Immunoreactivity of Intact Virions of Human Immunodeficiency Virus Type 1 (HIV-1) Reveals the Existence of Fewer HIV-1 Immunotypes than Genotypes (original) (raw)
Abstract
In order to protect against organisms that exhibit significant genetic variation, polyvalent vaccines are needed. Given the extreme variability of human immunodeficiency virus type 1 (HIV-1), it is probable that a polyvalent vaccine will also be needed for protection from this virus. However, to understand how to construct a polyvalent vaccine, serotypes or immunotypes of HIV must be identified. In the present study, we have examined the immunologic relatedness of intact, native HIV-1 primary isolates of group M, clades A to H, with human monoclonal antibodies (MAbs) directed at epitopes in the V3, C5, and gp41 cluster I regions of the envelope glycoproteins, since these regions are well exposed on the virion surface. Multivariate analysis of the binding data revealed three immunotypes of HIV-1 and five MAb groups useful for immunotyping of the viruses. The analysis revealed that there are fewer immunotypes than genotypes of HIV and that clustering of the isolates did not correlate with either genotypes, coreceptor usage (CCR5 and CXCR4), or geographic origin of the isolates. Further analysis revealed distinct MAb groups that bound preferentially to HIV-1 isolates belonging to particular immunotypes or that bound to all three immunotypes; this demonstrates that viral immunotypes identified by mathematical analysis are indeed defined by their immunologic characteristics. In summary, these results indicate (i) that HIV-1 immunotypes can be defined, (ii) that constellations of epitopes that are conserved among isolates belonging to each individual HIV-1 immunotype exist and that these distinguish each of the immunotypes, and (iii) that there are also epitopes that are routinely shared by all immunotypes.
The coding sequences of the human immunodeficiency virus type 1 (HIV-1) envelope have been used extensively to classify these viruses genetically into at least 11 clades, designated A to K, in group M (major) and into groups O (outlier) and N (new) (18, 19, 22, 26, 30, 33, 44). However, several studies have shown that the immune response to HIV infection is neither clade specific nor clade restricted (14, 23, 36, 37, 48, 50, 51). Sera and monoclonal antibodies (MAbs) derived from HIV-1-infected persons cross-react with HIV-1 isolates or proteins from different clades, suggesting that despite the genetic diversity that distinguishes these viruses, they have common antigenic epitopes (37, 51). The results of these studies indicate that antigenic regions that are conserved across different HIV clades and those that are distinct could be important for designing a polyvalent vaccine potent against a broad spectrum of HIV isolates. However, in order to understand how to construct a polyvalent HIV-1 vaccine, immunologically defined groups of HIV-1 isolates need to be identified along with the shared and distinguishing immunogenic epitopes that induce protective immunity.
Immunologic classifications of HIV-1 have been published. Those based on studies with sera from HIV-1-infected subjects defined HIV-1 “serotypes,” while classifications based on studies with MAbs defined HIV-1 “immunotypes.” For example, studies comparing genetic and immunologic classifications of HIV-1 by examining isolates of group M and group O with homologous and heterologous sera revealed that HIV-1 could be grouped immunologically and that genetic subtypes did not correlate with neutralization serotypes (39, 47). Similarly, multivariate analysis of a neutralization matrix comprised of 14 sera and 16 primary isolates identified eight “neutralization serotypes” (37). However, since these cluster analyses were based on data generated with polyclonal sera, the conserved epitopes characterizing each serotype could not be deciphered.
In order to identify conserved epitopes specific for each immunologically defined group of viruses, MAbs that are directed at defined epitopes would be needed. Using MAbs, such HIV-1 immunotypes were determined by multivariate analysis of data from the immunochemical reactivities of 1,176 combinations of human anti-V3 MAbs and V3 peptides which were representative of 56 viruses from eight clades (A to H) obtained from patients around the world (51). The analysis revealed seven immunologically defined groups of peptides, each containing peptides from more than one clade. Inspection of the amino acid sequences of the peptides in each of the peptide clusters revealed unique “signature sequences” that suggested structural motifs characteristic of each V3-based immunotype.
In recent studies using anti-HIV-1 gp120 and gp41 MAbs derived from patients infected with HIV-1 clade B or E, we studied the antigenic conservation of epitopes expressed on the surface of intact, native primary HIV-1 isolates of group M, clades A to H, obtained from patients around the world (34, 36). In these studies we observed that epitopes located in the V3 and C5 regions of gp120 and in the cluster I region of gp41 are shared and well exposed compared to the CD4-binding domain (CD4bd), V2, and C2 regions of gp120 and the cluster II region of gp41. This work confirmed previous studies showing that HIV-1 genetic clades do not correspond with serotypes or immunotypes and suggested that HIV-1 isolates could be grouped according to immunotypes that could enlighten our approach to designing a polyvalent HIV vaccine.
In the present study, we again applied a multivariate method of data analysis to the matrix of data generated with anti-HIV-1 human MAbs binding with intact, native HIV-1 primary isolates of group M, clades A to H. The analysis revealed three immunotypes of HIV-1 and the antigenic epitopes that are specific for each.
MATERIALS AND METHODS
HIV-1 isolates.
The 26 HIV-1 group M isolates of clades A to H studied have been described previously (36). The clade to which each isolate belongs is shown in Fig. 1. These HIV-1 isolates were obtained from patients in Cameroon (CA1, CA4, CA5, CA13, and CA20), Belgium (VI191), Uganda (92UG021 and 92UG001), France (BX08), the United States (MNp, IIIB, and JR-FL), Senegal (SG2728), Zimbabwe (2036), Rwanda (92RW021), Zambia (ZB18), the Ivory Coast (CI13), Zaire (MAL), Brazil (93BR019 and 93BR029), Thailand (92THA009, 92THA011, BK131, and CM235), and Gabon (VI525 and VI526). HIV-1 viral stocks were prepared as previously described (38). Briefly, 1 ml of p24-positive HIV-1 culture supernatants was used to infect 3-day phytohemagglutinin-stimulated HIV-negative donor peripheral blood mononuclear cells (PBMCs) (38, 39). After 2 to 3 weeks of culture, the culture supernatants from infected PBMCs were aliquoted (1 ml/tube) and stored in liquid nitrogen until use. The p24 concentration in each virus stock was quantitated using a noncommercial p24 enzyme-linked immunosorbent assay (ELISA) (34).
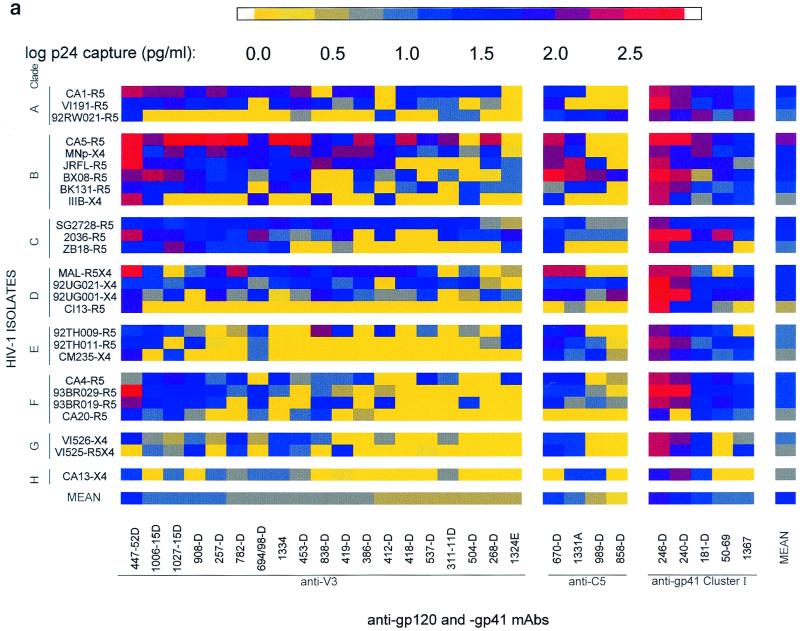
FIG. 1.
Binding profiles of human MAbs and HIV-1 isolates (a) grouped according to MAb specificities in the various envelope regions (anti-V3, anti-C5, and anti-gp41 cluster I) and virus clades (A to H) and (b) classified according to MAb groups (I to V) and virus immunotypes (1 to 3). The binding capacity of each MAb to each virus was measured by ELISA by assaying the amount of p24 released from MAb-bound virus after lysis. The p24 levels detected are represented as log-transformed data and were computed from the data presented in Table 1. Means shown were computed by adding a value of 1 to the amount of p24 captured by each MAb-virus combination (shown in Table 1). Subsequently, the values were transformed logarithmically and the means were calculated. For clarity, these logarithmic values have been color coded. Color ranges between 0.0 and 1.04 log (yellow-gray) correspond to lack of binding. Color ranges from >1.04 log (blue–dark blue–red) correspond to weak to strong binding. In both panels, the identity of the isolate is followed by a suffix indicating its coreceptor usage. In panel b, the prefix of the virus designation denotes the virus clade, and the prefix of the MAb designation denotes the MAb specificity.
Human anti-HIV MAbs.
Twenty-eight human anti-HIV-1 MAbs were used to elucidate the existence of immunotypes among the 26 HIV-1 isolates. These MAbs included 19 specific for the V3 region of gp120 (MAbs 447-52D, 419-D, 694/98-D, 838-D, 412-D, 1006-15D, 504-D, 257-D, 537-D, 311-11D, 386-D, 418-D, 1334, 782-D, 453-D, 908-D, 1027-15D, 268-D, and 1324E), 4 specific for the C5 region of gp120 (MAbs 670-D, 1331A, 858-D, and 989-D), and 5 specific for the cluster I region of gp41 (MAbs 50-69, 246-D, 240-D, 1367, and 181-D). The immunochemical specificity of each of these MAbs has previously been described (3, 14–16, 34, 49, 51, 52). All MAbs except 1324E were produced using PBMCs from HIV-1 clade B-infected persons. MAb 1324E was made from the cells of a clade E-infected individual (14). Human MAbs 860-55D to parvovirus B19 (11) and 246-D to gp41 (49) were used as negative and positive controls, respectively, as explained below. Human anti-p24 MAb 91-5 (13) was used in a noncommercial p24 ELISA to measure virus capture.
Virus-binding assay.
The virus-binding assay has been described in detail in previous studies (34, 36). Briefly, ELISA wells were coated at 4°C overnight with 100 μl of MAb at 10 μg/ml. After washing with phosphate-buffered saline (PBS) and blocking with 0.2 ml of 3% bovine serum albumin (BSA) in PBS (BSA-PBS), virus was added (100 μl/well at 100 ng of p24 per ml). After incubation at 37°C for 1 h, each well was washed with RPMI 1640 to remove unbound virus, and the bound virus was lysed with 250 μl of 1% Triton X-100. Positive and negative controls were performed with each isolate in each experiment. The amount of p24 captured in each MAb-virus test combination was quantified using human anti-p24 MAb 91-5 (13) in a noncommercial p24 ELISA (4).
To determine whether a particular MAb-virus combination displayed binding, the amount of captured p24 was compared to a threshold value (34). The threshold was calculated as the mean value plus six standard deviations for p24 from viruses captured with the negative control anti-parvovirus MAb 860-55D. The threshold value (T) was calculated as 11 pg of p24 per ml (log10 T = 1.04), and any value exceeding T was considered positive binding.
Cluster analysis.
The multivariate method of analysis has been used extensively and described in studies analyzing neutralization using HIV-1 isolates of different clades and sera from HIV-1-infected persons (37), in studies of the dynamics of neutralization escape mutants in a chimpanzee naturally infected with SIVcpz (35), and in analyses of the immunoreactivity of V3 peptides representing different HIV-1 clades with human anti-HIV-1 MAbs (51). Independent studies have employed this method to study the interaction between drugs and receptors and between viruses and antiviral compounds (2, 25). In the context of the study below, the analysis is based on the principle of grouping MAbs and viruses with similar specificities and profiles of reactivity.
This multivariate analysis was applied to the data matrix consisting of the amounts of p24 captured from the 26 viruses with each of 28 MAbs. To each p24 value, a value of 1 was added in order to eliminate values of 0; subsequently, the values were transformed logarithmically, and the log values were double centered by subtracting from the binding value for each MAb-virus combination the average binding value for all 26 isolates with that MAb and the average binding value for all 28 MAbs with that virus isolate. Each resulting value in the 26 by 28 matrix of data reflects the specific binding for each MAb-virus combination. Singular value decomposition of the doubly centered logarithmic matrix was computed, and the resulting transformed data were then used to determine the principal components, from which a dendogram was produced and the clusters of MAbs and isolates were identified by model-based clustering. Briefly, the dendograms were constructed by bottom-up hierarchical clustering of the data points obtained by singular value decomposition, from the isolate binding profile of each MAb (for the MAb dendogram), and from the MAb binding profile of each isolate (for the isolate dendogram). One begins with as many clusters as there are data points to cluster; there is at this time one point per cluster. The two clusters (both single points) that are closest are merged into a single cluster, thus reducing the number of clusters by one. Then, employing a notion of distance between clusters, the two closest clusters are again merged, thus again reducing the number of clusters by one more. The distance between clusters was defined as the maximum distance between pairs of points. This procedure is iterated until all the clusters have been merged into a single cluster. The dendogram displays the sequence of these merging steps. The clusters were then defined by model-based clustering, which is based on fitting probabilistic mixture models to the data and choosing the best-fitting model; this is accomplished by the method of maximum likelihood. The number of clusters is then chosen as the number of components of the best-fitting mixture model. These methods are described and explained in detail in the SPLUS statistical package and elsewhere (5, 6, 31, 32).
RESULTS
Binding patterns of anti-gp120 and anti-gp41 MAbs to intact, native primary HIV-1 isolates of group M, clades A to H.
In our previous work, we examined the ability of MAbs directed at epitopes in the V2, C2, V3, CD4bd, and C5 regions of gp120 and at clusters I and II of gp41 to bind to intact, native HIV-1 primary isolates of group M, clades A to H (36). The studies revealed that MAbs directed at epitopes in the V3 and C5 regions of gp120 and in the cluster I region of gp41 bound better than MAbs to epitopes in the V2, C2, and CD4bd regions of gp120 and in the cluster II region of gp41. These data demonstrated that the former regions are better exposed on HIV-1 primary isolates, and therefore 28 MAbs to V3 and C5 of gp120 and to cluster I of gp41 were used here to identify HIV-1 immunotypes. Table 1 shows the binding values for the 28 MAbs directed to these regions on 26 intact, native primary HIV-1 viruses of group M. The various HIV-1 isolates tested are listed according to their clades, and the MAbs are listed according to their specificities. Only binding levels of >11 pg of p24 per ml are considered positive (see above). Twenty-four of the 26 isolates (92%) gave mean binding levels with the 28 MAbs of >11 pg of p24/ml (shown in the right-hand column of Table 1), and 23 of the 28 MAbs (82%) gave mean binding levels with the 26 virus isolates of >11 pg of p24/ml (shown in the bottom row of Table 1).
TABLE 1.
Binding patterns of anti-gp120 and anti-gp41 MAbs with HIV-1 isolates
| Isolatea | Clade | Binding (pg of p24/ml) | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-V3 MAbs | Anti-C5 MAbs | Anti-gp41 MAbs | Meanb | |||||||||||||||||||||||||||
| 447-52D | 1006-15D | 1027-15D | 908-D | 257-D | 782-D | 694/98-D | 1334 | 453-D | 838-D | 419-D | 386-D | 412-D | 418-D | 537-D | 311-11D | 504-D | 268-D | 1324E | 670-D | 1331A | 989-D | 858-D | 246-D | 240-D | 181-D | 50-69 | 1367 | |||
| CA1-R5 | A | 148 | 100 | 120 | 69 | 118 | 57 | 52 | 80 | 108 | 0 | 75 | 55 | 0 | 42 | 21 | 17 | 19 | 17 | 0 | 19 | 25 | 0 | 0 | 245 | 141 | 80 | 70 | 16 | 61 |
| VI191-R5 | A | 39 | 49 | 61 | 25 | 50 | 20 | 0 | 33 | 67 | 37 | 4 | 27 | 0 | 30 | 8 | 6 | 0 | 4 | 0 | 60 | 0 | 0 | 0 | 252 | 129 | 10 | 66 | 10 | 35 |
| 92RW021-R5 | A | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 13 | 0 | 0 | 9 | 9 | 0 | 0 | 15 | 44 | 20 | 77 | 86 | 61 | 92 | 7 | 95 | 20 |
| CA5-R5 | B | 390 | 121 | 545 | 538 | 248 | 461 | 80 | 517 | 525 | 55 | 50 | 226 | 37 | 216 | 80 | 100 | 0 | 206 | 0 | 157 | 74 | 0 | 0 | 308 | 311 | 96 | 66 | 25 | 194 |
| MNp-X4 | B | 299 | 16 | 122 | 39 | 122 | 67 | 75 | 21 | 88 | 65 | 90 | 126 | 71 | 25 | 6 | 39 | 4 | 21 | 1 | 138 | 25 | 0 | 0 | 134 | 65 | 87 | 12 | 25 | 64 |
| JR-FL-R5 | B | 370 | 72 | 72 | 26 | 16 | 40 | 83 | 37 | 42 | 103 | 57 | 63 | 72 | 0 | 0 | 0 | 0 | 13 | 6 | 100 | 197 | 18 | 0 | 164 | 67 | 65 | 68 | 3 | 63 |
| BX08-R5 | B | 103 | 203 | 136 | 69 | 11 | 24 | 3 | 35 | 52 | 0 | 0 | 26 | 5 | 24 | 0 | 7 | 0 | 1 | 7 | 252 | 216 | 120 | 67 | 386 | 155 | 2 | 66 | 21 | 71 |
| BK131-R5 | B | 68 | 39 | 50 | 73 | 29 | 27 | 0 | 65 | 43 | 0 | 0 | 14 | 0 | 9 | 30 | 0 | 15 | 6 | 8 | 4 | 27 | 21 | 0 | 209 | 96 | 13 | 9 | 12 | 31 |
| IIIB-X4 | B | 169 | 44 | 0 | 0 | 0 | 0 | 52 | 0 | 0 | 51 | 0 | 0 | 28 | 0 | 0 | 0 | 11 | 0 | 0 | 21 | 0 | 0 | 0 | 175 | 20 | 5 | 22 | 10 | 22 |
| SG2728-R5 | C | 29 | 45 | 16 | 25 | 31 | 45 | 31 | 69 | 43 | 71 | 32 | 50 | 32 | 45 | 25 | 43 | 26 | 4 | 2 | 13 | 28 | 4 | 4 | 109 | 38 | 26 | 19 | 19 | 33 |
| 2036-R5 | C | 150 | 75 | 57 | 23 | 30 | 10 | 92 | 8 | 3 | 7 | 85 | 0 | 33 | 0 | 0 | 37 | 13 | 14 | 51 | 22 | 8 | 6 | 6 | 314 | 289 | 77 | 240 | 10 | 60 |
| ZB18-R5 | C | 20 | 18 | 96 | 58 | 16 | 14 | 15 | 22 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 24 | 0 | 0 | 0 | 208 | 23 | 20 | 29 | 0 | 21 |
| MAL-R5X4 | D | 276 | 13 | 0 | 8 | 62 | 153 | 22 | 37 | 66 | 21 | 64 | 55 | 72 | 69 | 14 | 0 | 6 | 0 | 2 | 162 | 189 | 0 | 0 | 245 | 192 | 6 | 0 | 11 | 62 |
| 92UG021-X4 | D | 15 | 16 | 6 | 30 | 43 | 24 | 5 | 16 | 28 | 48 | 7 | 20 | 0 | 22 | 47 | 13 | 22 | 2 | 0 | 10 | 14 | 10 | 26 | 480 | 114 | 19 | 26 | 46 | 40 |
| 92UG001-X4 | D | 53 | 4 | 22 | 0 | 62 | 5 | 18 | 1 | 5 | 74 | 19 | 7 | 5 | 3 | 10 | 0 | 2 | 13 | 6 | 51 | 52 | 4 | 98 | 471 | 287 | 27 | 54 | 72 | 51 |
| CI13-R5 | D | 74 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 288 | 33 | 50 | 3 | 1 | 16 |
| 92TH009-R5 | E | 15 | 23 | 14 | 4 | 0 | 2 | 16 | 0 | 0 | 114 | 26 | 0 | 44 | 0 | 6 | 0 | 5 | 0 | 27 | 39 | 51 | 0 | 0 | 135 | 61 | 28 | 12 | 0 | 22 |
| 92TH011-R5 | E | 19 | 19 | 0 | 19 | 1 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 17 | 52 | 12 | 9 | 1 | 172 | 136 | 8 | 20 | 19 | 19 |
| CM235-X4 | E | 53 | 0 | 10 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 42 | 7 | 19 | 2 | 116 | 68 | 40 | 8 | 13 | 14 |
| CA4-R5 | F | 5 | 37 | 72 | 49 | 6 | 22 | 0 | 27 | 3 | 6 | 9 | 10 | 0 | 0 | 50 | 7 | 0 | 0 | 18 | 35 | 39 | 0 | 2 | 227 | 122 | 30 | 74 | 14 | 31 |
| 93BR029-R5 | F | 492 | 25 | 34 | 44 | 27 | 8 | 17 | 9 | 0 | 9 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 16 | 5 | 1 | 262 | 276 | 40 | 45 | 18 | 48 |
| 93BR019-R5 | F | 102 | 37 | 19 | 19 | 13 | 0 | 19 | 0 | 0 | 17 | 18 | 0 | 30 | 0 | 0 | 0 | 12 | 0 | 0 | 18 | 4 | 9 | 4 | 167 | 122 | 23 | 67 | 26 | 26 |
| CA20-R5 | F | 34 | 9 | 17 | 5 | 0 | 0 | 6 | 0 | 8 | 11 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 64 | 0 | 29 | 12 | 7 | 7 |
| VI526-X4 | G | 43 | 4 | 2 | 8 | 2 | 18 | 2 | 13 | 13 | 8 | 0 | 0 | 4 | 0 | 0 | 4 | 0 | 0 | 0 | 14 | 13 | 0 | 0 | 201 | 141 | 23 | 0 | 5 | 19 |
| VI525-R5X4 | G | 0 | 9 | 3 | 19 | 0 | 15 | 0 | 16 | 9 | 0 | 26 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 26 | 15 | 0 | 0 | 156 | 83 | 27 | 0 | 29 | 16 |
| CA13-X4 | H | 15 | 0 | 6 | 0 | 6 | 3 | 9 | 9 | 4 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 12 | 12 | 0 | 67 | 132 | 12 | 0 | 0 | 10 |
| Meanc | 115 | 38 | 57 | 44 | 34 | 39 | 24 | 39 | 43 | 27 | 22 | 26 | 17 | 19 | 12 | 11 | 6 | 12 | 6 | 50 | 41 | 10 | 11 | 217 | 122 | 36 | 38 | 20 | 41 |
Some isolates bind strongly to several MAbs and, consequently, show efficient virus capture with high average binding values, while other isolates bind weakly or not at all to most MAbs and as a result show weak average binding. For example, virus CA5 (clade B) was the most efficiently bound isolate tested, binding to 24 of 28 (86%) of the MAbs with an average binding value of 194 pg of p24/ml. Though CA5 is efficiently captured by many MAbs, it is poorly captured by MAbs such as 504-D (anti-V3) and 1367 (anti-gp41). Isolates like CA5 that are efficiently captured by particular MAbs bear epitopes that are accessible to such MAbs but do not possess or do not display the epitopes of the MAbs which fail to capture them. Isolate CA20 (clade F) is an example of a virus that bound only weakly to 5 of the 28 (18%) MAbs tested; the average binding value for this isolate to the 28 MAbs (7 pg of p24/ml) was below the cut-off value for positive binding.
Similarly, some MAbs bind strongly to several isolates and consequently show strong average binding, while other MAbs bind weakly or not at all to most isolates and as a result show weak average binding. For example, as shown in Table 1, MAb 447-52D (an anti-V3 MAb) binds to 24 of 26 isolates (92%) and shows a high mean binding value to the isolates tested (115 pg of p24/ml; bottom row, Table 1). Another anti-V3 MAb, 257-D, bound to only 14 of 26 viruses, mostly displaying relatively weak binding, and consequently had an average binding value for all isolates of 34 pg of p24/ml. The quantitative aspects of this assay reveal that the more strongly a MAb binds to an isolate or group of isolates, the more exposed is the epitope on such isolates. Weak or no binding is associated with poor exposure of the epitope or its absence from the virion under examination. Thus, the epitope to which MAb 447-52D is directed is better exposed and present on more isolates than the epitope recognized by MAb 257-D.
In Table 1, the average binding values for each isolate with all 28 MAbs, shown in the right-hand column, can be compared to the binding value for each individual MAb-virus combination. Similarly, the mean binding value for each MAb to all 26 isolates is shown in the bottom row. The deviation of the individual value from the mean values reflects the specificity of the reaction. The closer the individual MAb-virus binding value is to the mean values, the less specific is the reaction, while the greater the deviation from the mean values, the greater is the specificity of the individual MAb-virus interaction. Many examples of MAb-virus binding values that are similar (poorly specific) or dissimilar (highly specific) to the mean values can be found in Table 1. For example, the reaction of MAb 419-D (anti-V3) with clade G virus VI525 (26 pg of p24/ml) is similar to the mean reactivity of MAb 419-D with all 26 viruses (22 pg of p24/ml) and to the mean reactivity of VI525 with all 28 MAbs (16 pg of p24/ml). Therefore, little specificity is attributable to the recognition of VI525 by MAb 419-D. In contrast, the capture of clade B virus MNp (clade B) by MAb 1027-15D (anti-V3) results in 122 pg of p24/ml, which reflects high specificity, since the average reactivity of MAb 1027-15D with all viruses is 57 pg of p24/ml and the average reactivity of MNp with all 28 MAbs is 64 pg of p24/ml. These data also identify MAbs that react with most viruses equally well and therefore are not specific, e.g., MAb 246-D (anti-gp41), which binds to all viruses, and other MAbs that show differential binding to the various viruses and are thus highly specific, e.g., MAb 386-D (anti-V3), which binds to only 38% of viruses.
The data in Table 1 were transformed into logarithmic values and color coded according to the level of p24 capture and are displayed in Fig. 1a. By visual inspection of these data, grouped according to MAb specificity and virus clade, one can discern that there is extensive cross-reactivity between MAbs of different specificities and viruses of various clades, and no clade-specific reactivity can be observed. In order to discern if these data could reveal immunologically related groups of viruses, a method of analysis was employed that groups isolates and MAbs into clusters based on their profiles of specific binding.
Mathematical analysis of the data matrix.
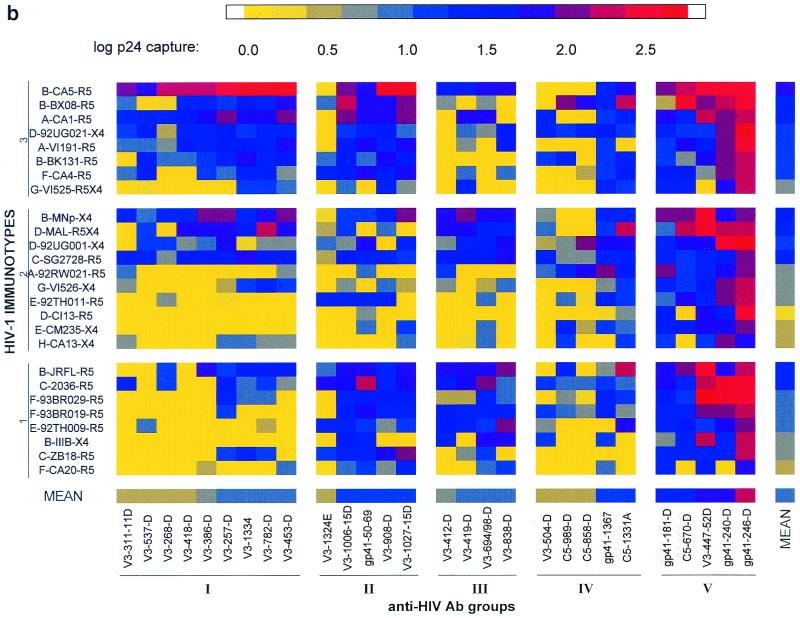
In previous studies, analysis of the immunoreactivity of various human anti-V3 MAbs with V3 peptides derived from 56 HIV-1 isolates was used to test the validity of the mathematical methods (51). The mathematical analyses classified the MAbs studied into groups which correlated well with their immunochemical properties. Given the concordance of the mathematically and immunochemically derived groupings of the MAbs, we used the same mathematical techniques to analyze for the presence of immunologically defined clusters among the 28 anti-HIV-1 MAbs on the basis of the profiles of their reactivities with the 26 intact, native HIV-1 isolates, i.e., on the pattern of specific binding of each MAb to each of the 26 isolates. Similarly, analyses were done to group the 26 viruses into immunotypes on the basis of their profiles of reactivities with the 28 MAbs. The results of the mathematical clustering of the 28 MAbs and the 26 viruses are displayed in Fig. 1b and as dendograms in Fig. 2 and 3. The 28 MAbs were classified into five Ab groups, and the 26 viruses were grouped into three immunotypes. The prefix of each MAb listed in Fig. 1b and 2 identifies the region on the viral envelope to which the MAb is directed, and the prefix of each isolate in Fig. 1b and 3 identifies the clade to which the virus belongs.
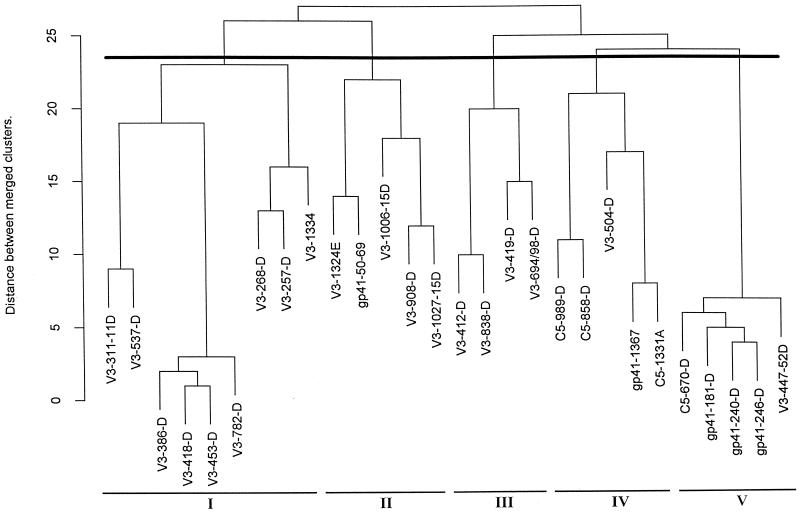
FIG. 2.
Dendrogram of 28 MAbs showing their immunologic relationships defined by binding with 26 intact, primary HIV-1 isolates. The dark line denotes the point in the tree at which model-based clustering delineates the most probabilistic number of clusters—five in this case. Each MAb group (cluster) is numbered I to V. The distance between merged groups can be determined on the scale shown on the y axis.
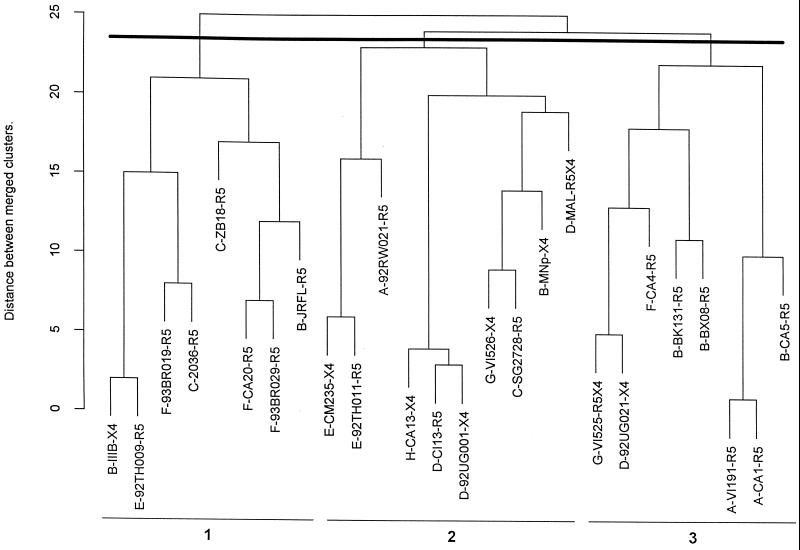
FIG. 3.
Dendogram of 26 intact, primary HIV-1 isolates showing their immunologic relationships defined by binding with 28 MAbs. The dark line denotes the point in the tree at which model-based clustering delineates the most probabilistic number of clusters—three in this case. Each viral immunotype is numbered 1 to 3. The distance between merged immunotypes can be determined on the scale shown on the y axis.
The analysis revealed binding profiles of MAb-virus combinations that defined HIV-1 immunotypes which could not have been identified by visual inspection. Thus, Fig. 1a, grouped by clade and MAb specificity, shows no consistent profile which could characterize any specific group of MAbs or viruses. But when the data in Fig. 1a were subjected to the multivariate cluster analysis and the MAbs and virus isolates were rearranged based on the similarities in their binding profiles, five groups of MAbs and three virus immunotypes were identified (Fig. 1b, 2, and 3).
Cluster analysis of the MAbs.
The five groups of MAbs and their relationships to one another, as revealed by the multivariate analysis, are shown in the dendogram in Fig. 2. The analysis defined Ab groups which were either homogeneous or heterogeneous with respect to the specificities of the MAbs they contained. Three of five Ab groups (I, II, and III) contained anti-V3 MAbs (with the one exception of MAb 50-69 to gp41 in Ab group II). The other two Ab groups (IV and V) contained mixtures of V3, C5, and gp41 MAbs.
The homogeneous Ab groups (I, II, and III) could distinguish between the HIV immunotypes, while the heterogeneous Ab groups (IV and V) revealed no specificity for the immunotypes. Thus, Fig. 1b shows that MAbs belonging to Ab group V exhibited the strongest overall binding and MAbs belonging to Ab group IV exhibited the weakest overall binding. Neither of these groups was specific for viruses in any of the three virus immunotypes (defined below). Conversely, though MAbs belonging to Ab group I bound poorly or not at all with most isolates in immunotypes 1 and 2, they did bind strongly to most isolates in virus immunotype 3 (Fig. 1b). Similarly, MAbs in Ab groups II and III also differentiated between virus immunotypes.
Cluster analysis of the isolates.
In order to define virus immunotypes, the dendogram displaying the results of the analysis of the grouping of isolates on the basis of their reactivities with the MAbs was cut at the same height as the MAb dendogram (Fig. 2). Only three HIV-1 immunotypes were identified (Fig. 3). The clustering of the isolates did not correlate with the viruses' genetic subtypes (denoted by the prefixes of the isolates in Fig. 3), with coreceptor usage (denoted by the suffixes of the isolates, Fig. 3), or with the geographic area from which the isolates were derived. Thus, none of the three HIV-1 immunotypes contained isolates from a single country or continent. For example, HIV-1 immunotype 1 comprises isolates from patients in Africa, the United States, Asia, and South America. These results are consistent with earlier work showing that HIV-1 serotypes identified on the basis of neutralization of HIV-1 isolates by sera and HIV immunotypes identified on the basis of V3 peptide reactivity with MAbs do not correlate with genetically defined clades (37, 51). The results reported here, like the previously published conclusions, suggest that isolates of different clades that belong to the same immunotype share distinguishing epitopes. These results also suggest that despite the genetic variability that marks the HIV-1 family of viruses, there are certain immunologic characteristics that are widely shared among virus isolates and others that divide the viruses into immunologically identifiable groups.
Reactivity and specificity profiles of HIV-1 immunotypes with Ab groups.
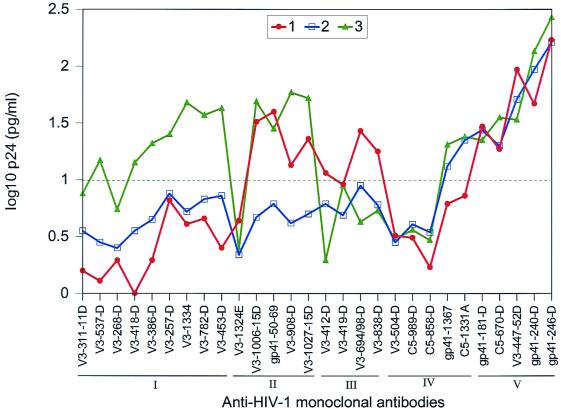
In order to identify epitopes (i) that are conserved on isolates belonging to the same HIV-1 immunotypes, (ii) that differentiate the members of the three immunotypes, and (iii) that are shared among all immunotypes, the average reactivity of viruses in each HIV-1 immunotype with each of the individual MAbs was examined. The results are shown in Fig. 4. The data show that MAbs belonging to Ab group I bound preferentially to HIV-1 isolates belonging to HIV-1 immunotype 3 rather than to isolates of HIV-1 immunotypes 1 and 2. This can also be observed by examining the overall pattern of binding in Fig. 1b. In contrast, MAbs of Ab group II bound better to isolates of both HIV-1 immunotypes 1 and 3 than to viruses of immunotype 2 (Fig. 4 and 1b), and MAbs of Ab group III bound preferentially to isolates of HIV-1 immunotype 1 (though overlap with some isolates in immunotype 2 was observed). While the MAbs of Ab group IV generally bound poorly to the HIV-1 isolates and the MAbs of Ab group V generally bound strongly, the MAbs in these two Ab groups did not distinguish among the three immunotypes.
FIG. 4.
Average p24 capture profiles by each MAb for the isolates in the three viral immunotypes. The MAbs are arranged according to their respective groups (I to V) on the x axis, and their reactivity profiles with the various viral immunotypes (1 to 3) are shown in the graph. The reactivity of each individual MAb with all isolates belonging to each viral immunotype is plotted in the graph. The broken line represents the cut-off (1.04 logs), as described in the text. Only values above the broken line represent significant positive binding.
The results clearly indicate that viral immunotypes identified by the mathematical analysis are defined by their immunologic characteristics. The preferential reactivity of MAbs from distinct Ab groups with specific HIV-1 immunotypes suggests (i) that constellations of specific epitopes are conserved among isolates belonging to each of the HIV-1 immunotypes, (ii) that there are epitopes that distinguish the immunotypes, and (iii) that there are epitopes that are commonly present or routinely shared by all immunotypes.
We also determined the specificity of each Ab group with each of the HIV-1 immunotypes. The specificity data presented in Table 2 are derived from the log-transformed, double-centered p24 binding values. Like the data shown in Fig. 4, the specificity analysis shows that the members of Ab group I demonstrate preferential binding with isolates of HIV-1 immunotype 3 versus isolates of HIV-1 immunotypes 1 and 2 (Table 2). Similarly, there is a high specificity of Ab group II for HIV-1 immunotype 1 versus HIV-1 immunotypes 2 and 3 and for Ab group III with virus immunotype 1, while Ab groups IV and V do not distinguish among the viral immunotypes.
TABLE 2.
Specificities of MAb groups with HIV-1 immunotypesa
| Ab group | Binding specificity for HIV-1 immunotype: | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| I | −0.31 | 0.01 | 0.30 |
| II | 0.27 | −0.32 | 0.13 |
| III | 0.40 | 0.04 | −0.44 |
| IV | −0.09 | 0.18 | −0.13 |
| V | 0.06 | 0.09 | −0.17 |
DISCUSSION
In order to protect against organisms that exhibit significant genetic variation, polyvalent vaccines are needed. Genetic variations among microorganisms such as Streptococcus pneumoniae and poliovirus have been documented, and the antigenic relationships of each have been studied (8, 17, 21, 29), resulting in definition of immunologic characteristics that distinguish each into serotypes. In the case of poliovirus, for example, three serotypes have been documented and proven critical to polio vaccine development (9, 20, 42).
Given the extreme variability of HIV-1, it is probable that a polyvalent vaccine will be needed for protection against the large number of strains. However, in order to understand how to construct such a polyvalent vaccine that will be useful against a maximum number of strains, serotypes or immunotypes of HIV-1 must be identified.
It is clear from several independent studies that HIV-1 genotypes do not generally correlate with immunotypes, although clades B and E appear to represent a special case (28). The poor correlation between HIV-1 genotypes and immunotypes is a consistent finding whether HIV peptides or recombinant proteins were analyzed with sera or MAbs. Even sera from individuals immunized with vaccine constructs based solely on clade B CXCR4-tropic strains have been found to cross-react with V3 peptides from diverse clades and to neutralize HIV-1 primary isolates that are from clades B and C which are CXCR4-tropic, CCR5-tropic, or dual-tropic (46), and cross-clade reactive cell-mediated immunity has also been induced with vaccines (10). These studies suggest that selection of vaccine components should not be based entirely on the choice of immunogens representing distinct HIV-1 genotypes, but rather should rely on selecting representatives of HIV immunotypes. However, studies aimed at defining HIV-1 serotypes or immunotypes, and the epitopes that distinguish them, have been sparse. Those studies that have been performed were based on the use of polyclonal sera (which made the definition of critical epitopes an impossible task [37]) or on peptides or recombinant envelope proteins that do not mimic the native structure of the virus (51).
The data presented above show that the patterns of binding of MAbs to intact virions do not correspond with the country or continent of origin of the virus or with the clade or coreceptor which characterizes the isolate. Yet many of these viruses have common antigenic epitopes that could be useful for selecting the minimum number of HIV-1 strains to be combined into a polyvalent vaccine in order to represent and protect against the maximum number of HIV-1 isolates.
The best-exposed epitopes that occur on the surface of intact virions are found in the V3 and C5 regions of gp120 and in antigenic cluster I of gp41 (spanning amino acids 579 to 613 of the envelope) (36). Therefore, the presence of these epitopes on the surface of virions of 26 isolates was examined immunologically and then analyzed using a mathematical approach, previously described, for identifying how to best classify diverse groups of HIV-1 isolates and MAbs (51). This method, applied to the interaction of MAbs and intact virions, revealed that these 26 HIV-1 isolates, from diverse clades and geographic origins, could be divided into a small number of immunotypes. This is not dissimilar to results derived from other studies and mathematical analyses based on data matrices generated (i) by neutralization assays using polyclonal sera from HIV-1-infected subjects with isolates from HIV-1 groups M and O (37), (ii) with immunochemical data matrices generated with human anti-HIV-1 MAbs and V3 peptides (51), or (iii) by cluster analysis of V3 peptides and polyclonal sera from HIV-infected patients from around the world (41). In each case, these independent studies revealed that there are fewer HIV-1 immunotypes than genotypes and that these immunotypes do not correlate with clades or coreceptor usage (37, 41, 51).
While studies of HIV-1 neutralization with polyclonal sera revealed the presence of neutralization serotypes (37), the epitopes involved could not be identified, although the complex nature of the humoral immune response would suggest that Abs of multiples specificities might contribute to the findings. Interestingly, the study presented above demonstrates that virus immunotypes were distinguished primarily on the basis of anti-V3 MAbs. Thus, of the MAbs that best distinguished among the three virus immunotypes, 17 of 18 were directed against V3 (Fig. 4). The role of the anti-V3 response in protective immunity against primary HIV-1 isolates is still highly controversial: only one human MAb, 447-52D, has been shown to have significant neutralizing activity for primary isolates (7). However, there is growing evidence that Abs to the conformational epitopes in V3 may be quite potent (40, 43; P. F. Zhang, X. Chen, J. B. Margolick, J. E. Robinson, S. Zolla-Pazner, M. N. Flora, and G. V. Quinnan, Jr., submitted for publication). While none of the anti-V3 MAbs identified here as MAbs that can distinguish among the three HIV-1 immunotypes have been shown to have broad or potent neutralizing activity when tested separately, these Abs could have biological effects when present in combination with each other, with Abs in the other two Ab groups, or with Abs not tested in this study. Indeed, many examples of the additive and synergistic interaction of Abs against HIV-1 exist in the literature (24, 27, 45), and the data presented here provide an indication as to which of the myriad of possible combinations of MAbs might be useful to test in synergy experiments to determine which combinations might most potently enhance neutralization of primary isolates and which combinations might give the broadest neutralization among diverse isolates.
To date, HIV-1 vaccine constructs have used immunogens derived from, at most, two HIV-1 clades, B and E (1). While the results of these trials are not yet available, it is unlikely that such a vaccine will protect against the majority of all HIV-1 viruses. It is equally unlikely that the preparation of a polyvalent vaccine including immunogens derived from all the many HIV-1 clades will be feasible. Thus, it is imperative to generate data which will help to estimate how many isolates, and which ones, need to be included in a vaccine that will give the maximum breadth of protection. For this, identification of HIV-1 immunotypes is a critical step.
The results of the study presented above clearly demonstrate that immunotypes of HIV-1 can be defined, as they have been for other microorganisms, and that each HIV-1 immunotype comprises isolates of different genetic subtypes from different geographic origins and of strains with different coreceptor tropisms. These findings give rise to a testable hypothesis, that polyvalent vaccines comprising representatives of a few HIV-1 immunotypes may give rise to broader immunity than polyvalent vaccines comprising representatives of the many HIV-1 genotypes.
Meanwhile, the different HIV-1 immunotypes, as defined here, can be defined on the basis of both common and distinguishing epitopes. Shared epitopes are recognized on most virions by MAbs that belong to Ab group V that are specific for antigenic determinants at the tip of the V3 loop, in the C-terminal portion of gp120, and at the apex of the immunodominant loop of gp41. In contrast, immunotypes can be distinguished primarily on the basis of the recognition by MAbs of what appear to be distinct shapes in the V3 regions of the three viral immunotypes. These shapes are dictated, only in part, by the V3 amino acid sequence; as shown previously, these shapes are also profoundly influenced by conformational parameters to which the rest of the gp120 molecule contributes (12, 51). Thus, immunoreactivity to V3 peptides is only of limited value. The ability to probe the antigenic nature of the intact virus particle, both immunochemically and functionally, thus promises to yield critical information pertinent to the human antiviral immune response and to the generation of broad protective immunity.
ACKNOWLEDGMENTS
This study was supported in part by grants from the National Institutes of Health (AI 44302, AI 32424, AI 36085, AI47053, and HL 59725) and by research funds from the Department of Veterans Affairs, through the VA Research Center for AIDS and HIV Infection.
We thank John Sullivan for the primary HIVMN isolate (MNp) and Guido van der Groen for the isolates from Cameroon, Ivory Coast, Belgium, and Gabon used in this study.
REFERENCES
- 1.Altman L K. FDA authorizes first full testing for HIV vaccine. New York, N.Y: New York Times; 1998. [PubMed] [Google Scholar]
- 2.Andries K, Dewindt B, Snoeks J, Wouters L, Moereels J, Lewi P J, Janssen P A J. Two groups of rhinoviruses revealed by a panel of antiviral compounds present sequence divergence and differential pathogenicity. J Virol. 1990;64:1117–1123. doi: 10.1128/jvi.64.3.1117-1123.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandres J C, Wang Q F, O'Leary J, Baleaux F, Amara A, Hoxie J, Zolla-Pazner S, Gorny M K. Human immunodeficiency virus (HIV) envelope binds to CXCR4 independently of CD4, and binding can be enhanced by interaction with soluble CD4 or by HIV envelope deglycosylation. J Virol. 1998;72:2500–2504. doi: 10.1128/jvi.72.3.2500-2504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastiani L, Laal S, Zolla-Pazner S, Kim M. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J Virol. 1997;71:3444–3450. doi: 10.1128/jvi.71.5.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryk A S, Raudenbush S W. Hierarchial linear models: applications and data analysis methods. Newbury Park, N.J: Sage Publications; 1992. [Google Scholar]
- 6.Carlin B P, Louis T A. Bayes and empirical Bayes methods for data analysis. London, United Kingdom: Chapman and Hall; 1996. [Google Scholar]
- 7.Conley A J, Gorny M K, Kessler II J A, Boots L J, Lineberger D, Emini E A, Ossorio M, Koenig S, Williams C, Zolla-Pazner S. Neutralization of primary human immunodeficiency virus type 1 virus isolates by the broadly reactive anti-V3 monoclonal antibody 447-52D. J Virol. 1994;68:6994–7000. doi: 10.1128/jvi.68.11.6994-7000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crainic R, Kew O. Evolution and polymorphism of poliovirus genomes. Biologicals. 1993;21:379–384. doi: 10.1006/biol.1993.1099. [DOI] [PubMed] [Google Scholar]
- 9.D'Angio C T, Maniscalco W M, Pichichero M E. Immunologic response of extremely premature infants to tetanus, Haemophilus influenzae, and polio immunizations. Pediatrics. 1995;96:18–22. [PubMed] [Google Scholar]
- 10.Ferrari G, Humphrey W, McElrath M J, Excler J-L, Duliege A M, Clements M L, Corey L C, Bolognesi D P, Weinhold K J. Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers. Proc Natl Acad Sci USA. 1997;94:1396–1401. doi: 10.1073/pnas.94.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gigler A, Dorsch S, Hemauer A, Williams C, Kim S, Young N S, Zolla-Pazner S, Wolf H, Gorny M K, Modrow S. Generation of neutralizing human monoclonal antibodies against parvovirus B19 proteins. J Virol. 1999;73:1974–1979. doi: 10.1128/jvi.73.3.1974-1979.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorny M K, Conley A J, Karwowska S, Buchbinder A, Xu J-Y, Emini E A, Koenig S, Zolla-Pazner S. Neutralization of diverse HIV-1 variants by an anti-V3 human monoclonal antibody. J Virol. 1992;66:7538–7542. doi: 10.1128/jvi.66.12.7538-7542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorny M K, Gianakakos V, Sharpe S, Zolla-Pazner S. Generation of human monoclonal antibodies to HIV. Proc Natl Acad Sci USA. 1989;86:1624–1628. doi: 10.1073/pnas.86.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorny M K, Mascola J R, Israel Z R, VanCott T C, Williams C, Balfe P, Hioe C, Brodine S, Burda S, Zolla-Pazner S. A human monoclonal antibody specific for the V3 loop of HIV type 1 clade E cross-reacts with other HIV type 1 clades. AIDS Res Hum Retroviruses. 1998;14:213–221. doi: 10.1089/aid.1998.14.213. [DOI] [PubMed] [Google Scholar]
- 15.Gorny M K, VanCott T C, Hioe C, Israel Z R, Michael N L, Conley A J, Williams C, Kessler II J A, Chigurupati P, Burda S, Zolla-Pazner S. Human monoclonal antibodies to the V3 loop of HIV-1 with intra- and interclade cross-reactivity. J Immunol. 1997;159:5114–5122. [PubMed] [Google Scholar]
- 16.Gorny M K, Xu J-Y, Karwowska S, Buchbinder A, Zolla-Pazner S. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol. 1993;150:635–643. [PubMed] [Google Scholar]
- 17.Huovilainen A, Mulders M N, Agboatwalla M, Poyry T, Stenvik M, Hovi T. Genetic divergence of poliovirus strains isolated in the Karachi region of Pakistan. J Gen Virol. 1995;76:3079–3088. doi: 10.1099/0022-1317-76-12-3079. [DOI] [PubMed] [Google Scholar]
- 18.Janssens W, Heyndrickx L, Fransen K, Mitte J, Peeters M, Nkengasong J N, Ndumbe P M, Delaporte E, Perret J-L, Atende C, Piot P, van der Groen G. Genetic and phylogenetic analysis of env subtypes G and H in Central Africa. AIDS Res Hum Retroviruses. 1994;10:877–879. doi: 10.1089/aid.1994.10.877. [DOI] [PubMed] [Google Scholar]
- 19.Janssens W, Nkengasong J N, Heyndrickx L, Fransen K, Ndumbe P M, Delaporte E, Peeters M, Perret J-L, Ndoumou A, Atende C, Piot P, van der Groen G. Further evidence of the presence of genetically aberrant HIV-1 strains in Cameroon and Gabon. AIDS. 1994;8:1012–1013. doi: 10.1097/00002030-199407000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Khalak R, Pichichero M E, D'Angio C T. Three-year follow-up of vaccine response in extremely pre-term infants. Pediatrics. 1998;101:597–603. doi: 10.1542/peds.101.4.597. [DOI] [PubMed] [Google Scholar]
- 21.Kilpatrick D R, Nottay B, Yang C F, Yang S J, DaSilva E, Penaranda S, Pallansch M, Kew O. Serotype-specific identification of poliovirus by PCR using primers containing mixed-base or deoxyinosine residues at positions of codon degeneracy. J Clin Microbiol. 1998;36:352–357. doi: 10.1128/jcm.36.2.352-357.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostrikis L G, Bagdades E, Cao Y, Zhang L, Dimitriou D, Ho D D. Genetic analysis of human immunodeficiency virus type 1 strains from patients in Cyprus: identification of a new subtype designated subtype I. J Virol. 1995;69:6122–6130. doi: 10.1128/jvi.69.10.6122-6130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kostrikis L G, Cao Y, Ngai H, Moore J P, Ho D D. Quantitative analysis of serum neutralization of human immunodeficiency virus type 1 from subtypes A, B, C, D, E, F, and I: lack of direct correlation between neutralization serotypes and genetic subtypes and evidence for prevalent serum-dependent infectivity enhancement. J Virol. 1996;70:445–458. doi: 10.1128/jvi.70.1.445-458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laal S, Burda S, Gorny M K, Karwowska S, Buchbinder A, Zolla-Pazner S. Synergistic neutralization of human immunodeficiency virus type 1 by combinations of human monoclonal antibodies. J Virol. 1994;68:4001–4008. doi: 10.1128/jvi.68.6.4001-4008.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewi P J. Spectral mapping, a technique for classifying biological activity profiles of chemical compounds. Drug Res. 1976;26:1295–1300. [PubMed] [Google Scholar]
- 26.Louwagie J, McCutchan F E, Peeters M, Brennan T P, Sanders-Buell E, Eddy G A, van der Groen G, Fransen K, Gershy-Damet G-M, Deleys R, Burke D S. Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS. 1993;7:769–780. doi: 10.1097/00002030-199306000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Mascola J R, Louder M K, VanCott T C, Sapan C V, Lambert J S, Muenz L R, Bunow B, Birx D L, Robb M L. Potent and synergistic neutralization of human immunodeficiency virus type 1 (HIV-1) primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J Virol. 1997;71:7198–7206. doi: 10.1128/jvi.71.10.7198-7206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mascola J R, Louwagie J, McCutchan F E, Fischer C L, Hegerich P A, Wagner K F, Fowler A K, McNeil J G, Burke D S. Two antigenically distinct subtypes of HIV-1: viral genotype predicts neutralization immunotype. J Infect Dis. 1994;169:48–54. doi: 10.1093/infdis/169.1.48. [DOI] [PubMed] [Google Scholar]
- 29.Melnick J L. The discovery of the enterovirus and the classification of poliovirus among them. Biologicals. 1993;21:305–309. doi: 10.1006/biol.1993.1088. [DOI] [PubMed] [Google Scholar]
- 30.Myers G, MacInnes K, Korber B. The emergence of simian/human immunodeficiency viruses. AIDS Res Hum Retroviruses. 1992;8:373–386. doi: 10.1089/aid.1992.8.373. [DOI] [PubMed] [Google Scholar]
- 31.Nádas A. Binary classification by stochastic neural nets. Trans Neural Networks. 1995;6:488–491. doi: 10.1109/72.363484. [DOI] [PubMed] [Google Scholar]
- 32.Nádas A. Hidden Markov models and some connections with artificial neural nets. In: Smolensky E A P, editor. Mathematical perspectives on neural networks. Lawrence Erlbaum Associates, Inc.; 1995. pp. 603–605. [Google Scholar]
- 33.Nkengasong J N, Janssens W, Heyndrickx L, Fransen K, Ndumbe P M, Motte J, Leonaers A, Ngolle M, Ayuk J, Piot P, van der Groen G. Genetic subtypes of HIV-1 in Cameroon. AIDS. 1994;8:1405–1412. doi: 10.1097/00002030-199410000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Nyambi P N, Gorny M K, Bastiani L, van der Groen G, Williams C, Zolla-Pazner S. Mapping of epitopes exposed on intact HIV-1 virions: a new strategy for studying the immunologic relatedness of HIV-1. J Virol. 1998;72:9384–9391. doi: 10.1128/jvi.72.11.9384-9391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nyambi P N, Lewi P, Peeters M, Janssens W, Heyndrickx L, Fransen K, Andries K, Haesevelde M V, Heeney J, Piot P, van der Groen G. Study of the dynamics of neutralizing escape mutants in a chimpanzee naturally infected with the simian immunodeficiency virus (SIVcpz-ant) J Virol. 1997;71:2320–2330. doi: 10.1128/jvi.71.3.2320-2330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nyambi P N, Mbah H A, Burda S, Williams C, Gorny M K, Nàdas A, Zolla-Pazner S. Conserved and exposed epitopes on intact, native, primary human immunodeficiency virus type 1 virions of group M. J Virol. 2000;74:7096–7107. doi: 10.1128/jvi.74.15.7096-7107.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nyambi P N, Nkengasong J, Lewi P, Andries K, Janssens W, Fransen K, Heyndrickx L, Piot P, van der Groen G. Multivariate analysis of human immunodeficiency virus type 1 neutralization data. J Virol. 1996;70:6235–6243. doi: 10.1128/jvi.70.9.6235-6243.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nyambi P N, Nkengasong J, Peeters M, Simon F, Eberle J, Janssens W, Fransen K, Willems B, Vereecken K, Heyndrickx L, Piot P, van der Groen G. Reduced capacity of antibodies from patients infected with human immunodeficiency virus type-1 (HIV-1) group O to neutralize primary isolates of HIV-1 group M viruses. J Infect Dis. 1995;172:1228–1237. doi: 10.1093/infdis/172.5.1228. [DOI] [PubMed] [Google Scholar]
- 39.Nyambi P N, Willems B, Janssens W, Fransen K, Nkengasong J, Peeters M, Vereecken K, Heyndrickx L, Piot P, van der Groen G. The neutralization relationship of HIV type 1, HIV type 2, and SIVcpz is reflected in the genetic diversity that distinguishes them. AIDS Res Hum Retroviruses. 1996;13:7–17. doi: 10.1089/aid.1997.13.7. [DOI] [PubMed] [Google Scholar]
- 40.Park E J, Gorny M K, Zolla-Pazner S, Quinnan G V., Jr A global neutralization resistance phenotype of human immunodeficiency virus type 1 is determined by distinct mechanisms mediating enhanced infectivity and conformational change of the envelope complex. J Virol. 2000;74:4183–4191. doi: 10.1128/jvi.74.9.4183-4191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plantier J-C, Le Pogam S, Poisson F, Buzelay L, Lejeune B, Barin F. Extent of antigenic diversity in the V3 region of the surface glycoprotein gp120 of human immunodeficiency virus type 1 group M and consequences for serotyping. J Virol. 1998;72:677–683. doi: 10.1128/jvi.72.1.677-683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rennels M B, Edwars K M, Keyserling H L, Reisinger K S, Hogerman D A, Madore D V, Chang I, Paradiso P R, Malinoski F J, Kimura A. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics. 1998;101:604–611. doi: 10.1542/peds.101.4.604. [DOI] [PubMed] [Google Scholar]
- 43.Schreiber M, Wachsmuth C, Muller H, Odemuyiwa S, Schmitz H, Meyer S, Meyer B, Schneider-Mergener J. The V3-directed immune response in natural human immunodeficiency virus type 1 infection is predominately directed against a variable, discontinuous epitope presented by the gp120 V3 domain. J Virol. 1997;71:9198–9205. doi: 10.1128/jvi.71.12.9198-9205.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon F, Mauclere P, Roques P, Loussert-Ajaka I, Muller-Trutwin M C, Saragosti S, Georges-Courbot M C, Barre-Sinoussi F, Brun-Vezinet F. Identification of a new human immunodeficiency virus type 1 distinct from group M and group O. Nat Med. 1998;4:1032–1037. doi: 10.1038/2017. [DOI] [PubMed] [Google Scholar]
- 45.Tilley S A, Honnen W J, Racho M E, Chou T-C, Pinter A. Synergistic neutralization of HIV-1 by human monoclonal antibodies against the V3 loop and the CD4-binding site of gp120. AIDS Res Hum Retroviruses. 1992;8:461–467. doi: 10.1089/aid.1992.8.461. [DOI] [PubMed] [Google Scholar]
- 46.Verrier F, Burda S, Belshe R, Duliege A-M, Excler J-L, Klein M, Zolla-Pazner S. A human immunodeficiency virus prime-boost immunization regimen in humans induces antibodies that show interclade cross-reactivity and neutralize several X4-, R5-, and dualtropic clade B and C primary isolates. J Virol. 2000;74:10025–10033. doi: 10.1128/jvi.74.21.10025-10033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogel T, Kurth R, Norley S. The majority of neutralizing Abs in HIV-1 infected patients recognize linear V3 loop sequences. J Immunol. 1994;153:1895–1904. [PubMed] [Google Scholar]
- 48.Weber J, Fenyo E-M, Beddows S, Kaleebu P, Bjorndal A the WHO Network for HIV Isolation Characterization. Neutralization serotypes of HIV-1 field isolates are not predicted by genetic subtype. J Virol. 1996;70:7827–7832. doi: 10.1128/jvi.70.11.7827-7832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu J-Y, Gorny M K, Palker T, Karwowska S, Zolla-Pazner S. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J Virol. 1991;65:4832–4838. doi: 10.1128/jvi.65.9.4832-4838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zolla-Pazner S, Gorny M K, Nyambi P N. The implications of antigenic diversity for vaccine development. Immunol Lett. 1999;66:159–164. doi: 10.1016/s0165-2478(98)00176-x. [DOI] [PubMed] [Google Scholar]
- 51.Zolla-Pazner S, Gorny M K, Nyambi P N, VanCott T C, Nádas A. Immunotyping of HIV-1: an approach to immunologic classification of HIV. J Virol. 1999;73:4042. doi: 10.1128/jvi.73.5.4042-4051.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zolla-Pazner S, O'Leary J, Burda S, Gorny M K, Kim M, Mascola J, McCutchan F E. Serotyping of primary human immunodeficiency virus type 1 isolates from diverse geographic locations by flow cytometry. J Virol. 1995;69:3807–3815. doi: 10.1128/jvi.69.6.3807-3815.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]