The High Mobility Group Protein 1 Is a Coactivator of Herpes Simplex Virus ICP4 In Vitro (original) (raw)
Abstract
ICP4 is an activator of herpes simplex virus early and late gene transcription during infection and in vitro can efficiently activate the transcription of a core promoter template containing only a TATA box and an initiator element. In this study, we noted that the extent of activation by ICP4 in vitro was highly dependent on the purity of TFIID when recombinant TFIIB, TFIIE, and TFIIF were used as sources of these factors. ICP4 efficiently activated transcription with a crude TFIID fraction. However, when immunoaffinity-purified TFIID was used in place of the less pure TFIID, ICP4 activated transcription to a significantly lesser extent. This finding indicated that the crude TFIID fraction may contain additional factors that serve as coactivators of ICP4. To test this hypothesis, the crude TFIID preparation was further fractionated by gel filtration chromatography. The TFIID that eluted from the column lacked the hypothesized coactivator activity. A fraction well separated from TFIID contained an activity that when added with the TFIID fraction resulted in higher levels of transcription in the presence ICP4. Further purification of the coactivator-containing fraction resulted in the isolation of a single 30-kDa polypeptide (p30). p30 was also shown to serve as a coactivator of ICP4 with immunoaffinity-purified TFIID; however, p30 had no effect on basal transcription. Amino acid sequence analysis revealed that p30 was the high mobility group protein 1, which has been shown to facilitate the formation of higher-order DNA-protein complexes.
The herpes simplex virus (HSV) infected cell protein 4 (ICP4) is the major transcriptional activator of viral early and late gene expression (9, 16, 18, 19, 34, 42). This protein also serves as a transcriptional repressor of some HSV promoters, including the ICP4 gene promoter (9, 35). The 175-kDa ICP4 polypeptide exists as a dimeric nuclear phosphoprotein (8, 32, 39, 48) that binds to DNA (17) and possesses discrete functional domains (11, 37, 38, 46). Although the DNA-binding activity of ICP4 appears to be required for activation (38, 46), several studies have shown that specific binding sites are not required (4, 14, 15, 23, 50). How the functional domains of ICP4 collectively function to regulate the transcription of different HSV promoters is not completely understood.
All HSV genes are transcribed from promoters recognized by the RNA polymerase II (Pol II) transcription machinery (1, 5), which consists of at least seven general transcription factors (GTFs): TFIIA, -B, -D, -E, -F, and -H and Pol II itself (reviewed in reference 36). The GTFs form a preinitiation complex on class II promoters that enable Pol II to accurately initiate transcription (reviewed in reference 36). It has been shown that a core Pol II promoter from the glycoprotein C (gC) gene, containing only a TATA box and initiator element (Inr), can be efficiently activated by ICP4 in vitro (21). Although ICP4 can activate promoters containing only a TATA box, maximal levels of ICP4-activated transcription are seen when both a TATA box and Inr are present (7, 21), indicating that factors recognizing these elements are involved in ICP4-activated transcription.
The observation that ICP4 interacts with TFIID (2), and activates promoters with a TATA box possessing a low affinity for TATA box-binding protein (TBP) to a greater extent than promoters with a TATA box possessing a high affinity for TBP, suggests that ICP4 may facilitate TFIID binding to the promoter (6, 25). It has also been shown that activation of promoters by ICP4 is enhanced by the addition of an Inr element, both in vivo (7) and in vitro (21). Furthermore, ICP4 contacts TFIID through the TBP-associated factor TAF250 in a manner dependent on the C-terminal region of ICP4 (2, 10, 11), and this region of ICP4 is required for efficient activation of transcription (2, 10, 11). TAF250 is an integral part of the TFIID complex (3, 45, 55) and has been shown to interact with Inr elements (33, 52). How the interactions between TBP, TAF250, and their respective _cis_-acting sites in combination with ICP4 result in activation is not known. It is likely that additional cell factors that may function at start site regions and/or facilitate the formation of ICP4 containing transcription complexes are involved in activation.
In this study, we determined whether additional factors were involved in ICP4-activated transcription by observing the efficiency with which ICP4 activated transcription in vitro, using purified GTFs and ICP4 as well as HeLa transcription factor preparations differing in purity. Using this system, we purified the cellular high mobility group protein 1 (HMG 1) from a crude HeLa transcription factor preparation based on its ability to augment ICP4 activation.
MATERIALS AND METHODS
GTFs and ICP4.
Pol II and TFIID were obtained by fractionating HeLa nuclear extract sequentially on phosphocellulose and DEAE-Sephacel as previously described (12, 43). The DB fraction was further fractionated by Superose 6 gel filtration chromatography (Pharmacia) as described below, and TFIID-containing fractions were identified by slot blot analysis with a polyclonal antibody to TBP (Upstate Biotechnology). Hemagglutinin (HA)-tagged TFIID (_e_TFIID) from the HeLa cell line LTR α3 was immunoaffinity purified to homogeneity by using the anti-HA monoclonal antibody 12CA5 (Boehringer Mannheim) covalently coupled to protein A-Sepharose (57). Recombinant human TFIIB (rTFIIB) was purified from Escherichia coli according to the method of Ha et al. (22). The recombinant 34- and 56-kDa subunits of TFIIE were each purified from E. coli and the rTFIIE was reconstituted in vitro (40). The recombinant TFIIF subunits, RAP30 and RAP74, were each purified from E. coli and reconstituted to rTFIIF in vitro (53, 54).
ICP4 was purified from human embryonic lung fibroblast nuclei infected with the wild-type HSV strain KOS as previously described (24, 28).
HMG 1 was purified by first applying the HeLa fraction DB (3 mg of protein) to a 30-ml Superose 6 (Pharmacia) column in buffer D (20 mM HEPES [pH 7.9], 20% glycerol, 0.2 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol) with 0.1 M KCl. Fractions were assayed for the ability to enhance ICP4-activated transcription in an in vitro transcription assay reconstituted with purified basal transcription factors as described below. Fractions were also subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% gel) and silver stained. Fractions exhibiting the greatest enhancement of ICP4-activated transcription were pooled and applied to a 0.5-ml mini-Mono Q ion-exchange column (Pharmacia) in buffer D with 0.1 M KCl. The column was eluted with a 5-ml linear gradient from 0.1 to 0.5 M KCl in buffer D. Fractions were collected and dialyzed to 0.1 M KCl in buffer D. Fractions were assayed for enhancement of ICP4-activated transcription by in vitro transcription analysis as described below. A portion of the peak ICP4 activation-enhancing fraction was also subjected to SDS-PAGE (15% gel) and Coomassie blue stained. The 30-kDa polypeptide present in this fraction was subjected to amino-terminal sequence analysis by Edman degradation (University of Michigan protein and carbohydrate structure facility).
In vitro transcription analysis.
Transcription reactions were set up in a final volume of 30 μl containing 3 μl of fraction CC which contains Pol II, 0.3 μg of rTFIIB, 0.16 μg of reconstituted rTFIIF, 10.5 ng of rTFIIE p56, 6.43 ng of rTFIIE p34, 50 to 100 ng of ICP4, 10 fmol of template DNA, and either 1 μl of fraction DB, 4 μl of immunoaffinity-purified TFIID, or 8 μl of Superose 6-fractionated TFIID (called Superose TFIID). The amount of TFIID in each preparation was normalized according to the relative abundance of TBP present. This was determined by Western analysis using a polyclonal antibody directed against TBP. Superose 6 and Mono Q column fractions were assayed by adding 5 and 2 μl, respectively, from the column fractions to transcription reaction mixtures by using the Superose TFIID. The transcription template consisted of superhelical plasmid DNA containing the gC core promoter with either a wild-type (wt) or functionally mutant (mut) Inr. The mut Inr plasmid contains a linker scanning mutation changing positions +1 to +6 from ACTACC to GAGCTC.
Transcription reactions were performed in a solution containing 40 mM HEPES (pH 7.9), 60 mM KCl, 12% glycerol, 8.3 mM MgCl2, 0.6 mM ribonucleoside triphosphates, 12 U of RNasin, and 0.3 mM dithiothreitol. The reactions were incubated for 1 h at 30°C mixtures, and the reactions were stopped with 70 μl of transcription stop buffer containing 150 mM sodium acetate, 15 mM EDTA, and 150 μg of tRNA per ml. Samples were then phenol extracted, chloroform extracted, and ethanol precipitated.
For primer extension analysis, the pellets were resuspended in 10 μl of a primer annealing mixture containing 10 mM Tris-HCl (pH 7.5), 250 mM KCl, and 3 to 6 ng of 5′ 32P-end-labeled primer complementary to the nucleotides from +42 to +77 downstream of the transcription start site. The primer was annealed by heating at 65°C for 30 min and then slowly cooled to room temperature. The annealed transcripts were subjected to primer extension in a solution containing 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol, 1 mM deoxynucleoside triphosphates, 12 U of RNasin, 50 μg of actinomycin D per ml, and 300 U of Moloney murine leukemia virus reverse transcriptase in a final volume of 40 μl. Reaction mixtures were incubated at 42°C for 1 h, and the reactions were stopped by adding 60 μl of a solution containing 1 M ammonium acetate and 20 mM EDTA. The samples were phenol extracted and ethanol precipitated. The pellets were resuspended in 5 μl of gel loading buffer containing 96% formamide, 10 mM EDTA, 0.01% bromophenol blue and xylene cyanol, and 10 mM NaOH. The samples were separated on 6% sequencing gels, fixed, dried, and exposed to X-ray film.
RESULTS
Previous studies have shown that ICP4 efficiently activates transcription in vitro with crude HeLa transcription factor fractions on a simple promoter containing only a TATA box and an Inr (21). Mutation of the Inr impaired the ability of ICP4 to efficiently activate transcription. Therefore, we hypothesized that factors present in the HeLa transcription factor fractions and dependent on an intact start site region were serving as ICP4 coactivators. In this study, we demonstrated the presence of an ICP4 coactivator in a crude HeLa TFIID preparation and then proceeded to purify and identify the activity.
A crude TFIID preparation contains an ICP4 coactivator.
TFIID is routinely prepared by fractionating HeLa nuclear extract on phosphocellulose and DEAE-Sephacel (12, 43). This preparation is called the DB fraction. Although this fraction possesses TFIID activity, it remains in a relatively crude state. In this study, we first determined whether this fraction contained an ICP4 coactivator activity that is separable from TFIID. As depicted in Fig. 1, we performed an in vitro transcription experiment to compare the efficiencies of activation by ICP4 with immunoaffinity-purified TFIID versus the DB fraction. TFIIB, -E, and -F were added to all reaction mixtures as recombinant proteins purified from E. coli, while Pol II was added within HeLa CC fraction. The template used in this experiment was the gC core promoter template, which contains only a TATA box and either the wt or mut Inr. By using the wt and mut Inr templates to compare transcription efficiencies, we were able to assess the role of this site in basal and activated transcription as a function of the crude and purified TFIID preparation.
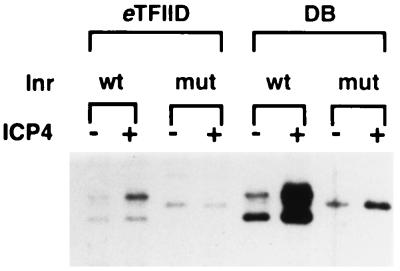
FIG. 1.
Abilities of different TFIID preparations to support ICP4-activated transcription. In vitro transcription reactions were performed with the Pol II fraction CC, rTFIIB, -E, and -F, and either immunoaffinity-purified _e_TFIID or the crude TFIID fraction, DB, in the absence and presence of purified ICP4. The quantity of each TFIID preparation was normalized as described in Materials and Methods. These conditions were tested on a gC core promoter template containing a TATA box and either a wt or a mut Inr. Shown are the primer extension products from reverse-transcribed RNA synthesized in the in vitro reactions.
With this set of GTFs, ICP4 activated transcription, albeit poorly, using immunoaffinity-purified TFIID (Fig. 1; compare lanes 1 and 2) in an Inr-dependent manner (compare lanes 1 and 2 with lanes 3 and 4). This mutation in the Inr also reduced the number of transcription start sites from three to one (compare lanes 1 and 3). With the DB fraction, ICP4 activated transcription four- to fivefold better than with purified TFIID (compare lanes 5 and 6 with lanes 1 and 2), also in an Inr-dependent manner (compare lanes 5 and 6 with lanes 7 and 8). These results indicate that a factor(s) present in DB allows ICP4 to activate more efficiently. In a previous report, we demonstrated efficient ICP4-activated transcription when immunoaffinity-purified TFIID was substituted for the DB TFIID fraction (2). However, in this study we used a relatively crude HeLa TFIIE and -F preparation in comparison to the purified recombinant TFIIE and -F used for Fig. 1. It is likely that this crude TFIIE and -F fraction also contains coactivators that contribute to ICP4’s ability to efficiently activate transcription. Also note that in lanes 5 and 6 it was not necessary to add TFIIA and -H to obtain efficient ICP4 activation. In the case of TFIIA, this was first observed in a previous study (21).
ICP4 also activated transcription on the mut Inr template using the DB fraction, although poorly in comparison to that seen on the wt template (Fig. 1; compare lanes 5 and 6 with lanes 7 and 8). This low level of activation is reflective of ICP4-activated transcription levels observed with the purified TFIID (compare lanes 1 and 2 with lanes 7 and 8), suggesting that a factor(s) not associated with TFIID but present in the DB fraction and dependent on the start site region serves as an ICP4 coactivator.
One study has demonstrated that this crude TFIID preparation contains activities that function through the Inr (28). It is believed that these activities allow TFIID to nucleate preinitiation complexes through the Inr. However, these factors are not associated with the TFIID multiprotein complex. The effect of this activity is evident in the experiment in Fig. 1. With the crude TFIID preparation, the Inr had a threefold effect on basal transcription (compare lane 5 with 7), indicating that a factor(s) in this fraction augmented basal transcription in an Inr-dependent manner. When purified TFIID was substituted for the DB TFIID preparation, this effect was not observed (compare lanes 1 and 3), indicating that the Inr factor(s) is not associated with TFIID.
Separation of TFIID from coactivator functions.
To clearly demonstrate that this coactivator and basal Inr factor were activities independent of TFIID, we further fractionated the DB fraction by gel filtration chromatography on Superose 6. Since TFIID is approximately 700 kDa, we reasoned that gel filtration would resolve TFIID from the many other proteins present in the DB preparation. The fractions containing TFIID were identified by slot blot analysis with an antibody directed against TBP. As expected, TFIID resolved early in the elution of the column. As shown in Fig. 2A, the Superose TFIID (lanes 5 and 6), like the immunoaffinity-purified TFIID (lanes 1 and 2), lacked the basal Inr activity that was apparent when the crude DB fraction was used (lanes 3 and 4). ICP4-activated transcription was also reduced with the Superose TFIID preparation (Fig. 2B, lanes 7 to 9), reflective of the levels of activation observed with immunoaffinity-purified TFIID (compare lanes 7 to 9 with lanes 1 to 3). The apparent lower level of activation seen with Superose TFIID relative to _e_TFIID is simply a consequence of the lower level of transcription with this preparation (lane 7). As expected, the DB fraction supported efficient ICP4-activated transcription (lanes 4 to 6). The experiments in Fig. 2 were performed with equivalent amounts of TFIID, based on TBP content as determined by Western analysis. These results indicate that the basal Inr and ICP4 coactivator activities are independent of TFIID, since TFIID fractionated on the basis of size lacks both of these activities.
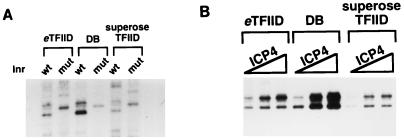
FIG. 2.
Purified TFIID lacks basal Inr activity and less efficiently supports ICP4 activation. The DB fraction was applied to a Superose 6 gel filtration column. The fractions containing TFIID were identified by slot blot analysis of column fractions with an antibody directed against TBP. The TFIID eluting from this column is designated Superose TFIID. In vitro transcriptions were performed with Pol II (CC), rTFIIB, -E, and -F, and either immunoaffinity-purified TFIID, DB fraction, or Superose TFIID. The quantity of each TFIID preparation was normalized as described in Materials and Methods. (A) Basal Inr activity of three different TFIID preparations. Each TFIID preparation was tested on the gC core promoter containing a TATA box and either a wt or a mut Inr. (B) ICP4-activated transcription using the three different TFIID preparations. Each reaction mixture received either 0, 50, or 100 ng of purified ICP4. Transcription reactions in lanes 1, 4, and 7 represent the basal levels of transcription with each of the three TFIID preparations.
It is clear that the more highly purified TFIID preparations were not as efficient in supporting activated transcription as the less purified DB preparation and that there may be proteins in the DB preparation that facilitate activation. To test this hypothesis, portions of each fraction from the Superose 6 column described above containing eluate subsequent to that containing TFIID were assayed for their effects on ICP4-activated transcription in vitro with the recombinant GTFs, Pol II, and TFIID obtained from the earlier-eluting fractions. Figure 3A shows the in vitro transcription results with fractions 23 to 28 of the 30 fractions tested. Peak enhancement of ICP4-activated transcription was apparent in fraction 25.
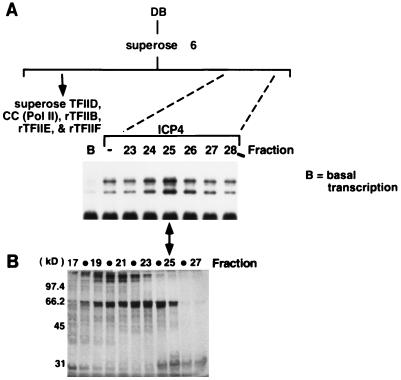
FIG. 3.
Gel filtration chromatography of the DB fraction further fractionates an ICP4 coactivator. (A) Transcription in the presence of ICP4 and gel filtration chromatography fractions. Superose 6 gel filtration fractions were assayed by adding a portion of each fraction to in vitro transcription reaction mixtures containing Pol II, rTFIIB, -E, and -F, Superose TFIID, and the wt gC core promoter template. ICP4 was included in each reaction except in lane B, which represents basal transcription levels with this combination of GTFs. The second lane indicates the level of ICP4-activated transcription without the addition of any column fractions. Only a subset (fractions 23 to 28) of the fractions tested are shown. A total of 30 fractions were collected from the column, with TFIID eluting in fraction 4. (B) Silver-stained gel after SDS-PAGE analysis of Superose 6 fractions. A portion of each fraction was loaded onto an SDS–10% polyacrylamide gel and silver stained. Shown are fractions 17 to 27.
The Superose 6 fractions were also subjected to SDS-PAGE and silver stained (Fig. 3B). Fraction 25 contained two polypeptides with apparent molecular masses of 66 and 30 kDa. Although reduced in abundance, these polypeptides and coactivator activity were also apparent in fraction 24. Fractions 24 and 25 were pooled and further fractionated by Mono Q ion-exchange chromatography. Forty fractions were collected, and aliquots of the flowthrough and gradient were analyzed by using the in vitro transcription assay described above. Shown in Fig. 4 are the results of the assay with the flowthrough and fractions 18 to 24. The enhancement of ICP4-activated transcription exhibited an elution profile from fractions 19 to 23, with the maximum appearing in fraction 23.
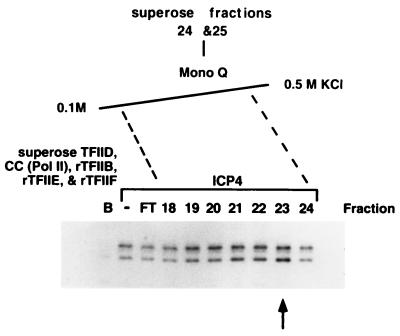
FIG. 4.
Ion-exchange chromatography of Superose 6 ICP4 coactivator fractions. Superose 6 fractions 24 and 25 were loaded onto a Mono Q ion-exchange column and eluted with a linear 0.1 M to 0.5 M KCl gradient. Fractions were assayed by adding a portion of each fraction to in vitro transcription reaction mixtures containing Pol II, rTFIIB, -E, and -F, Superose TFIID, and the wt gC core promoter template. ICP4 was included in each reaction except in lane B, which represents basal transcription levels with this combination of GTFs. The second lane indicates the level of ICP4 activated transcription without the addition of any column fractions. Flowthrough (FT) represents the material that did not bind the column at 0.1 M KCl. Shown are the results with fractions 18 to 24.
HMG 1 potentiates ICP4 activation with purified TFIID.
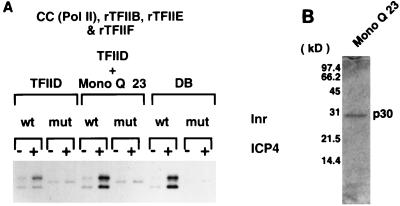
We next determined whether Mono Q fraction 23 exhibited coactivator activity with ICP4 and whether this fraction’s activity was dependent on a functional Inr using the immunoaffinity-purified TFIID. As shown in Fig. 5A, ICP4 poorly activated transcription using purified TFIID (lanes 1 and 2). The addition of Mono Q fraction 23 significantly enhanced the ability of ICP4 to activate transcription (lanes 5 and 6). This level of activation was comparable to that observed when DB was substituted for purified TFIID and Mono Q fraction 23 (compare lanes 5 and 6 with lanes 9 and 10). Mono Q fraction 23 did not affect basal transcription (lanes 1 and 5). ICP4-activated transcription with the Mono Q fraction 23 was also dependent on a functional Inr (compare lanes 5 and 6 with lanes 7 and 8). This was also reflective of the Inr-dependent nature of ICP4 activation with DB (compare lanes 9 and 10 with lanes 11 and 12). However, this fraction did not display any Inr activity under basal transcription conditions (compare lanes 5 and 7), suggesting that the Inr activity present in DB may have eluted elsewhere in either the Superose 6 or Mono Q column. Thus, Mono Q fraction 23 contains an activity that helps ICP4 activate transcription dependent on an intact Inr sequence and does not affect basal transcription.
FIG. 5.
A 30-kDa polypeptide possesses ICP4 coactivator activity. (A) In vitro transcription analysis of Mono Q fraction 23 with immunoaffinity-purified TFIID. In vitro transcription reactions were performed with Pol II, rTFIIB, -E, and -F, and either immunoaffinity-purified _e_TFIID or the crude TFIID fraction, DB, in the absence and presence of purified ICP4 and Mono Q fraction 23. The quantity of each TFIID preparation was normalized as described in Materials and Methods. Each condition was tested on a gC core promoter template containing a TATA box and either a wt or a mut Inr. (B) SDS-PAGE analysis of Mono Q fraction 23. A portion of Mono Q fraction 23 was loaded onto an SDS–15% polyacrylamide gel and stained with Coomassie blue. No additional bands were observed in this preparation when an overloaded gel was stained with silver (data not shown).
SDS-PAGE analysis of Mono Q fraction 23 revealed the presence of a single polypeptide with an apparent molecular mass of 30 kDa, designated p30 (Fig. 5B). p30 did not interact with ICP4 in an immunoaffinity assay using an HA-tagged ICP4 and the anti-HA monoclonal antibody 12CA5 (data not shown). Amino-terminal sequence analysis through 39 residues revealed that the sequence of p30 identically matched the amino-terminal sequence of HMG 1 (Fig. 6). Therefore, HMG 1 can serve as a coactivator of ICP4 in the presence of an intact Inr sequence.
FIG. 6.
p30 is HMG 1. p30 (Mono Q fraction 23) was subjected to amino-terminal sequence analysis through the first 39 amino acids. Shown is an amino acid sequence alignment comparing HMG 1 and p30.
DISCUSSION
In this study, we showed that the HSV activator ICP4 can activate transcription from a core promoter element by using a relatively simple set of cellular transcription factors. The level of activation was lower than that observed with a less pure set of factors and was dependent on a functional Inr element. This implied that there were proteins present in the less pure system that helped ICP4 activate transcription. One such protein was identified when the crude TFIID-containing fraction (DB) was substituted for immunoaffinity-purified TFIID in the transcription system. This resulted in substantially higher levels of activation, indicating that coactivators in addition to TAFs were present in this fraction and were enhancing ICP4-activated transcription. With further fractionation of the crude TFIID-containing fraction, it was possible to separate TFIID from an activity that enhanced ICP4 activation. This activity was purified to homogeneity based on the presence of a single 30-kDa protein on SDS-PAGE profiles. This protein was shown to enhance ICP4 activation by using the purest set of transcription factors, which had previously supported only low levels of activation. Amino-terminal sequence analysis of the 30-kDa polypeptide identified it as HMG 1. Thus, we concluded that HMG 1 can serve as a coactivator of ICP4.
HMG 1 is member of a ubiquitous family of nonhistone chromosomal proteins that share a DNA-binding region known as the HMG domain (reviewed in reference 20). HMG 1 belongs to the HMG 1/2 subclass that characteristically contains multiple HMG domains and binds DNA nonspecifically (reviewed in reference 20). The other subclass of HMG domain proteins contains only one HMG domain and binds DNA more specifically. HMG 1 has been shown to facilitate the formation of higher-order nucleoprotein complexes, which is believed to be the result of a DNA-bending activity shared by all HMG proteins. The role of HMG 1 as a coactivator, as reported here, is not unprecedented. HMG 1 has been shown to enhance Gal4-VP16-mediated activation in vitro (49) and p53 activation in transient transfection experiments (26). Thus, it has been hypothesized that HMG 1/2 proteins, through DNA bending, remodel promoter DNA conformation such that the interactions between activators and GTFs occur more efficiently (49).
A model for ICP4-activated transcription.
While many HSV promoters are considerably more complex than the core gC promoter, and many do not contain a strong match to the consensus Inr element, the studies described in this and previous reports (7, 21) allow us to propose a mechanism for how ICP4 may function to activate a relatively simple promoter. In an earlier study, we demonstrated that ICP4 interacts with TFIID through TAF250 and that this interaction is dependent on the C-terminal region of ICP4 (2). ICP4 did not interact with any other GTFs in that study (2). Additionally, it has been shown that the ICP4 C-terminal region is important for activated transcription both in vitro and in vivo (2, 10, 11). Since TFIID is the only GTF present in the simplified transcription system used in the present study shown to interact with ICP4, we believe that the weak but reproducible level of activation is due to the interaction between ICP4 and TAF250. Interestingly, this weak activation was dependent on a functional Inr sequence (21), although Inr activity was not observed for basal transcription. Therefore, ICP4 may exploit the Inr for activation by a mechanism different from that utilized by the cell for basal transcription.
How the cell utilizes the Inr element is unclear, and utilization may occur by several mechanisms, involving a variety of cellular factors (13, 27, 30, 31, 41, 44, 51, 56, 57), one of which may be TAF250 (33, 52). From DNase I footprinting and photo-cross-linking experiments, TAF250 has been shown to contact the Inr region (33, 52). Furthermore, in Drosophila, TAF250 along with Drosophila TAF150 (dTAF150) has been shown to be required for TFIID Inr-directed transcription (52). Unlike dTAF150, the human homolog of dTAF150, known as CIF150, is not a stable component of TFIID (28, 29). In the absence of TAF150/CIF150, TFIID Inr-directed transcription does not occur. Thus, one hypothesis is that TAF150/CIF150, although not recognizing the Inr directly, somehow stabilizes TAF250 recognition of the Inr and thereby promotes TFIID Inr-directed transcription (29). Similarly, one hypothesis for Inr-dependent ICP4 activation in the simplified transcription system is that ICP4 through its interaction with TAF250 allows TFIID to more efficiently bind and/or operate at the promoter through the Inr. By this scenario, ICP4 would be functioning like TAF150/CIF150, which would explain the lack of Inr function in this system in the absence of ICP4. Whether the addition of Inr-facilitating functions provided by molecules such as TAF150/CIF150 further augments the activation function of ICP4 is an open question that is currently under investigation.
As described above, HMG 1 was shown to enhance activation by ICP4 and has also been shown to serve as a coactivator in other studies (26, 49). These studies proposed that HMG 1, through its DNA-bending activity, facilitates multiprotein complex formation on DNA by remodeling the promoter conformation. A change in the DNA conformation induced by HMG 1 may allow ICP4 to more efficiently interact with TFIID through TAF250 and possibly other transcription factors. Facilitation of the ICP4-TFIID interaction by HMG 1 may enhance TFIID binding or further stabilize TFIID on the TATA box and Inr. This, in turn, would lead to increased preinitiation complex formation and increased synthesis of RNA.
ACKNOWLEDGMENTS
We thank Patricia Bates, Benoit Grondin, and William Hobbs for helpful discussions and comments on the manuscript.
This work was supported by NIH grant AI30612.
REFERENCES
- 1.Alwine J, Steinhart W L, Hill C W. Transcription of herpes simplex type 1 DNA in nuclei isolated from infected Hep-2 and KB cells. Virology. 1974;60:302–307. doi: 10.1016/0042-6822(74)90390-0. [DOI] [PubMed] [Google Scholar]
- 2.Carrozza M J, DeLuca N A. Interaction of the viral activator protein ICP4 with TFIID through TAF250. Mol Cell Biol. 1996;16:3085–3093. doi: 10.1128/mcb.16.6.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J-L, Donatella Attardi L, Verrijzer C P, Yokomori K, Tjian R. Assembly of recombinant TFIID reveals differential coactivator requirements for distinct transcriptional activators. Cell. 1994;79:93–105. doi: 10.1016/0092-8674(94)90403-0. [DOI] [PubMed] [Google Scholar]
- 4.Coen D M, Weinheimer S P, McKnight S L. A genetic approach to promoter recognition during trans induction of viral gene expression. Science. 1986;234:53–59. doi: 10.1126/science.3018926. [DOI] [PubMed] [Google Scholar]
- 5.Constanzo F, Campadelli-Fiume G, Foa-Tomasi L, Cassai E. Evidence that herpes simplex virus DNA is transcribed by cellular RNA polymerase B. J Virol. 1977;21:996–1001. doi: 10.1128/jvi.21.3.996-1001.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook W J, Gu B, DeLuca N A, Moynihan E B, Coen D M. Induction of transcription by a viral regulatory protein depends on the relative strengths of functional TATA boxes. Mol Cell Biol. 1995;15:4998–5006. doi: 10.1128/mcb.15.9.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook W J, Lin S M, DeLuca N, Coen D M. Initiator elements and regulated expression of the thymidine kinase gene. J Virol. 1995;69:7291–7294. doi: 10.1128/jvi.69.11.7291-7294.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courtney R J, Benyesh-Melnick M. Isolation and characterization of a large molecular weight polypeptide of herpes simplex virus type 1. Virology. 1974;62:539–551. doi: 10.1016/0042-6822(74)90414-0. [DOI] [PubMed] [Google Scholar]
- 9.DeLuca N A, Schaffer P A. Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Mol Cell Biol. 1985;5:1997–2008. doi: 10.1128/mcb.5.8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLuca N A, Schaffer P A. Activities of herpes simplex virus type 1 (HSV-1) ICP4 genes specifying nonsense peptides. Nucleic Acids Res. 1987;15:4491–4511. doi: 10.1093/nar/15.11.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLuca N A, Schaffer P A. Physical and functional domains of the herpes simplex virus transcriptional regulatory protein ICP4. J Virol. 1988;62:732–743. doi: 10.1128/jvi.62.3.732-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Digman J D, Lebovitz R M, Roeder R G. Accurate transcription by RNA polymerase II in soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du H, Roy A L, Roeder R G. Human transcription factor USF stimulates transcription through the initiator elements of the HIV-1 and the AdML promoters. EMBO J. 1993;12:501–511. doi: 10.1002/j.1460-2075.1993.tb05682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenberg S P, Coen D M, Mcknight S L. Promoter domains required for expression of plasmid-borne copies of the herpes simplex virus thymidine kinase gene in virus-infected mouse fibroblasts and microinfected frog oocytes. Mol Cell Biol. 1985;5:1940–1947. doi: 10.1128/mcb.5.8.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everett R D. A detailed analysis of an HSV-1 early promoter: sequences involved in trans-activation by viral immediate-early gene products are not early gene specific. Nucleic Acids Res. 1984;12:3037–3056. doi: 10.1093/nar/12.7.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everett R D. Transactivation of transcription by herpes virus product: requirement for two HSV-1 immediate early polypeptides for maximum activity. EMBO J. 1984;3:3135–3141. doi: 10.1002/j.1460-2075.1984.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faber S W, Wilcox K W. Association of the herpes simplex virus regulatory protein ICP4 with specific nucleotide sequences. Nucleic Acids Res. 1986;14:6067–6083. doi: 10.1093/nar/14.15.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelman I H, Silverstein S. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc Natl Acad Sci USA. 1985;82:5265–5269. doi: 10.1073/pnas.82.16.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godowski P J, Knipe D M. Transcriptional control of herpes virus gene expression: gene functions required for positive and negative regulation. Proc Natl Acad Sci USA. 1986;83:256–260. doi: 10.1073/pnas.83.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosschedl R, Giese K, Pagel J. HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet. 1994;10:94–100. doi: 10.1016/0168-9525(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 21.Gu B, Deluca N. Requirements for activation of the herpes simplex virus glycoprotein C promoter in vitro by the viral regulatory protein ICP4. J Virol. 1994;68:7953–7965. doi: 10.1128/jvi.68.12.7953-7965.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ha I, Lane W S, Reinberg D. Cloning of a human gene encoding the general transcription initiation factor IIB. Nature. 1991;532:689–695. doi: 10.1038/352689a0. [DOI] [PubMed] [Google Scholar]
- 23.Halpern M E, Smiley J R. Effects of deletions on expression of the herpes simplex virus thymidine kinase gene from the intact viral genome: the amino terminus of the enzyme is dispensable for catalytic activity. J Virol. 1984;50:733–738. doi: 10.1128/jvi.50.3.733-738.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imbalzano A N, Shepard A A, DeLuca N A. Functional relevance of specific interactions between herpes simplex virus type 1 ICP4 and sequences from the promoter-regulatory domain of the viral thymidine kinase gene. J Virol. 1990;64:2620–2631. doi: 10.1128/jvi.64.6.2620-2631.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imbalzano A N, DeLuca N A. Substitution of a TATA box from a herpes simplex virus late gene in the viral thymidine kinase promoter alters ICP4 inducibility but not temporal expression. J Virol. 1992;66:5453–5463. doi: 10.1128/jvi.66.9.5453-5463.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jayaraman L, Moorthy N C, Murthy K G K, Manley J L, Bustin M, Prives C. High mobility group protein-1 (HMG-1) is a unique activator of p53. Genes Dev. 1998;12:462–472. doi: 10.1101/gad.12.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufmann J, Smale S T. Direct recognition of initiator elements by a component of the transcription factor IID complex. Genes Dev. 1994;8:821–829. doi: 10.1101/gad.8.7.821. [DOI] [PubMed] [Google Scholar]
- 28.Kaufmann J, Verrijzer C P, Shao J, Smale S T. CIF, an essential cofactor for TFIID-dependent initiator function. Genes Dev. 1996;10:873–886. doi: 10.1101/gad.10.7.873. [DOI] [PubMed] [Google Scholar]
- 29.Kaufmann J, Ahrens K, Koop R, Smale S T, Muller R. CIF150, a human cofactor for transcription factor IID-dependent initiator function. Mol Cell Biol. 1998;18:233–239. doi: 10.1128/mcb.18.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez E, Chiang C-M, Gui H, Roeder R G. TATA-binding protein-associated factors in TFIID function through the initiator to direct basal transcription from a TATA-less class II promoter. EMBO J. 1994;13:3115–3126. doi: 10.1002/j.1460-2075.1994.tb06610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Means A L, Slansky J E, McMahon S L, Knuth M W, Farnham P J. The HIP-1 binding site is required for growth regulation of the dihydrofolate reductase gene promoter. Mol Cell Biol. 1992;12:1054–1063. doi: 10.1128/mcb.12.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metzler D W, Wilcox K W. Isolation of herpes simplex virus regulatory protein ICP4 as a homodimeric complex. J Virol. 1985;55:329–337. doi: 10.1128/jvi.55.2.329-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oelgeschlager T, Chiang C-M, Roeder R G. Topology and reorganization of a human TFIID-promoter complex. Nature. 1996;382:735–738. doi: 10.1038/382735a0. [DOI] [PubMed] [Google Scholar]
- 34.O’Hare P, Hayward G S. Evidence for a direct role for both the 175,000- and 110,000-molecular-weight immediate-early proteins of herpes simplex virus in the transactivation of delayed-early promoters. J Virol. 1985;53:751–760. doi: 10.1128/jvi.53.3.751-760.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Hare P, Hayward G S. Three trans-acting regulatory proteins of herpes simplex virus modulate immediate-early gene expression in a pathway involving positive and negative feedback regulation. J Virol. 1985;56:723–733. doi: 10.1128/jvi.56.3.723-733.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orphanides G, Lagrange T, Reinberg D. The general transcription factors of RNA polymerase II. Genes Dev. 1996;10:2657–2683. doi: 10.1101/gad.10.21.2657. [DOI] [PubMed] [Google Scholar]
- 37.Paterson T, Everett R D. Mutational dissection of the HSV-1 immediate-early protein Vmw175 involved in transcriptional transactivation and repression. Virology. 1988;166:186–196. doi: 10.1016/0042-6822(88)90160-2. [DOI] [PubMed] [Google Scholar]
- 38.Paterson T, Everett R D. The regions of the herpes simplex virus type 1 immediate early protein Vmw175 required for site specific DNA binding closely correspond to those involved in transcriptional regulation. Nucleic Acids Res. 1990;16:11005–11025. doi: 10.1093/nar/16.23.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira L, Wolff M H, Fenwick M, Roizman B. Regulation of herpes virus macromolecular synthesis. V. Properties of a polypeptide made in HSV-1 and HSV-2 infected cells. Virology. 1977;77:733–749. doi: 10.1016/0042-6822(77)90495-0. [DOI] [PubMed] [Google Scholar]
- 40.Peterson M G, Inostroza J, Maxon M E, Flores O, Admon A, Reinberg D, Tjian R. Structure and functional properties of human general transcription factor IIE. Nature. 1991;354:369–373. doi: 10.1038/354369a0. [DOI] [PubMed] [Google Scholar]
- 41.Purnell B A, Emanuel P A, Gilmour D S. TFIID sequence recognition of the initiator and sequences farther downstream in Drosophila class II genes. Genes Dev. 1994;8:830–842. doi: 10.1101/gad.8.7.830. [DOI] [PubMed] [Google Scholar]
- 42.Quinlan M, Knipe D. Stimulation of expression of a herpes simplex virus DNA-binding protein by two viral functions. Mol Cell Biol. 1985;5:957–963. doi: 10.1128/mcb.5.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinberg D, Roeder R. Factors involved in specific transcription by mammalian RNA polymerase II: purification and functional analysis of initiation factors IIB and IIE. J Biol Chem. 1987;262:3310–3321. [PubMed] [Google Scholar]
- 44.Roy A L, Malik S, Meisterernst M, Roeder R G. An alternative pathway for transcription initiation involving TFII-I. Nature. 1993;365:355–359. doi: 10.1038/365355a0. [DOI] [PubMed] [Google Scholar]
- 45.Ruppert S, Wang E H, Tjian R. Cloning and expression of human TAFII250: a TBP-associated factor implicated in cell-cycle regulation. Nature. 1993;362:175–179. doi: 10.1038/362175a0. [DOI] [PubMed] [Google Scholar]
- 46.Shepard A A, Imbalzano A N, DeLuca N A. Separation of primary structural components conferring autoregulation, transactivation, and DNA-binding properties to the herpes simplex virus transcriptional regulatory protein ICP4. J Virol. 1989;63:3714–3728. doi: 10.1128/jvi.63.9.3714-3728.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shepard A A, DeLuca N A. Activities of heterodimers composed of DNA-binding- and transactivation-deficient subunits of the herpes simplex virus regulatory protein ICP4. J Virol. 1991;65:299–307. doi: 10.1128/jvi.65.1.299-307.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shepard A A, Tolentino P, DeLuca N A. Transdominant inhibition of the herpes simplex virus transcriptional regulatory protein ICP4 by heterocomplex formation. J Virol. 1990;64:3916–3926. doi: 10.1128/jvi.64.8.3916-3926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shykind B M, Kim J, Sharp P A. Activation of the TFIID-TFIIA complex with HMG-2. Genes Dev. 1995;9:1354–1365. doi: 10.1101/gad.9.11.1354. [DOI] [PubMed] [Google Scholar]
- 50.Smiley J R, Johnson D C, Pizer L I, Everett R D. The ICP4 binding sites in the herpes simplex virus type 1 glycoprotein (gD) promoter are not essential for efficient gD transcription during virus infection. J Virol. 1992;66:623–631. doi: 10.1128/jvi.66.2.623-631.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Usheva A, Shenk T. TATA-binding protein independent initiation: YY1, TFIIB and RNA polymerase II direct basal transcription on supercoiled template DNA. Cell. 1994;76:1115–1121. doi: 10.1016/0092-8674(94)90387-5. [DOI] [PubMed] [Google Scholar]
- 52.Verrijzer C P, Chen J-L, Yokomori K, Tjian R. Binding of TAFs to core elements directs promoter selectivity by RNA polymerase II. Cell. 1995;81:1115–1125. doi: 10.1016/s0092-8674(05)80016-9. [DOI] [PubMed] [Google Scholar]
- 53.Wang B Q, Kostrub C F, Finkelstein A, Burton Z F. Production of human RAP30 and RAP74 in bacterial cells. Protein Expr Purif. 1993;4:207–214. doi: 10.1006/prep.1993.1027. [DOI] [PubMed] [Google Scholar]
- 54.Wang B Q, Lei L, Burton Z F. Importance of codon preference for production of human RAP74 and reconstitution of the RAP30/74 complex. Protein Expr Purif. 1994;5:476–485. doi: 10.1006/prep.1994.1067. [DOI] [PubMed] [Google Scholar]
- 55.Weinzierl R O J, Dynlacht B D, Tjian R. Largest subunit of Drosophila transcription factor IID directs assembly of a complex containing TBP and a coactivator. Nature. 1993;362:511–517. doi: 10.1038/362511a0. [DOI] [PubMed] [Google Scholar]
- 56.Weis L, Reinberg D. Accurate positioning of RNA polymerase II on a natural TATA-less promoter is independent of TATA-binding protein-associated factors and initiator-binding proteins. Mol Cell Biol. 1997;17:2973–2984. doi: 10.1128/mcb.17.6.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Q, Leiberman P M, Boyer T G, Berk A J. Holo-TFIID supports transcriptional stimulation by diverse activators and from a TATA-less promoter. Genes Dev. 1992;6:1964–1974. doi: 10.1101/gad.6.10.1964. [DOI] [PubMed] [Google Scholar]