Binding of Sindbis Virus to Cell Surface Heparan Sulfate (original) (raw)
Abstract
Alphaviruses are arthropod-borne viruses with wide species ranges and diverse tissue tropisms. The cell surface receptors which allow infection of so many different species and cell types are still incompletely characterized. We show here that the widely expressed glycosaminoglycan heparan sulfate can participate in the binding of Sindbis virus to cells. Enzymatic removal of heparan sulfate or the use of heparan sulfate-deficient cells led to a large reduction in virus binding. Sindbis virus bound to immobilized heparin, and this interaction was blocked by neutralizing antibodies against the viral E2 glycoprotein. Further experiments showed that a high degree of sulfation was critical for the ability of heparin to bind Sindbis virus. However, Sindbis virus was still able to infect and replicate on cells which were completely deficient in heparan sulfate, indicating that additional receptors must be involved. Cell surface binding of another alphavirus, Ross River virus, was found to be independent of heparan sulfate.
The alphaviruses belong to a genus of enveloped RNA viruses which can replicate in insects, birds, and mammals, including humans (60). They have a wide geographic distribution and pose a serious threat to human health in certain regions, causing fever, rash, arthralgia, myalgia, and fatal encephalitis. In mammals, some alphaviruses have tropisms for specific cell types such as muscle, neurons, and lymphatic cells. A better knowledge of the cellular receptors used by alphaviruses would have obvious implications for understanding of the different cellular tropisms and pathogeneses of these viruses, as well as applications to the design of safe live-attenuated vaccines.
Alphavirus virions have a simple structure, with a single strand of positive-sense RNA enclosed in an icosahedral capsid, which is surrounded by a lipid envelope derived from the host plasma membrane. The envelope contains two viral glycoproteins, E1 and E2, which are organized in spikes. During the initiation of infection, E2 is mainly responsible for binding to cellular receptors. Following endocytosis, a low-pH-dependent rearrangement of the glycoproteins occurs, triggering the membrane fusion activity of E1 and allowing entry of the capsid into the cytoplasm.
Alphaviruses cycle alternately between vertebrates and hematophagous insects (usually mosquitoes), suggesting either that virions bind to receptors that are highly conserved between species or that the virus can use multiple receptors. A previous study has identified a role for the 67-kDa high-affinity laminin receptor in binding of Sindbis virus (SV) to rodent and monkey cells but not to avian cells (68). Another study using Venezuelan equine encephalitis (VEE) virus identified a 32-kDa receptor in mosquito cells which also appears to be a laminin receptor (34). Other studies have identified unknown 74- and 110-kDa proteins as possible receptors for SV on mouse neuroblastoma cells (66) and a 63-kDa protein on chicken cells (69).
The normal in vivo role of glycosaminoglycans (GAGs) is to bind a diverse group of growth factors, chemokines, enzymes, and matrix components (20). In addition, however, these carbohydrates are important in the cell surface binding of a number of bacteria, parasites, and viruses (52). GAGs are unbranched polysaccharides present ubiquitously on cell surfaces and in the extracellular matrix and are usually found covalently attached to core proteins (proteoglycans) (31, 61). Some common types of GAG include heparan sulfate (HS), chondroitin sulfate (CS), dermatan sulfate, and keratan sulfate. GAGs acquire a net negative charge through N and O sulfation, and GAG-binding domains of proteins are typically positively charged regions containing arginine and lysine. Importantly, GAGs are found in a wide variety of vertebrate and invertebrate species, including insects (7).
The first virus found to bind HS was herpes simplex virus (HSV) (70), and since then a number of other herpesviruses have been demonstrated to use HS as an initial receptor (43, 45, 58). In addition, recent work has shown that HS is also involved in the binding of human immunodeficiency virus type 1, foot-and-mouth disease virus, respiratory syncytial virus, dengue virus, and adeno-associated virus type 2 (9, 21, 32, 47, 62). It should be noted, however, that additional receptors besides HS are involved in binding and entry of many, perhaps all, of these viruses.
There is suggestive evidence that GAGs may be involved in the binding of alphaviruses to cells. Binding appears to involve electrostatic interactions between ionizable groups on the virus and the cell membrane—it is highly dependent on the pH of the medium, and binding is reduced in medium of elevated ionic strength (15, 18, 34, 37, 38, 49). Studies have also shown that polyanions, including sulfated polysaccharides such as heparin, can influence binding of alphaviruses. When present during viral absorption, polyanions reduce the number of plaques formed in plaque assays (40), and pretreatment of certain cells with heparin can increase binding of SV (63). A sulfated polysaccharide contained in agar has been known for many years to inhibit the growth and decrease the plaque size of alphaviruses (4, 11, 57). Finally, the finding that treatment of cells with heparinase reduces the plaque-forming efficiency of SV (40) directly suggests that SV might bind to HS.
MATERIALS AND METHODS
Chemicals and antibodies.
The following were obtained from Sigma (St. Louis, Mo.): heparin (183 U/mg, from porcine intestinal mucosa), chondroitin sulfate A (CS-A) (bovine trachea), CS-B (porcine skin), CS-C (shark cartilage), dextran (molecular weight, 500,000), heparinase I (EC 4.2.2.7), and chondroitinase ABC (EC 4.2.2.4; affinity-purified). Dextran sulfate (17% S) and DEAE-dextran were obtained from Pharmacia (Piscataway, N.J.). The following were obtained from Seikagaku America Inc. (Rockville, Md.): HS (bovine kidney; 5.0 to 6.0% S), N-desulfated, N-acetylated heparin (<0.2% NS, >8.0% S); completely desulfated N-acetylated heparin (<0.1% NS, <1.5% S); completely desulfated N-sulfated heparin (>4.5% NS, 4.5 to 7.0% S).
Monoclonal antibodies were obtained as ascites from BALB/c mice. The following monoclonal antibodies were used: 202 immunoglobulin G3 (IgG3) against SV E2 epitope ab (42), R6 IgG2a against SV E2 epitope c (46), and the control antibody 3E1, an IgG1 recognizing HSV (HB-8067, from the American Type Culture Collection [Manassas, Va.]). IgG was purified from ascites with the Pierce T-Gel purification kit. Purified IgG was stored in frozen aliquots until use. Fab fragments were prepared by papain digestion with the Pierce Immunopure Fab preparation kit and were separated from Fc and undigested IgG with a protein A-Sepharose column. Concentrations of antibody were determined by optical densitometry at 280 nm.
Viruses and cells.
Chinese hamster ovary (CHO-K1) cells (CCL-61) and the GAG-deficient CHO derivatives pgsA-745 (CRL-2242), pgsD-677 (CRL-2244), and pgsE-606 (CRL-2246) (2, 13, 33) were obtained from the American Type Culture Collection and grown in Ham’s F-12 medium supplemented with 10% fetal calf serum and 50 μg of gentamicin per ml. BHK-21 cells were grown in Dulbecco’s modified Eagle medium with the same supplements. SV strain Toto 1101 (51) and Ross River virus (RRV) strain T48 (27) were grown and titered on BHK-21 cells.
For 35S-labeled viral stocks, BHK cells were infected for 1 h at a multiplicity of infection of approximately 2. Three hours later, cells were rinsed and the medium was replaced with Met–Cys-free Dulbecco’s modified Eagle medium containing 1% fetal calf serum and 35 μCi of [35S]Met-Cys per ml. Supernatant fluid was collected at 24 h postinfection and clarified by centrifugation. A one-third volume of 40% polyethylene glycol 8000 in 2 M NaCl was added, and the mixture was rocked overnight at 4°C. Virus was precipitated by centrifugation at 18,000 × g for 1 h and resuspended in a small volume of phosphate-buffered saline (PBS). Virus was applied to the top of a linear 15 to 40% (wt/vol) potassium tartrate gradient in PBS and centrifuged for 1.5 h at 190,000 × g. The viral band was collected and pelleted by centrifugation through a 15% sucrose cushion for 30 min at 240,000 × g. Virus was resuspended in a small volume of binding buffer: PBS (pH 7.2) supplemented with 0.5 mM MgCl2, 0.5 mM CaCl2 and 0.5% bovine serum albumin (BSA). Virus was stored in aliquots at −70°C. The counts per minute (cpm)/PFU ratio for SV Toto 1101 was 9.0 × 10−4. For RRV T48, the ratio was 3.8 × 10−3.
Plaque assays.
Virus was diluted in PBS supplemented with 0.5 mM MgCl2 and 0.5 mM CaCl2. Polyanion inhibitors were added as noted in Results. The medium was removed from confluent BHK monolayers in 35-mm wells, and 200 μl of virus was added. Cells were infected for 1 h in a humidified 5% CO2 atmosphere at 37°C, with occasional rocking. Cells were then overlaid with warm modified Eagle medium containing 0.6% Bacto Agar (Difco, Detroit, Mich.) and 1% fetal calf serum and lacking phenol red. Plaques were stained at 2 days with neutral red. When CHO cells (or their derivatives) were used for plaque assays, agarose was used instead of agar (to increase plaque diameter), and the overlay was supplemented with nonessential amino acids.
Cell-binding assay.
Cells were plated at 4 × 105 per well in 12-well plates and used the following day. Medium was removed from the cells, which were then rinsed twice on ice with ice-cold binding buffer (PBS [pH 7.2], 0.5 mM MgCl2, 0.5 mM CaCl2, 0.5% BSA). Approximately 104 cpm of 35S-labeled virus was added to each well in 150 μl of binding buffer, and plates were rocked at 4°C for varying lengths of time. Virus was removed and monolayers were rapidly rinsed twice with ice-cold binding buffer. Cells were lysed in 1% sodium dodecyl sulfate, and cpm were assayed by liquid scintillation.
In some experiments, cell monolayers were pretreated with heparinase or chondroitinase. Enzyme incubations were performed in binding buffer at room temperature with constant shaking for 1 h. Monolayers were rinsed twice, and virus binding was assayed as described above at 4°C.
Polyanion-binding assay.
Because GAGs bound poorly to plastic plates, we adapted the method of Yang et al. (71) to allow covalent coupling of polysaccharides through their carboxyl groups. Ninety-six-well plates precoated with reactive hydrazide linkers (Corning Costar, Wilkes Barre, Pa.) were incubated with 25 μg of polysaccharide per well in 100 μl of 100 mM 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide and 50 mM borate, pH 5.2. Incubation was at room temperature overnight with constant shaking. Plates were rinsed three times with 0.5 M sodium acetate, pH 4.0, and incubated for 30 min in this solution. Plates were rinsed three times with PBS and blocked with PBS containing 0.5% BSA for 30 min. Virus (5 × 103 to 1 × 104 cpm) in 100 μl of PBS (pH 7.0) with 0.5% BSA was added to each well. All incubations were performed at room temperature with constant shaking. After 2 h, 50 μl from each well was collected and counted by liquid scintillation. The amount bound to the plate was calculated by subtracting the resulting cpm from the cpm in an uncoated well. When antibody was used to block binding, virus and antibody were mixed for 30 min at room temperature before being added to the 96-well plate.
Heparin-Sepharose chromatography.
Prepacked 1-ml HiTrap heparin-Sepharose columns (Pharmacia) were equilibrated at room temperature with 10 ml of 100 mM NaCl–5 mM phosphate (pH 7.5)–0.5% BSA at a flow rate of 1 ml/min. Approximately 105 cpm of 35S-labeled virus was added in 1 ml of the same buffer, followed by 4 ml of buffer. The virus was eluted with a 40-ml linear gradient from 100 to 500 mM NaCl containing 5 mM phosphate (pH 7.5) and 0.5% BSA. One-milliliter fractions were collected and counted by liquid scintillation. Any remaining virus was removed from the column by using 0.5% sodium dodecyl sulfate. The NaCl concentration of each fraction was determined by measuring the conductivity of a 50-fold-diluted aliquot.
Statistical analysis.
All results are expressed as means ± standard deviations. Error bars in graphs represent standard deviations. Unless otherwise noted, results were tested for significance by analysis of variance (ANOVA), followed by the Tukey test to determine differences among groups. Results having P values of <0.05 were considered significant.
RESULTS
Inhibition of plaque formation by GAGs.
It has previously been reported that certain polyanions, including some GAGs, decrease the number of SV plaques when present in the medium during the binding step of plaque assays (40). This might be interpreted as a type of competition experiment; an excess of polyanions in the medium could be preventing binding of the virus to a cell surface polyanion.
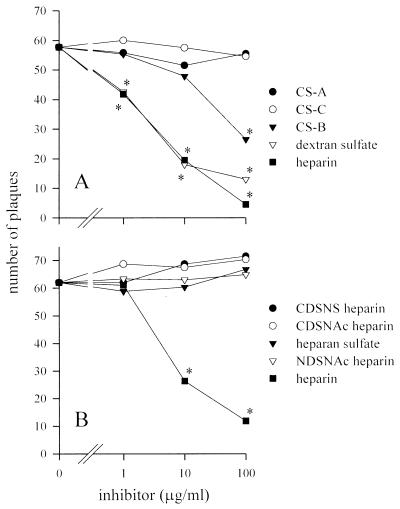
The major GAGs found on most cells are HS and CS. We examined this blocking phenomenon further in plaque assays on BHK cells by using heparin (a highly sulfated version of HS) and the three forms of CS found on the cell surface: CS-A, CS-B (also known as dermatan sulfate), and CS-C. Heparin and dextran sulfate (an artificial, highly sulfated polysaccharide) inhibited plaque formation to similar degrees (Fig. 1A). CS-A and CS-C had no effect, but CS-B inhibited plaque formation when present in high concentrations.
FIG. 1.
Inhibitory effects of polysaccharides on plaque formation by SV. SV strain Toto 1101 was used throughout this study. (A) SV was mixed with various GAGs or the highly sulfated polysaccharide dextran sulfate and incubated on monolayers of BHK cells for 1 h at 37°C before being overlaid with agar. (B) The requirement for high sulfation was shown by the inability of HS or various forms of desulfated heparin to inhibit SV plaque formation. Abbreviations: CDSNS, completely desulfated, N sulfated; CDSNAc, completely desulfated, N acetylated; NDSNAc, N desulfated, N acetylated. Error bars are omitted for clarity of presentation. Each point is the mean of three or more measurements. ∗, P of <0.05 versus no inhibitor.
Recognition of GAG-binding sites by proteins is a function both of saccharide sequence and the position and degree of sulfation. HSV-1, for example, shows strong preferences for certain motifs in HS (14). Although heparin inhibited plaque formation in our assay, HS did not (Fig. 1B). Heparin has two- to threefold-more sulfates per residue than HS (16), and it is not unusual for HS preparations of low sulfate content to show no inhibitory effect on HS-binding viruses (9, 36). Because heparin and HS contain the same saccharides and differ only in the degree of sulfation, a large amount of sulfation must be critical to the ability to inhibit SV plaque formation. Further experiments with three different types of desulfated heparin, none of which were able to inhibit plaque formation, indicated that both N and O sulfation are mandatory for the inhibitory activity of heparin (Fig. 1B).
Studies with radiolabeled SV confirmed that heparin in the medium was able to decrease viral binding to cells (data not shown), although additional interference of heparin at later steps, such as viral entry, cannot be excluded.
Cell surface HS binds SV.
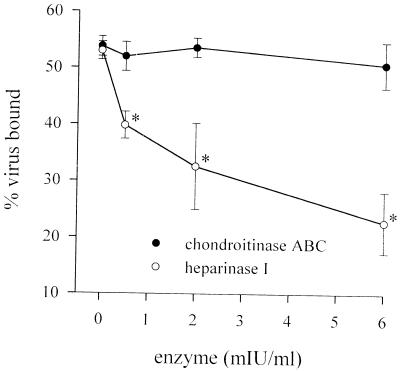
Cell surface GAGs can be removed enzymatically. Treatment of CHO cell monolayers with heparinase I, which degrades HS, caused a decrease in the binding of radiolabeled SV to the cell surface (Fig. 2). Treatment of GAG-deficient pgsA-745 cells with up to 6 mIU of heparinase per ml had no effect on binding of virus (data not shown), indicating that the effect of heparinase was not due to contaminating protease activity. Binding to CHO cells was unaffected by digestion with chondroitinase ABC, which degrades all three forms of CS (Fig. 2). Likewise, on BHK cells, heparinase I decreased binding of SV to a similar extent, and chondroitinase ABC again had no effect (data not shown). Pretreatment of BHK monolayers with heparinase was also able to reduce the number of plaques in a standard plaque assay (data not shown), in agreement with previous results (40). When binding of radiolabeled RRV was examined, however, treatment of BHK or CHO cells with up to 6 mIU of heparinase per ml had no effect on binding (data not shown).
FIG. 2.
Digestion of GAGs decreases the ability of cells to bind SV. CHO monolayers were predigested with various concentrations of heparinase I or chondroitinase ABC, and the amount of radiolabeled virus bound after 1 h at 4°C was measured. Each point is the mean of three measurements. ∗, P of <0.05 versus no enzyme.
As further confirmation that cellular HS binds SV, we examined binding to a series of CHO cell mutants which are impaired in GAG synthesis (Fig. 3A). Radiolabeled virus bound poorly to pgsA-745 cells, which do not synthesize either HS or CS because of a deficiency in xylosyltransferase (13), although binding was not completely abolished. SV bound equally poorly to pgsD-677 cells, which are deficient in _N_-acetylglucosaminyl- and glucuronosyltransferase (33) and have no HS but a threefold-higher level of CS on the cell surface. Together, these results indicate that HS is the major GAG involved in binding of SV. As further evidence of this, binding to pgsE-606 cells, which are deficient in _N_-sulfotransferase and produce a mixture of normal and undersulfated HS (2), was intermediate between binding to wild-type CHO cells and cell lines which are completely deficient in HS (Fig. 3A).
FIG. 3.
Binding of alphaviruses to GAG-deficient cells. (A) Binding of SV to wild-type CHO cells, pgsE cells with partially desulfated HS, pgsD cells lacking HS but having elevated CS, and pgsA cells with no HS or CS. At each time point, binding to CHO cells was significantly greater than binding to the three mutant cell lines. Binding to pgsE cells was also significantly greater than binding to pgsA and pgsD cells. There was no significant difference between binding to pgsD and binding to pgsA cells. (B) Binding of RRV. Binding to CHO cells was significantly greater than to the three mutant cell lines (but see text). Each point is the mean of three measurements.
RRV bound relatively well to CHO cells and to all three mutant cell lines (Fig. 3B). There was nevertheless a significant decrease in binding to pgsA-745 cells, a finding that was replicated in three additional experiments. However, statistical analysis also revealed that binding at 1, 2 and 3 h to pgsD-677 cells (which have CS but no HS) was significantly less than binding to pgsA-745 cells (which have neither CS nor HS). In addition, binding to pgsE-606 cells (which have normal CS but undersulfated HS) was also significantly less than binding to pgsA-745 cells at 2 and 3 h. It is clear that the small differences in binding of RRV to these cell lines must be due to other factors besides the presence or absence of GAGs on the cell surface. These cell lines were created with ethylmethane sulfonate mutagenesis (13), and it is possible that they have multiple mutations or that other small differences have arisen during the process of separately cloning and expanding these cell lines. Given that treating cells with heparinase did not decrease binding of RRV and RRV did not bind well to heparin (see below), the slight difference in binding between CHO and pgsA-745 cells is not sufficient evidence that RRV binds cell surface HS.
Plaque assays on CHO and pgsA-745 cells demonstrated that SV was able to infect and replicate on both cell lines. Plaque sizes on the two cell lines under agarose were similar, but the number of plaques on pgsA-745 cells was reduced to 11% ± 1.0% of the number on CHO cells (P < 0.001 [t test]), as would be expected because of the deficiency in viral binding. These results confirm that HS is important for binding of SV to cells but that other receptors besides HS are by themselves sufficient to allow binding and entry of virus, although at a greatly reduced level.
Curiously, RRV replicated very poorly on CHO and pgsA-745 cells and was unable to form plaques, even though it bound well to both cell lines. It is unclear whether this is analogous to the postentry block in replication seen with HSV-1 on CHO cells (59).
Binding of virus to immobilized GAGs.
In order to demonstrate that GAGs were by themselves sufficient to mediate binding of virus (in the absence of any other receptor), we immobilized various GAGs on plastic plates and examined the binding of radiolabeled virus. Sindbis virus bound at significant levels to heparin, dextran sulfate, and CS-B but not to HS, dextran, desulfated heparin, CS-A, or CS-C (Fig. 4A). This confirms that the degree of sulfation is a critical factor in binding. The result that SV bound well to CS-B was in agreement with the finding that CS-B was able to inhibit plaque formation (Fig. 1). RRV, in contrast, did not bind significantly to heparin or CS-B, although it bound at high levels to dextran sulfate (Fig. 4B).
FIG. 4.
Binding of alphaviruses to immobilized GAGs. Ninety-six-well plates were coated with various GAGs, and the amount of radiolabeled virus that bound was assessed. (A) Binding of SV. (B) Binding of RRV. Ten thousand counts per minute of virus per well were used. Each bar represents the mean of four measurements. ∗, P of <0.05 versus uncoated wells. See the legend to Fig. 1 for abbreviations.
Antibodies against the alphaviral glycoproteins, particularly against E2, are able to neutralize virus in plaque assays and reduce infection in vivo. It was of interest to determine whether monoclonal antibodies to E2 could block binding of SV to isolated GAGs. Heparin was chosen as a substrate because it bound virus well and was similar to the physiological HS substrate. Purified monoclonal antibodies against two different neutralizing epitopes on the E2 glycoprotein were able to decrease the binding of radiolabeled SV to immobilized heparin (Fig. 5). R6 IgG (against E2c) inhibited binding somewhat better than Fab fragments of R6, but both were able to completely block viral binding. Monoclonal antibody 202, which recognizes the E2ab epitope, was unable to completely inhibit binding but was still able to inhibit binding even at relatively low concentrations (Fig. 5). This might be related to cross-linking of the virus by 202, because Fab fragments of 202 did not show this effect and inhibited binding only at high concentrations.
FIG. 5.
Antibodies against the E2 glycoprotein can inhibit binding of SV to heparin. Ninety-six-well plates coated with heparin were incubated with 5,000 cpm of SV in the presence or absence of IgG or Fab fragments. R6 recognizes the E2c epitope, and 202 recognizes the E2ab epitope. 3E1 is a control antibody against HSV-1. Each point is the mean of two measurements. An additional experiment gave similar results.
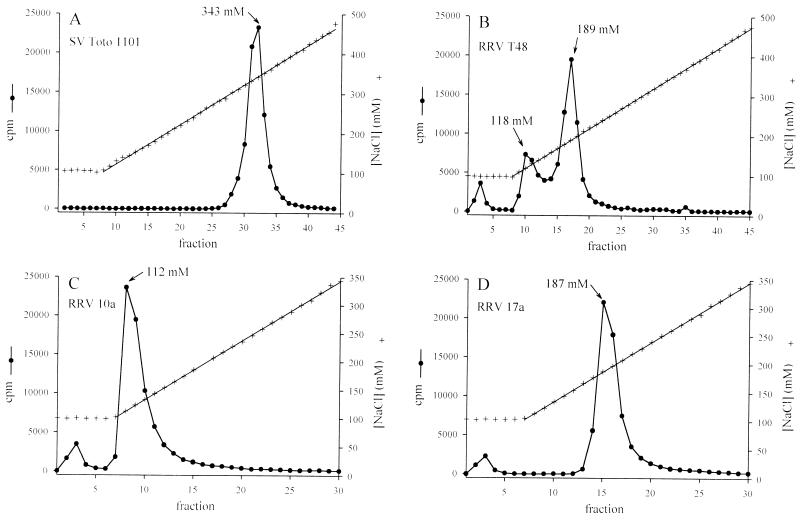
Sindbis virus also bound to heparin immobilized on Sepharose beads and could be eluted from heparin-Sepharose columns with increasing concentrations of NaCl (Fig. 6A). High concentrations of salt disrupt the electrostatic interactions between heparin and heparin-binding proteins, and this technique can yield sensitive information about the relative strength of binding. SV eluted as a single peak at 343 mM NaCl. RRV T48, however, eluted as three distinct peaks, at 100, 118, and 189 mM NaCl (Fig. 6B). Elution of RRV at lower salt concentrations than SV was consistent with the poor ability of RRV to bind to immobilized heparin (Fig. 4B). Peak fractions 3, 10, and 17 (Fig. 6B) were collected and plaqued on BHK cells. Fraction 3 contained no detectable infectious virus. The composition of this peak is unknown—it was observed in three separate gradient-purified preparations of RRV, where it accounted for roughly 5 to 8% of total cpm (Fig. 6B to D). Elution of material in 100 mM NaCl was never seen with SV (which was prepared in an identical manner).
FIG. 6.
Elution of alphavirus from heparin-Sepharose columns. Virus (70,000 to 100,000 cpm) was added to the column in 100 mM NaCl buffer and eluted with a gradient of from 100 to 500 mM NaCl. (A) SV. (B) RRV. Single viral clones from RRV peak fractions 10 and 17 were selected, grown, and radiolabeled. These viral clones eluted at salt concentrations similar to the peaks from which they were originally taken (C and D).
Individual plaques from RRV T48 fractions 10 and 17 were picked, passaged once on BHK cells, and used to make 35S-labeled purified virus. Both viruses grew to similar titers and had similar plaque sizes under agar overlays. Virus RRV 10a, originally obtained from the 118 mM peak, eluted at 112 mM NaCl (Fig. 6C). Virus RRV 17a, originally from the 189 mM peak, eluted at 187 mM NaCl (Fig. 6D). This indicates that our original preparation of RRV T48 contained two genetically distinct viral variants with different abilities to bind to heparin. The sequencing of such variants will help to map the regions of the glycoproteins which are responsible for binding to heparin.
Effects of sulfated polysaccharides on plaque size.
Studies performed several decades ago found that an inhibitor in agar influences the plaque sizes of viruses from many different families, and the inhibitor was eventually determined to be a sulfated polysaccharide (64). This inhibitor appears to slow the spread of viruses which bind well to it, resulting in plaques with reduced diameters. Agarose, a purified form of agar, does not contain sulfated polysaccharides, and consequently alphaviruses with small-plaque phenotypes under agar often have substantially increased plaque sizes under agarose (48, 57). We found that SV plaque size could be increased dramatically by using agarose overlays instead of agar, indicating that the sulfated polysaccharide in agar was inhibitory (Fig. 7A). As further confirmation of this, addition of the cationic polymer DEAE-dextran to agar led to increased plaque size, and addition of heparin to agarose decreased plaque size compared with agarose alone (Fig. 7A). Such manipulations had no effect on the plaque size of RRV (Fig. 7B), consistent with the lack of interaction between RRV and GAGs in other assays.
FIG. 7.
Effect of overlay on plaque diameter. Virus was absorbed to monolayers of BHK cells for 1 h at 37°C and then overlaid with 0.6% agar or agarose with or without additives. Plaque diameters were measured at 2 days to the nearest 0.5 mm. (A) Effect of overlay composition on SV strain Toto 1101. ∗, P of <0.05 versus agar plus DEAE-dextran; †, P of <0.05 versus agarose. (B) Overlay composition had no significant effect on the plaque size of RRV strain T48. Each bar is the mean of 15 or more measurements. Results were analyzed by ANOVA on ranks, followed by Dunn’s test.
DISCUSSION
We have shown that SV binds to cell surface HS based on a variety of evidence, including the ability of heparin in the medium to interfere with cell surface binding, decreased binding of SV to heparinase-treated cells, decreased binding to cells which are genetically deficient in HS, and direct binding of SV to immobilized heparin. In contrast, we were unable to show that RRV could bind to cell surface GAGs, and RRV had a relatively poor ability to bind to immobilized heparin.
Proteins which have affinity for GAGs bind to them largely through electrostatic interactions, with positively charged Arg and Lys side chains interacting with negatively charged sulfates (17). As in numerous other studies with HS-binding proteins, we found that highly sulfated heparin was the most effective substrate for binding, indicating that the degree of sulfation, more than the polysaccharide backbone, was the critical determinant for strength of binding. It has been observed for many years that cellular binding of a number of alphaviruses (SV, Semliki Forest virus, and eastern equine encephalitis, western equine encephalitis, and VEE viruses) is highly dependent on electrostatic interactions because it is influenced by the pH and ionic strength of the medium (15, 18, 34, 37, 38, 49). It therefore seems probable that other alphaviruses, in addition to SV, also bind to cell surface HS.
Other receptors.
As mentioned in the introduction, many of the viruses which are known to bind to GAGs also use other cell surface molecules, usually proteins, as receptors. The situation has been most carefully studied with HSV-1, where binding to HS is followed by binding to a more specific protein receptor (44). This is also true of HS-binding growth factors and signaling proteins such as fibroblast growth factor: signal transduction and endocytosis require binding to an additional, nonproteoglycan receptor (56). Because SV was able to bind to and replicate on GAG-deficient pgsA-745 cells, it is clear that other receptors must also be involved.
The nature of these additional receptors is not completely clear. It has previously been found that treating cells with either proteases or phospholipases decreases binding of SV (66). Both of these results could conceivably be explained by digestion or release of cell surface proteoglycans, which exist as a mixture of transmembrane proteins and glycosylphosphatidylinositol-linked proteins (6). Further studies on pgsA-745 cells in the absence of GAGs will be helpful in determining whether the remaining receptors are proteins, lipids, or carbohydrates.
The 67-kDa high-affinity laminin receptor has been identified as a possible SV receptor on some types of mammalian cells (68), and a 32-kDa probable laminin receptor has also been identified as a possible receptor for VEE virus on mosquito cells (34). There are many different types of laminin receptors on the mammalian cell surface, including the 67-kDa high-affinity receptor, another 67-kDa elastin-laminin receptor, a number of different integrin receptors, and a number of different members of the galactoside-binding lectin family (41). The high-affinity laminin receptor is a peculiar protein which has been the subject of intense interest because of its upregulation on metastatic tumor cells (8). The cell surface receptor is a 67-kDa glycoprotein, but the putative cDNA encodes only a 37-kDa protein. Overexpression of this cDNA leads to an increase in binding of SV (68). The 37-kDa protein appears to be a precursor of the 67-kDa protein on the basis of shared epitopes, partial amino acid sequencing of the 67-kDa protein, and pulse-chase analysis of its synthesis, but how it increases its mass is unclear. The 37-kDa protein contains neither a signal sequence for membrane translocation nor a transmembrane domain. The coding sequence is highly conserved among mammals, with only two differences out of 295 amino acids between hamsters and humans (68). Bizarrely, however, it has recently been discovered that the 37-kDa protein is also a ribosomal subunit (10, 65).
Interestingly, laminin itself is an HS-binding protein, with several independent binding sites on its three chains (41). It is not clear whether this is related to the ability of the high-affinity laminin receptor to bind SV.
Implications for viral behavior and virulence.
Alphavirus variants with reduced plaque sizes often have reduced virulence in vivo as well, although there are certainly exceptions to this rule, and fresh wild-type isolates frequently contain a mixture of large-plaque and small-plaque viruses. Repeated tissue culture passaging of alphaviruses can lead to decreased plaque size and decreased virulence (19, 39). Small-plaque and large-plaque alphavirus variants typically have different affinities for hydroxyapatite (a form of calcium phosphate), indicating changes in the surface charge of the glycoproteins (3, 23). It may be possible to reinterpret these findings in light of our demonstration that SV can bind to HS. We suggest that alphaviruses with a small-plaque phenotype under agar (indicating strong binding to the agar sulfated polysaccharide) may also bind better to HS and that strong binding to HS may decrease virulence in vivo.
The strain of SV used in this study, Toto 1101, is a relatively avirulent molecular clone derived from HRSP, a small-plaque virus (51). Like most laboratory strains of SV, HRSP has been passaged many times in tissue culture. Ongoing work indicates that other strains of SV also bind to HS. For example, strain AR339 elutes from heparin-Sepharose at an NaCl concentration of 338 mM and, like Toto 1101, binds markedly better to CHO cells than to GAG-deficient pgsA-745 cells (5). Further investigations with minimally passaged strains of SV will be necessary to determine the extent to which wild isolates bind HS. Nevertheless, it is clear that strains of SV in common laboratory usage bind significantly to HS.
How might altering the affinity of alphaviruses for HS affect virulence and pathogenesis? It is known that tissue culture passaging of another HS-binding virus, foot-and-mouth disease virus, selects for variants with increased heparin-binding ability, decreased plaque size, and decreased virulence (53). These changes are the result of a single amino acid substitution in the VP3 capsid protein, from His to Arg, and this probably leads to an increased heparin affinity through a direct interaction between the Arg and heparin (53). These heparin-binding variant viruses replicate poorly in animals and rapidly revert to virulent viruses with reduced heparin affinity through loss of the Arg or a spatially proximal Lys. In this case, at least, viruses which bind well to HS have a selective advantage in tissue culture but are disadvantaged in animals.
There is evidence in the literature that changes in affinity for HS could have implications for alphavirus pathogenesis. It is useful to first review the pharmacokinetics of HS-binding proteins: when injected intravenously, proteins with high heparin affinity are removed from the circulation and sequestered by cell surface HS within minutes (28, 67, 72). Most of the injected protein ends up in the liver, where the HS is unusually highly sulfated, having almost twice as many sulfates as HS in other tissues (35). Intravenous injection of heparin can increase the circulating half-life of intravenously injected HS-binding proteins and even release previously bound protein into the circulation. Heparin affinity can also control the tissue distribution of proteins. For example, the heparin-binding enzyme extracellular superoxide dismutase (EC-SOD) is normally sequestered by HS in tissue interstitial spaces and on endothelial cell surfaces. Roughly 2% of the human population has an allele for an EC-SOD variant with a single change from Arg to Gly, resulting in reduced affinity for heparin and a 10-fold-greater plasma concentration of EC-SOD (54). Likewise, when EC-SOD is injected subcutaneously or intramuscularly, normal EC-SOD is retained much longer at the injection site than truncated variants with reduced heparin affinity (29).
Remarkably, similar phenomena have been seen in a number of studies with alphaviruses, and we would put forward the hypothesis that this is a function of binding to HS. Following intravenous injection of virus, it has been observed that small-plaque variants of SV, VEE virus, and western equine encephalitis virus are cleared from the circulation much faster than large-plaque strains (22, 25, 50). For example, 30 min after injection of a radiolabeled small-plaque variant of VEE, more than 99% of the virus has disappeared from the plasma, but when a large-plaque variant is injected, less than 1% is cleared in the same time (25). Half of the cpm from the small-plaque virus can be found accumulated in the liver, and electron microscopic examination of the liver reveals binding and uptake of large amounts of virus. When injected subcutaneously, the small-plaque virus infects hamsters poorly. Most animals fail to seroconvert, and those that die have all developed revertant viruses with large-plaque morphologies (25).
As a second example, the major attenuating mutation in the TC-83 vaccine strain of VEE has been mapped to a change at E2 position 120 from Thr to Arg, a gain of a positively charged residue (30). This virus has a smaller plaque size and a higher affinity for hydroxyapatite than the parental TD strain (24) and absorbs better to BHK cells (55). TC-83 is cleared rapidly from the plasma after intravenous injection, unlike TD (26). After subcutaneous injection, TC-83 replicates more slowly than virulent VEE but eventually reaches slightly higher titers in the bone marrow and lymph nodes. However, the plasma viremia is much lower (1, 26). Interestingly, substitution of Lys at E2 120 also leads to attenuation (12).
These changes in plaque size and in vivo behavior suggest a possible role for HS binding. To prove this, such viral variants will need to be tested in vitro for heparin affinity and binding to cell surface HS. In vivo, it would be expected that concurrent intravenous injection of heparin would increase the circulating half-life of small-plaque variants, as is seen with other heparin-binding proteins. Sequencing of such variants will help to define the regions of the glycoproteins that are involved in binding to HS.
ACKNOWLEDGMENTS
This work was supported by a postdoctoral fellowship from the National Multiple Sclerosis Society and by grants R01-NS18596 and T32-AI07417 from the National Institutes of Health.
REFERENCES
- 1.Austin F J, Scherer W F. Studies of viral virulence. I. Growth and histopathology of virulent and attenuated strains of Venezuelan encephalitis virus in hamsters. Am J Pathol. 1971;62:195–210. [PMC free article] [PubMed] [Google Scholar]
- 2.Bame K J, Zhang L, David G, Esko J D. Sulphated and undersulphated heparan sulphate proteoglycans in a Chinese hamster ovary cell mutant defective in N-sulphotransferase. Biochem J. 1994;303:81–87. doi: 10.1042/bj3030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bose H R, Carl G Z, Sagik B P. Separation of Sindbis virus plaque-type variants by calcium phosphate chromatography. Arch Gesamte Virusforsch. 1970;29:83–89. doi: 10.1007/BF01253883. [DOI] [PubMed] [Google Scholar]
- 4.Brown L N, Packer R A. Some factors affecting plaque size of western equine encephalomyelitis virus. Am J Vet Res. 1964;25:487–493. [PubMed] [Google Scholar]
- 5.Byrnes, A. P., and D. E. Griffin. Unpublished data.
- 6.Carey D J, Evans D M. Membrane anchoring of heparan sulfate proteoglycans by phosphatidylinositol and kinetics of synthesis of peripheral and detergent-solubilized proteoglycans in Schwann cells. J Cell Biol. 1989;108:1891–1897. doi: 10.1083/jcb.108.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cássaro C M F, Dietrich C P. Distribution of sulfated mucopolysaccharides in invertebrates. J Biol Chem. 1977;252:2254–2261. [PubMed] [Google Scholar]
- 8.Castronovo V. Laminin receptors and laminin-binding proteins during tumor invasion and metastasis. Invasion Metastasis. 1993;13:1–30. [PubMed] [Google Scholar]
- 9.Chen Y, Maguire T, Hileman R E, Fromm J R, Esko J D, Linhardt R J, Marks R M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 10.Clausse N, Jackers P, Jarès P, Joris B, Sobel M E, Castronovo V. Identification of the active gene coding for the metastasis-associated 37LRP/p40 multifunctional protein. DNA Cell Biol. 1996;15:1009–1023. doi: 10.1089/dna.1996.15.1009. [DOI] [PubMed] [Google Scholar]
- 11.Colön J I, Idoine J B, Brand O M, Costlow R D. Mode of action of an inhibitor from agar on growth and hemagglutination of group A arboviruses. J Bacteriol. 1965;90:172–179. doi: 10.1128/jb.90.1.172-179.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis N L, Powell N, Greenwald G F, Willis L V, Johnson B J B, Smith J F, Johnston R E. Attenuating mutations in the E2 glycoprotein gene of Venezuelan equine encephalitis virus: construction of single and multiple mutants in a full-length cDNA clone. Virology. 1991;183:20–31. doi: 10.1016/0042-6822(91)90114-q. [DOI] [PubMed] [Google Scholar]
- 13.Esko J D, Stewart T E, Taylor W H. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci USA. 1985;82:3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feyzi E, Trybala E, Bergström T, Lindahl U, Spillmann D. Structural requirement of heparan sulfate for interaction with herpes simplex virus type 1 virions and isolated glycoprotein C. J Biol Chem. 1997;272:24850–24857. doi: 10.1074/jbc.272.40.24850. [DOI] [PubMed] [Google Scholar]
- 15.Fries E, Helenius A. Binding of Semliki Forest virus and its spike glycoproteins to cells. Eur J Biochem. 1979;97:213–220. doi: 10.1111/j.1432-1033.1979.tb13105.x. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher J T, Walker A. Molecular distinctions between heparan sulphate and heparin. Analysis of sulphation patterns indicates that heparan sulphate and heparin are separate families of N-sulphated polysaccharides. Biochem J. 1985;230:665–674. doi: 10.1042/bj2300665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gromm J R, Hilemann R E, Caldwell E E O, Weiler J M, Linhardt R J. Differences in the interaction of heparin with arginine and lysine and the importance of these basic amino acids in the binding of heparin to acidic fibroblast growth factor. Arch Biochem Biophys. 1995;323:279–287. doi: 10.1006/abbi.1995.9963. [DOI] [PubMed] [Google Scholar]
- 18.Hahon N, Cooke K O. Primary virus-cell interactions in the immunofluorescence assay of Venezuelan equine encephalomyelitis virus. J Virol. 1967;1:317–326. doi: 10.1128/jvi.1.2.317-326.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heydrick F P, Wachter R F, Hearn H J. Host influence on the characteristics of Venezuelan equine encephalomyelitis virus. J Bacteriol. 1966;91:2343–2348. doi: 10.1128/jb.91.6.2343-2348.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson R L, Busch S J, Cardin A D. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev. 1991;71:481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- 21.Jackson T, Ellard F M, Abu Ghazaleh R, Brookes S M, Blakemore W E, Corteyn A H, Stuart D I, Newman J W I, King A M Q. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J Virol. 1996;70:5282–5287. doi: 10.1128/jvi.70.8.5282-5287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jahrling P B. Virulence heterogeneity of a predominantly avirulent western equine encephalitis virus population. J Gen Virol. 1976;32:121–128. doi: 10.1099/0022-1317-32-1-121. [DOI] [PubMed] [Google Scholar]
- 23.Jahrling P B, Beall J L. Chromatographic separations of alphavirus strains by hydroxylapatite. J Clin Microbiol. 1977;6:238–243. doi: 10.1128/jcm.6.3.238-243.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jahrling P B, Eddy G A. Comparisons among members of the Venezuelan encephalitis virus complex using hydroxylapatite column chromatography. Am J Epidemiol. 1977;106:408–417. doi: 10.1093/oxfordjournals.aje.a112483. [DOI] [PubMed] [Google Scholar]
- 25.Jahrling P B, Gorelkin L. Selective clearance of a benign clone of Venezuelan equine encephalitis virus from hamster plasma by hepatic reticuloendothelial cells. J Infect Dis. 1975;132:667–676. doi: 10.1093/infdis/132.6.667. [DOI] [PubMed] [Google Scholar]
- 26.Jahrling P B, Scherer W F. Growth curves and clearance rates of virulent and benign Venezuelan encephalitis viruses in hamsters. Infect Immun. 1973;8:456–462. doi: 10.1128/iai.8.3.456-462.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karabatsos N. International catalogue of arboviruses including certain other viruses of vertebrates. 3rd ed. San Antonio, Tex: American Society for Tropical Medicine and Hygiene; 1985. pp. 867–868. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson K, Marklund S L. Plasma clearance of human extracellular-superoxide dismutase C in rabbits. J Clin Investig. 1988;82:762–766. doi: 10.1172/JCI113676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlsson K, Sandström J, Edlund A, Marklund S L. Turnover of extracellular-superoxide dismutase in tissues. Lab Investig. 1994;70:705–710. [PubMed] [Google Scholar]
- 30.Kinney R M, Chang G-J, Tsuchiya K R, Sneider J M, Roehrig J T, Woodward T M, Trent D W. Attenuation of Venezuelan equine encephalitis virus strain TC-83 is encoded by the 5′ noncoding region and the E2 envelope glycoprotein. J Virol. 1993;67:1269–1277. doi: 10.1128/jvi.67.3.1269-1277.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kjellén L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 32.Krusat T, Streckert H-J. Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells. Arch Virol. 1997;142:1247–1254. doi: 10.1007/s007050050156. [DOI] [PubMed] [Google Scholar]
- 33.Lidholt K, Weinke J L, Kiser C S, Lugemwa F N, Bame K J, Cheifetz S, Massagué J, Lindahl U, Esko J D. A single mutation affects both N-acetylglucosaminyltransferase and glucuronosyltransferase activities in a Chinese hamster ovary cell mutant defective in heparan sulfate biosynthesis. Proc Natl Acad Sci USA. 1992;89:2267–2271. doi: 10.1073/pnas.89.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ludwig G V, Kondig J P, Smith J F. A putative receptor for Venezuelan equine encephalitis virus from mosquito cells. J Virol. 1996;70:5592–5599. doi: 10.1128/jvi.70.8.5592-5599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luon M, Deakin J A, Gallagher J T. Liver heparan sulfate structure: a novel molecular design. J Biol Chem. 1994;269:11208–11215. [PubMed] [Google Scholar]
- 36.Lycke E, Johansson M, Svennerholm B, Lindahl U. Binding of herpes simplex virus to cellular heparan sulphate, an initial step in the adsorption process. J Gen Virol. 1991;72:1131–1137. doi: 10.1099/0022-1317-72-5-1131. [DOI] [PubMed] [Google Scholar]
- 37.Marker S C, Connelly D, Jahrling P B. Receptor interaction between eastern equine encephalitis virus and chicken embryo fibroblasts. J Virol. 1977;21:981–985. doi: 10.1128/jvi.21.3.981-985.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marker S C, Jahrling P B. Correlation between virus-cell receptor properties of alphaviruses in vitro and virulence in vivo. Arch Virol. 1979;62:53–62. doi: 10.1007/BF01314903. [DOI] [PubMed] [Google Scholar]
- 39.Marshall I D, Scrivani R P, Reeves W C. Variation in the size of plaques produced in tissue culture by strains of western equine encephalitis virus. Am J Hyg. 1962;76:216–224. [Google Scholar]
- 40.Mastromarino P, Conti C, Petruzziello R, Lapadula R, Orsi N. Effect of polyions on the early events of Sindbis virus infection of Vero cells. Arch Virol. 1991;121:19–27. doi: 10.1007/BF01316741. [DOI] [PubMed] [Google Scholar]
- 41.Mecham R P. Receptors for laminin on mammalian cells. FASEB J. 1991;5:2538–2546. doi: 10.1096/fasebj.5.11.1651264. [DOI] [PubMed] [Google Scholar]
- 42.Mendoza Q P, Stanley J, Griffin D E. Monoclonal antibodies to the E1 and E2 glycoproteins of Sindbis virus: definition of epitopes and efficiency of protection from fatal encephalitis. J Gen Virol. 1988;70:3015–3022. doi: 10.1099/0022-1317-69-12-3015. [DOI] [PubMed] [Google Scholar]
- 43.Mettenleiter T C, Zsak L, Zuckermann F, Sugg N, Kern H, Ben-Porat T. Interaction of glycoprotein gIII with a cellular heparinlike substance mediates adsorption of pseudorabies virus. J Virol. 1990;64:278–286. doi: 10.1128/jvi.64.1.278-286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 45.Neyts J, Snoeck R, Schols D, Balzarini J, Esko J D, van Schepdael A, de Clercq E. Sulfated polymers inhibit the interaction of human cytomegalovirus with cell surface heparan sulfate. Virology. 1992;189:48–58. doi: 10.1016/0042-6822(92)90680-n. [DOI] [PubMed] [Google Scholar]
- 46.Olmsted R A, Meyer W J, Johnston R E. Characterization of Sindbis virus epitopes important for penetration in cell culture and pathogenesis in animals. Virology. 1986;148:245–254. doi: 10.1016/0042-6822(86)90322-3. [DOI] [PubMed] [Google Scholar]
- 47.Patel M, Yanagishita M, Roderiquez G, Bou-Habib D C, Oravecz T, Hascall B C, Norcross M A. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res Hum Retroviruses. 1993;9:167–174. doi: 10.1089/aid.1993.9.167. [DOI] [PubMed] [Google Scholar]
- 48.Pattyn S R, de Vleesschauwer L. Plaque production by group A arboviruses. I. Influence of DEAE-dextran on plaques under agar and agarose. Plaque production under carboxymethylcellulose. Acta Virol. 1967;11:305–311. [PubMed] [Google Scholar]
- 49.Pierce J S, Strauss E G, Strauss J H. Effect of ionic strength on the binding of Sindbis virus to chick cells. J Virol. 1974;13:1030–1036. doi: 10.1128/jvi.13.5.1030-1036.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Postic B, Schleupner C J, Armstrong J A, Ho M. Two variants of Sindbis virus which differ in interferon induction and serum clearance. I. The phenomenon. J Infect Dis. 1969;120:339–347. doi: 10.1093/infdis/120.3.339. [DOI] [PubMed] [Google Scholar]
- 51.Rice C M, Levis R, Strauss J H, Huang H V. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987;61:3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rostand K S, Esko J D. Microbial adherence to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sa-Carvalho D, Rieder E, Baxt B, Rodatte R, Tanuri A, Mason P W. Tissue culture adaptation of foot-and-mouth disease virus selects viruses that bind to heparin and are attenuated in cattle. J Virol. 1997;71:5115–5123. doi: 10.1128/jvi.71.7.5115-5123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandström J, Nilsson P, Karlsson K, Marklund S L. 10-fold increase in human plasma extracellular superoxide dismutase content caused by a mutation in heparin-binding domain. J Biol Chem. 1994;269:19163–19166. [PubMed] [Google Scholar]
- 55.Scherer W F, Ellsworth C A, Ventura A K. Studies of viral virulence. II. Growth and adsorption curves of virulent and attenuated strains of Venezuelan encephalitis virus in cultured cells. Am J Pathol. 1971;62:211–219. [PMC free article] [PubMed] [Google Scholar]
- 56.Schlessinger J, Lax I, Lemmon M. Regulation of growth factor activation by proteoglycans: what is the role of the low affinity receptors? Cell. 1995;83:357–360. doi: 10.1016/0092-8674(95)90112-4. [DOI] [PubMed] [Google Scholar]
- 57.Schleupner C J, Postic B, Armstrong J A, Atchison R W, Ho M. Two variants of Sindbis virus which differ in interferon induction. II. Virological characterizations. J Infect Dis. 1969;120:348–355. doi: 10.1093/infdis/120.3.348. [DOI] [PubMed] [Google Scholar]
- 58.Secchiero P, Sun D, de Vico A L, Crowley R W, Reitz M S, Zauli G, Lusso P, Gallo R C. Role of the extracellular domain of human herpesvirus 7 glycoprotein B in virus binding to cell surface heparan sulfate proteoglycans. J Virol. 1997;71:4571–4580. doi: 10.1128/jvi.71.6.4571-4580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shieh M-T, WuDunn D, Montgomery R I, Esko J D, Spear P G. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992;116:1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stringer S E, Gallagher J T. Heparan sulphate. Int J Biochem Cell Biol. 1997;29:709–714. doi: 10.1016/s1357-2725(96)00170-7. [DOI] [PubMed] [Google Scholar]
- 62.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Symington J, Schlesinger M J. Characterization of a Sindbis virus variant with altered host range. Arch Virol. 1978;58:127–136. doi: 10.1007/BF01315405. [DOI] [PubMed] [Google Scholar]
- 64.Takemoto K K. Plaque mutants of animal viruses. Prog Med Virol. 1966;8:314–348. [PubMed] [Google Scholar]
- 65.Tohgo A, Takasawa S, Munakata H, Yonekura H, Hayashi N, Okamoto H. Structural determination and characterization of a 40 kDa protein isolated from rat 40 S ribosomal subunit. FEBS Lett. 1994;340:133–138. doi: 10.1016/0014-5793(94)80188-6. [DOI] [PubMed] [Google Scholar]
- 66.Ubol S, Griffin D E. Identification of a putative alphavirus receptor on mouse neural cells. J Virol. 1991;65:6913–6921. doi: 10.1128/jvi.65.12.6913-6921.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wallinder L, Peterson J, Olivecrona T, Bengtsson-Olivecrona G. Hepatic and extrahepatic uptake of intravenously injected lipoprotein lipase. Biochim Biophys Acta. 1984;795:513–524. doi: 10.1016/0005-2760(84)90181-4. [DOI] [PubMed] [Google Scholar]
- 68.Wang K-S, Kuhn R J, Strauss E G, Ou S, Strauss J H. High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J Virol. 1992;66:4992–5001. doi: 10.1128/jvi.66.8.4992-5001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang K-S, Schmaljohn A L, Kuhn R J, Strauss J H. Antiidiotypic antibodies as probes for the Sindbis virus receptor. Virology. 1991;181:694–702. doi: 10.1016/0042-6822(91)90903-o. [DOI] [PubMed] [Google Scholar]
- 70.WuDunn D, Spear P G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang B, Yang B L, Goetinck P F. Biotinylated hyaluronic acid as a probe for identifying hyaluronic acid-binding proteins. Anal Biochem. 1995;228:299–306. doi: 10.1006/abio.1995.1354. [DOI] [PubMed] [Google Scholar]
- 72.Yuge T, Furukawa A, Nakamura K, Nagashima Y, Shinozaki K, Nakamura T, Kimura R. Metabolism of the intravenously administered recombinant human basic fibroblast growth factor, trafermin, in liver and kidney: degradation implicated in its selective localization to the fenestrated type microvasculatures. Biol Pharm Bull. 1997;20:786–793. doi: 10.1248/bpb.20.786. [DOI] [PubMed] [Google Scholar]