Role of Active Efflux in Association with Target Gene Mutations in Fluoroquinolone Resistance in Clinical Isolates of Vibrio cholerae (original) (raw)
Abstract
Quinolones are among the drugs of choice in the management of cholera caused by Vibrio cholerae. In this study, we demonstrate that, in addition to mutations detected in the target genes gyrA and parC, proton motive force-dependent efflux is involved in quinolone resistance in clinical isolates of V. cholerae.
The targets of the quinolones are the type II topoisomerases DNA gyrase, a heterotetramer composed of two A and two B subunits encoded by the gyrA and gyrB genes, respectively, and topoisomerase IV, which is also composed of two subunits, ParC and ParE, encoded by the parC and parE genes, respectively (9, 10, 12). High-level flouroquinolone resistance is associated with the presence of multiple mutations in the quinolone resistance-determining regions (QRDRs) of the genes gyrA, gyrB, parC, and parE (1, 4, 8) and active efflux systems (3, 7, 14; J. Ahamed, J. Gangopadhyay, and M. Kundu, Letter, Antimicrob. Agents Chemother. **43:**2333-2334, 1999).
Quinolones possess excellent activity against Vibrio cholerae (2, 5, 17), the causative agent of cholera epidemics (15, 16). However, quinolone resistance has been reported recently in clinical isolates from Calcutta, India (6; P. Garg, S. Sinha, R. Chakraborty, S. K. Bhattacharya, G. B. Nair, and T. Ramamurthy, Letter, Antimicrob. Agents Chemother. **45:**1605-1606, 2001). In this report, we demonstrate some of the mechanisms of quinolone resistance.
Clinical isolates were recovered from hospitalized patients admitted to the Infectious Diseases Hospital, Calcutta, and grown at 37°C on a Gyrotory shaker in Luria broth (11) with 2% NaCl added to the medium when required.
MICs were determined according to guidelines of the National Committee for Clinical Laboratory Standards (13) by the standard agar dilution assay in Mueller-Hinton agar. Bacteria grown for 18 h in Luria broth were diluted to 2 × 106 CFU/ml. Control or drug-containing agar plates were incubated with one loopful (5 μl) of an inoculum (104 CFU per spot) and were incubated at 37°C for 20 h. The MIC was defined as the lowest drug concentration that prevented visible growth of bacteria. Susceptibility was tested in duplicate, and the tests were repeated three times.
Primer sequences were designed to amplify the fragment including the putative QRDR based on the genome sequence of V. cholerae available at http://www.tigr.org. For gyrA, the forward primer 5′-AATGTGCTGGGCAACGACTGG-3′ (nucleotide [nt] 157 to 177) and the reverse primer 5′-GTGCGCGATTTTCGACATACG-3′ (nt 376 to 396) were chosen to amplify the region encoding amino acids 53 to 132; for gyrB, the primers 5′-GGAAATGACTCGCCGTAAAGG-3′ (nt 1170 to 1190) and 5′-GTTGTGATAACGCAGTTTATCTGGG-3′ (nt 1455 to 1479) were chosen to amplify the region encoding amino acids 390 to 493; for parC, the primers 5′-GTCTGAGTTGGGTCTCTCGGC-3′ (nt 162 to 188) and 5′-AGAATCTCGGCAAACTTTGACAG-3′ (nt 388 to 411) were chosen to amplify the region encoding amino acids 54 to 137; and for parE, the primers 5′-ATGCGTGCCAGCAAGAAAGTG-3′ (nt 1138 to 1161) and 5′-TTATCGCTGTCAGGGTCAATCC-3′ (nt 1408 to 1428) were chosen to amplify the region encoding amino acids 379 to 467. The amplification procedure consisted of denaturation at 95°C for 2 min, followed by three cycles of denaturation for 30 s at 95°C, annealing for 30 s at 37°C, and polymerization for 1 min at 72°C. Finally, 30 cycles of denaturation for 30 s at 95°C, annealing for 30s at 45°C, and polymerization for 1 min at 72°C were carried out.
Accumulation of norfloxacin was studied as described previously (7). Briefly, V. cholerae cells were grown to mid-log phase, harvested, and suspended in 0.2 M MOPS (morpholinepropanesulfonic acid)-Tris buffer (pH 7.0) to an optical density at 600 nm of 20 per ml. Cells were energized with 0.2% glucose for 20 min. Norfloxacin was added at a concentration of 10 μM. Aliquots were taken at different time intervals, harvested, suspended in 1 ml of 100 mM glycine-HCl (pH 3.0), and shaken for 1 h at room temperature, and the fluorescence of the supernatant was measured with excitation at 277 nm and emission at 448 nm. Where indicated, the proton motive force uncoupler carbonyl cyanide _m-_chlorophenylhydrazone (CCCP) (7) was added to the assay mixture at a final concentration of 10 μM.
The MICs (Table 1) of the fluoroquinolones norfloxacin, ciprofloxacin, ofloxacin, and sparfloxacin indicated that different isolates showed various levels of susceptibility to fluoroquinolones. Based on the MICs, strains AS600, AS198, AS255, AS404, AS627, AS575, AS73, AS480, and AS150 are grouped together as group I; strains AS623, AS232, AS385, AS529, and AM107 are grouped together as group II; and strains AM54, AS28, and AS120 are grouped together as group III for comparison in the present study.
TABLE 1.
Susceptibility to quinolones, mutations in gyrA and parC, and accumulation of norfloxacin in clinical isolates of V. cholerae
| Strain | Type | Mutationa | MIC (μg/ml) ofb: | Accumulation of NOR (μg/mg [dry wt] of cells)c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GyrA at position: | ParC at position 85 (Ser) | |||||||||
| 83 (Ser) | 100 (Tyr) | Nor | CIP | SPX | OFL | Before addition of CCCP | After addition of CCCP | |||
| AM54 | Non-O1 | — | — | — | <0.1 | 0.1 | <0.1 | 0.1 | 0.35 ± 0.003 | 0.40 ± 0.004 |
| AS28 | O1 | Ile | Asp | — | 0.2 | 0.2 | 0.2 | 0.2 | 0.33 ± 0.003 | 0.39 ± 0.012 |
| AS120 | Non-O1 | Ile | — | Leu | 0.2 | 0.2 | 0.2 | 0.2 | 0.34 ± 0.004 | 0.40 ± 0.023 |
| AS623 | O1 | Ile | — | — | 0.8 | 0.8 | 0.8 | 0.8 | 0.21 ± 0.012 | 0.38 ± 0.034 |
| AS232 | Non-O1 | Ile | — | Leu | 0.8 | 0.8 | 0.8 | 0.8 | 0.23 ± 0.013 | 0.37 ± 0.021 |
| AS385 | Non-O1 | Ile | — | Leu | 1.6 | 1.6 | 1.6 | 1.6 | 0.22 ± 0.005 | 0.38 ± 0.022 |
| AS529 | Non-O1 | Ile | — | Leu | 1.56 | 1.6 | 1.6 | 1.6 | 0.23 ± 0.005 | 0.40 ± 0.033 |
| AM107 | Non-O1 | Ile | — | Leu | 1.6 | 1.6 | 1.6 | 1.6 | 0.22 ± 0.004 | 0.39 ± 0.022 |
| AS600 | Non-O1 | Ile | — | Leu | 6.25 | 6.25 | 6.25 | 6.25 | 0.13 ± 0.009 | 0.35 ± 0.013 |
| AS198 | Non-O1 | Ile | — | Leu | 6.25 | 6.25 | 6.25 | 6.25 | 0.12 ± 0.009 | 0.36 ± 0.060 |
| AS255 | Non-O1 | Ile | — | Leu | 6.25 | 6.25 | 6.25 | 6.25 | 0.12 ± 0.010 | 0.37 ± 0.030 |
| AS404 | Non-O1 | Ile | — | Leu | 6.25 | 6.25 | 6.25 | 6.25 | 0.13 ± 0.009 | 0.36 ± 0.012 |
| AS627 | Non-O1 | Ile | Asp | Leu | 12.5 | 12.5 | 12.5 | 12.5 | 0.11 ± 0.012 | 0.38 ± 0.031 |
| AS575 | Non-O1 | Ile | — | Leu | 12.5 | 12.5 | 12.5 | 12.5 | 0.12 ± 0.002 | 0.35 ± 0.025 |
| AS73 | O1 | Ile | — | Leu | 12.5 | 12.5 | 12.5 | 12.5 | 0.11 ± 0.009 | 0.34 ± 0.024 |
| AS480 | Non-O1 | Ile | — | Leu | 12.5 | 12.5 | 12.5 | 12.5 | 0.13 ± 0.012 | 0.35 ± 0.030 |
| AS150 | Non-O1 | Ile | — | Leu | 12.5 | 12.5 | 10 | 12.5 | 0.11 ± 0.005 | 0.34 ± 0.021 |
None of the strains possessed any mutation in gyrB or parE genes. Mutations in gyrA and parC were absent in only one strain, AM54. In the other 16 strains, a mutation at codon 83 (G→T transversion [underlined] in the codon AGT, resulting in the substitution of serine by isoleucine) was detected in gyrA. Two other strains, AS28 and AS627, had an additional mutation in codon 100 of gyrA. An A→C transversion in the codon GTA resulted in the substitution of aspartic acid for tyrosine. From the MICs, no correlation of this second mutation with increased resistance was apparent. In addition to the mutation in codon 83 of gyrA, 14 of the 17 strains harbored an additional mutation in codon 85 of parC, with a C→T transversion in codon TCG resulting in a substitution of serine by leucine. From Table 1, it is evident that strains harboring identical mutations in target genes did not necessarily show the same susceptibility to fluoroquinolones.
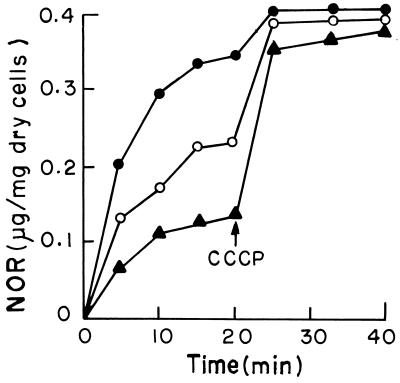
The steady-state accumulation of norfloxacin (after energization) was between three- and fourfold in group III strains, with MICs ranging from 0.1 to 0.2 μg/ml, compared to group I strains, with MICs ranging from 6.25 to 12.5 μg/ml, and twofold compared to the group II strains, suggesting that the high steady-state uptake of norfloxacin accounts for the greater susceptibility of group III strains to this drug. After the disruption of the efflux pump with the proton motive force uncoupler CCCP, the accumulation was almost at the same level in strains of all three groups. This clearly suggested the role of efflux pumps as one of the mediators in the reduced levels of drug accumulation in group I and II strains. The kinetics of norfloxacin accumulation before and after addition of CCCP were similar among strains classified in the same group. Figure 1 depicts the nature of accumulation of norfloxacin before and after addition of CCCP in three strains, AS150, AS232, and AM54, each representative of groups I, II, and III, respectively.
FIG. 1.
Accumulation of norfloxacin (NOR) in clinical isolates of V. cholerae. The accumulation of norfloxacin was studied as described in the text. ▴, group I strain; ○, group II strain; •, group III strain. CCCP was added at the time indicated by the arrow. The results shown are representative of three different experiments.
We demonstrate for the first time, that enhanced active efflux correlated with various degrees of quinolone resistance in clinical isolates of V. cholerae. Our study suggests that active efflux in combination with the presence of mutations in the target genes possibly accounts for high-level quinolone resistance in clinical isolates of V. cholerae.
Acknowledgments
This work was supported in part by grants from the Council of Scientific and Industrial Research and the Department of Science and Technology, Government of India, to M.K.
REFERENCES
- 1.Belland, R. J., S. G. Morrison, C. Ison, and W. M. Huang. 1994. Neisseria gonorrhoeae acquires mutations in analogous regions of GyrA and ParC in fluoroquinolone-resistant isolates. Mol. Microbiol. 14**:**371-380. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya, S. K., M. K. Bhattacharya, P. Dutta, D. Dutta, S. P. De, S. N. Sikdar, A. Maitra, A. Dutta, and S. C. Pal. 1990. Double-blind, randomized, controlled clinical trial of norfloxacin for cholera. Antimicrob. Agents Chemother. 34**:**939-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colmer, J. A., J. A. Fralick, and A. N. Hamood. 1998. Isolation and characterization of a putative multidrug resistance pump from Vibrio cholerae. Mol. Microbiol. 27**:**63-72. [DOI] [PubMed] [Google Scholar]
- 4.Deguchi, T., A. Fukuoka, M. Yasuda, M. Nakano, S. Ozeki, E. Kanematsu, Y. Nishino, S. Ishihara, Y. Ban, and Y. Kawada. 1997. Alterations in the GyrA subunit of DNA gyrase and the ParC subunit of topoisomerase IV in quinolone-resistant clinical isolates of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 41**:**699-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutta, D., S. K. Bhattacharya, M. K. Bhattacharya, A. Deb, M. Deb, B. Manna, A. Moitra, A. K. Mukhopadhyay, and G. B. Nair. 1996. Efficacy of norfloxacin and doxycycline for treatment of Vibrio cholerae O139 infection. J. Antimicrob. Agents Chemother. 37**:**575-581. [DOI] [PubMed] [Google Scholar]
- 6.Garg, P., S. Chakraborty, I. Basu, S. Datta, K. Rajendran, T. Bhattacharya, S. Yamasaki, S. K. Bhattacharya, Y. Takeda, G. B. Nair, and T. Ramamurthy. 2000. Expanding multiple antibiotic resistance among clinical strains of Vibrio cholerae isolated from 1992-7 in Calcutta, India. Epidemiol. Infect. 124**:**393-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh, A. S., J. Ahamed, K. K. Chauhan, and M. Kundu. 1998. Involvement of an efflux system in high-level fluoroquinolone resistance of Shigella dysenteriae. Biochem. Biophys. Res. Commun. 242**:**54-56. [DOI] [PubMed] [Google Scholar]
- 8.Heisig, P. 1996. Genetic evidence for a role of parC mutations in development of high-level fluoroquinolone resistance in Escherichia coli. Antimicrob. Agents Chemother. 40**:**879-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooper, D. C. 1998. Clinical applications of quinolones. Biochim. Biophys. Acta 1400**:**45-61. [DOI] [PubMed] [Google Scholar]
- 10.Kato, J., Y. Nishimura, R. Imamura, H. Niki, S. Hiraga, and H. Suzuki. 1990. New topoisomerase essential for chromosome segregation in E. coli. Cell 63**:**393-404. [DOI] [PubMed] [Google Scholar]
- 11.Lennox, E. S. 1955. Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1**:**190-206. [DOI] [PubMed] [Google Scholar]
- 12.Levine, C., H. Hiasa, and K. J. Marians. 1998. DNA gyrase and topoisomerase IV: biochemical activities, physiological roles during chromosome replication, and drug sensitivities. Biochim. Biophys. Acta 1400**:**29-43. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A5, 5th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Oethinger, M., W. V. Kern, A. S. Jellen-Ritter, L. M. McMurry, and S. B. Levy. 2000. Ineffectiveness of topoisomerase mutations in mediating clinically significant fluoroquinolone resistance in Escherichia coli in the absence of the AcrAB efflux pump. Antimicrob. Agents Chemother. 44**:**10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ries, A. A., D. J. Vugia, L. Beingolea, A. M. Palacios, E. Vasquez, J. G. Wells, N. G. Baca, D. L. Swerdlow, M. Pollack, N. H. Bean, L. Seminario, and R. V. Tauxe. 1992. Cholera in Piura, Peru: a modern urban epidemic. J. Infect. Dis. 166**:**1429-1433. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. 1992. Cholera in the Americas. Wkly. Epidemiol. Rec. 67**:**33-40.1536753 [Google Scholar]
- 17.Yamamoto, T., G. B. Nair, M. J. Albert, C. C. Parodi, and Y. Takeda. 1995. Survey of in vitro susceptibilities of Vibrio cholerae O1 and O139 to antimicrobial agents. Antimicrob. Agents Chemother. 39**:**241-244. [DOI] [PMC free article] [PubMed] [Google Scholar]