Reduction of Simian-Human Immunodeficiency Virus 89.6P Viremia in Rhesus Monkeys by Recombinant Modified Vaccinia Virus Ankara Vaccination (original) (raw)
Abstract
Since cytotoxic T lymphocytes (CTLs) are critical for controlling human immunodeficiency virus type 1 (HIV-1) replication in infected individuals, candidate HIV-1 vaccines should elicit virus-specific CTL responses. In this report, we study the immune responses elicited in rhesus monkeys by a recombinant poxvirus vaccine and the degree of protection afforded against a pathogenic simian-human immunodeficiency virus SHIV-89.6P challenge. Immunization with recombinant modified vaccinia virus Ankara (MVA) vectors expressing SIVmac239 gag-pol and HIV-1 89.6 env elicited potent Gag-specific CTL responses but no detectable SHIV-specific neutralizing antibody (NAb) responses. Following intravenous SHIV-89.6P challenge, sham-vaccinated monkeys developed low-frequency CTL responses, low-titer NAb responses, rapid loss of CD4+ T lymphocytes, high-setpoint viral RNA levels, and significant clinical disease progression and death in half of the animals by day 168 postchallenge. In contrast, the recombinant MVA-vaccinated monkeys demonstrated high-frequency secondary CTL responses, high-titer secondary SHIV-89.6-specific NAb responses, rapid emergence of SHIV-89.6P-specific NAb responses, partial preservation of CD4+ T lymphocytes, reduced setpoint viral RNA levels, and no evidence of clinical disease or mortality by day 168 postchallenge. There was a statistically significant correlation between levels of vaccine-elicited CTL responses prior to challenge and the control of viremia following challenge. These results demonstrate that immune responses elicited by live recombinant vectors, although unable to provide sterilizing immunity, can control viremia and prevent disease progression following a highly pathogenic AIDS virus challenge.
A safe and effective human immunodeficiency virus type 1 (HIV-1) vaccine is urgently needed to control the worldwide HIV-1 epidemic. A number of recent studies have demonstrated the importance of virus-specific CD8+ cytotoxic T lymphocytes (CTLs) in controlling HIV-1 replication in humans and simian immunodeficiency virus (SIV) replication in rhesus monkeys (18, 26, 27, 36). It is therefore widely believed that HIV-1 vaccine candidates should elicit potent virus-specific CTL responses in addition to neutralizing antibody (NAb) responses.
Live, attenuated virus vaccines have been shown to generate CTL and NAb responses capable of controlling a number of pathogenic viral challenges (10, 40, 41). However, significant safety concerns regarding this approach remain. Live, attenuated SIV vaccines have been shown to induce AIDS in neonatal and adult macaques (4, 5). More importantly, humans infected with _nef_-deleted HIV-1 have been reported to develop immunodeficiency and clinical disease (11, 14, 22).
Other vaccine strategies capable of eliciting virus-specific CTL responses are therefore being evaluated. Approaches that have generated considerable interest include plasmid DNA and recombinant live vectors. We have recently reported that plasmid DNA vaccination elicited high-frequency CTL responses that reduced setpoint viremia following an SIVsmE660 challenge in rhesus monkeys (12). We have also demonstrated that cytokine-augmented DNA vaccination elicited potent immune responses that effectively controlled viremia and prevented clinical disease progression following a pathogenic simian-human immunodeficiency virus SHIV-89.6P challenge (6, 7).
It remains to be determined whether other vaccination modalities, in particular live recombinant vectors, will provide a similar level of protection in monkeys challenged with the highly pathogenic virus SHIV-89.6P (32–34). A number of recombinant live poxviruses have been evaluated for their utility as HIV-1 vaccine candidates. Safety concerns regarding vaccinia virus (31) have led to the development of a number of attenuated poxviruses as vaccine vectors, including NYVAC, fowlpox, canarypox, and modified vaccinia virus Ankara (MVA) (8, 16, 28, 29, 37, 38). MVA is an attenuated form of vaccinia virus that has undergone 570 passages in primary chicken embryo fibroblasts and has genomic deletions that reduce its pathogenicity (23). We have recently reported that a recombinant MVA/gag-pol vaccine elicits SIV-specific CTL responses in rhesus monkeys (37). Following a pathogenic SIVsmE660 challenge, secondary CTL responses were detected associated with a partial control of viremia (38). In another study, vaccination with recombinant MVA/env, MVA/gag-pol, or MVA/gag-pol-env constructs reduced plasma viremia and increased survival following an SIVsmE660 challenge (28, 29).
In the present study, we investigate the ability of recombinant MVA vectors expressing SIV gag-pol and HIV-1 env derived from the primary patient isolate 89.6 to elicit CTL and NAb responses in rhesus monkeys. We also assess the protection afforded by these immune responses against a highly pathogenic SHIV-89.6P challenge.
MATERIALS AND METHODS
Construction of recombinant MVA vectors.
Open reading frames of SIVmac239 gag-pol and HIV-1 89.6 env truncated at amino acid 738 were inserted adjacent to the modified H5 promoter in the previously described plasmid transfer vectors pLW-9 and pLW-17, respectively (42, 43). Recombinant MVA/gag-pol and MVA/env vectors were each produced by homologous recombination, identified by immunostaining of live, infected cell foci, and clonally isolated. The purity of each recombinant virus was assessed by PCR and immunostaining. Expression of the recombinant proteins was determined by radioimmunoprecipitation. The production of Gag particles and surface expression and fusion competence of the expressed Env proteins were demonstrated.
Vaccination and challenge of rhesus monkeys.
Eight _Mamu-A*01_-positive rhesus monkeys were selected for inclusion in this study (20). The monkeys were immunized intramuscularly with 108 PFU of either control nonrecombinant MVA (n = 4) or recombinant MVA vectors expressing SIV gag-pol and HIV-1 89.6 env at weeks 0, 4, and 21. The monkeys were challenged at week 27 with a 1:500 dilution (estimated 100 50% monkey infective doses [MID50]) of the uncloned cell-free SHIV-89.6P stock (33, 34) by the intravenous (i.v.) route. Monkeys were maintained in accordance with National Institutes of Health and Harvard Medical School guidelines.
Tetramer staining.
Tetramer staining was performed with freshly isolated peripheral blood mononuclear cells (PBMC) from EDTA-anticoagulated whole blood specimens as described (3, 21). Briefly, soluble tetrameric Mamu-A*01 complexes folded around the SIV Gag p11C epitope (CTPYDINQM) (1, 24) were prepared. One microgram of phycoerythrin-labeled tetrameric Mamu-A*01/p11C complexes was used in conjunction with fluorescein isothiocyanate-labeled anti-human CD8α (Leu2a; Becton Dickinson), phycocerythrin-Texas Red (ECD)-labeled anti-human CD8αβ (2ST8-5H7; Beckman Coulter), and allophycocyanin-labeled anti-rhesus monkey CD3 (FN18) monoclonal antibodies to stain p11C-specific CD8+ T cells. A total of 100 μl of whole blood from the vaccinated or control monkeys was directly stained with these reagents, lysed, washed, and fixed. Samples were analyzed by four-color flow cytometry with a Becton Dickinson FACS Calibur system, and gated CD3+ CD8αβ+ T cells were examined for staining with tetrameric Mamu-A*01/p11C complexes.
CTL assays.
Functional chromium release cytotoxicity assays were performed as described (6). Briefly, 5 × 106 washed PBMC from rhesus monkeys were cultured in the presence of 10 μg of p11C peptide (CTPYDINQM)/ml (1, 24). On day 3 of culture, 20 U of human recombinant interleukin 2 (Hoffmann-La Roche)/ml was added. On day 12 of culture, peptide-stimulated PBMC were centrifuged over Ficoll (Ficoll-Paque) and assessed as effectors in standard 4-h 51Cr-release assays containing 104 target cells/well. Autologous B-lymphoblastoid cell lines pulsed with 1 μg of p11C peptide or p11B control peptide (ALSEGCTPYDIN)/ml and labeled overnight with 51Cr (100 μCi/ml) were used as targets. To measure spontaneous release of 51Cr, target cells were incubated with 100 μl of medium, and for maximum release target cells were incubated with 100 μl of 2% Triton X-100. Percent lysis was calculated as follows: [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100.
Neutralizing antibody assays.
Determination of antibody titers capable of neutralizing SHIV-89.6 and SHIV-89.6P was performed as described (9). Briefly, reduction of virus-induced cytopathic killing of MT-2 cells was measured by Finter's neutral red that is taken up by viable cells. A total of 50 μl of cell-free virus containing 500 50% tissue culture infective doses grown in human PBMC was added to multiple dilutions of test plasma in 100 μl of growth media in triplicate. These mixtures were incubated for 1 h before the addition of 5 × 104 MT-2 cells. Infection led to extensive syncytium formation and virus-induced cell killing in 4 to 6 days in the absence of neutralizing antibodies. Neutralization titers were calculated as the reciprocal dilution of plasma required to protect 50% of cells from virus-induced killing.
CD4+ T-lymphocyte counts and viral RNA levels.
CD4+ T-lymphocyte counts were determined by multiplying the total lymphocyte count by the percentage of CD3+ CD4+ T cells assessed by flow cytometry. Plasma viral RNA levels were measured by a real-time reverse transcriptase PCR amplification assay with a detection limit of 500 copies/ml as described (17) using gag primers and probes (39).
Statistical analyses.
Statistical analyses were performed with GraphPad Prism version 2.01 (GraphPad Software, Inc.). CD4+ T-lymphocyte counts and viral RNA levels were compared between groups by two-sided Wilcoxon rank sum tests. Day 70 setpoint values were chosen in order to analyze a complete data set prior to the death of any animals. Correlation of prechallenge vaccine-elicited CTLs and day 70 postchallenge setpoint viral RNA levels was assessed by a two-sided Spearman rank correlation test. In all cases, a P value of <0.05 was considered significant.
RESULTS
Vaccine trial design.
Eight rhesus monkeys (M. mulatta) expressing the major histocompatibility complex class I allele Mamu-A*01 were selected for inclusion in this study (20). These animals were immunized with the control nonrecombinant MVA (n = 4) or recombinant MVA vaccines expressing SIV gag-pol and HIV-1 env derived from the primary patient R5/X4 dual-tropic isolate 89.6 (n = 4). Animals received 108 PFU intramuscularly of control or recombinant MVA vectors at weeks 0, 4, and 21. At week 27, all eight animals were challenged i.v. with SHIV-89.6P. This highly pathogenic virus was derived by in vivo passage of the nonpathogenic virus SHIV-89.6 and has been shown to cause rapid CD4+ T-lymphocyte loss and clinical AIDS in the majority of naïve rhesus monkeys (7, 32–34).
Vaccine-elicited immune responses.
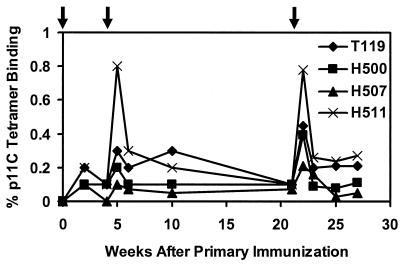
Staining CD8+ T cells with tetrameric MHC class I-peptide complexes followed by analysis by flow cytometry has proven to be an accurate method for quantitating epitope-specific CTLs in freshly isolated whole-blood specimens without the need for in vitro lymphocyte stimulation (3, 21). CTL responses specific for the _Mamu-A*01_-restricted immunodominant SIV Gag p11C epitope (CTPYDINQM) (1, 24) were measured by both tetramer staining and functional chromium release cytotoxicity assays. As shown in Fig. 1, p11C-specific CTLs were detected by tetramer staining in all vaccinated animals after the initial immunization. Higher levels were observed 1 week after the week 4 and week 21 boost immunizations, reaching a maximum of 0.2 to 0.8% of circulating CD3+ CD8+ T cells. Levels of CTLs following the second and third immunizations were comparable, consistent with the findings in our previous study of CTL responses elicited by recombinant MVA vectors in rhesus monkeys (37, 38). Following each boost immunization, there was a rapid expansion of p11C-specific CTLs followed by a rapid decline to steady-state plateau levels of 0.1 to 0.3% of circulating CD8+ T cells that persisted over time. Tetramer staining specific for the subdominant HIV-1 Env p41A epitope (YAPPISGQI) (13) was only detected in one animal (H507), and no tetramer staining specific for p11C or p41A was observed in the monkeys that received the control MVA (data not shown). As shown in Table 1, functional chromium release cytotoxicity assays confirmed these tetramer staining data. No NAb responses specific for SHIV-89.6 or SHIV-89.6P (<1:20 titer) were detectable in the control or vaccinated animals at peak immunity or prior to challenge (data not shown).
FIG. 1.
Vaccine-elicited CTL responses. _Mamu-A*01_-positive monkeys were immunized at weeks 0, 4, and 21 with recombinant MVA constructs expressing SIV gag-pol and HIV-1 89.6 env. Vaccine-elicited CD8+ T-cell responses specific for the immunodominant SIV Gag p11C (CTPYDINQM) epitope (1, 24) were measured by tetramer staining of freshly isolated PBMC (3, 21). The percent CD3+ CD8+ T cells that bound the Mamu-A*01/p11C tetramer is shown. Arrows indicate times of immunization.
TABLE 1.
Vaccine-elicited CTL responsesa
| Vaccination and monkey | Tetramer binding | Functional cytotoxicity (stimulated PBMC) | |
|---|---|---|---|
| Fresh PBMC | Stimulated PBMC | ||
| Control MVA | |||
| H544 | 0.0 | 0 | 0 |
| H547 | 0.0 | 0 | 1 |
| H549 | 0.0 | 0 | 1 |
| H561 | 0.0 | 0 | 0 |
| Recombinant MVA | |||
| T119 | 0.5 | 17 | 37 |
| H500 | 0.4 | 4 | 26 |
| H507 | 0.2 | 39 | 61 |
| H511 | 0.8 | 5 | 30 |
Immune responses following challenge.
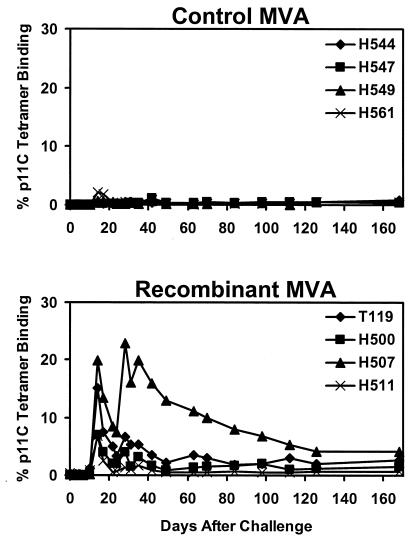
Six weeks after the final boost immunization, all eight animals were challenged i.v. with 100 MID50 of cell-free SHIV-89.6P. All animals were infected by this highly pathogenic viral challenge. As shown in Fig. 2, the control monkeys developed primary p11C-specific CTL responses, reaching a maximum of 0.2 to 2% of circulating CD8+ T cells on day 14 after challenge. In contrast, the vaccinated monkeys developed higher secondary p11C-specific CTL responses, reaching a maximum of 7 to 20% of circulating CD8+ T cells on day 14 after challenge. As shown in Table 2, the results of functional chromium release cytotoxicity assays confirmed these tetramer staining data.
FIG. 2.
Secondary CTL responses following challenge. Monkeys were challenged with SHIV-89.6P by the i.v. route on day 0. CD8+ T-cell responses specific for the SIV Gag p11C epitope were determined by tetramer staining of freshly isolated PBMC at multiple time points (3, 21). The percent CD3+ CD8+ T cells that bound the Mamu-A*01/p11C tetramer is shown.
TABLE 2.
CTL responses following challengea
| Vaccination and monkey | Tetramer binding | Functional cytotoxicity (stimulated PBMC) | |
|---|---|---|---|
| Fresh PBMC | Stimulated PBMC | ||
| Control MVA | |||
| H544 | 0.1 | 2 | 2 |
| H547 | 0.1 | 1 | 0 |
| H549 | 0.7 | 19 | 22 |
| H561 | 1.7 | 8 | 11 |
| Recombinant MVA | |||
| T119 | 7.5 | 48 | 29 |
| H500 | 3.9 | 49 | 37 |
| H507 | 13.5 | 59 | 34 |
| H511 | 2.5 | 42 | 28 |
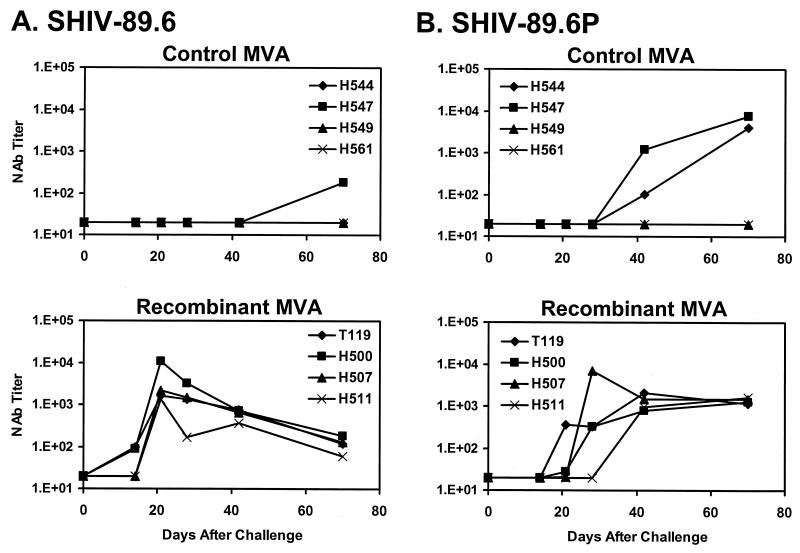
NAb responses specific for both SHIV-89.6 and SHIV-89.6P were assessed in MT-2 cell-killing assays (9). As shown in Fig. 3A, no SHIV-89.6-specific NAbs were detected in the plasma of the control animals, except for a low titer in monkey H547 on day 70 after challenge. In contrast, SHIV-89.6-specific NAbs were detected in the plasma of two vaccinated animals on day 14 after challenge, and high-titer NAbs (1,350 to 10,804) were observed in the plasma of all four vaccinated animals on day 21 following challenge. This rapid evolution of high-titer NAbs is consistent with a secondary SHIV-89.6-specific NAb response that was primed by the vaccine.
FIG. 3.
NAb responses following challenge. Plasma antibody titers capable of neutralizing SHIV-89.6 (A) and SHIV-89.6P (B) were measured by MT-2 cell-killing assays at multiple time points (9).
Since NAbs generated by SHIV-89.6 infection exhibit poor cross-neutralizing activity against SHIV-89.6P (9, 25), the vaccine expressing HIV-1 Env 89.6 would not be expected to prime for SHIV-89.6P-specific NAbs. As shown in Fig. 3B, only two control monkeys (H544 and H547) developed SHIV-89.6P-specific NAbs by day 42 after challenge. Surprisingly, all four vaccinated monkeys developed SHIV-89.6P-specific NAbs between days 21 and 42 after challenge. The six animals that developed detectable SHIV-89.6P-specific NAbs had similar peak titers.
CD4 counts, viral RNA levels, and clinical disease progression.
As shown in Fig. 4, the control monkeys developed a rapid and profound loss of CD4+ T lymphocytes between days 7 and 21 after challenge. Monkeys H549 and H561 demonstrated a complete loss of their CD4+ T lymphocytes, whereas H544 and H547 had significant but incomplete losses of their CD4+ T lymphocytes. In the vaccinated animals, monkeys H500 and H511 had completely stable CD4+ T-lymphocyte counts, whereas monkeys T119 and H507 exhibited partial declines in CD4+ T-lymphocyte counts by day 168 after challenge. On day 70 after challenge, a time by which the setpoint of viral replication is reached in SHIV-89.6P-infected rhesus monkeys, the CD4+ T-lymphocyte counts in the vaccinated monkeys were significantly higher than in the control monkeys (P = 0.028 by a two-sided Wilcoxon rank sum test).
FIG. 4.
CD4+ T-lymphocyte counts following challenge. CD4+ T-lymphocyte counts in peripheral blood were determined by multiplying the total lymphocyte count by the percentage of CD3+ CD4+ lymphocytes at multiple time points.
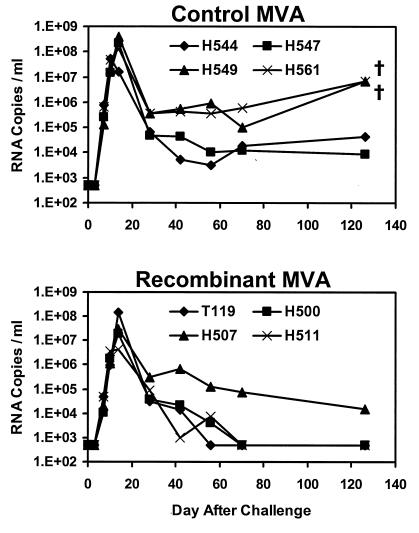
We next measured plasma viral RNA levels in the monkeys by a real-time amplification assay with a detection limit of 500 copies/ml (17, 39). As demonstrated in Fig. 5, the control monkeys developed high levels of peak primary viremia, reaching 5.4 × 107 to 3.8 × 108 copies/ml on day 10 or 14 after challenge. In the vaccinated monkeys, peak primary viremia was slightly lower, between 4.4 × 106 and 1.4 × 108 copies/ml. On day 70 after challenge, all the control animals had high-setpoint viral RNA levels of 1.2 × 104 to 5.9 × 105 copies/ml. In three of the four vaccinated monkeys (T119, H500, and H511), setpoint viremia was below the limit of detection of the assay (<500 copies/ml). Setpoint viremia in monkey H507, however, remained high. Interestingly, this animal had the lowest levels of vaccine-elicited CTLs prior to challenge. A trend toward a reduction in setpoint viremia was observed in the vaccinated animals compared with the control monkeys (P = 0.11 by a two-sided Wilcoxon rank sum test). The vaccinated animals had a 2.0-log reduction in geometric mean viral RNA levels after setpoint compared with the control animals.
FIG. 5.
Viral RNA levels following challenge. Plasma viral RNA levels were determined at multiple time points by a real-time amplification assay with a detection limit of 500 copies/ml (17, 39). †, death of the animal.
Significant clinical disease progression was observed in the two control animals (H549 and H561) that had complete depletion of their CD4+ T lymphocytes, persistent high viremia, and no SHIV-89.6P-specific NAbs. These two animals died at days 126 and 168 after challenge. In contrast, all the vaccinated animals remained healthy without evidence of clinical disease or mortality by day 168 after challenge. The rapid development of clinical AIDS and mortality in the control animals is comparable with our previous experience with SHIV-89.6P-infected monkeys (7, 34).
Immune correlates of protection.
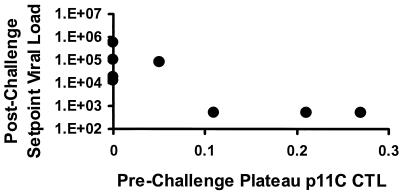
A scatter plot of data shown in Fig. 6 demonstrates a significant correlation between prechallenge vaccine-elicited plateau-phase p11C-specific CTL responses determined by tetramer staining and day 70 postchallenge setpoint viral RNA levels (P = 0.03 by a two-sided Spearman rank correlation test). This correlation is analogous to the correlation we observed in our recent study of immune responses and the protective efficacy elicited by DNA vaccination (7).
FIG. 6.
Correlation of prechallenge vaccine-elicited plateau-phase p11C-specific CTL responses as determined by tetramer staining and day 70 postchallenge setpoint viral RNA levels (P = 0.03).
DISCUSSION
In this study, virus-specific immune responses were elicited in rhesus monkeys using recombinant MVA vectors expressing SIV gag-pol and HIV-1 89.6 env. The kinetics and magnitude of the vaccine-elicited SIV Gag epitope-specific CTL responses were comparable to those observed in our previous study of recombinant MVA-vaccinated rhesus monkeys (37, 38). The levels of vaccine-elicited CTL responses in the present study were also comparable to those elicited by plasmid DNA vaccination in our prior studies, but were lower than those elicited by cytokine-augmented DNA vaccination (6, 7, 12).
As we reported previously (7), there was a statistically significant correlation between levels of prechallenge vaccine-elicited plateau-phase CTLs and setpoint viremia following challenge. The asymptotic appearance of the data from the scatter plots in both of these studies suggests that a level of vaccine-elicited plateau-phase CTLs may exist above which little additional benefit is obtained after challenge. The prechallenge plateau-phase CTL population presumably represents the vaccine-elicited memory pool of CTLs which expand upon exposure to virus to become functional effector CTLs. The correlation observed between levels of prechallenge plateau-phase CTLs and the control of viremia following challenge highlights the importance of CTLs in controlling AIDS virus replication.
Following the SHIV-89.6P challenge, secondary SIV Gag epitope-specific CTL responses were clearly evident in the vaccinated animals. The secondary CTL responses were maximal on day 14 and then rapidly declined to steady-state plateau levels. The magnitude of the secondary CTL responses following viral challenge reflected both the levels of vaccine-elicited CTL responses as well as the levels of viral antigen driving these responses. The vaccinated monkey that was unable to control viremia (H507) had persistently high levels of tetramer-binding CD8+ T cells following challenge, likely reflecting the high levels of antigen present in this animal.
NAbs specific for SHIV-89.6 and SHIV-89.6P were undetectable in the vaccinated monkeys at the time of peak vaccine-elicited immunity or on the day of challenge. However, high-titer SHIV-89.6-specific NAbs were detected in the vaccinated animals within 3 weeks after challenge. Since SHIV-89.6-specific NAbs are rarely detected in naïve animals prior to 6 weeks following infection with SHIV-89.6 or SHIV-89.6P (9, 24), the SHIV-89.6-specific NAb response observed here was most likely an anamnestic response primed by the vaccine. This secondary NAb response following challenge was presumably elicited by either shared epitopes between the Env 89.6 immunogen and the Env 89.6P on the challenge virus or a minor SHIV-89.6 quasispecies present in the SHIV-89.6P challenge stock.
NAbs specific for SHIV-89.6P were detected by day 21 to 42 after challenge in the vaccinated monkeys and considerably later or not at all in the control monkeys. It is unclear if the earlier emergence of SHIV-89.6P-specific NAbs in the vaccinated animals reflected de novo generation of NAbs facilitated by the preserved CD4+ T-cell help in these animals, affinity maturation of the SHIV-89.6-specific NAbs, or a secondary NAb response that was primed by the vaccine. This last possibility is perhaps least likely, since SHIV-89.6-specific NAbs have poor neutralizing activity against SHIV-89.6P (9, 25). Regardless of the mechanism, these data demonstrate that the rapid emergence of NAb responses specific for a highly pathogenic primary isolate-like challenge virus did not require immunization with a completely homologous Env construct.
On day 14 after challenge, at the time of peak primary viremia, secondary CTL responses were maximal and SHIV-89.6P-specific NAbs were undetectable, suggesting that the initial control of primary viremia in the vaccinated animals was mediated predominantly by CTLs. The subsequent control of viremia, however, likely reflects the effects of both cellular and humoral immune responses. In our prior study utilizing MVA vectors expressing env smH-4 and the related challenge virus SIVsmE660, the monkeys vaccinated with MVA/env developed secondary SIVsmH-4-specific NAbs after SIVsmE660 challenge but no convincing secondary SIVsmE660-specific NAbs (28, 29). It is possible that the absence of augmented SIVsmE660-specific NAbs following challenge accounted for the observation that MVA/gag-pol, MVA/env, and MVA/gag-pol-env vaccinations all provided comparable partial control of viremia in that study.
A significant limitation of the present study is the small number of monkeys, which precluded a statistical comparison of clinical disease end points and mortality. However, following the SHIV-89.6P challenge, the control animals developed low-frequency CTL responses, low-titer NAb responses, rapid loss of CD4+ T lymphocytes, high viral RNA levels, and clinical disease and death in two of the four animals in this group. The monkeys that received the recombinant MVA vaccines developed high-frequency CTL responses, high-titer NAb responses, partial preservation of CD4+ T lymphocytes, reduced viral RNA levels, and no evidence of clinical disease or mortality by day 168 after challenge. The 2.0-log reduction in mean setpoint plasma viremia observed in this study is similar to the 1.9-log reduction we have reported in SHIV-89.6P-challenged monkeys using plasmid DNA vaccination, although it is less striking than the 3.0-log reduction achieved using cytokine-augmented DNA vaccination (7). The results of the present study are also comparable with the results we obtained with recombinant MVA vaccination in conjunction with an SIVsmE660 challenge (37, 38).
The degree of protection achieved against SHIV-89.6P-induced AIDS by recombinant MVA vaccination and plasmid DNA vaccination should not be interpreted as evidence that SHIV-89.6P-induced disease is easy to ameliorate. In fact, several other vaccine modalities, including purified recombinant proteins and synthetic peptide vaccines, provide no discernible protection against SHIV-89.6P viremia or clinical disease progression in similarly conducted vaccine trials with rhesus monkeys (N. L. Letvin et al., unpublished data). The fact that SHIV-89.6P infection rapidly leads to immunodeficiency and AIDS in the majority of control monkeys makes this a useful challenge model for assessing the ability of vaccine candidates to provide protection against clinical disease progression in a relatively short time frame.
It is likely that a number of vaccine approaches will ultimately prove to have comparable efficacy in eliciting immune responses, controlling viremia, and preventing clinical sequelae of an AIDS virus infection. Such approaches are likely to include recombinant live vectors (38), plasmid DNA (7), and prime-boost approaches that involve boosting a DNA-primed immune response with a recombinant live vector (2, 15, 19, 35). Many of these vaccine strategies will be tested for their utility in humans over the next several years. If viral replication is similarly reduced in vaccinated humans who are subsequently infected with HIV-1, such individuals may demonstrate slowed disease progression and decreased HIV-1 transmission rates (30). Thus, a vaccine that elicits immunity that is not sterilizing but capable of reducing HIV-1 RNA levels following infection may still provide substantial clinical benefits to human populations.
ACKNOWLEDGMENTS
We thank Russell Byrum, Bioqual Inc., and Simoy Goldstein for generous assistance.
This study was supported in part by NIH grants AI-85343 and AI-26507.
REFERENCES
- 1.Allen T M, Sidney J, del Guercio M-F, Glickman R L, Lensmeyer G L, Wiebe D A, DeMars R, Pauza C D, Johnson R P, Sette A, Watkins D I. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from SIV. J Immunol. 1998;160:6062–6071. [PubMed] [Google Scholar]
- 2.Allen T M, Vogel T U, Fuller D H, Mothe B R, Steffen S, Boyson J E, Shipley T, Fuller J, Hanke T, Sette A, Altman J D, Moss B, McMichael A J, Watkins D I. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J Immunol. 2000;164:4968–4978. doi: 10.4049/jimmunol.164.9.4968. [DOI] [PubMed] [Google Scholar]
- 3.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 4.Baba T W, Jeong Y S, Penninck D, Bronson R, Greene M F, Ruprecht R M. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- 5.Baba T W, Liska V, Khimani A H, Ray N B, Dailey P J, Penninck D, Bronson R, Greene M F, McClure H M, Martin L N, Ruprecht R M. Live attenuated, multiple deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med. 1999;5:194–203. doi: 10.1038/5557. [DOI] [PubMed] [Google Scholar]
- 6.Barouch D H, Craiu A, Kuroda M J, Schmitz J E, Zheng X X, Santra S, Frost J D, Krivulka G R, Lifton M A, Crabbs C L, Heidecker G, Perry H C, Davies M-E, Xie H, Nickerson C E, Steenbeke T D, Lord C I, Montefiori D C, Strom T B, Shiver J W, Lewis M G, Letvin N L. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc Natl Acad Sci USA. 2000;97:4192–4197. doi: 10.1073/pnas.050417697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barouch D H, Santra S, Schmitz J E, Kuroda M J, Fu T-M, Wagner W, Bilska M, Craiu A, Zheng X X, Krivulka G R, Beaudry K, Lifton M A, Nickerson C E, Trigona W L, Punt K, Freed D C, Guan L, Dubey S, Casimiro D, Simon A, Davies M-E, Chastain M, Strom T B, Gelman R S, Montefiori D C, Lewis M G, Emini E A, Shiver J W, Letvin N L. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 8.Belyakov I M, Wyatt L S, Ahlers J D, Earl P, Pendleton C D, Kelsall B L, Strober W, Moss B, Berzofsky J A. Induction of a mucosal cytotoxic T-lymphocyte response by intrarectal immunization with a replication-deficient recombinant vaccinia virus expressing human immunodeficiency virus 89.6 envelope protein. J Virol. 1998;72:8264–8272. doi: 10.1128/jvi.72.10.8264-8272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawford J M, Earl P L, Moss B, Reimann K A, Wyand M S, Manson K H, Bilska M, Zhou J T, Pauza C D, Parren P W H I, Burton D R, Sodroski J G, Letvin N L, Montefiori D C. Characterization of primary isolate-like variants of simian-human immunodeficiency virus. J Virol. 1999;73:10199–10207. doi: 10.1128/jvi.73.12.10199-10207.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 11.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C, Lawson V A, Crowe S, Maerz A, Sonza S, Learmont J, Sullivan J S, Cunningham A, Dwyer D, Dowton D, Mills J. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 12.Egan M A, Charini W A, Kuroda M J, Voss G, Schmitz J E, Racz P, Tenner-Racz K, Manson K, Wyand M, Lifton M A, Nickerson C E, Fu T M, Shiver J W, Letvin N L. Simian immunodeficiency virus (SIV) gag DNA-vaccinated rhesus monkeys develop secondary cytotoxic T-lymphocyte responses and control viral replication after pathogenic SIV infection. J Virol. 2000;74:7485–7495. doi: 10.1128/jvi.74.16.7485-7495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan M A, Kuroda M J, Voss G, Schmitz J E, Charini W A, Lord C I, Forman M A, Letvin N L. Use of major histocompatibility complex class I/peptide/β2M tetramers to quantitate CD8+ cytotoxic T lympocytes specific for dominant and nondominant viral epitopes in simian-human immunodeficiency virus-infected rhesus monkeys. J Virol. 1999;73:5466–5472. doi: 10.1128/jvi.73.7.5466-5472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenough T C, Sullivan J L, Desrosiers R C. Declining CD4 T-cell counts in a person infected with nef-deleted HIV-1. New Engl J Med. 1999;340:236–237. doi: 10.1056/NEJM199901213400314. [DOI] [PubMed] [Google Scholar]
- 15.Hanke T, Samuel R V, Blanchard T J, Neumann V C, Allen T M, Boyson J E, Sharpe S A, Cook N, Smith G L, Watkins D I, Cranage M P, McMichael A J. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J Virol. 1999;73:7524–7532. doi: 10.1128/jvi.73.9.7524-7532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch V M, Fuerst T R, Sutter G, Carroll M W, Yang L C, Goldstein S, Piatak M, Elkins W, Alvord W G, Montefiori D C, Moss B, Lifson J D. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996;70:3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igarashi T, Endo Y, Englund G, Sadjadpour R, Matano T, Buckler C, Buckler-White A, Plishka R, Theodore T, Shibata R, Martin M. Emergence of a highly pathogenic simian/human immunodeficiency virus in a rhesus macaque treated with anti-CD8 mAb during a primary infection with a nonpathogenic virus. Proc Natl Acad Sci USA. 1999;96:14049–14054. doi: 10.1073/pnas.96.24.14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kent S J, Zhao A, Best S J, Chandler J D, Boyle D B, Ramshaw I A. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J Virol. 1998;72:10180–10188. doi: 10.1128/jvi.72.12.10180-10188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knapp L A, Lehmann E, Piekarczyk M S, Urvater J A, Watkins D I. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens. 1997;50:657–661. doi: 10.1111/j.1399-0039.1997.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuroda M J, Schmitz J E, Barouch D H, Criau A, Allen T M, Sette A, Watkins D I, Forman M A, Letvin N L. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J Exp Med. 1998;187:1373–1381. doi: 10.1084/jem.187.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Learmont J C, Geczy A F, Mills J, Ashton L J, Raynes-Greenow C H, Garsia R J, Dyer W B, McIntyre L, Oelrichs R B, Rhodes R I, Deacon N J, Sullivan J S. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. New Engl J Med. 1999;340:1715–1722. doi: 10.1056/NEJM199906033402203. [DOI] [PubMed] [Google Scholar]
- 23.Meyer H, Sutter G, Mayr A. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol. 1991;72:1031–1038. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- 24.Miller M D, Yamamoto H, Hughes A H, Watkins D I, Letvin N L. Definition of an epitope and MHC class I molecule recognized by Gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys. J Immunol. 1991;147:320–329. [PubMed] [Google Scholar]
- 25.Montefiori D C, Reimann K A, Wyand M S, Manson K, Lewis M G, Collman R G, Sodroski J G, Bolognesi D P, Letvin N L. Neutralizing antibodies in sera from macaques infected with chimeric simian-human immunodeficiency virus containing the envelope glycoproteins of either a laboratory-adapted variant or a primary isolate of human immunodeficiency virus type 1. J Virol. 1998;72:3427–3431. doi: 10.1128/jvi.72.4.3427-3431.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath M J. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. New Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 27.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 28.Ourmanov I, Bilska M, Hirsch V M, Montefiori D C. Recombinant modified vaccinia virus Ankara expressing the surface gp120 of simian immuodeficiency virus (SIV) primes for a rapid neutralizing antibody response to SIV infection in macaques. J Virol. 2000;74:2960–2965. doi: 10.1128/jvi.74.6.2960-2965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ourmanov I, Brown C R, Moss B, Carroll M, Wyatt L, Pletneva L, Goldstein S, Venzon D, Hirsch V M. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J Virol. 2000;74:2740–2751. doi: 10.1128/jvi.74.6.2740-2751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinn T C, Wawer M J, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan M O, Lutalo T, Gray R H. Viral load and heterosexual transmission of human immunodeficiency virus type 1. New Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 31.Redfield R R, Wright D C, James W D, Jones T S, Brown C, Burke D S. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. New Engl J Med. 1987;316:673–676. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]
- 32.Reimann K A, Li J T, Voss G, Lekutis C, Tenner-Racz K, Racz P, Lin W, Montefiori D C, Lee-Parritz D E, Lu Y, Collman R G, Sodroski J, Letvin N L. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J Virol. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reimann K A, Li J T, Veazey R, Halloran M, Park I-W, Karlsson G B, Sodroski J, Letvin N L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reimann K A, Watson A, Dailey P J, Lin W, Lord C I, Steenbeke T D, Parker R A, Axthelm M K, Karlsson G B. Viral burden and disease progression in rhesus monkeys infected with chimeric simian-human immunodeficiency viruses. Virology. 1999;256:15–21. doi: 10.1006/viro.1999.9632. [DOI] [PubMed] [Google Scholar]
- 35.Robinson H L, Montefiori D C, Johnson R P, Manson K H, Kalish M L, Lifson J D, Rizvi T A, Lu S, Hu S-L, Mazzara G P, Panicali D L, Herndon J G, Glickman R, Candido M A, Lydy S L, Wyand M S, McClure H M. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat Med. 1999;5:526–534. doi: 10.1038/8406. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 37.Seth A, Ourmanov I, Kuroda M J, Schmitz J E, Carroll M W, Wyatt L S, Moss B, Forman M A, Hirsch V M, Letvin N L. Recombinant modified vaccinia virus Ankara-simian immunodeficiency virus gag pol elicits cytotoxic T lymphocytes in rhesus monkeys detected by a major histocompatibility complex class I/peptide tetramer. Proc Natl Acad Sci USA. 1998;95:10112–10116. doi: 10.1073/pnas.95.17.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seth A, Ourmanov I, Schmitz J E, Kuroda M J, Lifton M A, Nickerson C E, Wyatt L, Carroll M, Moss B, Venzon D, Letvin N L, Hirsch V M. Immunization with a modified vaccinia virus expressing simian immunodeficiency virus (SIV) Gag-Pol primes for an anamnestic Gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge. J Virol. 2000;74:2502–2509. doi: 10.1128/jvi.74.6.2502-2509.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suryanarayana K, Wiltrout T A, Vasques G M, Hirsch V M, Lifson J D. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res Hum Retrovir. 1998;14:183–189. doi: 10.1089/aid.1998.14.183. [DOI] [PubMed] [Google Scholar]
- 40.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wyand M S, Manson K, Montefiori M D C, Lifson J F, Johnson R P, Desrosiers R C. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J Virol. 1999;73:8356–8363. doi: 10.1128/jvi.73.10.8356-8363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wyatt L S, Shors S T, Murphy B R, Moss B. Development of a replication-deficient recombinant vaccinia virus vaccine effective against parainfluenza virus 3 infection in an animal model. Vaccine. 1996;14:1451–1458. doi: 10.1016/s0264-410x(96)00072-2. [DOI] [PubMed] [Google Scholar]
- 43.Wyatt L S, Whitehead S S, Venanzi K A, Murphy B R, Moss B. Priming and boosting immunity to respiratory syncytial virus by recombinant replication-defective vaccinia virus MVA. Vaccine. 1999;18:392–397. doi: 10.1016/s0264-410x(99)00257-1. [DOI] [PubMed] [Google Scholar]