 1B and 2B, lanes 4–6). The accessibility of nucleotides in the loop also changed in vivo. Residues C106 and G107, which occupy the most exposed positions in vitro, were less extensively modified in vivo. In contrast, G110, which was fairly protected in vitro, exhibited the strongest methylation in vivo. This could be explained by a weak in vitro interaction between G110, A111 and U103, C104, which is disrupted in vivo.
During in vivo mapping of the CR3 region, several non-specific reverse transcriptase stops appeared after DMS treatment (Fig. 2C, lane 6). Nevertheless, the specific and highly reproducible signals obtained after in vitro and in vivo kethoxal modification (Fig. 2C, lanes 2 and 5) allowed us to draw conclusions about the architecture of this region of hTR (Fig. 1). Nearly all nucleotides in the CR3 region were accessible under in vitro conditions, indicating that they are not engaged in base pairing interactions. Upon in vivo modification, the 3′-part of CR3 (C180–C183) showed an even higher reactivity. For example, G182 in this region, together with G110 in the CR2 region, showed the strongest kethoxal modification in vivo in the 5′-domain of hTR. This strongly argues against formation of a stable in vivo interaction between the G107–C112 and G178–C183 regions of hTR.
Nucleotides U113–U115 and A174–A176 in the CR2 and CR3 regions, respectively, were also predicted to interact with each other (8). Under in vitro conditions residues A174–A176 and the neighbouring residues A171–A173 showed a weak and very uniform availability, making it unlikely that these nucleotides could belong to different structures. In vivo, nucleotides in the 5′-half of the CR3 region (A171–A176) were generally less reactive, indicating that they might be involved in hydrogen bonding. Since neither DMS nor kethoxal normally reacts with uridine, we cannot confirm the interaction between nucleotides U113–U115 and A171–A173. However, the appearance of new modifications at G98, C116 and A117 under in vivo conditions clearly indicates a new situation in living cells. One can envisage that either a pseudoknot structure is formed or a protein binds close to this part of hTR. Nucleotides in the neighbourhood of protein-binding sites frequently show an increased reactivity (15). The increased in vivo accessibility of G110 and A111 in the CR2 region may be a result of the same phenomenon.
Previously, structure probing of Tetrahymena telomerase RNA suggested that the pseudoknot structure is unfolded under in vitro conditions (15,16). In living Tetrahymena cells, however, nucleotides predicted to participate in pseudoknot formation were protected against DMS methylation (15), suggesting that a stable pseudoknot structure might be formed in vivo. In general, we found that nucleotides in the CR2 and CR3 regions of hTR were more reactive than expected for nucleotides engaged in stable helix formation (Fig. 1A and B). We concluded that in naked hTR the CR2 region forms a helix–terminal loop structure and the CR3 region is either free or interacts weakly with other regions of the RNA. In HeLa cells the long range interaction of CR2 and CR3 is either confined to the A174–A176 and U113–U115 regions or, according to an alternative scenario, only a fraction of cellular telomerase RNAs, for example the functionally active RNAs, feature the pseudoknot structure. Therefore, the dynamic interaction of CR2 and CR3 is not detectable by chemical modification.
Helices in the 5′-terminal template domain of hTR
Besides the CR2–CR3 pseudoknot helix, three additional stem structures, A62–C72/G136–U147, G78–U84/A126–C131 and C18–A37/U187–G208, have been predicted in the 5′-terminal template domain of hTR (8). Both in vitro and in vivo structure probing experiments confirmed formation of a stable A62–C72/G136–U147 helix (Fig. 1A and B). However, no evidence supported in vivo formation of the G78–U83/A126–C131 stem. As predicted by the high reactivity of residues A126–A129, this region of hTR is most probably breathing in living cells (Fig. 1B). Finally, the C18–A37 and U187–G208 sequences were predicted to form helix P1. The P1 stem is, however, not conserved in vertebrates. Rodent telomerase RNAs commence just 2 nt upstream of the template region and, therefore, they contain no P1-like structure. Accordingly, sequences preceding the template region of hTR were found to be dispensable for telomerase activity, supporting the idea that helix P1 does not represent an essential structural element of mammalian telomerase RNAs (30–32). However, alteration of the C180–G189 and C190–C199 sequences in the 3′-strand of the putative P1 helix of hTR abolished telomerase activity (30), suggesting that these sequences or local structures are functionally important. In in vivo structure probing experiments most residues in the C18–A37 and G188–G208 regions of hTR were highly resistant to single strand-specific chemical agents (Fig. 1B), leading to the conclusion that helix P1 is indeed formed in human telomerase RNP. Interestingly, G193 in the internal loop of the P1 stem (data not shown) and G185 downstream of the CR3 region were also protected in vivo (Fig. 2C, lane 5). Binding of a protein to this region of hTR might protect residues G185 and G193 and it may also explain the functional importance of these sequences (30). By the same token, appearance of a strong unusual in vivo modification at U195 might be interpreted by protein binding in its neighbourhood (Fig. 2C, lane 5).
Unfortunately, in vitro structure probing of the 5′-terminal region of hTR, including the CR1 region (see above), failed to provide conclusive results, since many nucleotides reacted with both single and double strand-specific agents (Fig. 1A). This strongly suggests that the 5′-terminal region of hTR acquires various alternative structures under in vitro conditions and that protein factors are essential for proper folding of hTR (15,35).
Structure of the 3′-terminal H/ACA snoRNA-like core domain of hTR
Vertebrate telomerase RNAs carry a large box H/ACA snoRNA-like 3′-terminal structure (8,9). Like canonical box H/ACA snoRNAs, the H/ACA-like domain of hTR is composed of two hairpin structures which are connected and followed by short single-stranded regions carrying the conserved H (ANANNA) and ACA box motifs (36,37). Moreover, the H/ACA-like domain of hTR binds authentic box H/ACA snoRNP proteins (13,20,38) which likely direct the 3′-end processing of precursor telomerase RNA, provide metabolic stability for the mature RNA and direct the intracellular trafficking of telomerase RNP (9,36,37,39–41).
The minimal core structure required for accumulation of box H/ACA snoRNAs includes the basal helixes of the 5′ and 3′ hairpins and the conserved H and ACA boxes (40). The H and ACA boxes of vertebrate telomerase RNAs are embedded in the evolutionarily conserved CR6 and CR8 regions, respectively (8). Intact H and ACA boxes are absolutely essential for accumulation of both H/ACA snoRNAs (36,37,40) and telomerase RNAs (9,34,39). The ACA box is invariantly located 3 nt from the 3′-terminus of both H/ACA snoRNAs and telomerase RNAs. Most probably, the ACA box delineates the correct 3′-terminus of the RNA by binding snoRNP proteins, which arrest the processing exonucleases (36,40). Unfortunately, our experimental approach failed to provide in vivo structural information about the 3′-terminal region of hTR (C431–C451), since this region was complementary to the most 3′-terminal primer used for primer extension.
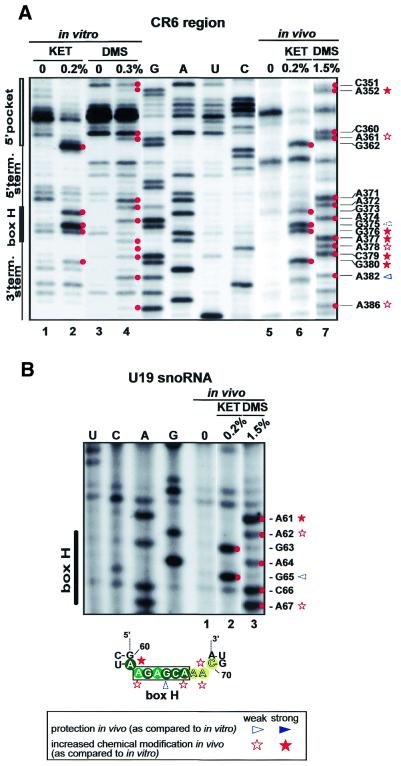
Structure probing of the hinge region containing the H motif (CR6 region) gave different results in vitro and in vivo (Fig. 3). In vitro, this region can be divided into an easily accessible 5′-part (A371–G375) and a slightly, if at all, accessible 3′-part (G376–G380), with an extremely strong modification at G375 as the transition point (Fig. 4A, lanes 1–4). However, when a truncated version of hTR (G207–C451) lacking the entire 5′-terminal template domain was mapped, a typical single-stranded conformation was observed for the whole hinge region (data not shown). Similar results were obtained previously during structural probing of the hinge regions of authentic box H/ACA snoRNAs (19,37). We therefore concluded that, in vitro, nucleotides G376–G380 in the 3′-part of the hinge region likely interact with some nucleotides in the misfolded 5′-terminal template domain of hTR (see above). In vivo, all nucleotides in the hinge region of hTR showed high reactivity with single strand-specific chemical agents (Fig. 4A, lanes 5–7). This observation was somewhat surprising, since at least the conserved A residues in the H box motif were hypothesised to specifically interact with box H/ACA snoRNP proteins. The A372, A374 and A377 residues in the H motif of hTR were, however, readily accessible for DMS methylation. To test whether this is a specific feature of telomerase H boxes, we also determined the in vivo modification pattern of the hinge region of human U19 box H/ACA snoRNA (Fig. 4B). Similar to the H box of hTR, all nucleotides in the H box of U19 were readily modified by DMS and kethoxal, indicating that snoRNP proteins do not protect the H box nucleotides of H/ACA snoRNAs and telomerase RNAs.
Figure 4.
Structure probing of the box H/ACA core domain of hTR and U19 snoRNA. (A) Secondary structure of the box H/ACA core domain of hTR was analysed under in vitro (lanes 1–4) and in vivo (lanes 5–7) conditions. (B) In vivo structure probing of the box H region of human U19 snoRNA. The modification pattern of the box H region of U19 is shown at the bottom. For other details, see the legend to Figure 2.
The H and ACA boxes work in concert with the basal helices of the 5′ and 3′ hairpins of box H/ACA snoRNAs. Formation of the 3′-terminal helix has been demonstrated to be crucial for accumulation of both box H/ACA snoRNAs (36,37) and telomerase RNAs (34,39). Therefore, it was not surprising that structure probing of hTR fully confirmed the double-stranded nature of this region both in vitro and in vivo (Fig. 3). In vitro, almost all nucleotides in the G381–C390 and G433–C443 regions were protected against single strand-specific agents and several nucleotides were recognised by RNase V1 (A382–G385 and C438), demonstrating the double-stranded structure of this region (Fig. 3A). In vivo, the 5′-strand of the 3′-terminal helix showed a slightly increased reactivity with DMS and kethoxal (Fig. 3B). This might result from protein binding to the helix and/or the ACA motif. No in vivo data are available for the 3′-terminal region of hTR, since it was complementary to the oligonucleotide primer. The 5′ helix of the H/ACA core motif, although dispensable for RNA accumulation, is required for telomerase function (39). In vitro and in vivo mapping experiments demonstrated that the 5′ helix of the H/ACA core represents one of the most stable helices of hTR. Nucleotides in this region were fully protected against single strand-specific nucleases and chemical agents (Fig. 3).
The putative 5′ and 3′ pseudouridylation pockets of hTR
Box H/ACA snoRNAs function as guide RNAs in the site-specific pseudouridylation of rRNAs and small nuclear RNAs (42–46). Internal loop structures in the 5′ and/or 3′ hairpin of the snoRNA, also known as pseudouridylation pockets, engage the target sequence by direct base pairing interaction. The substrate uridine destined for conversion into pseudouridine is positioned at the base of the upper stem, closing the pseudouridylation loop (43). The distance between the selected uridine and the H or ACA box of the snoRNA is ∼14 nt (42,43).
The 5′ and the 3′ hairpins of hTR are predicted to carry internal loops which, in principle, could function as pseudouridylation pockets. This idea is strengthened by the fact that, besides the Gar1, Nhp2 and Nop10 box H/ACA snoRNP proteins (13,20), mammalian telomerase RNAs are also associated with dyskerin, which most likely provides the pseudouridine synthase activity for box H/ACA snoRNPs (38). While the right strand of the putative 3′ pseudouridylation pocket of hTR could not be analysed in vivo, the left side of the loop showed high accessibility both in vitro and in vivo, indicating that this loop would be available for base pairing interaction with a putative target RNA (Fig. 3). However, neither the size nor the nucleotide composition of this loop is conserved in vertebrate telomerase RNAs. This strongly argues against a pseudouridylation guide function for the 3′-terminal hairpin of hTR. Previously, it was found that substitution of the variable C391–G399 sequence of hTR impaired RNA accumulation (39). This was an unexpected observation, since the base composition of the pseudouridylation loop has no effect on the processing and accumulation of box H/ACA snoRNAs (40). A possible interpretation of this observation could be that substitution of the C394–G399 region distorted the correct structure of the basal stem and/or the CR7 terminal stem–loop structure of the 3′ hairpin. Disruption of either of these elements could abolish in vivo accumulation of hTR (39).
The structure of the 5′ pocket seems to be more compact than was suggested earlier (38). Nucleotides from G353 to U357 in the right strand were highly protected both in vitro and in vivo. In the left side of the loop, nucleotides G219–C223 were also resistant to in vivo chemical treatment, suggesting that these regions form a stable 5 bp helix in living cells. In vitro, nucleotides in the 5′ strand showed a significantly higher reactivity. At U222 we observed strong single strand-specific cleavage and an extremely strong reaction with lead acetate, which suggests a well-exposed position of the molecule. Nevertheless, the highly protected in vivo architecture of the potential pseudouridylation guide domain in the 5′ hairpin of hTR does not support a function in RNA modification. Helices above the pseudouridylation guide loops of box H/ACA snoRNAs are essential for the pseudouridylation reaction (40). Structure probing experiments demonstrated that the upper parts of the putative pseudouridylation guide domains of hTR are followed by stable distal helices, although some nucleotides in these stems showed a slightly increased in vitro reactivity (e.g. G426 and C3450). It is noteworthy that neither in vitro nor in vivo mapping confirmed formation of the previously proposed unusual C401–A428 base pairing (8,9,39).
Telomerase RNA-specific elements in the 5′ and 3′ hairpins of hTR
The distal regions of the 5′ and 3′ hairpins of the H/ACA-like domain of hTR accommodate functionally essential telomerase-specific elements which are not found in canonical box H/ACA snoRNAs (33,34,39). The conserved CR7 element is located at the top of the 3′-terminal hairpin and it is required for in vivo accumulation of hTR (34,39). In vitro and in vivo probing of the CR7 region of hTR demonstrated that this element acquires a typical terminal stem–loop configuration, which consists of a 4 bp helix and an 8 nt long loop (Fig. 3).
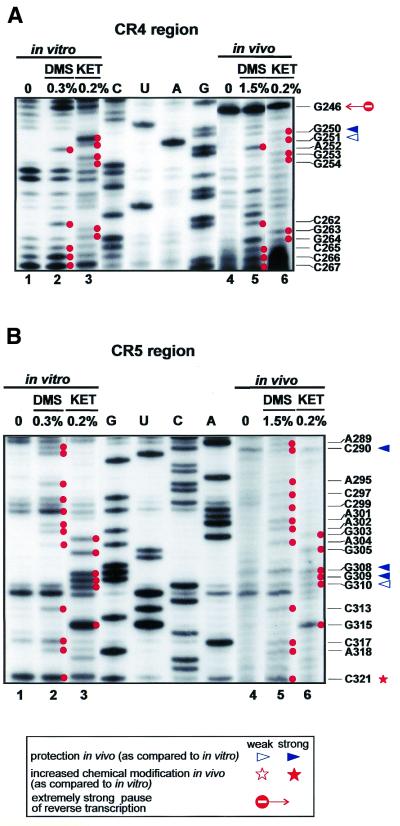
As compared to canonical box H/ACA snoRNAs, the 5′ hairpin of the H/ACA-like domain of vertebrate telomerase RNAs is largely extended. The upper part of this hairpin has been shown to carry structural elements essential for TERT binding and, thereby, for telomerase function (39). In vitro and in vivo structure probing of the 5′ hairpin of hTR resulted in very similar modification patterns which, in general, conformed well to the previously predicted structure of hTR (8,9). The 5′ hairpin of hTR is composed of several stable, highly GC-rich helices which are separated by asymmetrical internal loops or bulged nucleotides (Fig. 3). Most of the bulged (C262, A285 and G345) or unpaired (A252–G254 and A289) nucleotides were readily detectable both in vitro and in vivo. The top of the hairpin is stabilised by a 5 bp stem and a tetraloop structure. Elements crucial for TERT binding are located in the A241–G270/C286–C330 segment of the hairpin and include the conserved CR4 and CR5 regions (39). In this region significant differences were observed in the in vitro and in vivo modification patterns of the RNA. As expected, residue C290 was accessible for DMS modification and nuclease S1 cleavage in vitro, but was fully protected in telomerase RNP (Fig. 5B). Since substitution of the A289–U291 region reduced, and deletion of the C266–U291 region fully abolished, telomerase activity (39), we propose that this internal loop may contribute to the binding of TERT or another important protein component of telomerase holoenzyme. Both in vitro and in vivo mappings confirmed that the CR4 and CR5 regions form a 9 bp helix with a bulged C residue at position 262. The middle part of the CR5 region (A302–U314) was proposed to form a short branched stem–loop (8,9), although phylogenetic comparision of vertebrate telomerase RNAs failed to detect sequence co-variation confirming an interaction between the A302–G305 and the C311–U314 regions (8). Indeed, neither in vitro nor in vivo mappings provided unambiguous evidence for the formation of this helix. More interestingly, residues G308, G309 and G310 in the putative terminal loop of this stem were fully protected against in vivo kethoxal treatment, although they were accessible in naked RNA (Fig. 5B). Since the G303–C313 region was found to be essential for TERT binding and telomerase activity (38), residues G308–G310 might directly interact with TERT. Since the G308–G310 sequence is not conserved in vertebrates, it seems that the local secondary structure rather than the primary sequence is crucial for TERT binding. Finally, the increased in vivo protection of nucleotides G250, G251 and G319 indicates the existence of a duplex formed between nucleotides C248–G251 and U316–G319. This helix is apparently not formed in vitro, as indicated by the high reactivity of U249, G250 and U316. Whether this in vivo structural rearrangement is a consequence of protein binding to this region remains unclear.
Figure 5.
Secondary structure analysis of the evolutionarily conserved CR4 (A) and CR5 (B) regions of hTR. For other details, see the legend to Figure 2.
Concluding remarks
In vitro and in vivo secondary structure probing of hTR confirmed the existence of many, but not all, of the fundamental structural elements of hTR proposed previously on the basis of a comparative analysis of 35 vertebrate telomerase RNAs (8). In human telomerase RNP the 5′-terminal template domain of hTR folds into a long hairpin structure, in which formation of the previously predicted P1, P2a1 and P2b helices was confirmed. The top of the helix is closed by a terminal stem–loop, which accommodates the conserved CR2 region. In marked contrast to the predicted structure of hTR (8), structure probing experiments failed to provide any support for in vivo formation of the evolutionarily conserved P2a helix. Even more surprisingly, no evidence supported formation of the long range ‘pseudoknot’ interaction of hTR. This suggests that the evolutionarily conserved and functionally essential pseudoknot structure is formed only temporarily in the telomerase holoenzyme. This dynamic conformational rearrangement of telomerase RNA might have an important effect on the function of telomerase RNP. Nucleotides in the template region of hTR were readily modified by chemical agents. This might indicate that a major portion of cellular telomerase RNA is not telomere bound in HeLa cells.
The in vivo modification pattern of Tetrahymena telomerase RNA suggested that the reverse transcriptase might bind adjacent to the pseudoknot structure of the RNA (15). Intriguingly, we also observed strong in vivo protections in close proximity to the pseudoknot of hTR (residues G185, G188, C192 and G193). However, whether this region of hTR is protected by associated hTERT requires further investigation. Likewise, the strong in vivo protection of residues C290 and G308–G310 in two functionally essential regions of the H/ACA-like domain may also reveal protein binding sites of hTR.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr W. Filipowicz for providing us with a recombinant plasmid carrying hTR. Our work was supported by grants from the Hungarian Research Foundation (OTKA, T29042 and T31738) and la Ligue Nationale Contre le Cancer.
REFERENCES
- 1.Blackburn E.H. (1999) Telomerase. In Gestelend,R.F., Cech,T. and Atkins,J.F. (eds), The RNA World. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 609–635.
- 2.Nugent C.I. and Lundblad,V. (1998) The telomerase reverse transcriptase: components and regulation. Genes Dev., 12, 1073–1085. [DOI] [PubMed] [Google Scholar]
- 3.Bryan T.M. and Cech,T.R. (1999) Telomerase and the maintenance of chromosome ends. Curr. Opin. Cell Biol., 11, 318–324. [DOI] [PubMed] [Google Scholar]
- 4.Romero D.P. and Blackburn,E.H. (1991) A conserved secondary structure for telomerase RNA. Cell, 67, 343–353. [DOI] [PubMed] [Google Scholar]
- 5.ten Dam E., van Belkum,A. and Pleij,K. (1991) A conserved pseudoknot in telomerase RNA. Nucleic Acids Res., 19, 6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lingner J., Hendrick,L.L. and Cech,T.R. (1994) Telomerase RNAs of different ciliates have a common secondary structure and a permuted template. Genes Dev., 8, 1984–1998. [DOI] [PubMed] [Google Scholar]
- 7.McCormick-Graham M. and Romero,D.P. (1995) Ciliate telomerase RNA structural features. Nucleic Acids Res., 23, 1091–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J.L., Blasco,M.A. and Greider,C.W. (2000) Secondary structure of vertebrate telonerase RNA. Cell, 100, 503–514. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell J.R., Cheng,J. and and Collins,K. (1999) A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3′ end. Mol. Cell. Biol., 19, 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrington L., McPhail,T., Mar,V., Zhou,W., Oulton,R., Bass,M.B., Arruda,I. and Robinson,M.O. (1997) A mammalian telomerase-associated protein. Science, 275, 973–977. [DOI] [PubMed] [Google Scholar]
- 11.Nakayama J., Saito,M., Nakamura,H., Matsuura,A. and Ishikawa,F. (1997) TLP1: a gene encoding a protein component of mammalian telomerase is a novel member of WD repeats family. Cell, 88, 875–884. [DOI] [PubMed] [Google Scholar]
- 12.Holt S.E., Aisner,D.L., Baur,J., Tesmer,V.M, Dy,M., Ouellette,M., Trager,J.B., Morin,G.B., Toft,D.O., Shay,J.W., Wright,W.E. and White,M.A. (1999) Functional requirements of p23 and Hsp90 in telomerase complex. Genes Dev., 13, 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pogacic V., Dragon,F. and Filipowicz,W. (2000) Human H/ACA small nucleolar RNPs and telomerase share evolutionarily conserved proteins NHP2 and NOP10. Mol. Cell. Biol., 20, 9028–9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le S., Sternglanz,R. and Greider,C.W. (2000) Identification of two RNA-binding proteins associated with human telomerase RNA. Mol. Biol. Cell, 11, 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaug A.J. and Cech,T.R. (1995) Analysis of the structure of Tetrahymena nuclear RNAs in vivo: telomerase RNA, the self-splicing rRNA intron and U2 snRNA. RNA, 1, 363–374. [PMC free article] [PubMed] [Google Scholar]
- 16.Bhattacharyya A. and Blackburn,E.H. (1994) Architecture of telomerase RNA. EMBO J., 13, 5721–5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balzer M. and Wagner,R. (1998) A chemical modification method for the structural analysis of RNA and RNA-protein complexes within living cells. Anal. Biochem., 256, 240–242. [DOI] [PubMed] [Google Scholar]
- 18.Chabot B. (1994) Synthesis and purification of RNA substrates. In Higgins,S.J. and Hames,B.D. (eds), RNA Processing. IRL Press, Oxford, UK, pp. 1–29.
- 19.Kiss T., Bortolin,M.L. and Filipowicz,W. (1996) Characterization of the intron-encoded U19 RNA, a new mammalian small nucleolar RNA that is not associated with fibrillarin. Mol. Cell. Biol., 16, 1391–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dragon F., Pogacic,V. and Filipowicz,W. (2000) In vitro assembly of human H/ACA small nucleolar RNPs reveals unique features of U17 and telomerase RNAs. Mol. Cell. Biol., 20, 3037–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.England T.E., Bruce,A.G. and Uhlenbeck,O.C. (1980) Specific labelling of 3′ termini of RNA with T4 RNA ligase. Methods Enzymol., 65, 65–74. [DOI] [PubMed] [Google Scholar]
- 22.Efstratiadis A., Vournakis,J.N., Donis-Keller,H., Chaconas,G., Dougall,D.K. and Kafatos,F.C. (1977) End labeling of enzymatically decapped mRNA. Nucleic Acids Res., 4, 4165–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiss T., Antal,M. and Solymosy,F. (1987) Plant small nuclear RNAs. II. U6 RNA and a 4.5SI-like RNA are present in plant nuclei. Nucleic Acids Res., 15, 543–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciesiolka J., Michalowski,D., Wrzesinski,J., Krajewski,J. and Krzyzosiak,W.J. (1998) Patterns of cleavages induced by lead ions in defined RNA secondary structure motifs. J. Mol. Biol., 275, 211–220. [DOI] [PubMed] [Google Scholar]
- 25.Inoue T. and Cech,T.R. (1985) Secondary structure of the circular form of the Tetrahymena rRNA intervening sequence: a technique for RNA structure analysis using chemical probes and reverse transcriptase. Proc. Natl Acad. Sci. USA, 82, 648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moazed D., Stern,S. and Noller,H,F. (1986) Rapid chemical probing of conformation in 16S ribosomal RNA and 30S ribosomal subunit using primer extension. J. Mol. Biol., 187, 399–416. [DOI] [PubMed] [Google Scholar]
- 27.Autexier C. and Greider,C.W. (1998) Mutational analysis of the Tetrahymena telomerase RNA: identification of residues affecting telomerase activity in vivo. Nucleic Acids Res., 26, 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Licht J.D. and Collins,K. (1999) Telomerase RNA function in recombinant Tetrahymena telomerase. Genes Dev., 13, 1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilley D. and Blackburn,E.H. (1999) The telomerase RNA pseudoknot is critical for the stable assembly of a catalytically active ribonucleoprotein. Proc. Natl Acad. Sci. USA, 96, 6621–6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Autexier C., Pruzan,R., Funk,W.D. and Greider,C.W. (1996) Reconstitution of human telomerase activity and identification of a minimal functional region of the human telomerase RNA. EMBO J., 15, 5928–5935. [PMC free article] [PubMed] [Google Scholar]
- 31.Beattie T.L., Zhou,W., Robinson,M.O. and Harrington,L. (1998) Reconstitution of human telomerase activity. Curr. Biol., 8, 177–180. [DOI] [PubMed] [Google Scholar]
- 32.Tesmer V.M., Ford,L.P., Holt,S.E., Frank,B.C., Yi,X., Aisner,D.L., Ouellette,M., Shay,J.W. and Wright,W.E. (1999) Two inactive fragments of the integral RNA cooperate to assembly active telomerase with the human protein catalytic subunit (hTERT) in vitro. Mol. Cell. Biol., 19, 6207–6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachand F. and Autexier,C. (2001) Functional regions of human telomerase reverse transcriptase and human telomerase RNA required for telomerase activity and RNA-protein interactions. Mol. Cell. Biol., 21, 1888–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martín-Rivera L. and Blasco,M.A. (2001) Identification of functional domains and dominant negative mutations in vertebrate telomerase RNA using an in vivo reconstitution system. J. Biol. Chem., 276, 5856–5865. [DOI] [PubMed] [Google Scholar]
- 35.Uhlenbeck O.C. (1995) Keeping RNA happy. RNA, 1, 4–6. [PMC free article] [PubMed] [Google Scholar]
- 36.Balakin A.G., Smith,L. and Fournier,M.J. (1996) The RNA world of the nucleolus: two major families of small RNAs defined by different box elements with related functions. Cell, 86, 823–834. [DOI] [PubMed] [Google Scholar]
- 37.Ganot P., Caizergues-Ferrer,M. and Kiss,T. (1997) A family of box ACA small nucleolar RNAs is defined by a evolutionarily conserved secondary structure and ubiquitous sequence elements essential for RNA accumulation. Genes Dev., 11, 941–956. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell J., Wood,E. and Collins,K. (1999) A telomerase component is defective in the human disease dyskeratosis congenita. Nature, 402, 551–555. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell J.R. and Collins,K. (2000) Human telomerase activation requires independent interactions between telomerase RNA and telomerase reverse transcriptase. Mol. Cell, 6, 361–371. [DOI] [PubMed] [Google Scholar]
- 40.Bortolin M.L., Ganot,P. and Kiss,T. (1999) Elements essential for accumulation of small nucleolar RNAs directing site-specific pseudouridylation of ribosomal RNAs. EMBO J., 18, 457–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narayanan A., Lukowiak,A., Jády,B.E., Dragon,F., Kiss,T., Terns,R.M. and Terns,M. (1999) Nucleolar localisation signals of box H/ACA small nucleolar RNAs. EMBO J., 18, 5120–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ni J., Tien,A.L. and Fournier,M.J. (1997) Small nucleolar RNAs direct site-specific synthesis of pseudouridine in ribosomal RNAs. Cell, 89, 565–573. [DOI] [PubMed] [Google Scholar]
- 43.Ganot P., Bortolin,M.L. and Kiss,T. (1997) Site-specific pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell, 89, 799–809. [DOI] [PubMed] [Google Scholar]
- 44.Jády B.E. and Kiss,T. (2001) A small nucleolar guide RNA functions both in 2′-_O_-methylation and pseudouridylation of the U5 spliceosomal RNA. EMBO J., 20, 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith C.M. and Steitz,J.A. (1997) Sno storm in the nucleolus: new roles for myriad of small RNPs. Cell, 89, 669–672. [DOI] [PubMed] [Google Scholar]
- 46.Kiss T. (2001) Small nucleolar RNA-guided post-transcriptional modification of cellular RNAs. EMBO J., 20, 3617–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]