The Severity of Murray Valley Encephalitis in Mice Is Linked to Neutrophil Infiltration and Inducible Nitric Oxide Synthase Activity in the Central Nervous System (original) (raw)
Abstract
A study of immunopathology in the central nervous system (CNS) during infection with a virulent strain of Murray Valley encephalitis virus (MVE) in weanling Swiss mice following peripheral inoculation is presented. It has previously been shown that virus enters the murine CNS 4 days after peripheral inoculation, spreads to the anterior olfactory nucleus, the pyriform cortex, and the hippocampal formation at 5 days postinfection (p.i.), and then spreads throughout the cerebral cortex, caudate putamen, thalamus, and brain stem between 6 and 9 days p.i. (P. C. McMinn, L. Dalgarno, and R. C. Weir, Virology 220:414–423, 1996). Here we show that the encephalitis which develops in MVE-infected mice from 5 days p.i. is associated with the development of a neutrophil inflammatory response in perivascular regions and in the CNS parenchyma. Infiltration of neutrophils into the CNS was preceded by increased expression of tumor necrosis factor alpha and the neutrophil-attracting chemokine N51/KC within the CNS. Depletion of neutrophils with a cytotoxic monoclonal antibody (RB6-8C5) resulted in prolonged survival and decreased mortality in MVE-infected mice. In addition, neutrophil infiltration and disease onset correlated with expression of the enzyme-inducible nitric oxide synthase (iNOS) within the CNS. Inhibition of iNOS by aminoguanidine resulted in prolonged survival and decreased mortality in MVE-infected mice. This study provides strong support for the hypothesis that Murray Valley encephalitis is primarily an immunopathological disease.
Murray Valley encephalitis virus (MVE) is a member of the Flavivirus genus of the family Flaviviridae, a group of small, lipid-enveloped plus-strand RNA viruses (45), most of which are transmitted to vertebrate hosts by invertebrate vectors. Arthropod-borne flaviviruses are the causative agents of several diseases of public health significance, including yellow fever, dengue fever, Japanese encephalitis (JE), St. Louis encephalitis, and Murray Valley encephalitis. Flaviviruses are grouped into eight antigenic complexes on the basis of cross-reactivity in neutralization assays (6). MVE is a member of the JE virus serocomplex, a group of antigenically and genotypically related viruses (33) which are mosquito transmitted and encephalitogenic in humans. The known distribution of MVE is confined to certain regions of Australia and Papua New Guinea, where it is responsible for endemic cases of encephalitis in tropical northwestern Australia and for occasional epidemics in temperate southeastern Australia (24).
MVE causes age-dependent encephalitis in mice after peripheral inoculation (23) and provides a good model of arbovirus-mediated encephalitis in humans (27, 28). Despite this, the mechanism for development of encephalitis in mice infected with viruses of the JE virus serocomplex has not been elucidated. However, it is known that encephalitogenic flaviviruses replicate exclusively within neurons in the murine central nervous system (CNS) (15, 26, 30) and that disease severity and pathological changes within the CNS correlate directly with the distribution and relative quantity of virus in the MVE-infected mouse brain (5, 26).
Replication of flaviviruses within the murine CNS has been shown to provoke an intense meningeal, perivascular, and parenchymal inflammatory cell response (5, 14). Neuronal injury and destruction are associated with adherence of inflammatory cells (neutrophils and macrophages) to infected neurons (neuronophagia). The role of inflammation in the pathogenesis of flavivirus encephalitis has been further clarified by experiments in which mice were immunosuppressed prior to infection with virus. Immunosuppression of mice during infection with West Nile virus (7) or tick-borne encephalitis virus (36) resulted in prolonged survival, reduced mortality, and a reduction in CNS inflammatory cell infiltration compared to those for immunocompetent mice. Furthermore, it has been suggested that synthesis of cytokines and reactive oxygen and nitrogen intermediates by inflammatory cells infiltrating the CNS may cause toxic damage to neurons in several animal models of encephalitis (18), including flaviviruses (19). Thus, existing data support the hypothesis that flavivirus-mediated encephalitis is an immunopathological disease.
In this study, we have examined neutrophil inflammatory responses to MVE infection in the CNS by immunohistochemistry and have assessed the role of neutrophils in the pathogenesis of encephalitis by in vivo depletion with a cytotoxic antineutrophil monoclonal antibody (MAb). We have also examined expression of the proinflammatory cytokine tumor necrosis factor alpha (TNF-α), the murine neutrophil-attracting chemokine N51/KC, and the inflammatory cell-associated enzyme, inducible nitric oxide synthase (iNOS), within the murine CNS in response to MVE infection. We have investigated the role of the inflammatory mediator nitric oxide in the pathogenesis of encephalitis by inhibition of iNOS with the drug aminoguanidine. Finally, we have studied the development of apoptosis within MVE-infected neurons at increasing times postinfection (p.i.) by double labelling for viral RNA by in situ hybridization and for apoptosis by in situ terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labelling (TUNEL) assay.
MATERIALS AND METHODS
Virus strains and cells.
MVE BH3479 (25) was passaged once in suckling mouse brain and twice in C6/36 (Aedes albopictus) cells. Working stocks were culture supernatants of C6/36 cells. Cell culture media and cell and virus stocks were tested for the presence of endotoxin by Limulus amoebocyte lysate assay (E-Toxate; Sigma) before use in animal studies; measurable quantities of endotoxin were not detected in any of these reagents.
African green monkey kidney (Vero) cells (ATCC CCL 81), used between passage levels 124 and 128, were grown at 37°C in 20 mM HEPES-buffered M199 supplemented with 2 mM l-glutamine and 10% fetal calf serum (FCS) in 5% CO2–95% air. C6/36 cells (ATCC CRL 1660), used between passage levels 122 and 132, were grown at 28°C in M199 supplemented with 2 mM l-glutamine and 10% FCS.
Plaque assay.
Virus was assayed by plaque formation on Vero cell monolayers grown in 12-well plastic trays (tissue culture grade; CoStar Scientific Inc., Cambridge, Mass.). After 1 h of adsorption of serial 10-fold dilutions of virus, inoculum was removed and cells were overlaid with 1.75% methylcellulose in 2% FCS–M199. Following 5 days at 37°C in 5% CO2, monolayers were stained with 1% methylene blue–10% formalin and plaques were counted after overnight incubation. Infectivity titers were expressed as PFU per milliliter.
Virus infection of mice.
Groups of 21-day-old Swiss mice were inoculated in the left footpad or intraperitoneally (i.p.) with 104 PFU of virus. At the indicated times p.i., three to four mice from each group were anesthetized, killed by exsanguination through intracardiac puncture, and perfused with sterile phosphate-buffered saline (PBS), pH 7.4. Brains were dissected with the olfactory bulbs intact and prepared for frozen sectioning and RNA extraction as outlined below. All animal experiments were undertaken in accordance with protocols approved by the University of Western Australia Animal Experimentation Ethics Committee.
For viral growth studies, brains were dissected intact, snap frozen in liquid nitrogen, and stored at −80°C. The brains were subsequently dispersed in Dounce homogenizers and prepared as 10% suspensions in Hanks’ balanced salt solution, pH 8.0, containing 2% bovine serum albumin. Virus was titrated by plaque formation on Vero cell monolayers; the threshold for detection of virus was 100 PFU/g of tissue.
Extraction of RNA from virus-infected mouse brain.
RNA was extracted from MVE-infected or mock-infected mouse brain using RNAzol-B (Tel-Test Inc.) according to the manufacturer’s instructions. The brains were weighed before dispersal in Dounce homogenizers (2 ml of RNAzol-B per 100 mg of brain tissue). After dispersal, 10 μl of chloroform was added per 100 μl of homogenate, shaken for 15 s, and incubated on ice for 15 min. After centrifugation at 15,000 rpm (4°C) for 15 min in a microcentrifuge (Eppenderf), the aqueous layer was removed and the RNA was precipitated by the addition of an equal volume of isopropanol followed by centrifugation. The pellet was washed in 80% ethanol–20% diethyl pyrocarbonate (DEPC)-treated double-distilled water (ddH2O), air dried, and redissolved in 50 μl of DEPC-treated ddH2O. Contaminating DNA was digested with RQ1 RNase-free DNase (Promega) for 15 min at 37°C. RNA was then reprecipitated in 2 volumes of ethanol, washed, and resuspended in 50 μl of DEPC-treated ddH2O. The quantity of RNA in each sample was determined by spectrophotometry, and the RNA quality was assessed by agarose gel electrophoresis on 0.1% sodium dodecyl sulfate–1% agarose gels; RNA solutions were standardized to a concentration of 1 μg/μl in DEPC-treated ddH2O and stored at −80°C.
RT-PCR on mouse brain RNA preparations.
First-strand cDNA synthesis was undertaken on the MVE-infected and mock-infected mouse brain RNA samples. Each cDNA reaction mixture contained 1 mM deoxynucleoside triphosphates, 5 mM MgCl2, 16 U of RNase inhibitor (Promega), 25 U of Moloney murine leukemia virus reverse transcriptase (RT; Promega), 1 μg of mouse brain RNA, and 30 pmol of downstream primers for the constitutively expressed cellular gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase), MVE envelope (E) gene, TNF-α, N51/KC, or iNOS. The samples (20-μl volume) were incubated in a Perkin-Elmer GeneAmp 2400 thermocycler for 30 min at 37°C, and the reaction was stopped by heat inactivation at 95°C for 5 min.
The first-strand cDNA samples were made up to 100 μl of PCR mixture containing 50 mM Tris HCl (pH 8.3), 2 mM MgCl2, 0.2 mM deoxynucleoside triphosphates, 2 U of Taq polymerase (Promega), and 30 pmol of upstream primers. The primer pairs for GAPDH, N51/KC, iNOS, and TNF-α were designed to cross introns to avoid confusion between mRNA and genomic DNA. PCR conditions were optimized for each set of primers. After an initial denaturation step at 94°C for 2 min, the samples were subjected to 30 (TNF-α, N51/KC, and iNOS) or 35 (GAPDH and MVE E gene) cycles of denaturation at 94°C for 30 s, annealing at 45°C (GAPDH, MVE E gene, iNOS, and TNF-α) or 65°C (N51/KC) for 1 min, and extension at 72°C for 2 min. Samples then underwent a final incubation at 72°C for 10 min. After PCR, the amplified products were separated by electrophoresis on 1% agarose gels. The predicted sizes for the RT-PCR products are 520 bp for GAPDH, 427 bp for the MVE E gene, 374 bp for TNF-α, 539 bp for N51/KC, and 492 bp for iNOS. Nucleotide sequences of the oligonucleotide primers used in the RT-PCR assays are shown in Table 1.
TABLE 1.
Sequences of oligonucleotides used in RT-PCR and Southern hybridization
| Oligonucleotide | Nucleotide sequence (5′→3′) |
|---|---|
| GAPDH downstream | ACCACCATGGAGAAGGCTGG |
| GAPDH upstream | CTCAGTGTAGCCCAGGATGC |
| GAPDH probe | AAGGACTATGACCACAGTCCATGCC |
| MVE E gene downstream | CCAACTTCATATTTGATG |
| MVE E gene upstream | GCTCCTCGTTGCTCCTGC |
| MVE E gene probe | GAAACGTCTGACACAGTAGCTGCATAGCAG |
| TNF-α downstream | TTGACCTCAGCGCTGAGTTG |
| TNF-α upstream | CCTGTAGCCCACGTCGTAGC |
| TNF-α probe | CTGGAAGACTCCTCCCAGGTATATGGGCTC |
| N51/KC downstream | GTGGTTGACACTTAGTGGTCTC |
| N51/KC upstream | TCGCTTCTCTGTGCAGCGCT |
| N51/KC probe | GCTCTGATGGCACCGTCTGGTGAACGCTGG |
| iNOS downstream | CGGAATTCGAGCTTCGTGGCTTTGGGCTCCTC |
| iNOS upstream | CGGGATCCCTTCCGAAGTTTCTGGCAGCAGCG |
| iNOS probe | ACGTTCAGGACATCCTGCAAAAGCAGCTGG |
Southern hybridization of RT-PCR products.
After agarose gel electrophoresis, the cDNA contained in the gel was denatured for 45 min in denaturing solution (1.5 M NaCl, 0.5 M NaOH), rinsed in water, and soaked in neutralizing solution (1.5 M NaCl, 0.5 M Tris HCl [pH 7.5]) for 45 min. The cDNA was transferred overnight onto a nylon membrane (Hybond N+; Amersham) by capillary transfer in 20× SSC (3 M NaCl, 0.3 M sodium citrate).
Oligonucleotide probes complementary to the internal sequence of the RT-PCR products were end-labelled with [γ-32P]ATP in the presence of T4 polynucleotide kinase (Promega) at 37°C for 45 min, followed by heat inactivation for 10 min at 68°C, and the labelled probes were concentrated by ethanol precipitation. The nylon membrane was incubated in hybridization solution (7% sodium dodecyl sulfate, 0.5 M Na2HPO4–NaH2PO4 [pH 7.0], 1 mM EDTA, 1% bovine serum albumin) for 2 h at 65°C, followed by hybridization of 32P-labelled oligonucleotide probes (50 ng) in hybridization solution at 65°C for 12 to 14 h. Following overnight hybridization, the membrane was washed to remove unbound probe, air dried, and exposed to X-ray film overnight at −80°C. Oligonucleotide probe sequences are shown in Table 1.
The autoradiographs were scanned on a transmissive flatbed scanner, and the densities of the bands were quantified by using ImageQuaNT software (Molecular Dynamics). The relative densities of the bands for the MVE E gene, TNF-α, N51/KC, and iNOS mRNA were determined by reference to the density of the GAPDH mRNA band. The density of the GAPDH band at each time point varied no more than twofold.
Preparation of frozen tissue sections.
After dissection, the brains were covered in Cryo-M-Bed (Bright Instruments) embedding medium, snap frozen in liquid nitrogen, and stored at −80°C. Coronal or sagittal sections were cut on a cryostat and mounted onto slides which had been precoated with 3-aminopropyltriethoxy-silane (ICN Biochemicals). Tissue sections used for in situ hybridization (ISH) were cut to a thickness of 15 μm, air dried at room temperature for 30 min, fixed in phosphate-buffered 2% paraformaldehyde for 15 min, washed three times in PBS, and stored at 4°C in 70% ethanol–30% DEPC-treated water. Tissue sections used for immunohistochemistry were cut to a thickness of 8 μm, fixed in ice-cold acetone for 15 min, followed by phosphate-buffered 2% paraformaldehyde for 15 min, rinsed in distilled water for 3 min, and stored at −20°C.
ISH.
Tissue sections were treated with 0.2 N HCl for 20 min at room temperature, acetylated for 10 min in 0.25% acetic anhydride (ICN Biomedicals) containing 0.1 M triethanolamine (Sigma), and washed in PBS (three times). The sections were permeabilized by digestion in proteinase K (10 μg/ml; Boehringer Mannheim) for 15 min. Prehybridization was at room temperature for 2 to 3 h in 100 μl of hybridization buffer (50% formamide, 2× SSC, 0.01 M Tris HCl, 12.5% Denhardt’s solution, 0.1% Triton X-100, 250 μg of sheared, denatured salmon sperm DNA per ml, 5 mg of sodium pyrophosphate per ml). Hybridization solution was prepared by adding 200 ng of digoxigenin (DIG)-cRNA per ml of hybridization buffer, heated for 5 min at 85°C to denature the probe, and chilled on ice. Preparation of the MVE E gene probe for ISH was previously described by McMinn et al. (26). Prehybridization solution was removed, 100 μl of hybridization mixture was added, and hybridization was allowed to occur at 37°C for 18 to 20 h in a humidified chamber. After hybridization, the coverslips were removed by immersion in 4× SSC and the sections were washed twice in 2× SSC for 15 min at room temperature.
Upon completion of the hybridization step, the sections were incubated for 30 min with RNase A (1 μg/ml in 2× SSC) to digest unbound probe and washed twice in 0.2× SSC for 30 min at 37°C and once in 0.1× SSC for 15 min at room temperature. Tissue sections were then incubated in buffer solution (0.1 M Tris HCl [pH 7.5], 0.15 M NaCl) containing 0.5% (wt/vol) blocking reagent (Boehringer Mannheim) at room temperature for 30 min, followed by incubation with anti-DIG polyclonal antiserum alkaline phosphatase conjugate (1:500 in blocking buffer) (Boehringer Mannheim) for 1 h. Tissue sections were then washed twice in PBS (15 min each) at room temperature before commencing the TUNEL assay.
TUNEL assay.
Prior to commencing the TUNEL assay, endogenous peroxidase activity was blocked by immersion of the sections in 1% hydrogen peroxide-methanol for 10 min. This was followed by a 1-h incubation (at 37°C) in labelling mix (consisting of 50 mM cacodylate buffer [pH 6.8], 0.5 mM CoCl2, 0.05 mM dithiothreitol, 0.15 mM dATP, 40 U of terminal deoxynucleotidyltransferase [Promega], 0.6 μl of biotin-16-dUTP [Boehringer Mannheim]). After labelling, the sections were blocked with 2% normal sheep serum and 0.2% Triton X-100 in PBS, followed by a 30-min incubation with streptavidin horseradish peroxidase conjugate (Pierce) diluted 1:100 in PBS.
Visualization of the ISH and TUNEL signals in the tissue sections.
The ISH signal was detected by using the alkaline phosphatase substrate fast red TR/Naphthol (Sigma) to give a red cytoplasmic signal for viral RNA. The TUNEL signal was visualized by incubation with diaminobenzidine (Pierce) for 20 min, followed by incubation for 5 min in 0.5% copper sulfate in 0.15 M NaCl2, resulting in a dark brown nuclear signal. The double-labelled tissue sections were then washed several times in distilled water, lightly counterstained with hematoxylin, and mounted in CrystalMount (Biomeda), followed by DePeX (Gurr Scientific) and a glass coverslip. Specific neuroanatomical structures in the mouse brain were identified by reference to a stereotaxic atlas of the mouse brain (12).
Immunohistochemistry.
Tissue sections were rehydrated in PBS for 5 min, digested with trypsin (1 μg/ml) for 30 min at 37°C, and blocked with 5% normal rat serum for 30 min at room temperature. The sections were then incubated overnight (4°C) in undiluted cell culture supernatant of rat anti-mouse GR1 monoclonal antibody (RB6-8C5; a gift from R. Ashman, Department of Pathology, The University of Western Australia). The sections were then rinsed in PBS, and endogenous peroxidase activity was quenched by incubation in 1% hydrogen peroxide in PBS for 30 min. The sections were then incubated with biotinylated goat anti-rat antibody (Pierce) diluted 1:250 in PBS for 30 min and streptavidin horseradish peroxidase diluted 1:250 in PBS for 30 min. Bound primary antibody was detected by using a modified Hanker-Yates stain (13, 32). Sections were incubated in Hanker-Yates solution A (500 mg of _p_-phenylenediamine per liter, 1 g of catechol per liter, 4 g of ammonium nickel sulfate per liter, 6 g of cobalt chloride per liter in 0.1 M cacodylate buffer [pH 5.1]) for 5 min, washed once in PBS, and incubated in Hanker-Yates solution B (500 mg of _p_-phenylenediamine per liter, 1 g of catechol per liter, 0.003% H2O2 in 0.1 M cacodylate buffer [pH 5.1]) for 5 min. Sections were rinsed in tap water, counterstained in hematoxylin, dehydrated in ethanol, butan-1-ol, and xylene, and mounted in DePeX.
In vivo depletion of neutrophils in BALB/c and Swiss mice.
Cell culture supernatant containing the RB6-8C5 MAb (IgG2b) and normal rat serum (antibody isotype control) were concentrated by ammonium sulfate precipitation; the protein concentration was determined by using a spectrophotometer and was adjusted to 2 mg/ml with PBS. Groups of 21-day-old Swiss mice were injected i.p. every second day with 200 μg of RB6-8C5 MAb or with 200 μg of rat serum protein for 8 days. To assess the adequacy of neutrophil depletion, peripheral blood smears were collected daily by saphenous vein puncture, stained with Diff-Quik (Lab Aids), and examined by light microscopy. Neutrophils were identified by their characteristic morphology, and the total cell number in four high-power fields was determined; three mice were examined per time point.
The effectiveness of MAb RB6-8C5 in depleting neutrophils in mice with a peripheral neutrophilia was determined by injecting 20 mg of lipopolysaccharide (LPS; Escherichia coli serotype O55:B5; Sigma) per kg of body weight i.p. before commencing second daily i.p. injections of MAb. Peripheral neutrophil numbers were determined as outlined above.
Treatment of mice with aminoguanidine.
Groups of 21-day-old Swiss mice were treated twice daily by i.p. injection with 250 or 500 mg of aminoguanidine hemisulfate (Sigma) per kg in PBS for up to 14 days; 1% aminoguanidine (wt/vol) was provided continuously in the water supply throughout the experiment. Control mice were injected with PBS only. No aminoguanidine was added to the water supply of control mice.
To examine the effectiveness of aminoguanidine in the inhibition of iNOS activity in vivo, groups of 21-day-old mice which had received 250 or 500 mg of aminoguanidine per kg or placebo (as above) for 14 days were challenged with LPS (20 mg/kg, i.p.) and nitric oxide concentrations in serum samples were measured 12 h later by chemiluminescence by using a Sievers 280 NO analyzer (Sievers Instruments, Boulder, Colo.).
Statistical analysis.
Tests of statistical significance were used to compare differences in the average survival (Student’s t test) and percent mortality (χ2 test) of MVE-infected or mock-infected mice treated with the antineutrophil MAb RB6-8C5 or with the iNOS inhibitor aminoguanidine.
RESULTS
Time course of infection with MVE BH3479.
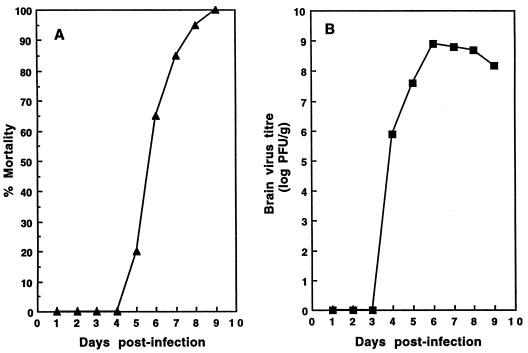
The model of MVE infection presented in this study involves peripheral inoculation (either footpad or i.p.) of a lethal dose of virus (104 PFU 100 times the i.p. or footpad 50% lethal dose) (26) to susceptible 21-day-old Swiss mice. A representative example of the outcome of MVE BH3479 infection in Swiss mice is presented in Fig. 1. The cumulative mortality of a group of mice following i.p. inoculation with 104 PFU of virus is shown in Fig. 1A. Mortality from BH3479 infection was first observed at 5 days p.i., and all mice had died from encephalitis by 9 days p.i. The average survival of mice infected with BH3479 was 6.9 (±1.2) days. Cumulative virus titers in the brains of infected mice are shown in Fig. 1B. Infectious virus was first observed in the brains of BH3479-infected mice at 4 days p.i. (average titer, 5 × 104 PFU/g) and peaked at 6 days p.i. (average titer, 1.5 × 109 PFU/g).
FIG. 1.
(A) Cumulative mortality profile of 21-day-old Swiss mice infected with MVE BH3479. Groups of 40 mice of either sex were inoculated i.p. with 104 PFU of BH3479 and were observed for 14 days of signs of illness and death. The percents cumulative mortality at the indicated time points are shown. No mock-infected mice died during the experiment. (B) Growth of MVE BH3479 in the brains of 21-day-old Swiss mice after peripheral inoculation. Groups of 50 mice of either sex were inoculated i.p. with 104 PFU of virus. At the indicated time points, mice were terminally anesthetized and the brains were removed and prepared as 10% suspensions in Hanks’ balanced salt solution. Infectivity titers within infected brains were determined by plaque formation on Vero cell monolayers. Infectivity titers are expressed as log10 PFU/g of tissue. Each time point represents the average of four mice; titers at each time point did not vary more than fourfold.
Distribution of TUNEL-positive cells within MVE-infected brain.
Recently published studies indicate that the neurovirulence of certain flaviviruses results from their ability to induce infected neurons to undergo apoptosis (10, 11, 22). Our initial experiments were designed to test the hypothesis that MVE causes encephalitis in mice by inducing apoptosis in infected neurons. Thus, we developed an assay in which viral RNA is detected by ISH and apoptosis is detected by in situ TUNEL assay in the same tissue section. This assay provided a clear picture of the proportion of virus-infected CNS cells which developed apoptosis during the time course of MVE infection in Swiss mice (see above).
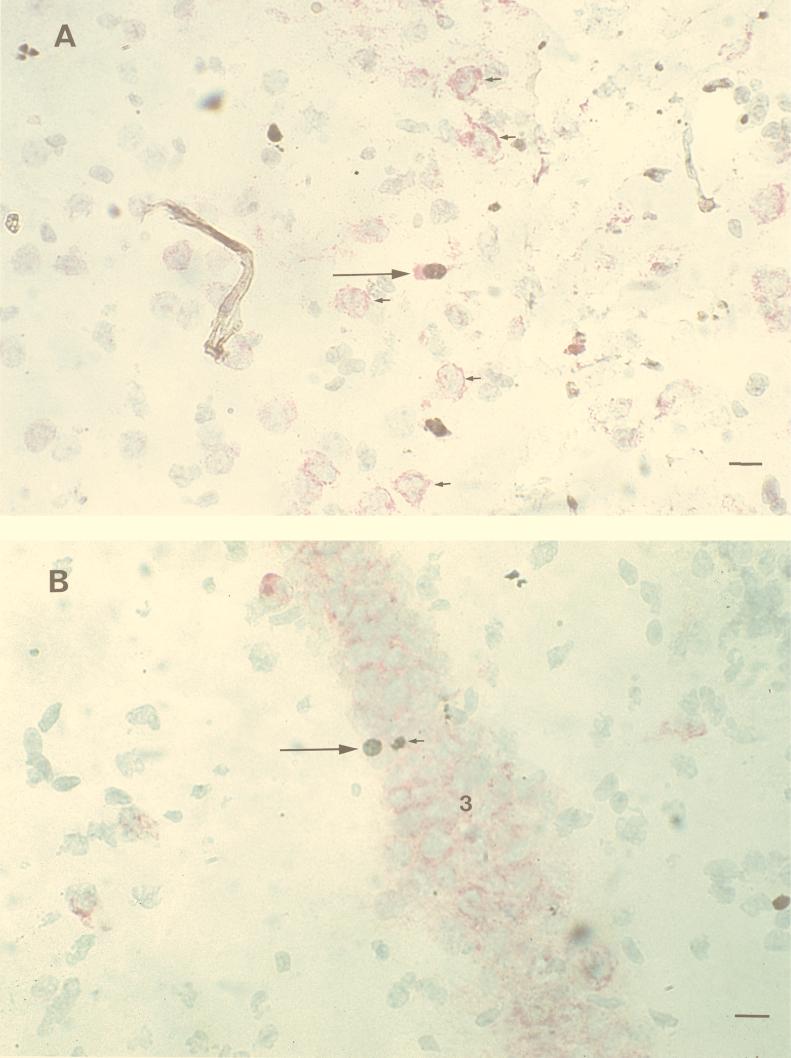
As noted previously (26), viral RNA was first detected in the olfactory bulb of BH3479-infected mice at 4 days p.i. and spread in a rostral-to-caudal direction over 4 to 5 days (data not shown). Viral RNA was widely distributed in the CNS from 6 days p.i. Double ISH and TUNEL staining in single cells was observed from 5 days p.i. in neurons of the anterior olfactory nucleus (Fig. 2A), pyriform cortex, dentate gyrus, proximal CA3 (Fig. 2B), distal CA1, caudate putamen, cerebral cortex, and midbrain. In all cases, viral RNA was detected at least 1 day before the appearance of TUNEL-positive nuclei. TUNEL-positive nuclei were never observed in uninfected neurons. Despite the majority of neurons within these structures showing viral RNA presence by 6 days p.i., fewer than 1 infected neuron per 1,000 showed evidence of apoptosis by in situ TUNEL assay.
FIG. 2.
Localization of MVE BH3479 RNA by ISH and of apoptotic cells by in situ TUNEL assay in the brains of Swiss mice. The ISH probe was DIG-labelled MVE E gene RNA of negative polarity; the hybridized probe was detected with anti-DIG alkaline phosphatase and fast red to produce a red cytoplasmic stain. TUNEL positivity was detected with biotinylated dUTP, streptavidin horseradish peroxidase, and diaminobenzidine to produce a brown nuclear stain. Frozen tissue sections (15 μm) were cut on a cryostat and counterstained with hematoxylin. (A) Coronal section through the anterior olfactory nucleus at 6 days p.i. A double-labelled neuron (long arrow) is seen in the center of the picture. Numerous MVE-infected neurons with TUNEL-negative nuclei are present (short arrows). Bar, 50 μm. (B) Coronal section through the hippocampal formation at 6 days p.i. A double-labelled neuron (long arrow) is seen within a heavily infected region of CA3 (indicated by the no. 3). A TUNEL-positive inflammatory cell is seen adjacent to the double-labelled neuron (short arrow). Bar, 50 μm.
A mixed-cell inflammatory infiltrate, in which neutrophils predominated, developed in the CNS of BH3479-infected mice between 5 and 6 days p.i. From 7 days p.i., inflammatory cells comprised the majority of TUNEL-positive cells in BH3479-infected mouse brain sections (data not shown). However, none of the TUNEL-positive inflammatory cells showed evidence of infection with MVE.
Time course and distribution of neutrophils in MVE-infected brain.
As noted above, only a small percentage of MVE-infected neurons appeared to undergo apoptosis, with most infected neurons showing no evidence of virus-induced cytopathology. However, a mixed-cell inflammatory infiltrate, predominated by neutrophils, was seen from 5 days p.i. and correlated with the onset of lethal encephalitis. This observation suggested the possibility that inflammatory responses to MVE infection may contribute to the severity of disease. Thus, we undertook experiments to identify the temporal and spatial distribution of neutrophils within the murine CNS in response to MVE infection.
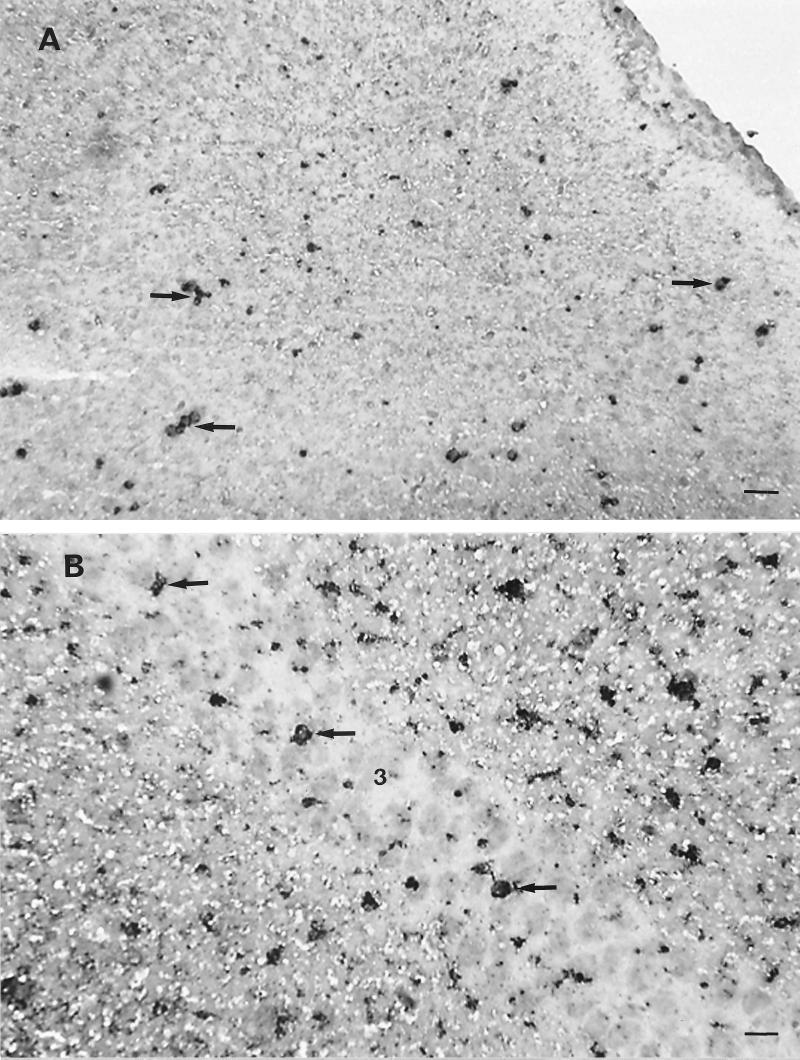
Groups of 21-day-old Swiss mice were infected i.p. with 104 PFU of BH3479, and brain tissue was collected daily between days 2 and 8 p.i. and prepared for immunohistochemistry as described above. Tissue sections were stained for the presence of neutrophils with the rat anti-mouse neutrophil MAb RB6-8C5 (17). Neutrophils were first identified at 4 days p.i. within and surrounding meningeal blood vessels. From 5 days p.i., neutrophils were observed within the parenchyma of the CNS and were often observed as distinct foci adherent to infected neurons (Fig. 3A). Neutrophil infiltration was heaviest in structures of the hippocampal formation, including the dentate gyrus, CA1, CA3 (Fig. 3B), and subiculum, and within midbrain structures, such as the thalamus. Neutrophil infiltration corresponded closely to areas of the CNS that were infected with virus and occurred concurrently or within 24 h of infection of particular CNS structures. After entering into the CNS parenchyma at 5 days p.i., neutrophil numbers increased progressively until 7 days p.i., after which no further increases in cell number were observed (data not shown).
FIG. 3.
Identification of infiltrating neutrophils in the brains of MVE BH3479-infected Swiss mice. Neutrophils were identified by using the rat anti-mouse neutrophil MAb RB6-8C5. Bound antibody was detected by using modified Hanker-Yates substrate (13, 32) to produce an opaque black deposit. Frozen tissue sections (8 μm) were cut on a cryostat and counterstained with hematoxylin. (A) Coronal section through the cerebral cortex at 6 days p.i. Many neutrophils can be seen adhering to neurons (neuronophagia; arrows). Bar, 80 μm. (B) Coronal section through the hippocampal formation at 7 days p.i. Foci of neutrophils (arrows) can be seen adhering to neurons within the CA3 (indicated by the no. 3) region. Bar, 50 μm.
Effect of neutropenia on the outcome of MVE infection in mice.
We have shown that the CNS parenchyma becomes infiltrated with neutrophils in response to MVE infection from 5 days p.i. Infiltrating neutrophils have been shown to inflict significant damage within the CNS during the acute inflammatory response to occlusion-reperfusion injury in mice (31). In order to determine if infiltrating neutrophils contribute to the pathogenesis of encephalitis, a comparison of the severity of MVE infection in neutropenic and nonneutropenic Swiss mice was undertaken.
Neutropenia was induced in mice by treatment with MAb RB6-8C5 (200 μg, i.p., every 2 days). Neutrophil numbers decreased from an average of 5 × 103 to 7 × 103/ml to 0.1 × 103 to 0.2 × 103/ml within 24 h of treatment, representing a drop of 96 to 98% compared to the numbers in rat serum protein-treated (antibody isotype) controls. The duration of neutrophil depletion during treatment with MAb RB6-8C5 was 4 days, after which neutrophil numbers rapidly returned to pretreatment levels (data not shown). The transient neutropenia induced by RB6-8C5 treatment before the return of normal cell numbers is consistent with the findings of earlier studies (9, 42, 43). The aim of the experiment described below was to determine the effect of MAb RB6-8C5-induced neutropenia on the severity of encephalitis during the CNS phase of MVE infection in Swiss mice, that is, between 5 and 9 days p.i. (Fig. 1).
Groups of 21-day-old Swiss mice were inoculated i.p. with 104 PFU of BH3479 and treated with MAb RB6-8C5 (200 μg, i.p.) or rat serum protein (200 μg, i.p.) at 5, 7, and 9 days p.i.; mice were observed for signs of illness and death until 21 days p.i. (Table 2). Mice treated with MAb RB6-8C5 had significantly prolonged survival (P < 0.05) and reduced mortality (P < 0.01) compared to the rat serum-treated controls. Immunohistochemical analysis indicated that neutrophils were undetectable in the CNS of MVE-infected, neutropenic mice between 5 and 9 days p.i., whereas mice treated with rat serum over the same time period had CNS neutrophil numbers comparable to sham neutrophil-depleted, MVE-infected mice (data not shown). Also, the prolonged survival of neutropenic, MVE-infected mice was not due to reduced viral neuroinvasion, as virus entered the CNS at 5 days p.i. in both neutrophil-depleted and mock-depleted mice and virus titers in the CNS of both groups were almost identical between 5 and 9 days p.i. (between 108 and 109 PFU/g).
TABLE 2.
Effect of neutrophil depletion on the outcome of MVE infection in mice
| Days mice treated ona | No. of mice | Antibody treatment | Mean days ± SD to death (P)b | Percent mortality (P)c |
|---|---|---|---|---|
| None | 20 | None | 8.0 ± 1.5 | 100 |
| 5, 7, 9 | 20 | Rat serum | 7.5 ± 2.6 (NS)d | 100 |
| 5, 7, 9 | 20 | RB6-8C5 | 11.0 ± 4.0 (<0.05) | 45 (<0.01) |
Expression of TNF-α, N51/KC, and iNOS mRNA in MVE-infected brain.
We have shown that neutrophils infiltrate the CNS parenchyma of MVE-infected mice between 5 and 9 days p.i. and appear to contribute to the severity of encephalitis. However, the stimulus for neutrophil infiltration into the CNS, and the mechanism by which neutrophils disturb CNS function during MVE infection, is not known. TNF-α is known to be expressed by resident CNS cells, especially astrocytes (44), in response to viral infection and to activate cerebral endothelial cells, resulting in leukocyte adhesion to the cerebral capillary wall (margination) (35). N51/KC is a murine homologue of human interleukin-8 (IL-8) and is a powerful neutrophil chemoattractant (40, 41). N51/KC is known to be expressed in the CNS (41) and stimulates the movement of marginated neutrophils into the CNS parenchyma. Neutrophils produce several substances that are toxic to neurons, including reactive oxygen and nitrogen intermediates (38). A high level of expression of iNOS (and thus nitric oxide) within the CNS is associated with the severity of encephalitis in several animal models (8, 18, 44). Thus, we were interested in determining if TNF-α, N51/KC, and iNOS were expressed within the CNS of MVE-infected mice during the encephalitic phase of infection.
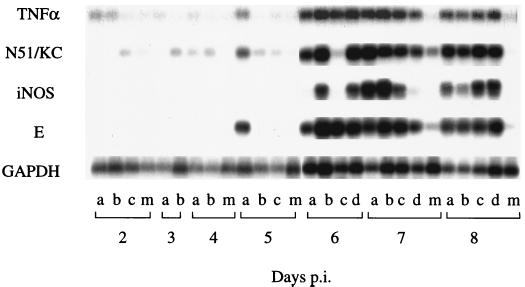
Groups of 21-day-old Swiss mice were infected i.p. with 104 PFU of BH3479, and brain tissue was collected from three or four mice between 2 and 8 days p.i., inclusive. These mice were part of the same experiment as the neutrophil distribution study (see above), allowing us to correlate the expression of TNF-α, N51/KC, and iNOS with that of neutrophil infiltration in the same animals. RNA extracted from brain tissue was used as a template for RT-PCR or Southern hybridization to identify mRNA for GAPDH, TNF-α, N51/KC, iNOS, and MVE E gene RNA (Fig. 4). GAPDH mRNA was used as a standard to allow determination of the relative quantities of MVE E gene RNA and TNF-α, N51/KC, and iNOS mRNA present in each sample by densitometry of the Southern blot bands (data not shown).
FIG. 4.
Representative RT-PCR and Southern blot analysis of GAPDH, TNF-α, N51/KC, and iNOS mRNA from MVE-infected mouse brains at 2 to 8 days after i.p. inoculation of 104 PFU of BH3479 (two to four mice per time point); individual mouse brain samples are marked (lanes a through d) at each time point. Negative controls consisted of distilled water and mock-infected mouse brain RNA during the RT-PCR; mock-infected mouse brain samples are labelled (lanes m) at each time point. Predicted sizes of the RT-PCR products are as follows: 520 bp for GAPDH, 427 bp for the MVE E gene, 374 bp for TNF-α, 539 bp for N51/KC, and 492 bp for iNOS. Bands of the appropriate size were amplified in each case, indicating that the PCR products had been amplified from mRNA templates and not from contaminating genomic DNA.
MVE E gene RNA was first detected in one of three infected mice at 5 days p.i. and was present in the brains of all mice at similar concentrations between 6 and 8 days p.i. TNF-α and N51/KC mRNA were detected at low levels in the CNS of mock-infected mice and MVE-infected mice between 2 and 5 days p.i. Levels of TNF-α and N51/KC mRNA increased six- to eightfold in the CNS of MVE-infected mice between 6 and 8 days p.i., concurrent with infiltration of neutrophils into the CNS of the same animals and with the onset of encephalitis (see above). By contrast, iNOS mRNA was not detected in the CNS of mock-infected or MVE-infected mice between 2 and 5 days p.i. but was expressed at a high level between 6 and 8 days p.i. As noted for TNF-α and N51/KC, expression of iNOS mRNA coincided with neutrophil infiltration into the CNS and with the onset of encephalitis.
Effect of aminoguanidine inhibition of iNOS on the outcome of MVE infection.
It has been suggested that the expression of nitric oxide by infiltrating inflammatory cells may disturb neuron function in virus-mediated encephalitis (18). Thus, it was of interest to study the effect of nitric oxide expression in the CNS on the outcome of MVE infection in mice.
Groups of 21-day-old Swiss mice were treated with aminoguanidine at a concentration of 250 or 500 mg/kg (twice daily, i.p., 15 days); a placebo group received diluent (PBS) only. Twenty-four hours after the commencement of aminoguanidine treatment, mice were infected or mock-infected with 104 PFU of BH3479 in the left footpad and were observed for signs of illness and death during the following 14 days. The percent mortality and average survival in groups of mice treated with aminoguanidine were compared with those of placebo-treated mice (Table 3). Percent mortalities in the two aminoguanidine-treated mouse groups differed significantly from those of the placebo-treated mice (P < 0.05). In addition, the average survival of mice treated with 250 mg of aminoguanidine per kg (8.7 ± 1.9 days; P < 0.01) or with 500 mg of aminoguanidine per kg (9.1 ± 1.9 days; P < 0.001) was significantly prolonged compared to the average survival of placebo-treated control mice (7.6 ± 1.5 days).
TABLE 3.
Effect of aminoguanidine on survival of MVE-infected mice
| Treatment given to micea | No. of miceb | Average days ± SD of survival (P)c | Percent mortality (P)d |
|---|---|---|---|
| None | 28 | 7.8 ± 1.8 | 82 |
| Placebo | 58 | 7.6 ± 1.5 (NS)e | 78 |
| Aminoguanidine (250 mg/kg) | 58 | 8.7 ± 1.9 (<0.01) | 59 (<0.05) |
| Aminoguanidine (500 mg/kg) | 58 | 9.1 ± 1.9 (<0.001) | 57 (<0.05) |
The effectiveness of iNOS inhibition by aminoguanidine was determined by comparing concentrations of nitric oxide in serum 12 h after LPS stimulation (20 mg/kg, i.p.) of mice that had been treated with 250 or 500 mg of aminoguanidine per kg or with placebo for 14 days. Mice treated with placebo but not given LPS stimulation had an average serum nitric oxide concentration of 0.49 ± 0.11 μM. In contrast, LPS stimulation of placebo-treated mice resulted in an average serum nitric oxide concentration of 3.51 ± 3.91 μM. Mice that had been treated with 250 and 500 mg of aminoguanidine per kg had average serum nitric oxide concentrations of 0.63 ± 0.36 μM and 0.42 ± 0.28 μM, respectively, 12 h after LPS stimulation, indicating that aminoguanidine treatment reduced serum nitric oxide levels to pre-LPS stimulation levels.
DISCUSSION
In this study, we have examined the pathological features of CNS infection with a virulent strain of MVE in weanling Swiss mice after peripheral inoculation. Virus entered the CNS via the olfactory bulb at 4 days p.i. and spread throughout the CNS in a rostral-to-caudal direction over a 4- to 5-day period, consistent with previous observations (26), and caused 95 to 100% mortality with an average survival of 6.9 ± 1.2 days. Infected mice developed encephalitis from 5 days p.i., associated with development of a mixed inflammatory cell infiltrate in which neutrophils predominated. The inflammatory cell infiltrate was distributed in perivascular regions and in virus-infected regions of the CNS parenchyma. Neutrophils were first seen in significant numbers in meningeal vessels at 4 days p.i. and within the CNS parenchyma at 5 days p.i. Neutrophil numbers steadily increased until 7 days p.i. and were seen only in areas of the CNS known to be infected with virus. Neutrophil infiltration coincided with increased expression of mRNA for TNF-α and the neutrophil-attracting chemokine N51/KC in the CNS. Depletion of neutrophils with a cytotoxic antineutrophil MAb during the CNS phase of infection resulted in significantly prolonged survival and reduced mortality compared to those of the controls. Induced expression of the inflammatory mediator iNOS mRNA within the CNS also correlated with the onset of encephalitis in infected mice. Treatment of MVE-infected mice with the iNOS inhibitor aminoguanidine resulted in significantly prolonged survival and reduced mortality compared to those of the placebo-treated controls.
The mechanism by which flaviviruses induce encephalitis in the host is still incompletely understood. Recent studies have shown that the flaviviruses dengue virus (10) and JE virus (22) induce apoptosis in infected neuroblastoma cells in vitro, suggesting a mechanism by which flavivirus infection may damage neurons within the CNS. Furthermore, the onset of encephalitis in dengue virus-infected mice is associated with the induction of apoptosis in infected neurons (11). However, we have shown that fewer than 0.1% of infected neurons develop apoptosis in MVE-infected Swiss mice, indicating that virus-induced cell injury is unlikely to be an important mechanism in the pathogenesis of MVE-mediated encephalitis in this model. In contrast, our study has provided a clear demonstration that host inflammatory responses to MVE infection in the CNS make a significant contribution to the pathogenesis of encephalitis. Depletion of neutrophils with a cytotoxic antineutrophil MAb was associated with prolonged survival of MVE-infected mice compared to that of mock-depleted controls, despite the presence of very similar titers of virus in the brains of neutrophil-depleted and control mice. Neutrophil recruitment into the CNS in response to insults such as infection, trauma, and infarction has been shown to have a deleterious effect on brain function (1, 2). This may be due to the release of toxic inflammatory mediators (31) or to a secondary effect of neutrophil-mediated breakdown of the blood-brain barrier (3), resulting in altered CNS homeostasis.
The neutrophil response to MVE infection in the CNS was associated with localized CNS expression of the proinflammatory cytokine TNF-α and the neutrophil chemoattractant N51/KC. Triggers for neutrophil infiltration into the CNS have recently been identified in mice and rats (40). CNS microglial cells and astrocytes activated by viral infection release IL-1β and TNF-α early in infection (44). TNF-α is known to activate cerebral endothelial cells and to promote leukocyte (neutrophil and monocyte) margination within cerebral capillaries (35). TNF-α also induces the expression of Cys-X-Cys (CXC) chemokines by CNS cells, including CINC-1 in rats (39) and N51/KC in mice (41). CXC chemokines provide a potent signal for neutrophil infiltration into the parenchyma of the CNS. Transgenic mice that constitutively express N51/KC in oligodendrocytes develop intense neutrophil infiltration within the CNS parenchyma, associated with disruption of the blood-brain barrier and chronic neurological dysfunction (41). Knockout (IL-1β−/−) mice develop significantly less paralysis and have lower mortality after infection with a neurovirulent strain of the alphavirus Sindbis virus than control mice of the same strain which express IL-1β (21). Interestingly, despite the differences in clinical outcome, no difference in the development of CNS neuronal apoptosis was observed between the two strains of mice. This suggests that the induction of apoptosis may be of lesser importance in the pathogenesis of Sindbis virus encephalitis in the mouse than was previously thought (20). Furthermore, these data suggest that Sindbis virus encephalitis in mice may result from the effect of inflammatory cytokine expression on CNS neuronal function, similar to that which we have observed in the MVE mouse model.
We have shown that expression of iNOS mRNA was induced in the CNS in response to MVE infection. It has been suggested that sustained, high-level expression of iNOS, and thus the potent inflammatory mediator nitric oxide, by infiltrating monocytes and neutrophils or by resident CNS cells, such as microglia and astrocytes, may be a determinant of disease severity in several animal models of viral encephalitis (18). This hypothesis is supported by the study of iNOS inhibition with aminoguanidine, which resulted in prolonged survival and decreased mortality of MVE-infected mice compared to those of placebo-treated controls. Aminoguanidine has a potent and highly specific inhibitory effect on iNOS at the concentrations used in this study (29). Interestingly, the reduced mortality and prolonged survival of aminoguanidine-treated mice was less marked than that resulting from neutrophil depletion, suggesting that other neutrophil-associated inflammatory mediators are involved in the pathogenesis of encephalitis. Our findings are supported by the work of Kreil and Eibl (19), who showed that tick-borne encephalitis virus-infected mice treated with aminoguanidine throughout infection had significantly prolonged survival compared to controls. Nitric oxide has been shown to disturb neuron function (8, 37), suggesting a mechanism by which high-level NO expression within the CNS may contribute to encephalitis. In addition, sustained expression of NO within the CNS increases permeability of the blood-brain barrier (4) and induces further expression of CXC chemokines, such as N51/KC, in the CNS (34).
We offer the following hypothesis for the development of encephalitis in MVE-infected immunocompetent mice. Viral replication and expression of viral antigen in the CNS of infected mice activate resident astrocytes and/or microglial cells, leading to localized expression of TNF-α (40). TNF-α induces the expression of leukocyte adhesion molecules on the surface of cerebral endothelial cells, resulting in margination of neutrophils and monocytes within cerebral capillaries (35). TNF-α also stimulates expression of the chemokine N51/KC by resident CNS cells (40, 41), resulting in chemotaxis of neutrophils across the cerebral endothelium (i.e., the blood-brain barrier) and into the CNS parenchyma. Upon entry into the CNS parenchyma, neutrophils release several inflammatory mediators, including nitric oxide, which disturb CNS homeostasis and neuronal function. The disturbance of CNS homeostasis in MVE infection may be exacerbated further by the combined effect of TNF-α and NO on the integrity of the blood-brain barrier (31, 41).
In conclusion, these studies provide clear evidence for an immunopathological mechanism in the pathogenesis of Murray Valley encephalitis in mice and may ultimately be of use in determining a role for anti-inflammatory agents in the management of flavivirus encephalitis in humans.
ACKNOWLEDGMENTS
D.M.A. and V.B.M. made an equal contribution to this study.
We thank Tulene Kendrick for preparation of the RB6-8C5 MAb supernatant and Josephine Ciputra for purification of RNA from mouse brain.
This work was undertaken with the support of grants from the Arthur Yeldham and Mary Raine Medical Research Foundation of Western Australia, the Australian Research Council, and the National Health and Medical Research Council.
REFERENCES
- 1.Anthony D, Dempster R, Fearn S, Clements J, Wells G, Perry V H, Walker K. CXC chemokines generate age-related increases in neutrophil-mediated brain inflammation and blood-brain barrier breakdown. Curr Biol. 1998;8:923–926. doi: 10.1016/s0960-9822(07)00373-9. [DOI] [PubMed] [Google Scholar]
- 2.Anthony D C, Bolton S J, Fearn S, Perry V H. Age-related effects of interleukin-1β on polymorphonuclear neutrophil-dependent increases in blood-brain barrier permeability in rats. Brain. 1997;120:435–444. doi: 10.1093/brain/120.3.435. [DOI] [PubMed] [Google Scholar]
- 3.Bell M D, Taub D D, Perry V H. Overriding the brain’s intrinsic resistance to leukocyte recruitment with intraparenchymal injection of recombinant chemokines. Neuroscience. 1996;74:283–292. doi: 10.1016/0306-4522(96)00083-8. [DOI] [PubMed] [Google Scholar]
- 4.Boje K M. Inhibition of nitric oxide synthase attenuates blood-brain barrier disruption during experimental meningitis. Brain Res. 1996;720:75–83. doi: 10.1016/0006-8993(96)00142-4. [DOI] [PubMed] [Google Scholar]
- 5.Boyle D B. Comparative studies of two related Australian flaviviruses: Murray Valley encephalitis and Kunjin. Ph.D. thesis. Canberra, Australia: Australian National University; 1979. [Google Scholar]
- 6.Calisher C H, Karabatsos N, Dalrymple J M, Shope R E, Porterfield J S, Westaway E G, Brandt W E. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyvalent antisera. J Gen Virol. 1989;70:37–43. doi: 10.1099/0022-1317-70-1-37. [DOI] [PubMed] [Google Scholar]
- 7.Camenga D L, Nathanson N. An immunopathologic component in experimental togavirus encephalitis. J Neuropathol Exp Neurol. 1975;34:492–500. doi: 10.1097/00005072-197511000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Campbell I L, Samimi A, Chiang C-S. Expression of the inducible nitric oxide synthase. J Immunol. 1994;153:3622–3629. [PubMed] [Google Scholar]
- 9.Cuprynski C J, Brown J F, Wagner R D, Sternberg H. Administration of antigranulocyte monoclonal antibody RB6-8C5 prevents expression of acquired resistance to Listeria monocytogenes infection in previously immunized mice. Infect Immun. 1994;62:5161–5163. doi: 10.1128/iai.62.11.5161-5163.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Despres P, Flamand M, Ceccaldl P, Deubel V. Human isolates of dengue type 1 virus induce apoptosis in mouse neuroblastoma cells. J Virol. 1996;70:4090–4096. doi: 10.1128/jvi.70.6.4090-4096.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Despres P, Frenkel M-P, Ceccaldi P-E, Dos Santos C D, Deubel V. Apoptosis in the mouse central nervous system in response to infection with mouse-neurovirulent dengue viruses. J Virol. 1998;72:823–829. doi: 10.1128/jvi.72.1.823-829.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franklin K, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego, Calif: Academic Press; 1997. [Google Scholar]
- 13.Hanker J S, Yates P E, Metz C B, Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977;9:789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- 14.Hase T, Dubois D R, Summers P L. Comparative study of mouse brains infected with Japanese encephalitis virus by intracerebral or intraperitoneal inoculation. Int J Exp Pathol. 1990;71:857–869. [PMC free article] [PubMed] [Google Scholar]
- 15.Hase T, Summers P L, Dubois D R. Ultrastructural changes of mouse brain neurons infected with Japanese encephalitis virus. Int J Exp Pathol. 1990;71:493–505. [PMC free article] [PubMed] [Google Scholar]
- 16.Hennekens C H, Buring J E. Epidemiology in medicine. Boston, Mass: Little, Brown and Co.; 1987. [Google Scholar]
- 17.Hestdal K, Ruscetti F W, Ihle J N, Jacobsen S E W, Dubois C M, Kopp W C, Longo D L, Keller J R. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991;147:22–28. [PubMed] [Google Scholar]
- 18.Koprowski H, Zheng Y M, Heber-Katz E, Fraser N, Rorke L, Fu Z F, Hanlon C, Dietzschold B. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci USA. 1993;90:3024–3027. doi: 10.1073/pnas.90.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreil T R, Eibl M M. Nitric oxide and viral infection: no antiviral activity against a flavivirus in vitro, and evidence for contribution to pathogenesis in experimental infection in vivo. Virology. 1996;219:304–306. doi: 10.1006/viro.1996.0252. [DOI] [PubMed] [Google Scholar]
- 20.Lewis J, Wesselingh S L, Griffin D E, Hardwick J M. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J Virol. 1996;70:1828–1835. doi: 10.1128/jvi.70.3.1828-1835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang X H, Goldman J E, Jiang H H, Levine B. Resistance of interleukin-1β-deficient mice to fatal Sindbis virus encephalitis. J Virol. 1999;73:2563–2567. doi: 10.1128/jvi.73.3.2563-2567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao C-L, Lin Y-L, Wang J-J, Huang Y-L, Yeh C-T, Ma S-H, Chen L-K. Effect of enforced expression of human bcl-2 on Japanese encephalitis virus-induced apoptosis in cultured cells. J Virol. 1997;71:5963–5971. doi: 10.1128/jvi.71.8.5963-5971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald F. Murray Valley encephalitis infection in the laboratory mouse. I. Influence of age on susceptibility to infection. Aust J Exp Biol Med Sci. 1952;30:319–324. doi: 10.1038/icb.1952.29. [DOI] [PubMed] [Google Scholar]
- 24.Mackenzie J S, Lindsay M D, Coelen R J, Broom A K, Hall R A, Smith D W. Arboviruses causing human disease in the Australian zoogeographic region. Arch Virol. 1994;136:447–461. doi: 10.1007/BF01321074. [DOI] [PubMed] [Google Scholar]
- 25.Marshall I D, Woodroofe G M, Hirsch S. Viruses recovered from mosquitoes and wildlife serum collected in the Murray Valley of south-eastern Australia, February 1974, during an epidemic of encephalitis. Aust J Exp Biol Med Sci. 1982;60:457–470. doi: 10.1038/icb.1982.51. [DOI] [PubMed] [Google Scholar]
- 26.McMinn P C, Dalgarno L, Weir R C. A comparison of the spread of Murray Valley encephalitis viruses of high or low neuroinvasiveness in the tissues of Swiss mice after peripheral inoculation. Virology. 1996;220:414–423. doi: 10.1006/viro.1996.0329. [DOI] [PubMed] [Google Scholar]
- 27.McMinn P C. The molecular basis of virulence of the encephalitogenic flaviviruses. J Gen Virol. 1997;78:2711–2722. doi: 10.1099/0022-1317-78-11-2711. [DOI] [PubMed] [Google Scholar]
- 28.Monath T P, Heinz F X. Flaviviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 961–1034. [Google Scholar]
- 29.Moore P K, Handy R L C. Selective inhibitors of neuronal nitric oxide synthase—is no NOS really good NOS for the nervous system? Trends Pharmacol Sci. 1997;18:204–211. doi: 10.1016/s0165-6147(97)01064-x. [DOI] [PubMed] [Google Scholar]
- 30.Murphy F A, Harrison A K, Gary G W, Whitfield M S, Forrester F T. St. Louis encephalitis virus infection of mice. Lab Investig. 1968;19:652–662. [PubMed] [Google Scholar]
- 31.Perry V H, Bell M D, Brown H C, Matyszak M K. Inflammation in the nervous system. Curr Opin Neurobiol. 1995;5:636–641. doi: 10.1016/0959-4388(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 32.Perry V H, Linden R. Evidence for dendritic competition in the developing retina. Nature. 1982;297:683–685. doi: 10.1038/297683a0. [DOI] [PubMed] [Google Scholar]
- 33.Poidinger M, Hall R A, Mackenzie J S. Molecular characterization of the Japanese encephalitis serocomplex of the Flavivirus genus. Virology. 1996;218:417–421. doi: 10.1006/viro.1996.0213. [DOI] [PubMed] [Google Scholar]
- 34.Remick D G, Villarette L. Regulation of cytokine gene expression by reactive oxygen and reactive nitrogen intermediates. J Leukoc Biol. 1996;59:471–475. doi: 10.1002/jlb.59.4.471. [DOI] [PubMed] [Google Scholar]
- 35.Selmaj K W. The role of cytokines in inflammatory conditions of the central nervous system. Semin Neurosci. 1992;4:221–229. [Google Scholar]
- 36.Semenov B F, Khozinsky V V, Vargin V V. The damaging action of cellular immunity in flavivirus infection of mice. Med Biol. 1975;53:331–336. [PubMed] [Google Scholar]
- 37.Shankar V, Kao M, Hamir A N, Sheng H, Koprowski H, Dietzschold B. Kinetics of virus spread and changes in levels of several cytokine mRNAs in the brain after intranasal infection of rats with borna disease virus. J Virol. 1992;66:992–998. doi: 10.1128/jvi.66.2.992-998.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith J A. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki H, Suematsu M, Miura S, Liu Y Y, Watanabe K, Miyasaka M. Rat CINC/Gro—a novel mediator for locomotive and secretagogue activation in neutrophils in vivo. J Leukoc Biol. 1994;55:652–657. doi: 10.1002/jlb.55.5.652. [DOI] [PubMed] [Google Scholar]
- 40.Tani M, Ransohoff R M. Do chemokines mediate inflammatory cell invasion of the central nervous system parenchyma? Brain Pathol. 1994;4:135–143. doi: 10.1111/j.1750-3639.1994.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 41.Tani M, Fuentes M E, Peterson J W, Trapp B D. Neutrophil infiltration, glial reaction, and neurological disease in transgenic mice expressing the chemokine N51/KC in oligodendrocytes. J Clin Investig. 1996;98:529–539. doi: 10.1172/JCI118821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas J, Gangappa S, Kanangat S, Rouse B T. On the essential involvement of neutrophils in the immunopathologic disease herpetic stromal keratitis. J Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]
- 43.Tumpey T M, Chen S-H, Oakes J E, Lausch R N. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J Virol. 1996;70:898–904. doi: 10.1128/jvi.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Dam A-M, Bauer J, Tilders F J H, Berkenbosch F. Endotoxin-induced appearance of immunoreactive interleukin-1β in ramified microglia in rat brain: a light and electron microscopic study. Neuroscience. 1995;65:815–826. doi: 10.1016/0306-4522(94)00549-k. [DOI] [PubMed] [Google Scholar]
- 45.Westaway E G, Brinton M A, Gaidamovich S Y, Horzinek M C, Igarashi A, Kaariainen L, Lvov D K, Porterfield J S, Russell P K, Trent D W. Flaviviridae. Intervirology. 1985;24:183–192. doi: 10.1159/000149642. [DOI] [PubMed] [Google Scholar]