Lipopolysaccharide activates nuclear factor κB in rat intestine: role of endogenous platelet-activating factor and tumour necrosis factor (original) (raw)
Abstract
- We examined the effect of lipopolysaccharide (LPS), a cell wall constituent of Gram negative bacteria, on nuclear factor κB (NF-κB) activation in the intestine and the roles of endogenous platelet-activating factor (PAF), tumour necrosis factor-α (TNF) and neutrophils. We also compared the time course of NF-κB activation in response to PAF and LPS.
- Ileal nuclear extracts from LPS (8 mg kg−1, IV)-injected rats were assayed for NF-κB-DNA-binding activity and identification of the subunits. Some rats were pretreated with WEB2170 (a PAF receptor antagonist), anti-TNF antibody, or anti-neutrophil antiserum. NF-κB p65 was localized by immunohistochemistry. An additional group was challenged with PAF (2 μg kg−1, IV) for comparison.
- LPS activates intestinal NF-κB, both as p50-p50 and p50-p65 dimers within 15 min, and the effect peaks at 2 h. The effect is slower and more sustained than that of PAF, which peaks at 30 min. Activated NF-κB was immunolocalized within epithelial and lamina propria cells. LPS effect was reduced by 41, 37 and 44%, respectively, in animals pretreated with WEB2170, anti-TNF antibody, or anti-neutrophil antiserum (P<0.05).
- LPS activates intestinal NF-κB in vivo and neutrophil activation is involved in its action. The LPS effect is mediated by both endogenous PAF and TNF.
Keywords: Lipopolysaccharide, inflammation, transcription factors, in vivo
Introduction
NF-κB is a key transcription factor in the inflammatory response due to its regulatory role on pro-inflammatory cytokines, enzymes and adhesion molecules (Baeuerle & Henkel, 1994). In many cells, NF-κB exists in the cytoplasm in an inactive state, linked to a potent inhibitor, inhibitor protein κB (IκB), and becomes active only upon stimulation by agents such as LPS, reactive oxygen species (ROS), cytokines, and phorbol esters (Baeuerle & Henkel, 1994; Kopp & Ghosh, 1995). These agents trigger NF-κB/IκB dissociation and nuclear translocation of NF-κB, which regulates the gene transcription of several cytokines and adhesion molecules (Baeuerle & Henkel, 1994; Kopp & Ghosh, 1995).
LPS, a cell wall constituent of gram-negative bacteria, plays a major role in the pathogenesis of septic shock. Administration of LPS in vivo produces shock and intestinal injury (Hsueh et al., 1987) and stimulates the production of IL1, IL6, chemokines (Baeuerle & Henkel, 1994; Kopp & Ghosh, 1995; Libermann & Baltimore, 1990; Couturier et al., 1992; Geng et al., 1993), adhesion molecules (Henninger et al., 1997) and TNF, a pivotal mediator in septic shock (Heumann et al., 1992; Tracey et al., 1986), which is known to activate NF-κB (Shakhov et al., 1990; Schutze et al., 1995; Sun et al., 1993). The transcription of many of these mediators is modulated by NF-κB. LPS also induces the production of PAF, a phospholipid mediator involved in shock (Benveniste, 1988; Handley, 1990) and bowel inflammation (Gonzalez-Crussi & Hsueh, 1983). PAF, when administered systemically, causes bowel necrosis (Gonzalez-Crussi & Hsueh, 1983) and leukocyte-endothelium adhesion (Sun et al., 1996; Kubes et al., 1990). Many of the in vivo effects of LPS are abrogated by pretreatment of the animal with anti-TNF antibody (Tracey et al., 1987) or PAF antagonists (Hsueh et al., 1987; Toth, 1990). These observations suggest that endogenous TNF and PAF mediate the adverse effects of LPS.
Several protein subunits (p50, p65, p52, cRel, RelB) compose the NF-κB complex, forming either a homo or a heterodimer. It is known that LPS activates p50-p65 in endothelial cells in vivo (Essani et al., 1996) and haematopoietic cells in vitro (Ziegler-Heithbrock et al., 1994; Sanceau et al., 1995), but has no effect on the intestinal T84 epithelial cell line (Savkovic et al., 1997). Very few in vivo studies on NF-κB activation have been done. LPS has been shown to induce p50-p65 in rat lung tissue (Blackwell et al., 1996). We recently showed that PAF upregulates the gene expression of NF-κB (Tan, et al., 1994) and activates NF-κB in the rat intestine 30 min after its injection, mainly as p50 homodimers (De Plaen, et al., 1998). Furthermore, we found that neutrophils were necessary for its activation. However, the in vivo effect of LPS on NF-κB activation in the intestine has not been examined to date.
The purpose of this study is to: (a) determine if LPS activates NF-κB in vivo in rat intestine; (b) examine the subunit composition and the time course of LPS-induced NF-κB activation; (c) determine if endogenous PAF and TNF mediate LPS effect on NF-κB activation; and (d) examine the role of polymorphonuclear leukocytes (PMN) in the process.
Methods
Materials
PAF (1-_O_-hexadecyl-2-acetyl-sn-glycero-3-phosphocholine, Biomol Research Lab, Plymouth Meeting, PA, U.S.A.) was prepared (in 5 mg ml−1 bovine serum albumin) as previously described (Tan et al., 1996). WEB2170, a PAF antagonist, was kindly provided by Dr H. Heuer, Boehringer-Ingelheim, Mainz, Germany and rabbit anti-mouse TNF antibody, which cross-reacts with rat TNF, by Dr D.G. Remick.
Animal experiments
Young male Sprague-Dawley rats (60–90 g) were anaesthetized with Nembutal, 65 mg kg−1, IP, and intubated. A carotid artery was catheterized for continuous blood pressure recording and blood sampling and a jugular vein for drug injection. The animals were grouped as follows: (a) sham-operated, vehicle (albumin-saline), 0.6 ml kg−1, IV; (b) LPS (8 mg kg−1, IV, Difco Co., Detroit, MI, U.S.A.); (c) WEB2170 (2 mg kg−1, IV) followed 20 min later by LPS (8 mg kg−1, IV); (d) 1 : 1 diluted rabbit anti-mouse-TNF antibody (10 ml kg−1, IP) followed 30 min later by LPS (8 mg kg−1, IV) or saline; (e) antiPMN antiserum (5 ml kg−1 d−1, IP, Accurate Chemical, Westbury, NY, U.S.A.) or saline for 2 days prior to LPS (8 mg kg−1, IV) or saline injection. Complete PMN depletion after antiserum treatment was confirmed by blood smear examination. Most experiments were terminated 2 h after LPS, and blood samples for hematocrit and leukocyte count were obtained just before LPS injection and 2 h afterwards. Hematocrit was measured as an index of capillary leak. In another set of experiments, the animals were injected with either LPS (8 mg kg−1, IV) or PAF (2 μg kg−1, IV), and the experiment was terminated after 30 min, 60 , 90 min and 2 h respectively. The respective doses of PAF and LPS were chosen so the agent would provoke a sustained (>1 h) pathophysiological change without causing severe, extensive bowel necrosis, which would hamper the extraction of intact nuclei.
At the end of the experiment, approximately 20 cm of ileum were collected and washed with 4°C-saline. Peyer's patches were resected. Tissues were immediately frozen in liquid nitrogen and nuclear extracts were prepared as explained below. The protocol was approved by the Institutional Animal Care and Use Committee.
Preparation of nuclear extracts
Nuclear extracts were prepared following a standard protocol (Deryckere & Gannon, 1994). About 500 mg of frozen ileum were grounded with a mortar in liquid nitrogen. The powder was suspended in a buffer (in mM): (NaCl 150, HEPES 10, pH 7.9, EDTA 1, PMSF 0.5, 0.6% Nonidet P-40) and briefly homogenized, and large debris were removed by centrifugation. The supernatant was incubated on ice for 5 min and then centrifuged at 5000 r.p.m. for 5 min. The pelleted nuclei were lysed by resuspension in 0.5 ml of buffer containing (in mM): HEPES 20 (pH 7.9), NaCl 420, MgCl21.2, EDTA 0.2, DTT 0.5, PMSF 0.5, benzamidine 2, 25% glycerol, and 5 μg ml−1 each of pepstatin, leupeptin and aprotinin. After centrifugation at 10,000×g to remove nuclear debris, nuclear extracts were stored at −80°C. Bradford's method was used to determine protein concentration (Bradford, 1976).
Determination of NF-κB-DNA binding activity by electrophoretic mobility shift assay (EMSA)
NF-κB consensus oligonucleotide (AGT-TGA-GGG-GAC-TTT-CCC-AGG) (Promega Co., Madison, WI, U.S.A.) was labelled with [γ-32P] ATP (3000 Ci mmol−1, 10 mCi ml−1, Amersham, Arlington Heights, IL, U.S.A.), using T4 polynucleotide kinase. Equal amounts of nuclear extract (10 μg/10 μl) were added to 4 μl of gel shift binding buffer in mM: (Tris HCl 10, pH 7.5, NaCl 50, EDTA 0.5, MgCl2 1, DTT 0.5, 40% glycerol, 10 μg ml−1 Poly dIdC) (15 min, room temperature). The mixture was incubated for 20 min with 1 μl of the 32P-labelled oligonucleotide probe. 1.5 μl of loading buffer was added and the sample electrophoresed in a 6% polyacrylamide gel (De Plaen et al., 1998).
The dried gel was analysed with the Storm 860 phosphorimaging system (Molecular Dynamics, Sunnyvale, CA, U.S.A.) and the intensity of the NF-κB complex was quantified by densitometry.
For supershift experiments, the oligonucleotide - nuclear protein binding reaction was followed by incubating the mixture overnight at 4°C with 1 μg of antibody against p50 (polyclonal IgG against p50 nuclear localizing signal, cat. #sc114X), p65 (cat. #sc109X), p52, cRel, and RelB subunits (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.). EMSA was then carried out as described above.
Immunohistochemical studies
Rat ileum was embedded in O.C.T. compound and snap-frozen in liquid nitrogen. Cryostat sections (5 μm) of ileum were air dried, post-fixed in 100% cold acetone for 5 min, and blocked for endogenous peroxidase. Nonspecific background staining was blocked by serum. The slides were then incubated with a mouse mAb specific against activated NF-κB p65 (Boehringer Mannheim) for 45 min at room temperature. A standard ABC technique (Vectastain Avidin-Biotin complex Kit) was used to visualize the reaction product. Sections were counterstained with hematoxylin and coverslipped for microscopic examination. Negative controls were done by replacing the primary antibody with an isotypic control antibody.
Statistical analysis
ANOVA (one way analysis of variance) was used to analyse the data of multiple groups and the difference between groups was considered significant if the P value was less than 0.05.
Results
LPS activates intestinal NF-κB; the effect is less rapid than PAF, but more sustained
LPS, 8 mg kg−1 IV, activates NF-κB in rat ileum as early as 15 min after injection. The activation further increases at 60 min, decreases slightly at 90 min and reaches a maximum at 2 h (Figure 1). The same trend was found in three independent sets of experiments. Therefore, the 2-h time interval was chosen for all other experiments. (Preliminary experiments were done to select the appropriate doses of LPS and PAF that activate NF-κB without causing severe shock and significant bowel necrosis, since massive tissue injury precludes the integrity of extracted nuclei for further NF-κB assay). Ileum was chosen because a preliminary study indicated that NF-κB DNA-binding activity is higher in the ileum than in the jejunum. Further, the injury induced by PAF or LPS occurs predominately in the ileum. The whole ileum tissue was used, since we previously found that the procedure for the isolation of epithelial cells activates NF-κB (De Plaen et al., 1998).
Figure 1.
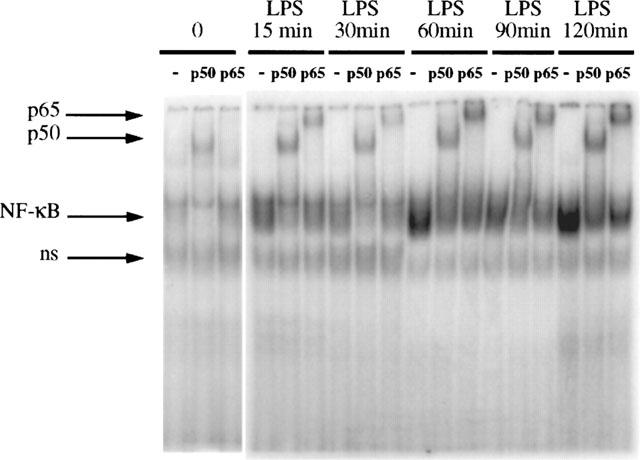
Time-course and subunit composition of LPS-induced NF-κB activation in the intestine. EMSA performed on ileal nuclear extracts of six animals (one sham operated, and rats injected with LPS (8 mg kg−1, IV) respectively at 15, 30, 60, 90, 120 min) is shown here with supershift experiments of each sample with anti-p50 Ab and anti-p65 Ab. Maximum activation occurs 2 h after LPS injection. Both p50 and p65 are found to be part of the complex, as indicated by the diminished density of the NF-κB complex and appearance of a slow band in the supershift experiment. The experiment was repeated on a total of 18 animals (three per time point) and showed consistent results.
Compared to LPS, the effect of PAF on NF-κB activation is shorter. As shown in Figure 2, the activation of NF-κB is maximal within 30 min after injection, subsides afterwards, and returns to near baseline at 2 h (Figure 2).
Figure 2.
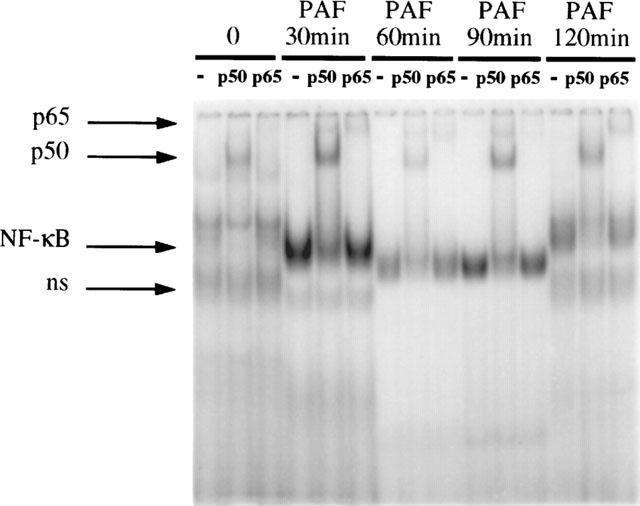
Time-course and subunit composition of PAF-induced NF-κB activation in the intestine. EMSA performed on ileal nuclear extracts of five animals (one sham operated, and rats injected with PAF (2 μg kg−1, IV)- respectively at 30, 60, 90, 120 min) is shown here with supershift experiments. Maximum activation of NF-κB, mainly as p50, is seen within 30 min after the injection. The experiment was performed on a total of 15 animals (three per time point) and show consistent results.
LPS-activated intestinal NF-κB is composed of both p50-p50 and p50-p65: comparison with PAF
In supershift experiments, antibodies against p50, p65, p52, RelB and cRel were used. In the ileum of sham-operated animals, a low constitutive level of NF-κB activation is found and mainly p50 is detected. Only a minimal amount of p65 could be observed as indicated by the supershift of the NF-κB complex by anti-p65 antibody (Figure 1). In LPS-treated animals, only p50 and p65 are found as part of the complex throughout the 2 h experimental period. This is indicated by the marked slowing down of the migration of the NF-κB-oligonucleotide complex (supershift) after the addition of anti-p50 and anti-p65 antibodies (Figure 1). Other antibodies do not produce any supershift at all time points, confirming the absence of p52, RelB and cRel in the NF-κB complex (data not shown). These findings differ from PAF-induced NF-κB activation, in which mainly p50 is detected at various time points examined (Figure 2). In our previous study, we have examined the subunit composition of NF-κB after PAF injection and found no p52, cRel and RelB throughout the experimental period (De Plaen et al., 1998). The specificity of the NF-κB binding was confirmed by a competitive experiment using unlabelled NF-κB oligonucleotide probe which completely blocked the labelled oligonucleotide-NF-κB complex formation, whereas the complex remained unchanged after addition of excess unlabelled AP-1 probe (data not shown).
The effect of LPS on intestinal NF-κB activation is largely dependent on the production of endogenous PAF and TNF
The NF-κB activation by LPS (8 mg kg−1) is partially but significantly reduced by pretreatment with IV injection of 2 mg kg−1 WEB2170, a PAF receptor antagonist (Figure 3). Pretreatment with anti-TNF antibody also significantly decreases NF-κB activation following LPS treatment (Figure 4). Pretreatment with PAF antagonists or anti-TNF antibody did not alter the subunit composition of NF-κB: supershift experiments show both p50 and p65 subunits in the NF-κB complex (data not shown).
Figure 3.
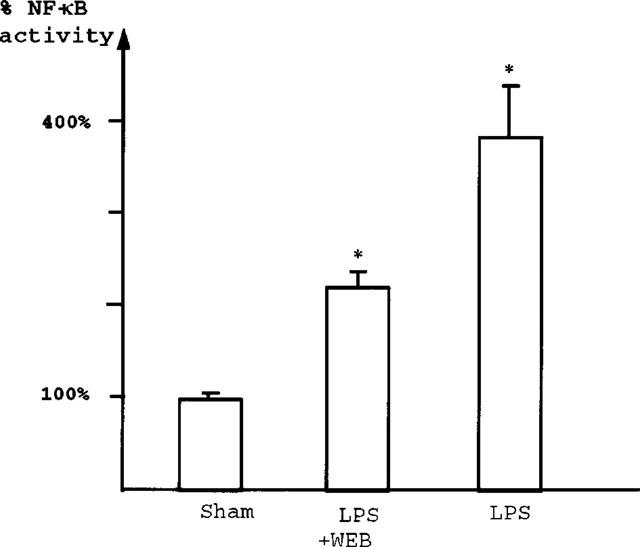
LPS-induced NF-κB activation is reduced by pretreatment with WEB2170, a PAF receptor antagonist. Comparison of NF-κB-DNA binding activity between sham-operated rats (_n_=5), rats injected with WEB2170 and LPS (_n_=5) and LPS alone (_n_=6), as quantified by densitometry using a phosphorimager (Storm 860). A mean value and s.e.mean are calculated from the five sham-operated animals. Results of the LPS and WEB+LPS groups are expressed as percentage of the sham value. NF-κB binding activity in nuclei of ileal cells increased 3.8±0.52 fold 2 h after LPS injection (P<0.005). This effect was significantly reduced (by 41%) following pretreatment with WEB2170 (2.24±0.15 fold of control value) (P<0.05).
Figure 4.
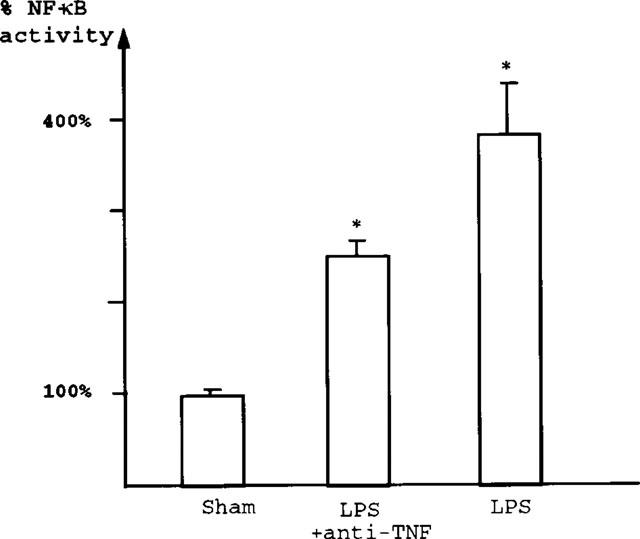
Anti-TNF antibody attenuates LPS-induced NF-κB activation; both p50 and p65 are reduced. Comparison of NF-κB-DNA binding activity, as quantified by densitometry. A mean value and s.e.mean are calculated from sham-operated animals. Results of the LPS and anti-TNF+LPS groups are expressed as percentage of the sham value. The 3.8 fold increase of NF-κB binding activity after LPS treatment was significantly reduced (by 37%) by pretreatment with anti-TNF antibodies (2.43±0.13 fold of control value; P<0.05).
Activation of NF-κB by LPS involves PMN activation
Anti-PMN antiserum partially but significantly decreases LPS-induced NF-κB activation in the intestine (Figure 5). This is in contrast to the complete inhibition of PAF-induced NF-κB activation in rat ileum by PMN depletion, as previously reported (De Plaen et al., 1998). Supershift experiments show that both p50 and p65 are found as part of the NF-κB complex in PMN-depleted animals injected with LPS (data not shown).
Figure 5.
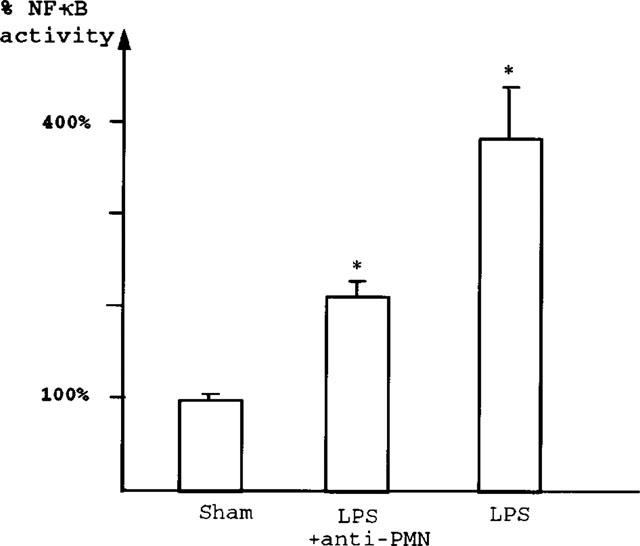
Neutrophil-depletion reduces LPS-induced intestinal NF-κB activation NF-κB-DNA binding activity measured by densitometry: Results of the LPS and anti-PMN+LPS groups are expressed as percentage of sham value. Pretreatment with anti-PMN antibodies significantly reduces (by 44%) the 3.8 fold increase of NF-κB binding activity induced by LPS (2.43±0.13 fold of control value) (P<0.05).
NF-κB p65 is localized in some epithelial cells as well as in lamina propria cells in small intestine
As shown in Figure 6A,B, there is a weak staining of NF-κB p65, localized mainly within the cytoplasm of some epithelial cells in both villi and crypts, as well as in some lamina propria cells. Two hours after LPS challenge, there is a strong staining of the nuclei of these cells, suggesting nuclear translocation and activation of NF-κB p65 (Figure 6C,D). The negative control (primary antibody replaced by an isotypic control antibody) shows no staining for NF-κB p65 (Figure 6E,F).
Figure 6.
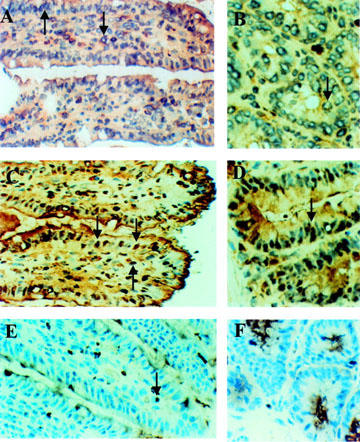
Immunohistochemical localization of NF-κB p65 subunit. NF-κB p65 is localized in the cytoplasm in some epithelial cells in the villi (A), and crypts (B), as well as in some lamina propria cells (A, B) in sham operated group. This is indicated by the perinuclear staining (arrows) of these cells. Two hours after LPS challenge, NF-κB p65 is translocated to the nuclei of these cells in villi (C) and crypts (D). This is indicated by the homogenous nuclear staining (arrows). No NF-κB staining is seen in the negative control (same ABC staining with isotypic primary antibody). Note cytoplasmic staining due to endogenous peroxidase activity (arrow) (E,F).
Physiological changes and intestinal injury induced by LPS
PAF induces an immediate hypotension which, in most animals, returned to baseline level after 60 min (De Plaen et al., 1998). LPS induces a more sustained, late onset hypotension, which begins around 45 min after the injection (Figure 7). Hypotension is improved by pretreatment with WEB2170, a PAF receptor antagonist, but not by PMN-depletion (Figure 7). Anti-TNF antibody seems to ameliorate the early phase of hypotension caused by LPS, but blood pressure still drops after 1 h, and the end blood pressure is not different from rats receiving LPS alone. LPS induced a 21.8±5.4% (P<0.05) (mean±s.e.mean) increase in hematocrit compared to baseline value (38±0.4). This increment was reduced to 11.7±3.4% (ns), if rats were pretreated with WEB2170; to 8±1% (P<0.05), if rats were pretreated with anti-TNF antibody; and to 8±4.6% (ns), if rats were made neutropenic by anti-PMN antiserum. LPS injection caused a drop in peripheral leukocyte count to 69±7.5% of baseline value (7140±516 in 1 mm3 blood, mean±s.e.mean), which was not significantly affected by pretreatment with WEB2170, anti-TNF antibody, or anti-PMN antiserum.
Figure 7.
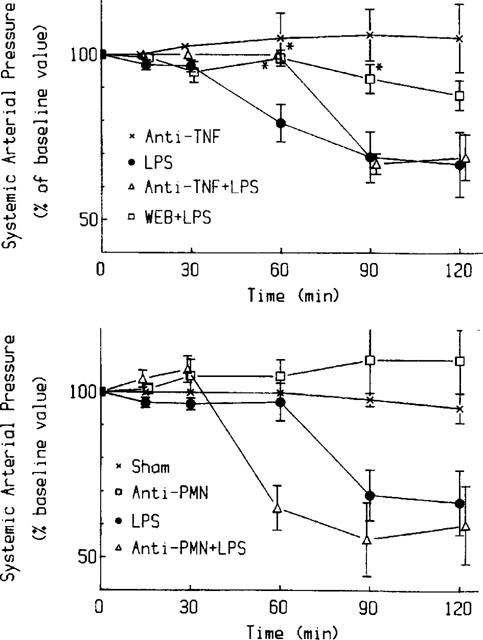
Effects of WEB2170, anti-PMN anti-serum, and anti-TNF antibody on LPS-induced systemic hypotension. Baseline mean blood pressure (100%) was 93±2.6 mmHg. Upper panel: LPS-induced hypotension (_n_=10) is reduced by WEB2170 (_n_=4). Anti-TNF antibody ameliorates only the early phase of the hypotension caused by LPS (_n_=3). Lower panel: Pretreatment with anti-PMN antiserum (_n_=3) did not improve the hypotension induced by LPS. * indicates statistically significant changes (P<0.05).
Discussion
NF-κB is composed of homo- or heterodimers of the subunits p50, p65, p52, cRel and RelB (Baeuerle & Henkel, 1994). Only p65, RelB and cRel have a transactivation domain and are thought to stimulate transcription (Baeuerle & Henkel, 1994; Miyamoto & Verma, 1995; Kopp & Ghosh, 1995). p50-p65 is the most frequently described form of NF-κB involved in the inflammatory response (Baeuerle & Henkel, 1994; Miyamoto & Verma, 1995; Kopp & Ghosh, 1995) and is activated in inflammatory processes (Barnes & Karin, 1997). Its role in septic shock and inflammatory diseases in vivo has been demonstrated in several recent studies: (a) in mice with colitis, intestinal inflammation was reduced after treatment with antisense phosphorothioate oligonucleotides to the p65 subunit (Neurath et al., 1996); (b) in a mouse model of endotoxaemia, in vivo transfer with an expression plasmid coding for IκB (a physiological inhibitor of NF-κB activation) increased survival (Bohrer et al., 1997); (c) NF-κB p50-p65 has been found to be activated in epithelial cells of inflamed intestinal mucosa of patients with inflammatory bowel disease (Rogler et al., 1998); and (d) NF-κB activation is associated with decreased survival in patients with sepsis (Bohrer et al., 1997).
Activation of p50-p65 by LPS has been shown in naive human monocyte cell lines (Ziegler-Heitbrock et al., 1994; de Wit et al., 1996) and in rat lung tissue (Blackwell et al., 1996). After LPS stimulation, p50-cRel has been identified in addition to p50-p65 in human monocytic THP-1 cells (Cordle et al., 1993), in rat liver (Essani et al., 1996) and in mouse macrophages (Ding et al., 1995).
Very few studies have been done on NF-κB in the intestine, a major immune organ of the body. We previously demonstrated that a low level of NF-κB (p50-p50) activity is constitutively present in the intestine and that PAF induces translocation of p50 into the nucleus of epithelial cells and of some cells in the lamina propria (De Plaen et al., 1998). In this study, we investigated LPS effect on NF-κB activation in the intestine. The intestine is constantly exposed to bacteria and their products, such as LPS, which may be crucial in eliciting an inflammatory response in the intestine. This is supported by the observation that colitis which develops in gene-targeted mice (Podolsky, 1997), and PAF-induced ischaemic necrosis failed to develop in germ-free animals (Sun et al., 1995). It has been previously shown that enteropathogenic E. coli, but not nonpathogenic E. coli, activates NF-κB in T84 cell line (a human intestinal cell line), mainly as p50-p50 homodimers (Savkovic et al., 1997). Interestingly, LPS was ineffective when directly added to these cells in vitro (Savkovic et al., 1997). In contrast, our present study demonstrates that in vivo administration of LPS activates NF-κB; its dimers are composed of both p50-p50 and p50-p65. The observations reported here underscore that the in vitro and in vivo effects of LPS on intestinal NF-κB activation differ markedly. This difference may be accounted for by the observed dependency of the in vivo effect of LPS on the production of secondary mediators, e.g., endogenous PAF and TNF, by inflammatory cells, as well as in vivo PMN activation.
It has been suggested that PAF is an important mediator of endotoxin shock (Benveniste, 1988) and necrotizing enterocolitis (Hsueh et al., 1998). Systemic injection of PAF causes shock and intestinal injury (Gonzalez-Crussi & Hsueh, 1983) and LPS-induced bowel injury can be prevented by pretreatment with PAF receptor antagonist (Hsueh et al., 1987), suggesting that endogenously produced PAF mediates LPS-induced bowel injury. The present study indicates that the LPS effect on NF-κB activation in the intestine is also partly mediated by PAF. Another central mediator of endotoxin shock is TNF (Tracey et al., 1987) which is known to activate p50-p65 and p50-c-Rel in vitro (Beg et al., 1994). Our observation suggests that part of the LPS effect on intestinal NF-κB activation is mediated via endogenous TNF production, since it is reduced by anti-TNF antibody. It is possible that the TNF involvement could be underestimated due to suboptimal delivery of the antiserum to the intestine (a rich source of endogenous TNF (Huang et al., 1994). Pretreatment of rats with anti-PMN antiserum decreases the NF-κB activation in intestinal nuclear extracts after LPS injection, while it completely inhibits NF-κB activation after PAF injection (De Plaen et al., 1998). How neutrophils are involved in intestinal NF-κB activation is not clear.
NF-κB activation by LPS, slower than PAF, peaks at 2 h after injection. This slowness may be due to the dependency of LPS on the production of endogenous secondary mediators such as PAF and TNF. The timecourse of LPS-induced NFκB activation in the intestine is similar to that previously reported in rat lungs (Blackwell et al., 1996); it seems to follow the development of hypotension in response to LPS. However, hypotension by itself does not cause activation of NF-κB, since PMN-depleted animals treated with either LPS or PAF display the same level of hypotension as non-PMN-depleted rats but with no (PAF-treated animals) or decreased (LPS-treated animals) NF-κB activation.
We have previously shown that PAF, at a dose (1.5 μg kg−1) below that causing prolonged systemic hypotension and gross intestinal injury, activates NF-κB in the rat ileum within 30 min, mainly as p50 homodimers (De Plaen et al., 1998). In the present study, we used a higher dosage of PAF in order to compare with LPS which has a prolonged time course. At this higher dose, mild intestinal injury often developed. However, the outcome of intestinal NF-κB was the same as that from low dose PAF: only p50-p50 homodimers were activated; p50-p65 heterodimers were either barely detectable or absent. These observations suggest that the activation of p50-p65 is not a prerequisite for PAF-induced bowel injury. P50-p50 is also the main complex of unstimulated monocytes (de Wit et al., 1996) and unstimulated rat intestine (De Plaen et al., 1998). In a human monocytic cell line made tolerant by pretreatment with LPS (Ziegler-Heitbrock et al., 1996), LPS activates p50-p50 predominantly. Most genes are probably regulated by the concurrent effect of several kinds of transcription factors interacting in a very complex manner (Ray & Ray, 1995; Smith et al., 1994).
Another interesting finding of this study is the observed NF-κB activation in the intestinal epithelium, in addition to some immune cells in the lamina propria. Intestinal epithelial cells are the first line of natural defense against bacterial infection. Previous publications have shown that gut epithelium is capable of synthesizing various cytokines and chemokines such as TNF, IL-8, MCP-1, GM-CSF (Jung et al., 1995) and IL-6 (McGee et al., 1995). The presence of this key immunoregulatory transcription factor in the epithelial cells supports an important role of these cells in immune response.
This study emphasizes the very different actions of LPS and of PAF on NF-κB activation in the intestine. Further, we showed that the effect of LPS is probably mediated, at least in part, by endogenous PAF and TNF, and is largely dependent on PMN activation. This dependence on secondary mediators and neutrophil activation may explain the discrepant results of in vivo experiments and in vitro studies.
Acknowledgments
This work was supported by NIH grants DK34574 and HD31840.
Abbreviations
EMSA
electrophoretic mobility shift assay
IκB
inhibitor protein κB
LPS
lipopolysaccharide
NF-κB
Nuclear factor κB
PAF
platelet-activating factor
PMN
polymorphonuclear leukocytes
ROS
reactive oxygen species
TNF
tumour necrosis factor-α
References
- BAEUERLE P.A., HENKEL T. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- BARNES P.J., KARIN M. Nuclear factor-kappa B: a pivotal transcription factor in chronic inflammatory diseases. New. Engl. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- BEG A.A., BALDWIN A.S.. , Jr Activation of multiple NF-kappa B/Rel DNA-binding complexes by tumour necrosis factor. Oncogene. 1994;9:1487–1492. [PubMed] [Google Scholar]
- BENVENISTE J. PAF-acether, an ether phospholipid with biological activity. Prog. Clin. Biol. Res. 1988;282:73–85. [PubMed] [Google Scholar]
- BLACKWELL T.S., BLACKWELL T.R., HOLDEN E.P., CHRISTMAN B.W., CHRISTMAN J.W. In vivo antioxidant treatment suppresses nuclear factor-κB activation and neutrophilic lung inflammation. J. Immunol. 1996;157:1630–1637. [PubMed] [Google Scholar]
- BOHRER H., QIU F., ZIMMERMANN T., ZHANG Y., JLLMER T, MANNEL D., BOTTIGER B.W., STERN D.M., WALDHERR R., SAEGER H.D., ZIEGLER R., BIERHAUS A., MARTIN E., NAWROTH P.P. Role of NFkappaB in the mortality of sepsis. J. Clin. Invest. 1997;100:972–985. doi: 10.1172/JCI119648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- CORDLE S., DONALD R., READ M.A., HAWIGER J. Lipopolysaccharide induces phosphorylation of MAD3 and activation of c-Rel and related NFκB proteins in human monocytic THP-1 cells. J. Biol. Chem. 1993;268:11803–11810. [PubMed] [Google Scholar]
- COUTURIER C., JAHNS G., KAZATCHKINE M.D., HAEFFNER-CAVAILLON N. Membrane molecules which trigger the production of interleukin-1 and tumor necrosis factor-alpha by lipopolysaccharide-stimulated human monocytes. Eur. J. Immunol. 1992;22:1461–1466. doi: 10.1002/eji.1830220619. [DOI] [PubMed] [Google Scholar]
- DE PLAEN I.G., TAN X.D., CHANG H., QU X.-W., LIU Q., HSUEH W. Intestinal NF-κB is activated, mainly as p50 homodimers, by platelet-activating factor. Biochim. Biophys. Acta. 1998;1392:185–192. doi: 10.1016/s0005-2760(98)00024-1. [DOI] [PubMed] [Google Scholar]
- DERYCKERE F., GANNON F. A one-hour minipreparation technique for extraction of DNA-binding proteins from animal tissues. Biotechniques. 1994;16:405. [PubMed] [Google Scholar]
- DE WIT H., HOOGSTRATEN D., HALIE R.M., VELLENGA E. Interferon-γ modulates the lipopolysaccharide-induced expression of AP-1 and NF-κB at the mRNA and protein level in human monocytes. Exp. Hematol. 1996;24:228–235. [PubMed] [Google Scholar]
- DING A., HWANG S., LANDER H.M., XIE Q.-W. Macrophages derived from C3H/HeJ (Lpsd) mice respond to bacterial lipopolysaccharide by activating NF-κB. J. Leukocyte Biol. 1995;57:174–179. doi: 10.1002/jlb.57.1.174. [DOI] [PubMed] [Google Scholar]
- ESSANI N.A., MCGUIRE G.M., MANNING A.M., JAESCHKE H. Endotoxin-induced activation of the nuclear transcription factor kappa B and expression of E-selectin messenger RNA in hepatocytes, Kupffer cells, and endothelial cells in vivo. J. Immunol. 1996;156:2956–2963. [PubMed] [Google Scholar]
- GENG Y., ZHANG B., LOTZ M. Protein tyrosine kinase activation is required for lipopolysaccharide induction of cytokines in human blood monocytes. J. Immunol. 1993;151:6692–6700. [PubMed] [Google Scholar]
- GONZALEZ-CRUSSI F., HSUEH W. Experimental model of ischemic bowel necrosis: The role of platelet-activating factor and endotoxin. Am. J. Pathol. 1983;112:127–135. [PMC free article] [PubMed] [Google Scholar]
- HANDLEY D.A.Platelet-activating factor as a mediator of endotoxin-related diseases Platelet-activating factor in Endotoxin and Immune Diseases 1990New York: Marcel Dekker; 451–496.In Handley, D.A., Saunders, R.N., Houlihan, W.J. & Tomasch J.C. eds [Google Scholar]
- HENNINGER D.D., PANES J., EPPIHIMER M., RUSSELL J., GERRITSEN M., ANDERSON D.C., GRANGER D.N. Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J. Immunol. 1997;158:1825–1832. [PubMed] [Google Scholar]
- HEUMANN D., GALLAY P., BARRAS C., ZAECH P., ULEVITCH R.J., TOBIAS P.S., GLAUSER M.P., BAUMGARTNER J.D. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J. Immunol. 1992;148:3505–3512. [PubMed] [Google Scholar]
- HSUEH W., CAPLAN M.S., TAN X., MACKENDRICK W., GONZALEZ-CRUSSI F. Necrotizing enterocolitis of the newborn: Pathogenetic concepts in perspective. Ped. Dev.Pathol. 1998;1:2–16. doi: 10.1007/s100249900002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSUEH W., GONZALEZ-CRUSSI F., ARROYAVE J. Platelet-activating factor: an endogenous mediator for bowel necrosis in endotoxemia. FASEB J. 1987;1:403–405. doi: 10.1096/fasebj.1.5.3678700. [DOI] [PubMed] [Google Scholar]
- HUANG L., TAN X., CRAWFORD S.E., HSUEH W. Platelet-activating factor and endotoxin induce tumour necrosis factor gene expression in rat intestine and liver. Immunology. 1994;83:65–69. [PMC free article] [PubMed] [Google Scholar]
- JUNG H.C., ECKMANN L., YANG S.K., PANJA A., FIERER J., MORZYCKA-WROBLEWSKA E., KAGNOFF M.F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOPP E.B., GHOSH S. NF-kappa B and rel proteins in innate immunity. Adv. Immunol. 1995;58:1–27. doi: 10.1016/s0065-2776(08)60618-5. [DOI] [PubMed] [Google Scholar]
- KUBES P., IBBOTSON G., RUSSELL J., WALLACE J.L., GRANGER D.N. Role of platelet-activating factor in ischemia/reperfusion-induced leukocyte adherence. Am. J. Physiol. 1990;259:300–305. doi: 10.1152/ajpgi.1990.259.2.G300. [DOI] [PubMed] [Google Scholar]
- LIBERMANN T.A., BALTIMORE D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell. Biol. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGEE D.W., BAMBERG T., VITKUS S.J., MCGHEE J.R. A synergistic relationship between TNF-alpha, IL-1 beta, and TGF-beta 1 on IL-6 secretion by the IEC-6 intestinal epithelial cell line. Immunology. 1995;86:6–11. [PMC free article] [PubMed] [Google Scholar]
- MIYAMOTO S., VERMA I.M. Rel/NFkappa B/I kappa B story. Adv. Cancer Res. 1995;66:255–292. [PubMed] [Google Scholar]
- NEURATH M.F., PETTERSSON S., MEYER K.-H., BUSCHENFELDE Z., STROBER W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-κB abrogates established experimental colitis in mice. Nature Medicine. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- PODOLSKY D.K. Lessons from genetic model of inflammation bowel disease. Acta Gastrol. Belg. 1997;60:163–165. [PubMed] [Google Scholar]
- RAY A., RAY B.K. Lipopolysaccharide-mediated induction of the bovine interleukin-6 gene in monocytes requires both NF-kappa B and C/EBP binding sites. DNA Cell Biol. 1995;14:795–802. doi: 10.1089/dna.1995.14.795. [DOI] [PubMed] [Google Scholar]
- ROGLER G., BRAND K., VOGL D., PAGE S., HOFMEISTER R., ANDUS T., KNUECHEL R., BAEUERLE P.A., SCHOLMERICH J., GROSS V. Nuclear Factor-κB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- SANCEAU J., KAISHO T., HIRANO T., WIETZERBIN J. Triggering of the human interleukin-6 gene by interferon-gamma and tumor necrosis factor-alpha in monocytic cells involves cooperation between interferon regulatory factor-1, NF kappa B, and Sp1 transcription factors. J. Biol. Chem. 1995;270:27920–27931. doi: 10.1074/jbc.270.46.27920. [DOI] [PubMed] [Google Scholar]
- SAVKOVIC S., LOUTSOURIS A., HECHT G. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia Coli. Am. J. Physiol. 1997;273:1160–1167. doi: 10.1152/ajpcell.1997.273.4.C1160. [DOI] [PubMed] [Google Scholar]
- SCHUTZE S., WIEGMANN K., MACHLEIDT T., KRONKE M. TNF-induced activation of NF-κB. Immunobiol. 1995;193:193–203. doi: 10.1016/s0171-2985(11)80543-7. [DOI] [PubMed] [Google Scholar]
- SHAKHOV A.N., COLLART M.A., VASSALLI P., NEDOSPASOV S.A., JONGENEEL C.V. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J. Exp. Med. 1990;171:35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH M.F., Jr, EIDLEN D., AREND W.D., GUTIERREZ-HARTMANN A. LPS-induced expression of the human IL-1 receptor antagonist gene is controlled by multiple interacting promoter elements. J. Immunol. 1994;153:3584–3593. [PubMed] [Google Scholar]
- SUN S.-C., GANCHI P.A., BALLARD D.W., GREENE W.C. NF-κB controls expression of inhibitor IκB-alpha: Evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- SUN X., MACKENDRICK W., TIEN J., HUANG W., CAPLAN M., HSUEH W. Endogenous bacterial toxins are required for the injurious action of platelet-activating factor in rats. Gastroenterology. 1995;109:83–88. doi: 10.1016/0016-5085(95)90271-6. [DOI] [PubMed] [Google Scholar]
- SUN X.M., QU X.-W., HUANG W., GRANGER D.N., BREE M., HSUEH W. The role of leukocyte beta 2-integrin in PAF-induced shock and intestinal injury. Am. J. Physiol. 1996;270:184–190. doi: 10.1152/ajpgi.1996.270.1.G184. [DOI] [PubMed] [Google Scholar]
- TAN X., SUN X., GONZALEZ-CRUSSI F.X., GONZALEZ-CRUSSI F., HSUEH W. PAF and TNF increase the precursor of NF-kappa B p50 mRNA in mouse intestine: quantitative analysis by competitive PCR. Biochim. Biophys. Acta. 1994;1215:157–162. doi: 10.1016/0005-2760(94)90105-8. [DOI] [PubMed] [Google Scholar]
- TAN X.D., WANG H., GONZALEZ-CRUSSI F.X., CHANG H., GONZALEZ-CRUSSI F., HSUEH W. Platelet-activating factor and endotoxin increase the enzyme activity and gene expression of type II phospholipase A2 in the rat intestine. Role of polymorphonuclear leukocytes. J. Immunol. 1996;156:2985–2990. [PubMed] [Google Scholar]
- TOTH P.D.The biological effects of PAF antagonists on endotoxemia Platelet-activating Factor in Endotoxin and Immune Diseases 1990New York: Marcel Dekker; 589–608.In: Handley, D.A., Saunders, R.N., Houlihan, W.J. & Tomesch, J.C. eds [Google Scholar]
- TRACEY K.J., BEUTLER B., LOWRY S.F., MERRYWAETHER J., WOLPE S., MILSARK I.W., HARIRI R.J., FAHEY T.J.3D, , ZENELLA A., ALBERT J.D., SHIRES G.T., CERAMI A. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- TRACEY K.J., FONG Y., HESSE D.G., MANOGUE K.R., LEE A.T., JUO G.C., LOWRY S.F., CERAMI A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- ZIEGLER-HEITBROCK H.W., WEDEL A., SCHRAUT W., STROBEL M., WENDELGASS P., STERNSDORF T., BAEUERLE P.A., HAAS J.G., RIETHMULLER G. Tolerance to lipopolysaccharide involves mobilization of nuclear factor kappa B with predominance of p50 homodimers. J. Biol. Chem. 1994;269:17001–17004. [PubMed] [Google Scholar]