Secondary Structure Analysis of a Minimal Avian Leukosis-Sarcoma Virus Packaging Signal (original) (raw)
Abstract
We previously identified a 160-nucleotide packaging signal, MΨ, from the 5′ end of the Rous sarcoma virus genome. In this study, we determine the secondary structure of MΨ by using phylogenetic analysis with computer modeling and heterologous packaging assays of point mutants. The results of the in vivo studies are in good agreement with the computer model. Additionally, the packaging studies indicate several structures which are important for efficient packaging, including a single-stranded bulge containing the initiation codon for the short open reading frame, uORF3, as well as adjacent stem structures. Finally, we show that the L3 stem-loop at the 3′ end of MΨ is dispensable for packaging, thus identifying an 82-nucleotide minimal packaging signal, μΨ, composed of the O3 stem-loop.
Retroviruses typically incorporate two copies of their RNA genome into viral particles. This process, referred to as RNA packaging, involves the specific recognition and binding of a sequence on the viral genome, called Ψ or E, by viral proteins. Viral assembly and packaging can occur in the absence of Pol and Env proteins, indicating that _trans_-acting packaging factors are found exclusively on the Gag polyprotein (19). RNA-packaging signals have been identified in the 5′ ends of most retroviral genomes. Structures formed within these sequences have been identified for several retroviruses, including Murine leukemia virus and Human immunodeficiency virus type 1 (reviewed in references 2 and 5).
We have previously identified a 160-nucleotide (nt) Rous sarcoma virus (RSV) packaging signal, MΨ, located between the primer binding site and Gag initiation codon, capable of conferring packaging of a heterologous RNA (4). This heterologous RNA is packaged only 2.6-fold less efficiently than is wild-type (wt) RSV genomic RNA (3). Computer models of MΨ secondary structure for several strains of avian leukosis-sarcoma virus (ALSV) predict two major stem-loops: O3 and L3 (4, 10, 13). Additionally, three smaller stem-loops, which we have named O3SLa, O3SLb, and O3SLc, extend from the O3 loop. Several groups have found that mutations disrupting base pairing of the O3 stem greatly reduce packaging of the RNA while compensatory mutations restore packaging to wt levels (4, 10, 14). In contrast, while deletions of the 3′ end of L3 reduce the packaging efficiency (3), maintenance of the predicted L3 stem structure is not required for efficient packaging (10).
ALSVs are unique among other retroviruses in that they contain three short open reading frames (ORFs) upstream of the Gag ORF. The third of these, uORF3, is located within MΨ. Interestingly, many but not all mutations which decrease the translation efficiency of uORF3 also decrease the packaging efficiency (8, 9, 17, 20). There is a debate in the literature about whether this correlation indicates a functional coupling of packaging and uORF3 translation (8, 9), or whether there are instead important RNA-packaging structures that overlap uORF3 and that are disrupted in these mutants (20).
In the present study, we determined the secondary structure of MΨ by phylogenetic analysis with computer modeling and heterologous packaging studies. Results of our in vivo experiments are in good agreement with the computer model. Additionally, the packaging studies indicate several regions which are important for efficient packaging, including the single-stranded bulge containing the uORF3 initiation codon, as well as adjacent stem structures. Finally, in the course of these experiments we found that the L3 stem-loop is dispensable for packaging. A heterologous RNA containing only the 82-nt O3 stem-loop is packaged as efficiently as a heterologous RNA containing the complete MΨ sequence.
MATERIALS AND METHODS
Sequence analysis.
An alignment of the 160-nt sequence corresponding to MΨ from 20 ALSV strains was performed by using Multalin (7) on the server located at http://www.toulouse.inra.fr/multalin.html. Secondary-structure analysis was performed with mfold version 2.3 (23, 25) on the server located at http://mfold1.wustl.edu/∼mfold/rna/form1.cgi. The strains used in the alignment, followed by their GenBank accession numbers are as follows: RSV-PrC (J02342); Y73SV (V01170); MHV2-E21 (M14008); FuSV (AF033810); MCV29-HBI (M11784); ASV-CT10 (Y00302); RAV-1 (M62407); RAV-2 (K02374); RSV-SRB (AF052428); RSV-SRA-V (U41731); ALV-SubJ (Z46390); UR2SV (M10455); AEV (X06197); EV-1 (M13103); MCV29 (J02247); MHV-2 (M16529); RSV-PrB (J02339); RSV-SRA (L29198); AMV (X51496); and AMAV (L10922).
Mutagenesis.
pASY194, which contains the 160 nt of RSV-PrC strain MΨ (GenBank accession no. J02342, nt 389 to 548) inserted in the _Mlu_I site of pASY161, a pCMVneo derivative (1, 4, 16), was used as the template for oligonucleotide-mediated site-directed mutagenesis, using the MORPH kit (5 Prime → 3 Prime, Inc., Boulder, Colo.). pΔL3, in which the L3 stem-loop is deleted from MΨ, was made by PCR amplification of the first 82 nt of MΨ, which was then inserted into the unique _Mlu_I site of pASY161, for use in the heterologous packaging assay.
Heterologous packaging assay.
The quail packaging cell line, Q2bn-4D (21) was grown in GM+D+CK (Ham's F10 medium containing 10% tryptose phosphate broth, 5% calf serum [Gemini Bio-Products], 1% heat-inactivated chick serum [GibcoBRL], and 1% dimethyl sulfoxide). Cells were maintained in 6% CO2 at 37°C. Plasmids were transfected by using the modified calcium phosphate method (6) on cells seeded in Dulbecco modified Eagle medium (DME) supplemented with 10% calf serum. Cells were selected for drug resistance with GM+D+CK containing 0.1 mg of G418 per ml. Mass cultures of drug-resistant cells were obtained after approximately 3 weeks under selection.
To radiolabel viruses, cells were plated at a density of 5 × 106 cells per 100-mm-diameter plate in GM+D+CK at least 18 h before labeling. The cells were washed twice with phosphate-buffered saline and once with serum-free DME minus methionine and cysteine (DME−Met−Cys). The cells were then labeled with 250 μCi of [35S]methionine-[35S]cysteine (EXPRESS 35S protein labeling mix; NEN Research Products) in 2 ml of DME−Met−Cys. After 5 h of incubation at 37°C in 6% CO2, 3 ml of DME−Met−Cys supplemented with 10% dialyzed fetal bovine serum was added. The following day, supernatants were collected and labeled viral particles were concentrated by high-speed centrifugation through a 20% sucrose cushion. Half of the concentrated virion were set aside for RNase protection analysis (RPA), and the remaining half was immunoprecipitated. The labeled viral particles were incubated for 90 min at room temperature in 1.0 ml of Ab-buffer (20 mM Tris [pH 7.4], 50mM NaCl, 1 mM EDTA [pH 8.0], 0.5% Nonidet P-40 [NP-40], 0.5% deoxycholic acid, 0.5% sodium dodecyl sulfate [SDS], 0.5% aprotinin) with 5 μl of polyclonal rabbit anti-RSV PRB antibody and 30 μl of protein A-Sepharose beads. The antigen-antibody complexes were washed twice in radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% NP-40, 1% deoxycholic acid, 0.1% SDS, 0.5% aprotinin), once in high-salt buffer (10 mM Tris-HCl [pH 7.4], 2 M NaCl, 1% NP-40, 0.5% deoxycholic acid), and once more in RIPA buffer. The bound proteins were eluted in SDS sample buffer and loaded onto an SDS–12.5% polyacrylamide gel. Following electrophoresis, the gel was dried and, after an overnight exposure, scanned directly by a Molecular Dynamics phosphorImager. Radioactive bands corresponding to the CA protein were quantitated, in machine units, by using ImageQuant (Molecular Dynamics) software.
RPAs were performed on viral and whole-cell lysates by using the Direct Protect kit (Ambion). For making antisense neo to detect the heterologous RNA, pASY185 (3) was linearized with _Rsr_II or _Nco_I and in vitro transcribed with T7 RNA polymerase to produce probes which protects 166 or 249 nt, respectively, of neo. For making antisense glyceraldehyde-3-phosphate dehydrogenase (gapdh) probe, pGEM1-GAPDH (11, 22) was linearized with _Hin_dIII and in vitro transcribed with T7 RNA polymerase to produce a probe which protects 169 nt of gapdh. The probes were gel purified on a 6% polyacrylamide gel. After RNase treatment, protected RNAs were separated on a 6% polyacrylamide gel. The dried gel was scanned directly by a Molecular Dynamics PhosphorImager after an overnight exposure. RNA bands were quantitated, in machine units, by using ImageQuant software.
Calculation of packaging efficiency.
Packaging efficiencies for the heterologous RNAs were determined by calculating the amount of neo RNA in virions (as measured by RPA) normalized to the number of virions (as measured by RIPA). To compare packaging efficiencies between different experiments, each time the assay was performed the calculated packaging efficiencies were normalized to that of CMVneo-MΨ.
RESULTS
Phylogenetic Analysis.
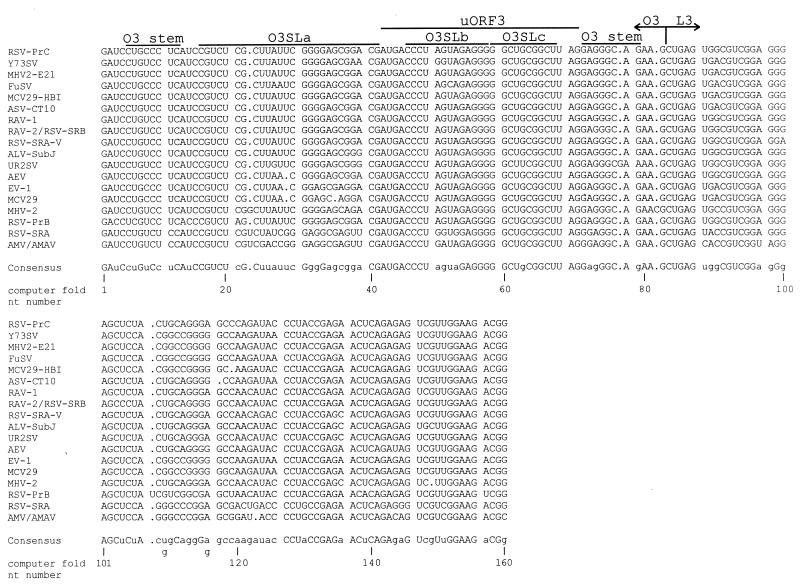
We obtained the nucleotide sequence corresponding to MΨ for 20 ALSV strains and isolates and aligned them by computer alignment. Several of these strains are defective transforming viruses and therefore require helper virus to replicate. We have included these viruses in our alignment since they are transferable by helper virus and therefore must have a functional packaging signal. Additionally, one of the strains, EV-1, is an endogenous virus. An endogenous virus would not necessarily have a functional packaging signal. However, we have tested the EV-1 MΨ sequence in a heterologous packaging assay and found that it can confer packaging of a heterologous RNA only 1.4-fold less efficiently than can the MΨ sequence from a replication competent strain, RSV-PrC (data not shown). The alignment is shown in Fig. 1, along with the consensus sequence. The percent similarity of the strains to the consensus sequence ranged from 77.5% (AMV and AMAV) to 99.4% (RAV-1). The strain used in previous packaging experiments in our laboratory, RSV-PrC, has 98.8% identity to the consensus sequence. Of the 160 nt, 95 nt (59.4%) are conserved in all 20 strains. For comparison, we performed an alignment of a 101-nt sequence from the 3′ untranslated region, the direct repeat, for 17 of these ALSV strains (data not shown). Only 48 nt (47.5%) of the 101 nt were conserved in all 17 strains.
FIG. 1.
Computer alignment of the MΨ sequence from 20 ALSV strains. Strains with identical MΨ sequence are shown on the same line. The sequences corresponding to uORF3 and the computer-predicted O3 and L3 stem-loops are outlined. Gaps in the alignment of the sequences are indicated by periods. The consensus sequence is shown below the alignment. Nucleotides conserved in all 20 strains are indicated by capital letters in the consensus. In cases where two nucleotides are equally conserved at a given position, both are indicated.
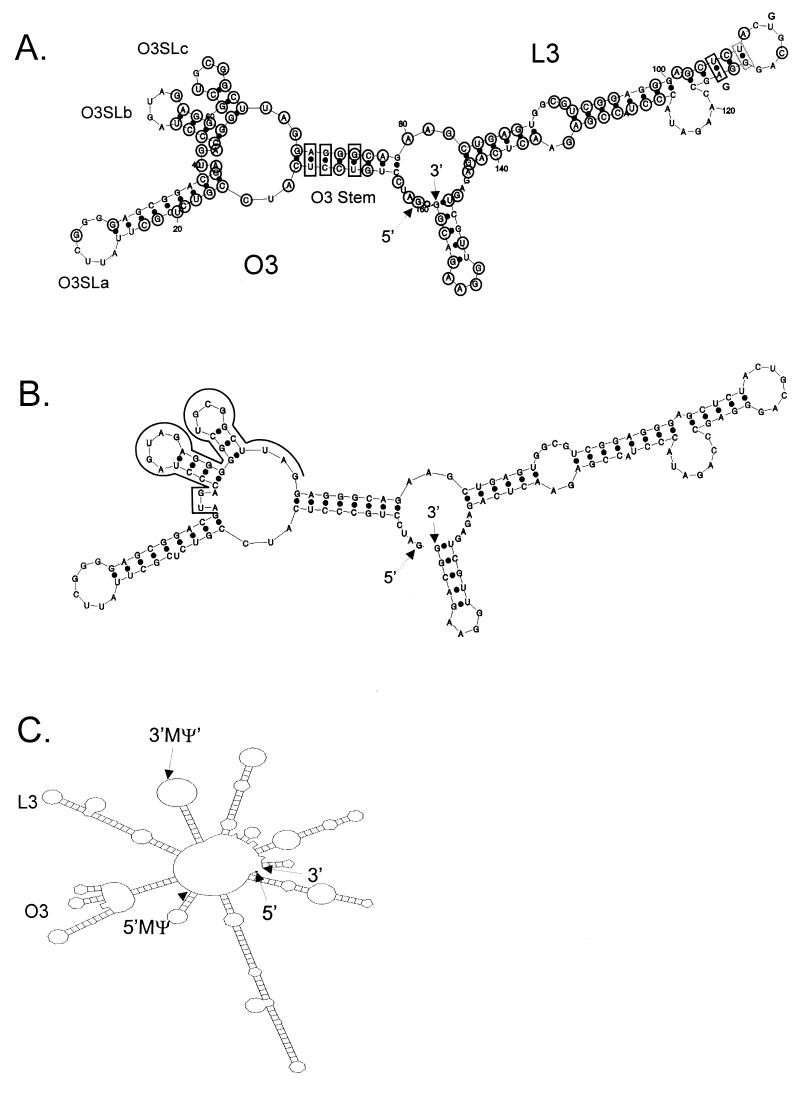
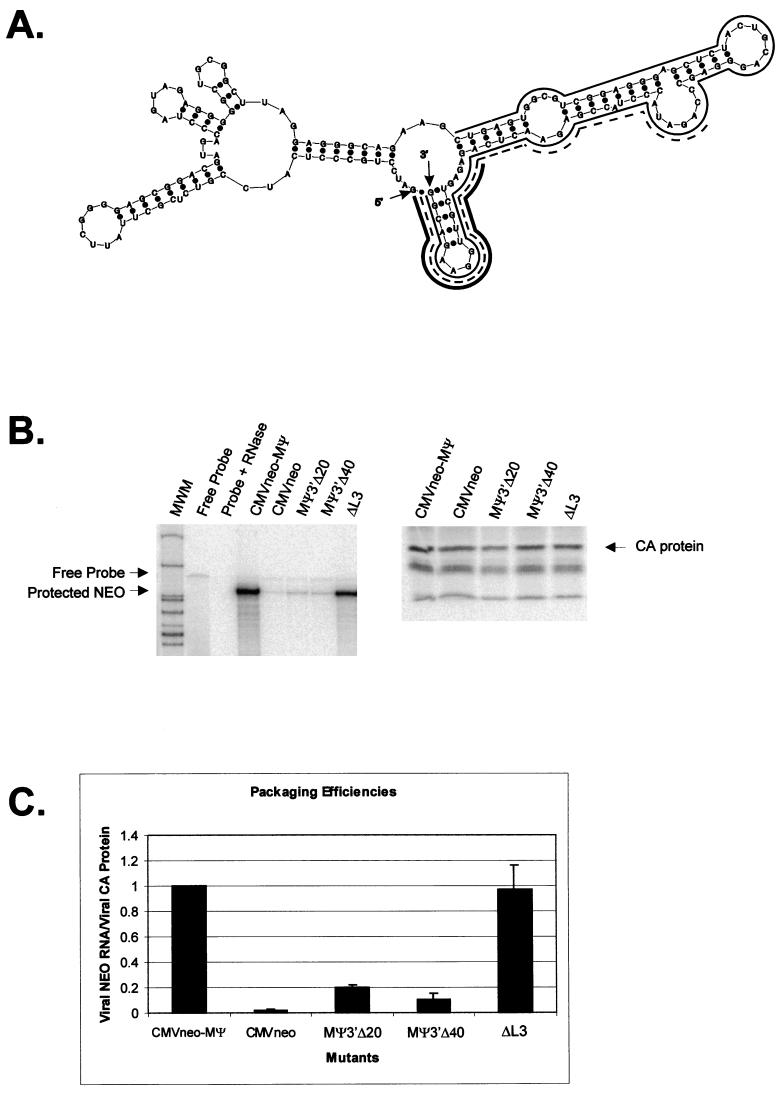
We next modeled the most stable fold of the consensus sequence by computer modeling, as shown in Fig. 2A. The predicted O3 and L3 stem-loops are labeled, as are the small stem-loops which extend from the O3 loop, which we have named O3SLa, O3SLb, and O3SLc. Base covariation, in which the primary sequence is not conserved but in all 20 strains the predicted base pairing is conserved, is seen at three positions in the O3 stem and at one position in the L3 stem. An additional base pair in the L3 stem has covariation in 19 of the strains. The most stable fold for the RSV-PrC strain is shown in Fig. 2B. The fold is identical to the consensus model, despite changes at two nucleotides. Importantly, the same structures are also predicted to form in the context of surrounding viral sequences. The most stable fold of the first 451 nt from the RSV-PrC genome is shown in Fig. 2C. All of the structures shown in Fig. 2B are found within Fig. 2C, with the exception of the 14-nt stem-loop at the 3′-most end of MΨ, which is predicted to be part of a much larger stem-loop in the viral context.
FIG. 2.
(A) mfold (23, 25) computer model of the most stable secondary structure of the MΨ consensus sequence (free energy, −56.2 kcal/mol). The 5′ and 3′ ends of the RNA are indicated. The two major stem-loop structures, O3 and L3, are indicated, as well as the smaller stem-loops extending from the O3 loop: O3SLa, O3SLb, and O3SLc. Nucleotides conserved in all 20 strains are circled. Black boxes indicate positions in which the precise nucleotides are not conserved but the predicted base pairing is conserved in all 20 strains. The gray box indicates a position in which the predicted base pairing is conserved in 19 of the 20 strains. (B) Computer model of the most stable secondary structure of the MΨ sequence of RSV-PrC (−59.0 kcal/mol). The 5′ and 3′ ends of the RNA are indicated, and the uORF3 sequence is outlined. (C) Computer model of the most stable secondary structure of the first 451 nt of the RSV-PrC genomic RNA (−159.6 kcal/mol). The 5′ and 3′ ends of the RNA are indicated, and the 5′ and 3′ ends of the sequence corresponding to MΨ are also shown.
In vivo analysis of O3SLa, O3SLb, and O3SLc.
We next confirmed this predicted MΨ secondary structure and determined the role of these structures, if any, in packaging, by using a heterologous packaging assay (3, 4). Nucleotide substitutions and deletions were made in specific regions of the RSV-PrC MΨ. These mutated packaging signals were inserted downstream of the neo coding sequence in pASY161, a derivative of pCMVneo (1, 4, 16). The plasmids were then transfected into the Q2bn avian packaging cell line (21), and mass cultures of G418-resistant cells were obtained. The level of expression of the heterologous RNAs in cells relative to a cellular mRNA, gapdh, was determined by RPA of cell lysates with antisense neo and gapdh probes. The expression level was similar for each heterologous RNA used in these studies (data not shown). The packaging efficiency for each mutant was calculated as the total heterologous RNA detected in virions (by RPA) normalized to the number of viral particles (as determined by RIPA). Each mutant was tested at least three times in the assay.
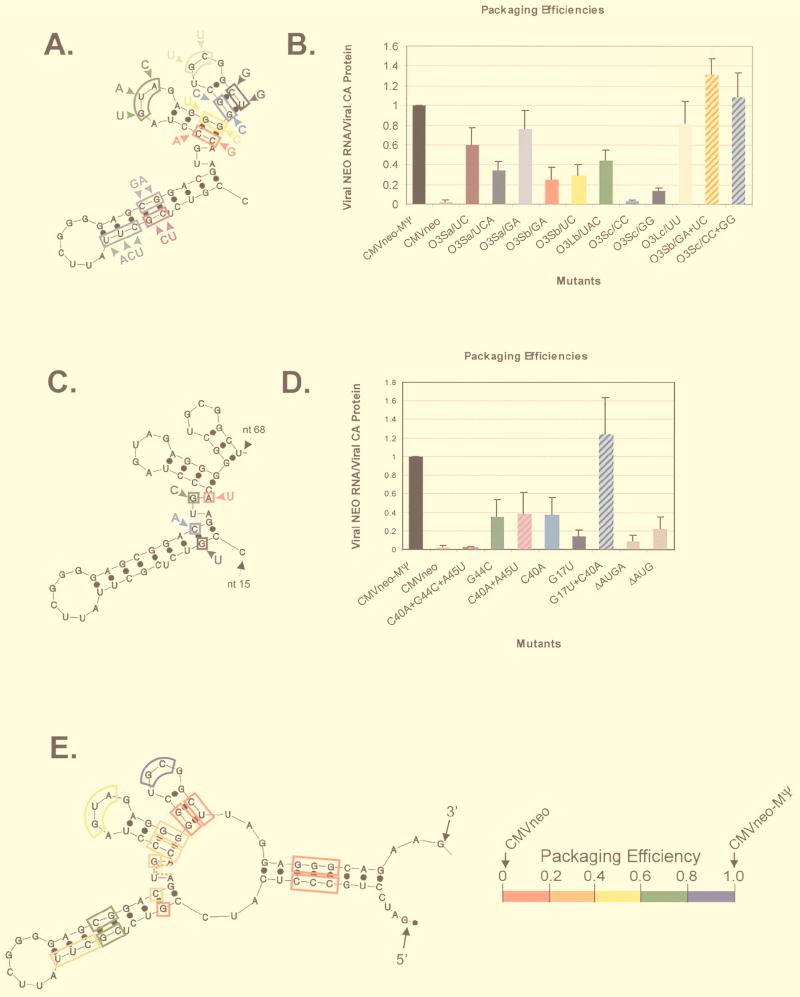
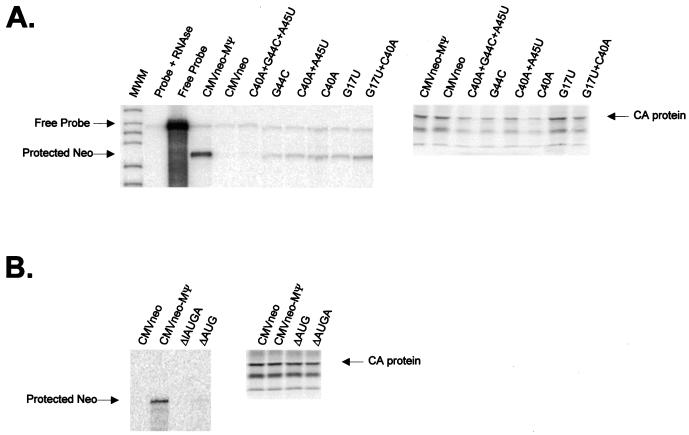
We first constructed three O3SLa mutants (O3Sa/UC, O3Sa/UCA, and O3Sa/GA), as shown in Fig. 3A, and tested them in the packaging assay (Fig. 4A). The average packaging efficiency from three repetitions of the assay is shown in Fig. 3B. Although none of the mutants was packaged as efficiently as the positive control, CMVneo-MΨ, which contains wt MΨ, the effects on packaging were modest. Thus, the upper portion of the O3SLa does not appear to play a critical role in packaging. We next constructed three O3SLb and O3SLc mutants (Fig. 3A) and tested them in the packaging assay (Fig. 4B). For each stem-loop, we made a mutation in one half of the stem (O3Sb/GA, O3Sb/UC, O3Sc/CC and O3Sc/GG) and in the loop region (O3Lb/UAC and O3Lc/UU). The average packaging efficiencies are shown in Fig. 3B. Mutation of either side of the O3SLb stem caused a more than threefold reduction in packaging. Mutation of the O3SLb loop caused a twofold reduction in packaging. In contrast, mutations on either side of the O3SLc stem caused more than a fivefold reduction in packaging. Mutation of the O3SLc loop caused less than a 1.3-fold reduction in packaging. To determine whether the reduction in the packaging of the O3SLb and O3SLc stem mutants was due to the changes in the primary sequence or the secondary structure, we constructed two compensatory mutations (O3Sb/GA+UC and O3Sc/CC+GG) and determined their packaging efficiencies (Fig. 4C). As shown in Fig. 3B, the packaging efficiency of both compensatory mutants is similar to that of CMVneo-MΨ, indicating that these regions are indeed base paired and that maintenance of these structures is important for efficient packaging.
FIG. 3.
(A) Summary of site-directed mutagenesis of O3SLa, O3SLb, and O3SLc. Each colored box represents a different mutant. The new sequence is indicated outside the box. (B) Average packaging efficiencies of the mutants shown in panel A from three repetitions of the packaging assay, relative to CMVneo-MΨ. Bar chart colors correspond to the mutant colors in panel A. Striped bars represent compensatory mutants. Error bars represent standard deviations. Packaging efficiencies were calculated as the ratio of neo RNA packaged into particles, as measured by RPA, to the number of viral particles, as measured by RIPA. (C) Summary of site-directed mutagenesis of the uORF3 initiation codon and surrounding nucleotides. (D) Packaging efficiencies of the mutants shown in panel C. (E) Computer fold of μΨ, the first 82 nt of MΨ, summarizing the site-directed mutagenesis studies. Each box represents a different mutant. The dashed line indicates a deletion mutant. The colors correspond to the packaging efficiency of that mutant, as shown at the right of the fold. The 5′ and 3′ ends of the RNA are indicated.
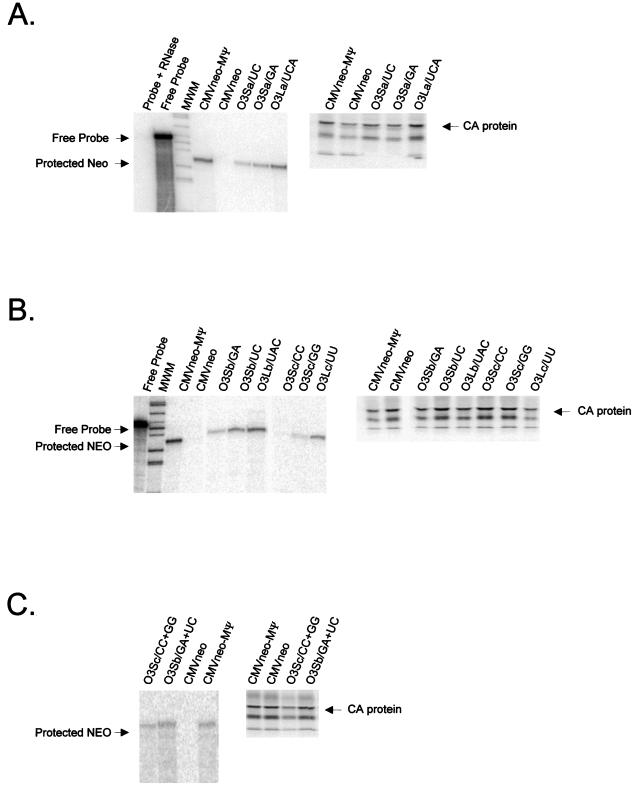
FIG. 4.
(A) RPA (left) and RIPA (right) of virions released from G418-resistant mass cultures of Q2bn cells transfected with plasmids expressing the O3SLa mutant RNAs (described in Fig. 3A) indicated above each lane. In the RPA, RNAs were protected with an antisense neo probe. The expected location of free probe and the protected neo bands are indicated. For the RIPA, proteins were precipitated with anti-PRB antibody. The expected size of the capsid (CA) band is indicated. (B) RPA (left) and RIPA (right) of virions released from cells transfected with plasmids expressing the O3SLb and O3SLc mutant RNAs (described in Fig. 3A) indicated above each lane. (C) RPA (left) and RIPA (right) of virions released from cells transfected with plasmids expressing the O3Sb and O3Sc compensatory mutation RNAs.
In vivo analysis of the uORF3 initiation codon.
Previous packaging studies have shown that mutations in the uORF3 initiation codon cause a reduction in packaging (8, 9, 17). The initiation codon is predicted by the computer fold to be located within a single-stranded bulge between O3SLa and O3SLb (Fig. 2A and B). To test the effect of mutations in and around the uORF3 initiation codon, we first constructed a new _Bgl_II restriction site in this region. This mutant, C40A+G44C+A45U, contains three substitutions, one within the initiation codon (G44C) and two surrounding the codon (C40A and A45U) (Fig. 3C). The packaging efficiency of this mutant was 50-fold lower than that of CMVneo-MΨ and was similar to that of the negative control, CMVneo, which contains no retroviral sequences (Fig. 3D and 5A). To determine the contribution of the individual nucleotide substitutions to the observed packaging defect, we constructed three additional mutants with the following mutations (Fig. 3C): G44C, C40A, and C40A+A45U. All three mutants had similar, intermediate packaging phenotypes (Fig. 3D and 5A). Thus, it appears that the large packaging defect seen in the triple mutant is a combination of the C40A and G44C mutations. Since the C40 nucleotide is predicted by the computer model to be base paired (Fig. 2A and B), we next determined the role of secondary structure in the C40A packaging defect. We constructed a mutant with a nucleotide change in the other half of the stem, G17U, and the compensatory mutant, with the C40A+G17U mutation (Fig. 3C). G17U was packaged 6.8-fold less efficiently than CMVneo-MΨ, while the compensatory mutant had a packaging phenotype close to that of wt (Fig. 3D and 5A), indicating that the secondary structure in this region, not the primary sequence, is required for efficient packaging. Finally, to further examine the role in packaging of the single-stranded region containing the initiation codon, we made two mutants: ΔAUG, which has a deletion of the initiation codon, and ΔAUGA, which has a deletion of the entire single-stranded region. The packaging efficiencies were reduced 4.7- and 12-fold, respectively, relative to CMVneo-MΨ (Fig. 3D and 5B).
FIG. 5.
(A) RPA (left) and RIPA (right) of virions released from G418-resistant mass cultures of Q2bn cells transfected with plasmids expressing the uORF3 mutant RNAs (described in Fig. 3C) indicated above each lane. In the RPA, RNAs were protected with an antisense neo probe. The expected locations of free probe and the protected neo bands are indicated. For the RIPA, proteins were precipitated with anti-PRB antibody. The expected size of the capsid (CA) band is indicated. (B) RPA (left) and RIPA (right) of virions released from cells transfected with plasmids expressing the uORF3 initiation codon deletion mutant RNAs.
In vivo analysis of L3.
We previously showed that deletion of sequences from the 3′ end of MΨ causes a reduction in packaging (3, 4). In contrast, Doria-Rose and Vogt have shown that several mutants retaining neither the primary nor the predicted secondary structure of L3 can be efficiently packaged (10). To reconcile these findings, we previously suggested that L3 may be dispensable for packaging or might instead serve to stabilize the O3 stem-loop, which might directly interact with _trans_-acting packaging factors (3). To test this hypothesis, we constructed a mutant, ΔL3, that contains a precise deletion of L3, as well as the remaining 18 nt from the 3′-end of MΨ (Fig. 6A). We tested the packaging efficiency of ΔL3 in parallel with our previously described 3′-end deletion mutants, MΨ3′Δ20 and MΨ3′Δ40 (Fig. 6A). Representative RPA and RIPA gels are shown in Fig. 6B. The average packaging efficiencies from three repetitions of the assay are shown in Fig. 6C. ΔL3 was packaged as efficiently as the positive control, CMVneo-MΨ, indicating that L3 is dispensable for packaging. We have named this 82-nt minimal packaging signal μΨ. When we modeled the secondary structure of μΨ by computer modeling, we found that the O3 sequences folded into structures identical to those in MΨ (Fig. 3E).
FIG. 6.
(A) Computer fold of MΨ, with lines shown next to nucleotides deleted in L3 mutants. The thin solid line indicates nucleotides deleted in ΔL3. The dashed line indicates nucleotides deleted in MΨ3′Δ40. The thick solid line indicates nucleotides deleted in MΨ3′Δ20. The 5′ and 3′ ends of the RNA are indicated. (B) RPA (left) and RIPA (right) of virions released from G418-resistant mass cultures of Q2bn cells transfected with plasmids expressing the L3 mutant RNAs indicated above each lane. In the RPA, RNAs were protected with an antisense neo probe. The expected locations of the free probe and the protected neo bands are indicated. For the RIPA, proteins were precipitated with anti-PRB antibody. The expected size of the capsid (CA) band is indicated. (C) Average packaging efficiencies of the mutants, from three repetitions of the packaging assay, relative to CMVneo-MΨ. Error bars represent standard deviations. Packaging efficiencies were calculated as the ratio of neo RNA packaged into particles, as measured by RPA, to the number of viral particles, as measured by RIPA.
DISCUSSION
Our alignment of 20 ALSV strains indicates that 59.4% of the nucleotides within MΨ are conserved in every strain. The covariation seen for several base pairs in the computer model provides good evidence that these secondary structures are conserved as well. Previously, phylogenetic and computer-modeling analysis of the secondary structure of the ALSV leader region, including the packaging sequence, was performed by Hackett et al. with a consensus sequence from 13 ALSV strains (13). Their secondary-structure model of MΨ is similar to ours, except for O3SLa and the immediately surrounding nucleotides. Their computer fold predicts both G17 and C40 to be single stranded. In our computer fold, these nucleotides are base paired with each other. Importantly, this base pairing was verified by the results obtained with our mutants G17U, C40A, and G17U+C40A. The consensus sequence of Hackett et al. has a few base changes relative to our consensus; however, the different folds appear to be due to the different computer algorithms used, because we obtained almost identical folds for both consensus sequences by using mfold program version 2.3 (data not shown).
The sequences within the computer-predicted O3SLa are not well conserved between ALSV strains, providing evidence that these sequences are not important for packaging (Fig. 2A). Nuclease-mapping studies performed in our laboratory indicate that many of the nucleotides in the upper portion of the computer-predicted stem are actually single stranded (data not shown). Indeed, mutations in this region had only modest effects on packaging in our heterologous packaging assay (Fig. 3A and B). Additionally, our laboratory has previously shown that mutation of the sequences in the loop of O3SLa causes only a modest reduction in packaging (15). The nucleotides composing the stems of O3SLb and O3SLc are conserved in all 20 ALSV strains (Fig. 2A). Additionally, mutations in these stems reduced packaging in our heterologous packaging assay (Fig. 3A and B). However, compensatory mutations restored packaging (Fig. 3B), indicating the importance of the secondary structure, not the primary sequence, in packaging. Mutagenesis of the sequence composing the O3SLc loop had only a modest effect on packaging (Fig. 3A and B). Our results with O3SLc are in agreement with those of Doria-Rose and Vogt (10). Their packaging studies were performed with the RSV-SRA strain, which, according to computer models, folds somewhat differently from RSV-PrC. However, their O3 mid-stem-loop is identical to that of O3SLc, with the exception of an additional base pair at the base of the stem. They found that in the viral context, when they randomized the sequences of the O3 mid-stem-loop, mutants that retained some base pairing in the stem were selectively packaged. Additionally, there was no packaging selection of particular loop sequences.
It has previously been shown that many mutations which decrease the translation efficiency of uORF3 also decrease the packaging efficiency of the RNA (8, 9, 17, 20). Donze et al. proposed that this correlation could indicate a functional coupling of the processes of uORF3 translation and RNA packaging (8). In a later study, however, Sonstegard and Hackett were unable to find a direct correlation between the efficiencies of uORF3 translation and RNA packaging (20). They concluded that uORF3 translation and RNA packaging are not functionally coupled but proposed that RNA secondary structures that promote packaging overlap with sequences in the uORF3. Indeed, our studies verify the presence of O3SLa just upstream of uORF3 and the presence of O3SLb and O3SLc within uORF3. Additionally, mutations in these stems reduced the packaging efficiency. On the other hand, our computer model indicates that the initiation codon for uORF3 is located within a single-stranded region between O3SLa and O3SLb (Fig. 2A and B). Furthermore, nuclease-mapping studies performed in our laboratory also indicate that this region is single stranded (data not shown). We made three constructs, G44C, ΔAUG, and ΔAUGA, which contain deletions or substitutions within this region. In computer models, the G44C and ΔAUG mutations are not predicted to affect the overall secondary structure of MΨ (data not shown). These mutants were all packaged less efficiently than CMVneo-MΨ. Since these mutations are predicted to reduce or abolish uORF3 translation, we cannot formally rule out the possibility that the reduction in packaging is directly related to the reduction in translation, as predicted by Donze et al (8). However, these mutants are all packaged more efficiently than the negative control, CMVneo, indicating that, at the very least, translation is not essential for packaging. It is also important to note that in our heterologous RNA, MΨ and therefore uORF3 are located more than 200 nt downstream of the neo ORF. Thus, uORF3 translation is probably very inefficient in this context. However, we have recently shown that CMVneo-MΨ is packaged only 2.6-fold less efficiently than wt RSV genomic RNA (3). Taking these results together, we currently believe uORF3 translation plays, at most, a minor role in RNA packaging.
We found that a heterologous RNA containing only the O3 stem-loop and several flanking nucleotides was packaged as efficiently as was CMVneo-MΨ. Thus, the L3 stem-loop is dispensable for packaging. We previously showed that deletion of sequences from the 3′ end of L3 caused a reduction in packaging (3, 4). We now believe that alternative structures formed in the absence of these L3 sequences disrupted the proper folding of O3 in the RNAs. Although L3 is not necessary for packaging in the heterologous system, it appears to play an important role in some step of the viral life cycle. A virus with a deletion of L3 has been shown to replicate very poorly; however, the replication step in which this virus is blocked was not determined (10). Interestingly, Fosse et al. have shown that palindromic sequences in the L3 loop play a critical role in ALSV dimer formation in vitro (12). Recently, however, it has been demonstrated that maintenance of palindromic sequences in the L3 loop is not absolutely required for infectivity (10). However, L3 palindromes may play some role in infectivity, since when they are mutated, they are gradually selected for over time (10). Although it has long been postulated that dimerization of genomic RNAs is necessary for retroviral encapsidation (reviewed in reference 5), this dependence has never been definitively shown in ALSV. If the L3 loop is solely responsible for RSV dimer formation, the efficient packaging of μΨ would provide further evidence that dimerization is not essential for packaging. However, we have not yet tested μΨ for its ability to form dimers. It is possible that additional sequences or structures within this minimal packaging signal can induce dimerization.
In Fig. 3E, we have summarized the results of the site-directed mutagenesis studies. As well as the mutants described in this paper, we have included two O3 stem mutants previously described by our laboratory (4). From the results we have obtained thus far, it is difficult to predict precisely how this structure may interact with Gag. Unlike DNA, the major groove of the RNA double helix is relatively inaccessible to binding proteins (18). Most RNA binding sites, therefore, are found in single-stranded loops and bulges and at the ends of helices, where loops and bulges can distort the geometry of the helix, opening the major groove (24). The packaging defect of every stem mutation we constructed could be rescued by a compensatory mutation. Thus, Gag does not appear to make base-specific contacts in these regions. Mutations in the loops of O3SLa, O3SLb, and O3SLc had modest effects on packaging. On the other hand, mutations and deletions in the bulge between O3SLa and O3SLb had a much greater effect on packaging. Thus, this region, in addition to its role in initiation of uORF3 translation, may be a binding site for Gag. It is unlikely to be the only binding site, since we were able to detect a low level of packaging of RNAs with a deletion of the entire bulge. We have not mutated the single-stranded regions between the O3 stem and O3SLa and O3SLc. These sequences are particularly good candidates for Gag binding because they are highly conserved. We are currently using a rapid genetic assay, the yeast three-hybrid system, to more precisely define both the _cis_- and _trans_-acting determinants involved in encapsidation.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Cancer Institute (CA 18282) to M.L.L. J.D.B. was supported by a National Science Foundation graduate fellowship.
We thank Bonnie Kealoha and Larry Baker for technical assistance and Volker Vogt and Mark Roth for their comments on the manuscript.
REFERENCES
- 1.Aronoff R, Linial M L. Specificity of retroviral RNA packaging. J Virol. 1991;65:71–80. doi: 10.1128/jvi.65.1.71-80.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks J D, Beemon K L, Linial M L. RNA regulatory elements in the genomes of simple retroviruses. Semin Virol. 1997;8:194–204. [Google Scholar]
- 3.Banks J D, Kealoha B O, Linial M L. An MΨ-containing heterologous RNA, but not env mRNA, is efficiently packaged into avian retroviral particles. J Virol. 1999;73:8926–8933. doi: 10.1128/jvi.73.11.8926-8933.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks J D, Yeo A, Green K, Cepeda F, Linial M L. A minimal avian retroviral packaging sequence has a complex structure. J Virol. 1998;72:6190–6194. doi: 10.1128/jvi.72.7.6190-6194.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkowitz R, Fisher J, Goff S P. RNA packaging. Curr Top Microbiol Immunol. 1996;214:177–218. doi: 10.1007/978-3-642-80145-7_6. [DOI] [PubMed] [Google Scholar]
- 6.Chen C, Okayama H. High efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donze O, Damay P, Spahr P F. The first and third uORFs in RSV leader RNA are efficiently translated: implications for translational regulation and viral RNA packaging. Nucleic Acids Res. 1995;23:861–868. doi: 10.1093/nar/23.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donze O, Spahr P F. Role of the open reading frames of Rous sarcoma virus leader RNA in translation and genome packaging. EMBO J. 1992;11:3747–3757. doi: 10.1002/j.1460-2075.1992.tb05460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doria-Rose N A, Vogt V M. In vivo selection of Rous sarcoma virus mutants with randomized sequences in the packaging signal. J Virol. 1998;72:8073–8082. doi: 10.1128/jvi.72.10.8073-8082.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dugaiczyk A, Haron J A, Stone E M, Dennison O E, Rothblum K N, Schwartz R J. Cloning and sequencing of a deoxyribonucleic acid copy of glyceraldehyde-3-phosphate dehydrogenase messenger ribonucleic acid isolated from chicken muscle. Biochemistry. 1983;22:1605–1613. doi: 10.1021/bi00276a013. [DOI] [PubMed] [Google Scholar]
- 12.Fosse P, Motte N, Roumier A, Gabus C, Muriaux D, Darlix J L, Paoletti J. A short autocomplementary sequence plays an essential role in avian sarcoma-leukosis virus RNA dimerization. Biochemistry. 1996;35:16601–16609. doi: 10.1021/bi9613786. [DOI] [PubMed] [Google Scholar]
- 13.Hackett P B, Dalton M W, Johnson D P, Petersen R B. Phylogenetic and physical analysis of the 5′ leader RNA sequences of avian retroviruses. Nucleic Acids Res. 1992;19:6929–6934. doi: 10.1093/nar/19.24.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knight J B, Si Z H, Stoltzfus C M. A base-paired structure in the avian sarcoma virus 5′ leader is required for efficient encapsidation of RNA. J Virol. 1994;68:4493–4502. doi: 10.1128/jvi.68.7.4493-4502.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee E-G, Yeo A, Kraemer B, Wickens M, Linial M L. The Gag domains for avian retroviral RNA encapsidation determined by two independent methods. J Virol. 1999;73:6282–6292. doi: 10.1128/jvi.73.8.6282-6292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linial M. Creation of a processed pseudogene by retroviral infection. Cell. 1987;49:93–102. doi: 10.1016/0092-8674(87)90759-8. [DOI] [PubMed] [Google Scholar]
- 17.Moustakas A, Sonstegard T S, Hackett P B. Alterations of the three short open reading frames in the rous sarcoma virus leader RNA modulate viral replication and gene expression. J Virol. 1993;67:4337–4349. doi: 10.1128/jvi.67.7.4337-4349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowakowski J, Tinoco I., Jr RNA Structure and Stability. Semin Virol. 1997;8:153–165. [Google Scholar]
- 19.Oertle S, Spahr P F. Role of the Gag polyprotein precursor in packaging and maturation of Rous sarcoma virus genomic RNA. J Virol. 1990;64:5757–5763. doi: 10.1128/jvi.64.12.5757-5763.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonstegard T S, Hackett P B. Autogenous regulation of RNA translation and packaging by Rous sarcoma virus Pr76gag. J Virol. 1996;70:6542–6552. [PMC free article] [PubMed] [Google Scholar]
- 21.Stoker A W, Bissell M J. Development of avian sarcoma and leukosis virus-based vector-packaging cell lines. J Virol. 1988;62:1008–1015. doi: 10.1128/jvi.62.3.1008-1015.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tikhonenko A T, Black D J, Linial M L. Viral Myc oncoproteins in infected fibroblasts down-modulate thrombospondin-1, a possible tumor suppressor gene. J Biol Chem. 1996;271:30741–30747. doi: 10.1074/jbc.271.48.30741. [DOI] [PubMed] [Google Scholar]
- 23.Walter A E, Turner D H, Kim J, Lyttle M H, Muller P, Mathews D H, Zuker M. Coaxial stacking of helixes enhances binding of oligoribonucleotides and improves predictions of RNA folding. Proc Natl Acad Sci USA. 1994;91:9218–9222. doi: 10.1073/pnas.91.20.9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weeks K M, Crothers D M. Major groove accessibility of RNA. Science. 1993;261:1574–1577. doi: 10.1126/science.7690496. [DOI] [PubMed] [Google Scholar]
- 25.Zuker M. On finding all suboptimal foldings of an RNA molecule. Science. 1989;244:48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]