Cytokine Responses in Gnotobiotic Pigs after Infection with Virulent or Attenuated Human Rotavirus (original) (raw)
Abstract
To understand the role of cytokines during rotavirus infection, we assessed the kinetics of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) (proinflammatory), IL-12 (Th1 inducer), gamma interferon (IFN-γ) (Th1), IL-4 and IL-10 (Th2), and transforming growth factor β (Th3) cytokine responses by enzyme-linked immunosorbent assay in serum and intestinal contents of neonatal gnotobiotic pigs and IL-12, IFN-γ, IL-4, and IL-10 cytokine-secreting cell (CSC) responses of mononuclear cells from ileum, spleen, and blood by ELISPOT. Pigs received the virulent Wa P1A[8]G1 strain of human rotavirus (HRV) (VirHRV), attenuated Wa HRV (AttHRV), or mock (controls). The TNF-α levels peaked earlier and remained elevated in serum of the VirHRV group but peaked later in the AttHRV group. In serum, IL-6 was significantly elevated at postinoculation day (PID) 1 in the VirHRV group and at PID 3 in both HRV groups. The IL-12 was detected in serum of all pigs including controls with significantly elevated peaks in both HRV-infected groups, indicating a role for IL-12 in the induction of immune responses to rotavirus infection. Only low and transient IFN-γ responses occurred in serum and intestinal contents of the AttHRV-infected pigs, compared to significantly higher and prolonged IFN-γ responses in the VirHRV-infected pigs. This observation coincides with the diarrhea and viremia induced by VirHRV. The number of IFN-γ-secreting cells was significantly higher in the ileum of the VirHRV group than in that of the controls. The number of IL-4 CSCs was significantly higher in ileum of both HRV groups than in that of the controls. Significantly higher levels of IL-10 in the serum occurred early in the VirHRV group, compared to lower levels in the AttHRV group. However, the number of IL-10 CSCs was significantly higher later in ileum and spleen of the AttHRV than in the VirHRV group, suggesting a delayed initiation of a Th2 response induced by AttHRV. A significantly higher percentage of pigs had IFN-γ and IL-10 responses in serum after VirHRV infection than after AttHRV infection or in controls. These data indicate a balanced Th1/Th2 response during rotavirus infection, with higher cytokine levels early after infection with VirHRV compared to that with AttHRV. Mapping the kinetics and patterns of cytokine responses after rotavirus infection has important implications for induction of protective immunity by HRV vaccines. Higher protection rates may be associated with more balanced Th1- and Th2-type responses, but induction of higher earlier IFN-γ (Th1) and proinflammatory cytokines triggered by VirHRV may also play an important role in the higher intestinal immunoglobulin A responses and protection rates induced by VirHRV.
Immune responses can be differentiated according to patterns of cytokine production during a viral or bacterial infection. The first cytokines to be produced are the proinflammatory cytokines, such as interleukin-1 (IL-1), IL-6, IL-8, and tumor necrosis factor alpha (TNF-α), and later the Th1 cytokines, such as IL-2 and gamma interferon (IFN-γ), and the Th2 cytokines IL-4, IL-5, IL-13, and IL-10. The late cytokines promote T- and B-cell differentiation and clonal expansion (22). It is important to control T-cell responses to self-antigens, infectious organisms, and foreign proteins to prevent chronic inflammation and tissue pathology. This function is exerted by regulatory cytokines such as transforming growth factor β (TGF-β) secreted by Th3 and IL-10 secreted by T regulatory (Treg) cells (28, 42).
The biological functions of cytokines and the Th1/Th2 paradigm of immune responses are established mostly from studies of mice. Three cytokines seem to be central to the initial development of Th1 and Th2 cells. Interleukin-12 and IL-4 influence the development of antigen-activated CD4+ T cells into Th1 or Th2 cells, respectively (36). The Th1 cytokines such as IFN-γ, IL-12, and IL-18 promote cell-mediated immunity and are required for effective responses to intracellular pathogens including viruses. Interleukin-12 is secreted by antigen-presenting cells (APCs) and binds to natural killer (NK) cells and Th0 cells, inducing rapid synthesis of IFN-γ (29). IFN-γ plays a major role in the defense against virus infection. Macrophage activation induced by T lymphocytes is mediated by IFN-γ, which also contributes to endothelial cell activation, Th1 cell development, and upregulation of major histocompatibility complex expression on both professional APCs and non-APCs (8). The Th2 cytokines such as IL-4, IL-5, and IL-10 mediate production of neutralizing antibodies (immunoglobulin G [IgG] and IgA) and the mast cell/eosinophil degranulating antibody IgE and induce membrane expression of major histocompatibility complex class II molecules on macrophages (30). Interleukin-4 is produced by a variety of cells including mast cells, Th2 effector cells, and NK cells. The major functions of IL-4 include promoting development of the Th2 subset of T cells and blocking most of the macrophage-activating effects of IFN-γ (27). Interleukin-10 secreted by Treg cells inhibits T-cell-mediated immune inflammation by inhibiting cytokine production by macrophages (i.e., TNF-α, IL-1, and IL-12) and Th1 cells (i.e., IFN-γ and granulocyte-macrophage colony-stimulating factor) (2, 37, 43). IL-10 induces IgA production in naïve (IgD+) B cells. TGF-β/CD40L is thought to promote class switching from IgM to IgA in humans, whereas IL-10 initiates B-cell differentiation and growth (33). In the gut TGF-β has been reported to induce further expansion and differentiation of B cells into IgA-committed plasma cells (24). Interleukin-6 stimulates IgA B-cell development in vitro. IL-6 has positive effects on cytotoxic T lymphocytes and Th cell-dependent activities (32) and is essential for control of some viral, bacterial, and fungal infections, primarily through its effects on inflammatory and cell-mediated immune responses. TGF-β mediates T-cell differentiation to the Th3 type for the induction of oral tolerance (44). TNF-α levels have been associated with the severity of rotavirus disease in children (6).
Recent studies have shown that the Th1/Th2 paradigm also applies to swine. Porcine T cells exposed to dendritic cells treated with egg white lysozyme express high levels of IL-13 and moderate levels of IL-10 and IFN-γ, suggesting a Th2 bias. Porcine T cells exposed to killed Mycobacterium tuberculosis expressed increased IFN-γ and decreased IL-10, suggesting a Th1 bias to this antigen (31). Based on limited numbers of studies of swine, the cytokine response patterns induced by infection (virus, bacteria, or parasites) are very similar to those of humans (18, 38, 47). The anti-inflammatory effect of IL-10 was recently demonstrated in swine with bacterial pneumonia by pretreating pigs with adenovirus type 5-expressed IL-10 (26). The pathogenesis of influenza virus is very similar between humans and swine, and the major cytokines associated with flu symptoms in swine (IFN-α, IL-1, and IL-6) were also found in human volunteers infected with influenza virus (20). Studies of cytokine responses in pigs experimentally infected with parasites that infect both humans and pigs (Ascaris suum and Toxoplasma gondii) suggest a Th1/Th2-like paradigm similar to that in mice. A. suum induced a Th2 response with the expression of IL-4, IL-5, and IL-13, whereas T. gondii elicited a Th1 response with increased levels of IFN-γ and TNF-α (14).
The immune responses to rotavirus infection or vaccination have been assessed mainly by measuring antibody responses in serum and fecal samples and cellular immunity (3, 48-51). Only a few investigators have described selected cytokine responses to rotavirus infection and their potential roles in pathogenicity and immunity. In humans, during acute rotavirus infection, IL-10 and TNF-α were elevated in the plasma of children and high levels of IFN-γ correlated with prolonged and acute diarrhea (5, 6, 23). In mice, a mixed Th1/Th2 cytokine pattern including IFN-γ, IL-5, and IL-10 was observed after in vitro rotavirus stimulation of lymphocytes from mice infected with rotavirus (19).
The neonatal gnotobiotic pig model has been used to study humoral and cellular immunity to human rotavirus (HRV) and HRV pathogenicity (3, 45, 46, 51); however, there is no information about the cytokine profiles in this model during infection and in association with pathogenicity or immunity. The purpose of this study was to examine cytokine profiles, kinetics, and duration of cytokine responses after infection with virulent HRV (VirHRV; mimicking natural infection) or oral vaccination with attenuated HRV (AttHRV; mimicking live oral vaccine) in serum, intestinal contents (IC), and lymphoid tissues of gnotobiotic pigs.
MATERIALS AND METHODS
Virus.
The attenuated cell-culture adapted strain of Wa HRV (P1A[8]G1), derived from the 27th passage in African green monkey kidney cells (MA104), was used for inoculation of gnotobiotic pigs using a dose of 5 × 107 fluorescent focus-forming units (51). The virulent Wa HRV from pooled intestinal contents of gnotobiotic pigs was used to infect the pigs with 106 median infectious doses. The median infectious dose of the virulent Wa HRV inoculum for gnotobiotic pigs was previously determined to be at least 1 focus-forming unit (45).
Inoculation and challenge of gnotobiotic pigs.
Near-term pigs were derived by surgery and maintained in gnotobiotic isolator units as described previously (25). At 3 to 5 days of age, pigs were assigned to one of three groups and inoculated as follows: (i) AttHRV (n = 31 pigs), inoculated with 1 oral dose of attenuated Wa HRV; (ii) VirHRV (n = 30 pigs), inoculated with 1 oral dose of virulent Wa HRV; or (iii) controls (n = 22 pigs), inoculated with 1 oral dose of minimum essential medium (MEM). Subsets of pigs from each group were euthanized at postinoculation day (PID) 3 (AttHRV, n = 5; VirHRV, n = 5; control, n = 3), PID 5 (AttHRV, n = 4; VirHRV, n = 3; control, n = 3), PID 7 (AttHRV, n = 5; VirHRV, n = 5; control, n = 3), PID 14 (AttHRV, n = 5; VirHRV, n = 4; control, n = 4), PID 21 (AttHRV, n = 6; VirHRV, n = 6; control, n = 5), and PID 28 (AttHRV, n = 6; VirHRV, n = 8; control, n = 4). MEM-inoculated controls provided the baseline cytokine levels for comparison with the infected pigs.
Assessment of diarrhea.
Rectal swabs were collected for 7 days after inoculation to assess diarrhea. Fecal consistency was scored as follows: 0, normal; 1, pasty; 2, semiliquid; and 3, liquid, as described previously (51).
Detection of viremia by ELISA.
Serum processed from blood samples collected on PID 1, 3, 5, and 7 was analyzed by antigen-capture enzyme-linked immunosorbent assay (ELISA) to detect HRV antigen, as previously described (34).
Isolation of MNC for cytokine ELISPOT assay.
For the isolation of mononuclear cells (MNC), the small intestine (ileum), spleen, and blood were collected from euthanized pigs and processed as previously described (40, 51). A cytokine ELISPOT assay for IFN-γ, IL-10, IL-12, and IL-4 was conducted as follows: Multiscreen-IP sterile 96-well plates (Millipore, Bedford, MA) were coated with anti-porcine IFN-γ (5 μg/ml), anti-porcine IL-10 (5 μg/ml) (Biosource, Camarillo, CA), anti-porcine IL-12 (1 μg/ml), and anti-porcine IL-4 (1 μg/ml) (R&D Systems, Minneapolis, MN) overnight at room temperature (RT). Prior to use, the plates were blocked with Roswell Park Memorial Institute (RPMI 1640) medium containing 10% fetal bovine serum for 2 h at RT, and then cell suspensions from each tissue were added in concentrations of 5 × 105 and 5 × 104 cells per well. Cells were stimulated with 50 μg/ml of semipurified (ultracentrifugation through 35% sucrose cushions) AttHRV or 10 μg of phytohemagglutinin (positive control) or RPMI (negative control). The optimal time and dose for in vitro antigen restimulation were determined in preliminary experiments. After 72 h of incubation at 37°C and 5% CO2, the cells were removed by washing and the following reagents were added: biotinylated monoclonal antibodies to porcine IFN-γ (1 μg/ml), IL-10 (1 μg/ml) (Biosource), IL-12 (0.2 μg/ml), or IL-4 (1 μg/ml) (R&D Systems). Plates were incubated overnight at 4°C. Horseradish peroxidase-conjugated streptavidin (Biosource) was added at a concentration of 0.3 μg/ml, and plates were incubated for 2 h at RT. The spots were developed with H2O2 and 3-amino-9-ethylcarbazole (AEC) (Sigma-Aldrich, St. Louis, MO). The numbers of cytokine-secreting cells (CSC) were counted using an ImmunoSpot series 3A analyzer (Cellular Technology Ltd., Cleveland, OH) based on the number of spots per 5 × 105 MNC. Because some of the cytokine reagents were not available at the beginning of the study, the cytokine ELISPOT assay was conducted only for IFN-γ, IL-10, IL-4, and IL-12. The residual cytokine production resulting from in vivo stimulation was assessed by stimulating cells with only RPMI medium; these cell counts were subtracted from the HRV-stimulated CSC.
Detection of cytokine levels by ELISA in serum and intestinal contents.
Blood was collected from pigs at PID 0, 1, 3, 5, 7, 10, 14, 21, and 28, and intestinal contents were collected at euthanasia. Serum samples were processed (without heat inactivation) and stored at −20°C until tested. IC were diluted 1:2 in MEM (without fetal bovine serum). Protease inhibitor cocktail was added to the IC samples to prevent cytokine degradation (3), and the samples were stored at −20°C until tested. Analysis of the different cytokines was dependent upon availability of serum samples, as depicted in Table 1.
TABLE 1.
Percentage of gnotobiotic pigs inoculated with AttHRV or VirHRV or mock infected that developed cytokine responses in serum from PID 1 to 28 detected by ELISAa
| Group | Proinflammatory | Th1 | Th2 | Th3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | TNF-α | IFN-γ | IL-12 | IL-10 | IL-4 | TGF-β | ||||||||
| Pos/totalb | %c | Pos/total | % | Pos/total | % | Pos/total | % | Pos/total | % | Pos/total | % | Pos/total | % | |
| VirHRV | 20/22 | 90A | 10/24 | 60A | 20/30 | 67A | 30/30 | 100 | 21/30 | 70A | 17/30 | 57A | 15/15 | 100 |
| AttHRV | 18/24 | 75A | 12/24 | 50A | 12/31 | 39B | 31/31 | 100 | 9/31 | 29B | 20/31 | 65A | 22/22 | 100 |
| Control | 1/12 | 8B | 2/13 | 15B | 0/22 | 0C | 22/22 | 100 | 0/22 | 0C | 6/22 | 27B | 15/15 | 100 |
The ELISA was conducted as follows: Nunc Maxisorp 96-well plates were coated with anti-porcine IFN-γ (1.5 μg/ml), anti-porcine IL-10 (2 μg/ml), anti-porcine TGF-β (1.5 μg/ml) (Biosource), anti-porcine IL-12 (0.75 μg/ml), anti-porcine IL-4 (1.5 μg/ml), anti-porcine IL-6 (0.8 μg/ml), or anti-porcine TNF-α (2 μg/ml) (R&D Systems) overnight at RT. Prior to use, the plates were blocked with phosphate-buffered saline (PBS)-0.1% Tween-0.5% bovine serum albumin (BSA) for 2 h at RT. Samples were added to the wells in a volume of 50 μl plus 50 μl of PBS-1% BSA. Control samples were diluted in PBS-1% BSA. Plates were incubated for 2 h at RT and then were washed five times with PBS-0.1% Tween, and the following reagents were added: biotinylated monoclonal antibodies to porcine IFN-γ (0.5 μg/ml), IL-10 (0.5 μg/ml), TGF-β (0.75 μg/ml) (Biosource), IL-12 (0.2 μg/ml), IL-4 (0.25 μg/ml), IL-6 (0.1 μg/ml), or TNF-α (0.2 μg/ml) (R&D Systems). Plates were incubated for 2 h at RT. Horseradish peroxidase-conjugated streptavidin (Biosource) was added at a concentration of 0.1 μg/ml, and plates were incubated for 45 min at RT for IFN-γ, IL-10, and IL-12; for IL-4, plates were incubated for 1 h at RT. Standard curves were generated using recombinant porcine IFN-γ and IL-10 (Biosource); IL-12, IL-4, IL-6, and TNF-α (R&D Systems); and human recombinant TGF-β (Biosource). The standard curves were calculated using a computer-generated four-parameter curve-fit for each cytokine. Sensitivities for these ELISAs were 7.5 pg/ml for IFN-γ, IL-10, IL-4, and IL-12 and 15 pg/ml for IL-6, TNF-α, and TGF-β.
Recovery rate of exogenous cytokines in the intestinal contents of gnotobiotic pigs.
To evaluate the stability of cytokines in intestinal contents, cytokine standards were added to the intestinal contents of mock-infected control pigs that previously tested negative for cytokines. Cytokines were added at the following concentrations: 1.0 ng/ml of IFN-γ, IL-10, IL-4, and IL-12 and 4.0 ng/ml of TNF-α. The intestinal contents were then incubated for 30 min at 37°C and tested by ELISA to estimate the recovery rates of the cytokines added.
Statistical analysis.
The CSC numbers and concentrations of cytokines in serum and intestinal contents were compared for PID 3, 5, 7, 14, 21, and 28 among and within the groups using the Kruskal-Wallis rank sum test (nonparametric). Proportions of pigs that secreted cytokines among groups were compared using Fisher's exact test. Statistical significance was assessed at P < 0.05. Spearman correlation coefficients were used to evaluate the correlation between severity of diarrhea and levels of serum IFN-γ, IL-6, and TNF-α.
RESULTS
A higher percentage of VirHRV-inoculated gnotobiotic pigs than of AttHRV pigs and controls developed Th1 (IFN-γ) and Th2 (IL-10) cytokines in serum.
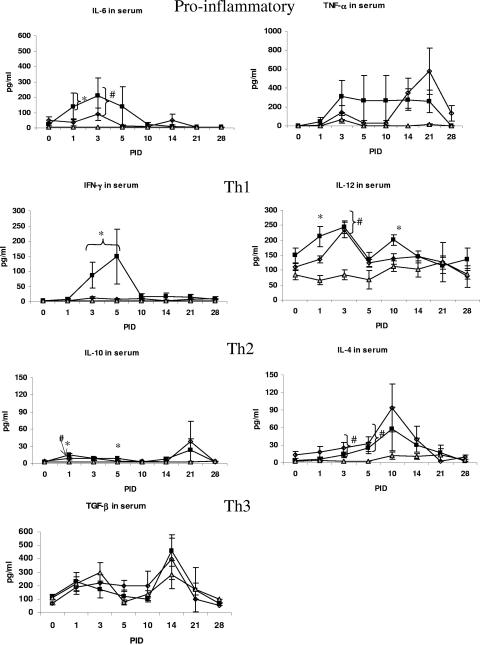
The overall percentages of pigs positive for each detectable cytokine in the serum are summarized in Table 1, and the levels of each cytokine at different time points are depicted in Fig. 1. Pigs that had at least one serum sample with cytokine levels higher than the detection limit of the assay were considered positive responders in calculating the overall cytokine response percentage. A significantly higher percentage of pigs had IFN-γ and IL-10 responses (67 and 70%, respectively) after VirHRV infection than after AttHRV infection (39 and 29%, respectively) or in controls (0%). Both groups infected with HRV had significantly higher percentages than the control group of pigs that developed TNF-α, IL-6, and IL-4 responses. All pigs, regardless of inoculation group, had IL-12 and TGF-β in the serum.
FIG. 1.
Cytokine levels in serum of gnotobiotic pigs. Symbols: squares, VirHRV; diamonds, AttHRV; triangles, control. Statistical symbols for P < 0.05: *, higher than AttHRV and controls; #, higher than controls.
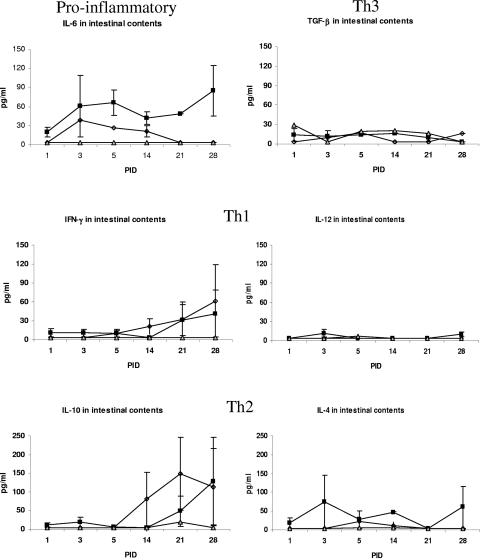
Most cytokines lacked stability in intestinal contents (except IL-12); nevertheless, higher cytokine levels were detected in intestinal contents of gnotobiotic pigs inoculated with VirHRV than in IC of AttHRV or control group pigs.
We first examined degradation of each cytokine in intestinal contents to assess their stability and their potential loss due to proteolysis and impact on assay results. Exogenous cytokines added to the intestinal contents showed low recovery rates for all cytokines tested, except for IL-12. Ten percent of IFN-γ (10 pg/ml) was recovered, and less than 10% of IL-10, IL-4, and TNF-α was recovered. However, more than 90% of IL-12 was recovered (910 pg/ml). Thus, actual levels of IFN-γ, IL-10, IL-4, and TNF-α are likely underestimated, but their relative levels in AttHRV- and VirHRV-infected pigs should not be differentially affected.
The VirHRV-infected pigs had consistently higher intestinal cytokine levels (IFN-γ, IL-10, IL-4, and IL-6) early at PID 3 and 5, but especially at PID 3, than did the AttHRV-infected pigs, but no significant differences were detected between the two HRV pig groups or with the controls (Fig. 2). Low levels of IL-12 were transiently detected only in the intestinal contents of the VirHRV-infected pigs.
FIG. 2.
Cytokine levels in intestinal contents of gnotobiotic pigs. Symbols: ▪, VirHRV; ⋄, AttHRV; ▵, control.
The VirHRV-inoculated gnotobiotic pigs developed a higher, earlier mean level of proinflammatory cytokine responses in serum than did the AttHRV pigs or controls.
The VirHRV-infected pigs had 1.5- to 8-fold-higher (although not significantly), earlier TNF-α levels in the serum at PID 3 to 10 than did the AttHRV-infected pigs (Fig. 1; Table 2). Both VirHRV and AttHRV groups had higher levels of TNF-α at PID 14 and 21 than did the controls. Similarly, IL-6 was significantly elevated at PID 1 in serum of the VirHRV group and at PID 3 in both groups compared to the controls (17- to 57-fold higher, Table 2). The IL-6 levels in intestinal contents were consistently two- to sixfold higher in VirHRV pigs than in the AttHRV group.
TABLE 2.
Cytokine responses (fold increase over controlsa) to rotavirus infection in serum
| Group | Proinflammatory | Th1 | Th2 | |||
|---|---|---|---|---|---|---|
| TNF-α | IL-6 | IFN-γ | IL-12 | IL-4 | IL-10 | |
| VirHRV | 8 | 57 | 40 | 2.6 | 6.3 | 1.8 |
| AttHRV | 6 | 17 | 3 | 2.5 | 9.5 | 1.8 |
| Control | 1 | 1 | 1 | 1 | 1 | 1 |
VirHRV-inoculated gnotobiotic pigs developed higher and earlier Th1 and Th2 cytokines in serum than did AttHRV or control group pigs.
For the Th1 responses, the levels of IFN-γ were significantly elevated at PID 3 and 5 in the serum of the VirHRV group (10-fold higher) compared to the AttHRV group (Fig. 1; Table 2). Although IL-12, the Th1 inducer, was detected in the serum of all pigs at each time point, a significantly elevated peak occurred early at PID 1 in the VirHRV group and again at PID 10 compared to the AttHRV and control groups, and also at PID 3 in both HRV groups compared to the controls. Interestingly, IFN-γ responses were detected at peak levels later in the intestinal contents of both groups. The IL-12 responses in intestinal contents were low for both groups. It is noteworthy that the peak of Th1 cytokine responses was greater in serum than in intestinal contents.
For Th2 responses, however, both groups had peak serum levels at PID 21, and significantly higher levels of IL-10 were detected early in serum at PID 1 and 5 in the VirHRV group compared to the AttHRV and control groups (Fig. 1). For intestinal contents, the VirHRV pigs had a transient low peak at PID 3 and then another higher peak at PID 28. The AttHRV pigs had a single peak late at PID 21. The late peak in intestinal content response was higher than the serum response. Both HRV groups developed significantly higher early levels of IL-4 in serum at PID 3 and 5 compared to the control group. However, higher, earlier IL-4 levels were detected at PID 3 in intestinal contents of VirHRV pigs, and they persisted at higher levels at all PIDs except PID 21. Interestingly, the peak of IL-10 and IL-4 responses of the VirHRV pigs in intestinal contents was higher than in serum.
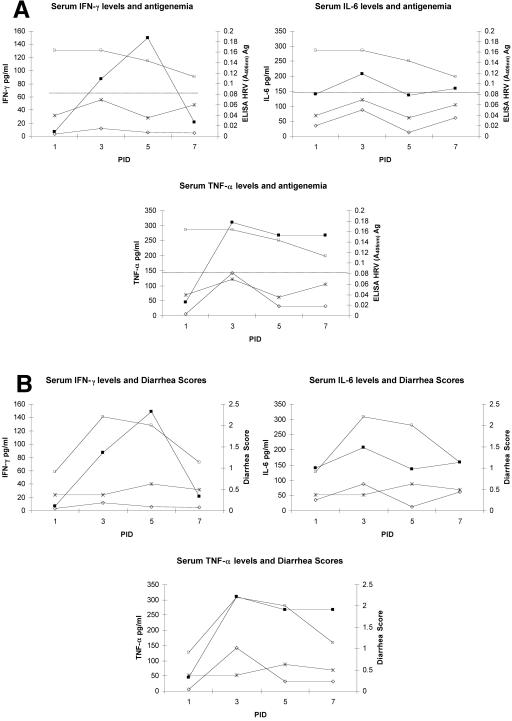
Peak levels of IFN-γ in serum of the VirHRV-infected pigs coincided with antigenemia, and early IL-6 response strongly correlated with severity of diarrhea.
The levels of IFN-γ, IL-6, and TNF-α in serum at PID 1, 3, 5, and 7 were compared with the HRV antigen ELISA titer (_A_405) in serum (antigenemia) and the diarrhea scores observed in both AttHRV and VirHRV groups (Fig. 3a and b). The peak levels of IL-6 and TNF-α coincided with peaks of diarrhea scores and antigenemia detected in the VirHRV group. Positive correlations were found between diarrhea and levels of serum TNF-α (r = 0.67, P = 0.05) and serum IL-6 (r = 0.93, P = 0.0002) in the VirHRV group; however, they were restricted to PID 1. Unlike TNF-α and IL-6, IFN-γ peaked at PID 5 shortly after the peaks of diarrhea and viremia (PID 1 to PID 3) in the VirHRV group. The AttHRV-inoculated pigs showed low diarrhea scores; no antigenemia (_A_405 < 0.08); and lower IFN-γ, TNF-α, and IL-6 levels at PID 1 and 3.
FIG. 3.
(A) IFN-γ, IL-6, and TNF-α and antigenemia in gnotobiotic piglets inoculated with HRV. Symbols: ▪, serum IFN-γ levels in VirHRV group; □, HRV antigen in VirHRV group; ⋄, serum IFN-γ levels in AttHRV group; *, HRV antigen in AttHRV group. Dotted line represents cutoff value (ELISA = 0.08). (B) IFN-γ, IL-6, and TNF-α and diarrhea in gnotobiotic piglets inoculated with HRV. Symbols: ▪, IFN-γ in VirHRV group; □, diarrhea score in VirHRV group; ⋄, IFN-γ in AttHRV group; *, diarrhea score in AttHRV group.
The number of cytokine-secreting cells was similar for Th1 and Th2 responses between VirHRV- and AttHRV-inoculated gnotobiotic pigs.
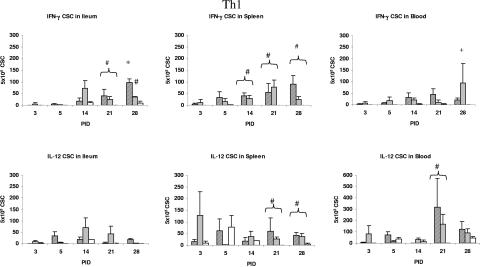
Cytokine-secreting cell responses detected by cytokine-ELISPOT assays are depicted in Fig. 4 (Th1) and 5 (Th2). For Th1 responses, the numbers of IFN-γ CSC were significantly higher in the ileum of VirHRV-infected pigs than in that of the AttHRV-infected pigs at PID 28. The numbers of IFN-γ CSC in both HRV groups were higher or significantly higher than those in the control group at PID 21 in ileum and from PID 14 to 28 in spleen and blood. Significantly higher numbers of IL-12 CSC were observed in the spleen at PID 21 and 28 and in the blood at PID 21 of both HRV groups compared to the control group.
FIG. 4.
Th1 (IFN-γ and IL-12) cytokine-secreting cell responses in ileum, spleen, and PBMCs of gnotobiotic pigs inoculated with VirHRV, AttHRV, or controls. Due to higher IL-12 numbers in PBMCs, the PBMC graph is shown using a different scale. Symbols: ▨, VirHRV; ░⃞, AttHRV; □, controls. Statistical symbols for P < 0.05: *, higher than AttHRV and controls; +, higher than VirHRV and controls; #, higher than controls.
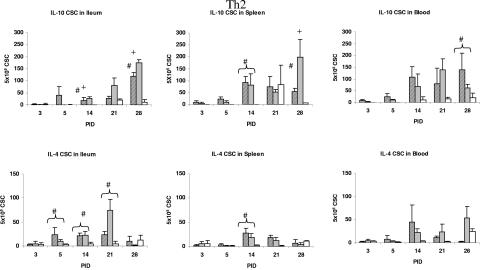
FIG. 5.
Th2 (IL-10 and IL-4) cytokine-secreting cells in ileum, spleen, and blood of gnotobiotic piglets inoculated with HRV. Symbols: ▨, VirHRV; ░⃞, AttHRV; □, controls. Statistical symbols for P < 0.05: *, higher than AttHRV and controls; +, higher than VirHRV and controls; #, higher than controls.
For Th2 responses, the numbers of IL-10 CSC were significantly higher later in the ileum of the AttHRV-infected pigs at PID 14 and 28 and also in the spleen at PID 28 compared to the VirHRV-infected and control pigs. The numbers of IL-10 CSC in both HRV groups were higher or significantly higher than those in the control group from PID 14 to 28 in the blood. The numbers of IL-4 CSC were significantly higher in the ileum of pigs from both HRV groups compared to the control group at PID 5, 14, and 21 and in the spleen at PID 14. The later elevated IL-10 CSC responses in the AttHRV group suggest again a delayed initiation of a Th2 response induced by the AttHRV. These results indicate a balanced Th1/Th2 response in the HRV-infected pigs.
DISCUSSION
Delineating cytokine responses to a viral infection is important to understanding viral pathogenicity and immunity. The pair of virulent and attenuated Wa HRV strains provides a valuable tool to study the relationship between cytokine profiles, kinetics, and magnitude of responses and their relationships to the pathogenicity and immunity induced by these two rotavirus strains that differ in virulence (34, 35). The pathogenicity of and immune responses to VirHRV and AttHRV have been well defined in gnotobiotic pigs (3, 4, 45, 46, 49, 51). The VirHRV infection caused diarrhea and villous atrophy and 100% rectal and nasal virus shedding, with the presence of viral antigen within villous epithelial cells and infectious rotavirus in the serum (viremia) (4, 45). Strong intestinal and systemic B- and T-cell responses were generated against VirHRV, and pigs that recovered from VirHRV infection were fully protected against homotypic rotavirus reinfection (46, 49, 51). In comparison, AttHRV does not cause diarrhea or villous atrophy (45). The AttHRV-infected pigs had limited fecal virus shedding (5 to 17%) but a higher rate of nasal virus shedding (79%), with no detectable viremia (4). Cytokine responses induced by VirHRV versus AttHRV during the course of infection may play an important role in the differences in the pathogenicity and immunogenicity of these two rotavirus strains.
In this study, we analyzed cytokine profiles, including proinflammatory cytokines (TNF-α and IL-6) and Th1 inducer (IL-12), Th1 (IFN-γ), Th2 (IL-4 and IL-10), and Th3 (TGF-β) cytokines, in the serum and intestinal contents of pigs infected with VirHRV or AttHRV or mock infected. We also determined IFN-γ, IL-12, IL-4, and IL-10 CSC in intestinal and systemic lymphoid tissues. The cytokine levels detected in serum and intestinal contents represent the in vivo cytokine production and profiles in the extracellular fluids, which reflect the cumulative amounts of each cytokine secreted by lymphocytes and all other cell types. On the other hand, CSC detected by ELISPOT in lymphoid tissues represent the precursor cytokine-secreting cells among MNC after in vitro restimulation with HRV antigen. For quantitation of the CSC, the MNC were stimulated with HRV antigen in vitro for about 72 h; thus, the CSC responses represent mostly the activated antigen-specific effectors or memory Th1 or Th2 lymphocytes. The discrepancies seen between cytokine concentration and kinetics in the serum or IC and the CSC numbers in the lymphoid tissues were also observed by Diaz and Mateu (16) in a study of the evolution of the immune response in conventional pigs and in studies of antigen-induced and spontaneous IL-4 responses in humans (15, 17). These discrepancies are also similar to the lack of correlation between the serum antibody titer and the antibody-secreting cells detected in lymphoid tissues as reported in previous studies (10, 39).
Higher levels of TNF-α were detected in serum of the VirHRV-infected pigs than in that of the AttHRV-infected pigs at PID 1 to 10. This finding agrees with the work of Azim et al. (6), who found an association of TNF-α levels with the severity of rotavirus disease in children. Our finding also agrees with their earlier study (5), in which they observed increased levels of TNF-α during the first week of rotavirus infection. The transient (PID 3, 5, and 10) higher levels of TNF-α in the serum of the VirHRV-infected pigs may correspond to the intestinal lesions induced within the first days in the VirHRV pigs; no intestinal lesions were detected in the AttHRV-infected pigs (45). The similar elevated levels of TNF-α observed later (PID 14 and 21) in both HRV groups likely reflect a general response to rotavirus infection and the absence of extensive inflammation-induced infiltration of polymorphonuclear cells in the intestinal tissues after VirHRV or AttHRV infection (45). Significantly higher concentrations of IL-6 were detected early in the VirHRV pigs (PID 1) compared to the AttHRV or control pigs, followed by elevated concentrations in both virus-infected groups at PID 3 and 5. Jiang et al. (23) reported significantly higher concentrations of IL-6 in acute-phase serum of children after rotavirus infection and correlated the IL-6 concentrations with fever. In gnotobiotic pigs, the elevation of IL-6, together with TNF-α, strongly correlated with the severity of diarrhea observed in the VirHRV pigs.
The Th1 inducer IL-12 was detected in serum of all pigs including controls at each time point, with significantly elevated peaks at PID 1 only for VirHRV and at PID 3 for both HRV groups. Although there were no significant differences in the numbers of IL-12 CSC in any tissue at any time point between VirHRV- and AttHRV-infected pigs, the significantly higher IL-12 level in the serum at PID 3 and the IL-12 CSC responses in blood and spleen of both HRV groups at PID 21 or 28 compared to the controls may indicate a role for IL-12 in the initiation and development of the Th1 immune responses to rotavirus infection contributing to viral clearance. Production of IL-12 by macrophages and B cells results in the increased production of IFN-γ (12). Supporting this concept, in our study, IL-12 peaked in serum at PID 1 to 3 in the VirHRV group followed by an IFN-γ peak at PID 3 to 5 in the serum. In agreement with the upregulated serum IL-12 levels was the observation of significantly higher numbers of IL-12 CSC in the spleen and blood but not in the ileum or intestinal contents of VirHRV- or AttHRV-infected pigs, suggesting a more systemic distribution of IL-12 production.
The magnitude of the IFN-γ (Th1) response to rotavirus infection is associated with the magnitude of the T-cell immune responses. In the VirHRV-infected pigs, we observed an earlier-onset, significantly higher, and more prolonged serum IFN-γ response than in the AttHRV-infected pigs. We also observed significantly higher IFN-γ CSC numbers at PID 28 in the ileum of the VirHRV-infected pigs but generally lower IFN-γ responses in the AttHRV-infected pigs. These observations are consistent with the significantly higher intestinal T-cell responses in the VirHRV-infected pigs than in the AttHRV-infected pigs as measured by a lymphocyte proliferation assay in a previous study (46). Higher numbers of IFN-γ CSC in the ileum of the VirHRV group at PID 28 may represent the stronger homing of virus-specific memory T cells to their induction site.
IFN-γ production is known to be a major contributing factor against infections by a variety of cytopathic viruses (52). However, whether IFN-γ contributes directly to the protection against challenge by VirHRV infection of pigs (49, 51) is unclear. The IFN-γ levels in the serum and intestinal contents at PID 21 (the time of challenge in a previous study [51]) did not differ significantly between the VirHRV and AttHRV groups. Yet one dose of VirHRV induces almost complete protection against rotavirus shedding, whereas one dose of AttHRV induces protection at low rates (49, 51). Studies of mice have shown the lack of a role for IFN-γ in the resolution of rotavirus-induced diarrhea and infection of IFN-γ−/− mice (1, 41), although an in vitro study has shown that IFN-γ inhibited rotavirus entry into epithelial cells (7).
The kinetics and magnitude of IFN-γ responses have also been related to the pathogenicity of the rotavirus strains. In this study, IFN-γ levels were elevated in pigs with viremia and diarrhea after VirHRV infection, which is in agreement with the observations from studies of children (5, 6, 23). Jiang et al. (23) described higher levels of IFN-γ, IL-6, and IL-10 in acute-phase sera after rotavirus infection of children. Azim et al. (5) observed that significantly elevated plasma IFN-γ levels were associated with subsequent development of persistent diarrhea compared to those with acute diarrhea or uninfected children, suggesting that IFN-γ concentrations may correlate with a more severe or prolonged pathogenesis of rotavirus infection. In a subsequent study, Azim et al. (6) compared cytokines (TNF-α, IFN-γ, IL-8, and IL-10) in supernatants from unstimulated peripheral blood mononuclear cells (PBMCs) of the children with rotaviral diarrhea to the controls (children with diarrhea from nonenteric pathogens). They found that IFN-γ was the only cytokine that was significantly higher in children with rotavirus diarrhea than in the control diarrheal children (6). IFN-γ can be induced in response to early cytokines such as IL-12, IL-18, and IFN-α/β after viral infection, the latter reflective of innate immune responses. In this study, IL-12 was significantly elevated before IFN-γ in the VirHRV pigs. It is difficult to determine if IFN-γ plays a role in the pathogenesis of rotavirus disease or if it is produced in response to the elevated proinflammatory cytokines seen after virus replication and viremia in VirHRV pigs. However, we observed that the peak of the IFN-γ response (PID 5) occurred shortly after the peaks of diarrhea and viremia in the pigs (PID 1 to 3). It is likely that IL-12 and IFN-γ play a protective role in rotavirus infection by diminishing viral replication whereas TNF-α and IL-6 may play a role in the manifestation of diarrhea.
We found increased IL-10 (Th2 and Treg) cytokine responses in serum of gnotobiotic pigs inoculated with VirHRV, with significantly higher rates of responders (70% for VirHRV versus 29% for AttHRV) and higher serum levels during the acute phase of infection (PID 1 to 5), compared to those in the AttHRV group. This finding corresponds to significantly higher IgA antibody responses in the VirHRV-infected pigs than in the AttHRV-infected pigs (39, 51). Azim et al. (5) also found that IL-10 levels in the plasma of children with rotavirus infection were increased. Although the numbers of IL-10 CSC in the ileum of VirHRV-infected pigs were higher early (PID 5), they were lower or significantly lower than in the AttHRV-infected pigs from PID 14 to 28. This may be related to the significantly higher IFN-γ serum levels and CSC in ileum of the VirHRV-infected pigs and the impact of IFN-γ as a downregulator of IL-10 expression (21). The IL-4 responses in both HRV groups were similar, indicating that IL-4 is not likely to play a major role in differences in pathogenicity and immune responses between the VirHRV- and AttHRV-infected pigs. However, IL-4 is secreted at lower levels than Th1 cytokines. It has been demonstrated that, even after polyclonal stimulation, IL-4 concentrations were 1,000-fold lower than the concentrations of IFN-γ. Dhiman et al. (15) demonstrated low levels of IL-4 in cell supernatants by ELISA and a low frequency of IL-4 CSC by conventional ELISPOT compared to use of an IL-4 receptor-blocking assay.
Although in this study we detected cytokines in the intestinal contents, the levels were likely underestimated due to the instability of certain cytokines to the pH and proteolytic enzymes present in the intestine, as indicated by the low recovery rates. However, these low cytokine levels may also reflect lower inflammatory responses in the intestine during HRV infection (45). Further investigations are needed to confirm the absence of IL-12 in intestinal contents (despite its stability to intestinal secretions) and the generally lower numbers of IL-12 CSC (<50 CSC per 5 × 105 MNC) seen in ileum compared to spleen and PBMCs. Interleukin-12 was the only cytokine that had a recovery rate from intestinal contents higher than 90%; nevertheless, IL-12 was undetectable in the intestinal contents of infected pigs. The low numbers of IL-12 CSC in ileum compared to spleen and PBMCs further confirms a lack of or low secretion of IL-12 in the gut lumen (intestinal contents) during rotavirus infection. This finding suggests a higher regulatory control of IL-12 secretion in the gut, as a way to maintain equilibrium between immune responses and oral tolerance. In addition to these findings, a comparison of the levels of Th1 and Th2 cytokines in intestinal contents showed higher levels of Th2-type than Th1-type cytokines. At early PIDs, IL-4 was present in intestinal contents at levels up to seven times higher than those of IFN-γ (VirHRV group) but similar to those of IL-6. Interleukin-4 seemed to be later replaced by IL-10, which was also present at higher levels than IFN-γ and also higher levels than IL-6. This higher Th2-type intestinal cytokine response may be indicative of the type of immunity needed for protection against rotavirus diarrhea, which in studies of both pigs and children after experimental or natural rotavirus infection has been associated with intestinal or stool IgA antibodies (3, 13, 51).
In summary, our findings indicate that both Th1- and Th2-type cytokines are induced during infection with VirHRV or AttHRV, but with an earlier, higher magnitude of both Th1 (IFN-γ) and Th2 (IL-10) cytokine responses generally induced by VirHRV. The Th1/Th2 dichotomy has been well established in a number of investigations of mice, but it appears less clear for other species, such as cattle (11). Our data indicate that, for rotavirus infection in swine, a mixed Th1 and Th2 response was generated by AttHRV or VirHRV infections. Fromantin et al. (19) also showed that spleen cells from suckling mice orally inoculated with highly replicative homologous or weakly replicative heterologous strains of rotavirus produced a mixed Th1/Th2 pattern of cytokines after in vitro restimulation of the spleen cells with rotavirus. Accumulating evidence suggests that these Th1 and Th2 responses during viral and intracellular bacterial infections are not mutually exclusive and that the dual Th1-Th2 nature of the antiviral immune response is a product of cytokine cross-regulatory mechanisms. The coinduction of Th2 immunity during Th1-mediated immunity maintains the immune homeostasis, facilitating recovery from infection and an avoidance of unbalanced Th1- or Th2-mediated pathology or sequelae (9).
Our findings provide the first comprehensive delineation of cytokine profiles after HRV infection of gnotobiotic pigs and a basis for comparisons with those in rotavirus-infected children. A balanced Th1/Th2 response but with significantly higher, earlier IFN-γ, IL-10, and proinflammatory cytokines induced by VirHRV infection was associated with complete protection against rotavirus reinfection in pigs as observed in previous studies (51). Thus, such cytokine response patterns may serve as an indicator of protective immunity for evaluation of rotavirus vaccines. Higher protection rates may be associated with more balanced Th1- and Th2-type responses, but induction of higher, earlier IFN-γ and the proinflammatory cytokines triggered by VirHRV may also play an important role in the induction of immunity by VirHRV. Understanding the pattern of cytokine secretion in response to specific antigenic stimulation and association of cytokines with immune responses is important to understand the function of cytokines and the use of cytokines as markers of pathogenicity and immunity or as possible adjuvants.
Acknowledgments
We thank Juliette Hanson, Richard McCormick, Peggy Lewis, Ana Azevedo, and Shane Clark for technical assistance.
This work was supported by grants from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants RO1A133561 and RO1A137111). Salaries and research support were provided by state and federal funds provided to the Ohio Agricultural Research and Development Center (OARDC) of The Ohio State University. Marli S. P. Azevedo was a fellow (1999 to 2003) of Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq), Brasilia, Brazil.
REFERENCES
- 1.**Angel, J., M. A. Franco, H. B. Greenberg, and D. Bass.1999. Lack of a role for type I and type II interferons in the resolution of rotavirus-induced diarrhea and infection in mice. J. Interferon Cytokine Res. 19:**655-659. [DOI] [PubMed] [Google Scholar]
- 2.**Asseman, C., S. Mauze, M. W. Leach, R. L. Coffman, and F. Powrie.1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190:**995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.**Azevedo, M. S., L. Yuan, C. Iosef, K. O. Chang, Y. Kim, T. V. Nguyen, and L. J. Saif.2004. Magnitude of serum and intestinal antibody responses induced by sequential replicating and nonreplicating rotavirus vaccines in gnotobiotic pigs and correlation with protection. Clin. Diagn. Lab Immunol. 11:**12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.**Azevedo, M. S., L. Yuan, K.-I. Jeong, A. Gonzalez, T. V. Nguyen, P. S., M. Gochnauer, W. Zhang, A. Azevedo, and L. J. Saif.2005. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J. Virol. 79:**5428-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.**Azim, T., S. M. Ahmad, E. K. Sefat, M. S. Sarker, L. E. Unicomb, S. De, J. D. Hamadani, M. A. Salam, M. A. Wahed, and M. J. Albert.1999. Immune response of children who develop persistent diarrhea following rotavirus infection. Clin. Diagn. Lab. Immunol. 6:**690-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.**Azim, T., M. H. Zaki, G. Podder, N. Sultana, M. A. Salam, S. M. Rahman, E. K. Sefat, and D. A. Sack.2003. Rotavirus-specific subclass antibody and cytokine responses in Bangladeshi children with rotavirus diarrhoea. J. Med. Virol. 69:**286-295. [DOI] [PubMed] [Google Scholar]
- 7.**Bass, D. M.1997. Interferon gamma and interleukin 1, but not interferon alfa, inhibit rotavirus entry into human intestinal cell lines. Gastroenterology 113:**81-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.**Billiau, A.1996. Interferon-gamma: biology and role in pathogenesis. Adv. Immunol. 62:**61-130. [DOI] [PubMed] [Google Scholar]
- 9.**Bot, A., K. A. Smith, and M. von Herrath.2004. Molecular and cellular control of T1/T2 immunity at the interface between antimicrobial defense and immune pathology. DNA Cell Biol. 23:**341-350. [DOI] [PubMed] [Google Scholar]
- 10.**Brown, K. A., J. A. Kriss, C. A. Moser, W. J. Wenner, and P. A. Offit.2000. Circulating rotavirus-specific antibody-secreting cells (ASCs) predict the presence of rotavirus-specific ASCs in the human small intestinal lamina propria. J. Infect. Dis. 182:**1039-1043. [DOI] [PubMed] [Google Scholar]
- 11.**Brown, W. C., A. C. Rice-Ficht, and D. M. Estes.1998. Bovine type 1 and type 2 responses. Vet. Immunol. Immunopathol. 63:**45-55. [DOI] [PubMed] [Google Scholar]
- 12.**Chan, S. H., B. Perussia, J. W. Gupta, M. Kobayashi, M. Pospisil, H. A. Young, S. F. Wolf, D. Young, S. C. Clark, and G. Trinchieri.1991. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J. Exp. Med. 173:**869-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.**Coulson, B. S., K. Grimwood, I. L. Hudson, G. L. Barnes, and R. F. Bishop.1992. Role of coproantibody in clinical protection of children during reinfection with rotavirus. J. Clin. Microbiol. 30:**1678-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.**Dawson, H. D., E. Beshah, S. Nishi, G. Solano-Aguilar, M. Morimoto, A. Zhao, K. B. Madden, T. K. Ledbetter, J. P. Dubey, T. Shea-Donohue, J. K. Lunney, and J. F. Urban, Jr.2005. Localized multigene expression patterns support an evolving Th1/Th2-like paradigm in response to infections with Toxoplasma gondii and Ascaris suum. Infect. Immun. 73:**1116-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.**Dhiman, N., I. G. Ovsyannikova, R. C. Howe, J. E. Ryan, R. M. Jacobson, and G. A. Poland.2004. Interleukin-4 induced by measles virus and measles-derived peptides as measured by IL-4 receptor-blocking ELISA. J. Immunol. Methods 287:**217-225. [DOI] [PubMed] [Google Scholar]
- 16.**Diaz, I., and E. Mateu.2005. Use of ELISPOT and ELISA to evaluate IFN-gamma, IL-10 and IL-4 responses in conventional pigs. Vet. Immunol. Immunopathol. 106:**107-112. [DOI] [PubMed] [Google Scholar]
- 17.**Ekerfelt, C., J. Ernerudh, and M. C. Jenmalm.2002. Detection of spontaneous and antigen-induced human interleukin-4 responses in vitro: comparison of ELISPOT, a novel ELISA and real-time RT-PCR. J. Immunol. Methods 260:**55-67. [DOI] [PubMed] [Google Scholar]
- 18.**Fossum, C., E. Wattrang, L. Fuxler, K. T. Jensen, and P. Wallgren.1998. Evaluation of various cytokines (IL-6, IFN-alpha, IFN-gamma, TNF-alpha) as markers for acute bacterial infection in swine—a possible role for serum interleukin-6. Vet. Immunol. Immunopathol. 64:**161-172. [DOI] [PubMed] [Google Scholar]
- 19.**Fromantin, C., L. Piroth, I. Petitpas, P. Pothier, and E. Kohli.1998. Oral delivery of homologous and heterologous strains of rotavirus to BALB/c mice induces the same profile of cytokine production by spleen cells. Virology 244:**252-260. [DOI] [PubMed] [Google Scholar]
- 20.**Hayden, F. G., R. Fritz, M. C. Lobo, W. Alvord, W. Strober, and S. E. Straus.1998. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J. Clin. Investig. 101:**643-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.**Herrero, C., X. Hu, W. P. Li, S. Samuels, M. N. Sharif, S. Kotenko, and L. B. Ivashkiv.2003. Reprogramming of IL-10 activity and signaling by IFN-gamma. J. Immunol. 171:**5034-5041. [DOI] [PubMed] [Google Scholar]
- 22.**Hughes, P. A., and L. A. Babiuk.**1995. Potentiation of the immune response by cytokines, p. 183-202. In M. J. Myers and M. P. Murtaugh (ed.), Cytokines in animal health and disease. Marcel Dekker, Inc., New York, N.Y.
- 23.**Jiang, B., L. Snipes-Magaldi, P. Dennehy, H. Keyserling, R. C. Holman, J. Bresee, J. Gentsch, and R. I. Glass.2003. Cytokines as mediators for or effectors against rotavirus disease in children. Clin. Diagn. Lab. Immunol. 10:**995-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.**Lamm, M. E., and J. M. Phillips-Quagliata.2002. Origin and homing of intestinal IgA antibody-secreting cells. J. Exp. Med. 195:**F5-F8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.**Meyer, R. C., E. H. Bohl, and E. M. Kohler.1964. Procurement and maintenance of germ-free swine for microbiological investigations. Appl. Microbiol. 12:**295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.**Morrison, D. F., D. L. Foss, and M. P. Murtaugh.2000. Interleukin-10 gene therapy-mediated amelioration of bacterial pneumonia. Infect. Immun. 68:**4752-4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.**Murphy, K. M.1998. T lymphocyte differentiation in the periphery. Curr. Opin. Immunol. 10:**226-232. [DOI] [PubMed] [Google Scholar]
- 28.**Nakamura, K., A. Kitani, and W. Strober.2001. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor β. J. Exp. Med. 194:**629-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.**Ohshima, Y., and G. Delespesse.1997. T cell-derived IL-4 and dendritic cell-derived IL-12 regulate the lymphokine-producing phenotype of alloantigen-primed naive human CD4 T cells. J. Immunol. 158:**629-636. [PubMed] [Google Scholar]
- 30.**Paludan, S. R.1998. Interleukin-4 and interferon-gamma: the quintessence of a mutual antagonistic relationship. Scand. J. Immunol. 48:**459-468. [DOI] [PubMed] [Google Scholar]
- 31.**Raymond, C. R., and B. N. Wilkie.2004. Th-1/Th-2 type cytokine profiles of pig T-cells cultured with antigen-treated monocyte-derived dendritic cells. Vaccine 22:**1016-1023. [DOI] [PubMed] [Google Scholar]
- 32.**Romani, L., A. Mencacci, E. Cenci, R. Spaccapelo, C. Toniatti, P. Puccetti, F. Bistoni, and V. Poli.1996. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. J. Exp. Med. 183:**1345-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.**Rousset, F., E. Garcia, T. Defrance, C. Peronne, N. Vezzio, D. H. Hsu, R. Kastelein, K. W. Moore, and J. Banchereau.1992. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. USA 89:**1890-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.**Saif, L., L. Yuan, L. Ward, and T. To.1997. Comparative studies of the pathogenesis, antibody immune responses, and homologous protection to porcine and human rotaviruses in gnotobiotic piglets. Adv. Exp. Med. Biol. 412:**397-403. [DOI] [PubMed] [Google Scholar]
- 35.**Saif, L. J., L. A. Ward, L. Yuan, B. I. Rosen, and T. L. To.1996. The gnotobiotic piglet as a model for studies of disease pathogenesis and immunity to human rotaviruses. Arch. Virol. Suppl. 12:**153-161. [DOI] [PubMed] [Google Scholar]
- 36.**Seder, R. A., and W. E. Paul.1994. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu. Rev. Immunol. 12:**635-673. [DOI] [PubMed] [Google Scholar]
- 37.**Suvas, S., A. K. Azkur, B. S. Kim, U. Kumaraguru, and B. T. Rouse.2004. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J. Immunol. 172:**4123-4132. [DOI] [PubMed] [Google Scholar]
- 38.**Svensson, H., B. Cederblad, M. Lindahl, and G. Alm.1996. Stimulation of natural interferon-alpha/beta-producing cells by Staphylococcus aureus. J. Interferon Cytokine Res. 16:**7-16. [DOI] [PubMed] [Google Scholar]
- 39.**To, T. L., L. A. Ward, L. Yuan, and L. J. Saif.1998. Serum and intestinal isotype antibody responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J. Gen. Virol. 79:**2661-2672. [DOI] [PubMed] [Google Scholar]
- 40.**VanCott, J. L., T. A. Brim, R. A. Simkins, and L. J. Saif.1993. Isotype-specific antibody-secreting cells to transmissible gastroenteritis virus and porcine respiratory coronavirus in gut- and bronchus-associated lymphoid tissues of suckling pigs. J. Immunol. 150:**3990-4000. [PubMed] [Google Scholar]
- 41.**Vancott, J. L., M. M. McNeal, A. H. Choi, and R. L. Ward.2003. The role of interferons in rotavirus infections and protection. J. Interferon Cytokine Res. 23:**163-170. [DOI] [PubMed] [Google Scholar]
- 42.**van Ginkel, F., H. Nguyen, and J. McGhee.2000. Vaccines for mucosal immunity to combat emerging infectious diseases. Emerg. Infect. Dis. 6:**123-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.**Vieira, P. L., J. R. Christensen, S. Minaee, E. J. O'Neill, F. J. Barrat, A. Boonstra, T. Barthlott, B. Stockinger, D. C. Wraith, and A. O'Garra.2004. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J. Immunol. 172:**5986-5993. [DOI] [PubMed] [Google Scholar]
- 44.**Wahl, S. M., and W. Chen.2003. TGF-beta: how tolerant can it be? Immunol. Res. 28:**167-179. [DOI] [PubMed] [Google Scholar]
- 45.**Ward, L. A., B. I. Rosen, L. Yuan, and L. J. Saif.1996. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J. Gen. Virol. 77:**1431-1441. [DOI] [PubMed] [Google Scholar]
- 46.**Ward, L. A., L. Yuan, B. I. Rosen, T. L. To, and L. J. Saif.1996. Development of mucosal and systemic lymphoproliferative responses and protective immunity to human group A rotaviruses in a gnotobiotic pig model. Clin. Diagn. Lab. Immunol. 3:**342-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.**Wattrang, E., P. Wallgren, and C. Fossum.1998. Actinobacillus pleuropneumonia serotype 2—effects on the interferon-alpha production of porcine leukocytes in vivo and in vitro. Comp. Immunol. Microbiol. Infect. Dis. 21:**135-154. [DOI] [PubMed] [Google Scholar]
- 48.**Yuan, L., A. Geyer, D. C. Hodgins, Z. Fan, Y. Qian, K. O. Chang, S. E. Crawford, V. Parreno, L. A. Ward, M. K. Estes, M. E. Conner, and L. J. Saif.2000. Intranasal administration of 2/6-rotavirus-like particles with mutant Escherichia coli heat-labile toxin (LT-R192G) induces antibody-secreting cell responses but not protective immunity in gnotobiotic pigs. J. Virol. 74:**8843-8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.**Yuan, L., C. Iosef, M. S. Azevedo, Y. Kim, Y. Qian, A. Geyer, T. V. Nguyen, K. O. Chang, and L. J. Saif.2001. Protective immunity and antibody-secreting cell responses elicited by combined oral attenuated Wa human rotavirus and intranasal Wa 2/6-VLPs with mutant Escherichia coli heat-labile toxin in gnotobiotic pigs. J. Virol. 75:**9229-9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.**Yuan, L., S. Y. Kang, L. A. Ward, T. L. To, and L. J. Saif.1998. Antibody-secreting cell responses and protective immunity assessed in gnotobiotic pigs inoculated orally or intramuscularly with inactivated human rotavirus. J. Virol. 72:**330-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.**Yuan, L., L. A. Ward, B. I. Rosen, T. L. To, and L. J. Saif.1996. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J. Virol. 70:**3075-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.**Zinkernagel, R. M.1996. Immunology taught by viruses. Science 271:**173-178. [DOI] [PubMed] [Google Scholar]