Skeletal Muscle-Selective Knockout of LKB1 Increases Insulin Sensitivity, Improves Glucose Homeostasis, and Decreases TRB3 (original) (raw)
Abstract
LKB1 is a tumor suppressor that may also be fundamental to cell metabolism, since LKB1 phosphorylates and activates the energy sensing enzyme AMPK. We generated muscle-specific LKB1 knockout (MLKB1KO) mice, and surprisingly, found that a lack of LKB1 in skeletal muscle enhanced insulin sensitivity, as evidenced by decreased fasting glucose and insulin concentrations, improved glucose tolerance, increased muscle glucose uptake in vivo, and increased glucose utilization during a hyperinsulinemic-euglycemic clamp. MLKB1KO mice had increased insulin-stimulated Akt phosphorylation and a >80% decrease in muscle expression of TRB3, a recently identified Akt inhibitor. Akt/TRB3 binding was present in skeletal muscle, and overexpression of TRB3 in C2C12 myoblasts significantly reduced Akt phosphorylation. These results demonstrate that skeletal muscle LKB1 is a negative regulator of insulin sensitivity and glucose homeostasis. LKB1-mediated TRB3 expression provides a novel link between LKB1 and Akt, critical kinases involved in both tumor genesis and cell metabolism.
LKB1 is a serine/threonine kinase that links a diverse array of cellular processes, including cancer, cellular polarity, and metabolism. Originally identified as the tumor suppressor protein mutated in Peutz-Jeghers syndrome (17, 21), LKB1 has since been shown to regulate polarity in a number of systems, including Caenorhabditis elegans (48), Drosophila melanogaster (28), Xenopus (33), and mammalian cells (3). Biochemically, LBK1 can phosphorylate and activate at least 13 members of the AMP-activated protein kinase (AMPK) subfamily of protein kinases (20, 27) when associated with two regulatory proteins essential for catalytic activity, STE20-related adapter protein and mouse protein 25 (MO25) (15). AMPK, the most studied of LKB1's downstream substrates, is a conserved serine/threonine kinase that functions in the regulation of energy metabolism (16, 18, 29). Studies with cell culture (15, 40, 51) or conditional knockouts of LKB1 in skeletal muscle (38), cardiac muscle (39), or liver (41) have found that LKB1 regulates AMPK activity both in vitro and in vivo. Other than AMPK and the microtubule affinity-regulating kinase (MARK) proteins, which have been implicated in the control of cellular polarity (9), relatively little is known about the function of the AMPK-related kinases. The well-established role of AMPK in metabolism, however, directly implicates LKB1 in the maintenance of energy balance.
The protein kinase Akt functions both in the control of cell proliferation and as a critical node in insulin signaling, appearing to mediate most of the metabolic effects of insulin (44). Akt is activated by phosphorylation of Thr308 within the T loop of the catalytic domain and Ser473, located in a C-terminal, noncatalytic region of the enzyme (1). A mammalian homolog of D. melanogaster tribbles, TRB3, was recently identified as a negative regulator of Akt activity in human embryonic kidney 293 (HEK293) cells and mouse liver (10). In HEK293 cells and liver, TRB3 binds Akt and inhibits insulin-stimulated Akt phosphorylation on Thr308 and Ser473. However, the function of TRB3 on Akt activity in skeletal muscle has not been studied. While the regulation of TRB3 is not fully understood, a recent study found that PGC1α induces the expression of TRB3 in a PPARα-dependent manner in the liver (25).
In this study, we have examined the role of LKB1 in skeletal muscle by creating skeletal muscle-specific LKB1 knockout mice. Mice lacking LKB1 in skeletal muscle (MLKB1KO) showed lower fasting blood glucose and serum insulin levels, improved glucose tolerance, and enhanced insulin-stimulated glucose uptake and insulin signaling. In exploring the mechanisms underlying the observed enhancement of insulin signaling, we found that PGC1α, PPARα, and TRB3 were significantly decreased in muscle from MLKB1KO mice. Furthermore, overexpression of TRB3 in C2C12 myoblasts resulted in blunted insulin-stimulated Akt phosphorylation, establishing that TRB3 inhibits insulin-stimulated Akt phosphorylation in skeletal muscle cells. This unexpected role for skeletal muscle LKB1 in the negative regulation of glucose homeostasis contrasts with the positive function of LKB1 in the regulation of glucose homeostasis in the liver (41) and points to tissue-specific roles for LKB1.
MATERIALS AND METHODS
Animals.
Mice carrying a floxed LKB1 gene on the FVB background (4) were crossed with MCK-Cre transgenic mice on the FVB background (6). The floxed LKB1 and MCK-Cre genotypes were determined by PCR analysis of genomic DNA extracted from the tail (4, 6). Ten- to twelve-week-old mice were used for all experiments. Males were used for glucose uptake and transport studies, whereas males and females were used for all other experiments with no differences between sexes. Experiments were in accordance with NIH guidelines and approved by the Joslin Institutional Animal Care and Use Committee.
Analytical procedures.
Mice were killed via decapitation and exsanguinated. Serum free fatty acid, insulin, and adiponectin were measured with a NEFA-C kit (Wako), radioimmunoassay (Linco), and enzyme-linked immunosorbent assay (Linco), respectively. Triglycerides, total cholesterol, and glycogen were determined using kit assays (Sigma).
Western blot analysis and in vitro kinase assays.
Following sacrifice, tissues were rapidly dissected, frozen in liquid nitrogen, and homogenized as previously described (12). Antibody for TRB3 was raised against the human TRB3 antigenic region 27-40: TERPVQKRARSGPQ. Antibodies purchased from commercial sources included those for LKB1 and PPARα (Santa Cruz); GLUT1 and GLUT4 (Chemicon); AMPKα1, AMPKβ2, acetyl-coenzyme A carboxylase (ACC), MARK2/3, Akt, and P-ACC-Ser79 (Upstate); insulin receptor β subunit, MARK4, P-Akt-Thr308, P-Akt-Ser437, and P-AMPK-Thr172 (Cell Signaling); and PGC1α (Calbiochem); horseradish peroxidase (HRP)-conjugated antirabbit (Amersham); HRP-conjugated antimouse (Upstate); and HRP-conjugated antigoat (Promega). Antibodies to AMPKα2, γ3, and pan-α were generated in our laboratory (31, 53). Isoform-specific AMPK (31), MARK2/3, MARK4 (36), and Akt (37) activities were determined as described previously. We were unable to measure QIK and QSK activities due to an inability to obtain suitable antibodies.
In vitro muscle incubations.
Mice were sacrificed, and soleus and exterior digitorum longus (EDL) muscles were dissected, mounted, and incubated in Krebs-Ringer bicarbonate buffer (KRB), pH 7.4, containing 2 mM pyruvate for 20 min. Following preincubation, muscles were incubated in KRB buffer in the presence or absence of 2 mM 5-aminoimidazole-4-carboxamide-1-β-d-ribofuxonoside (AICAR) for 20 min or contracted for 10 min, and glucose transport activity was measured (16).
Recombinant adenoviruses and infection in C2C12 cells.
Adenoviral vectors containing green fluorescent protein (GFP) or human TRB3 were cloned into the pCMV6-XL5 vector. An adenovirus expressing GFP was used to monitor viral infection efficiency and was used as a control. After 2 days of differentiation, C2C12 cells were infected by adenoviruses with 1.25 × 107 PFU/9.7 cm2 well.
Metabolic parameters.
Fasted (14 h) or fed mice were subjected to a glucose tolerance test (GTT) and AICAR tolerance test, respectively. Mice received glucose (2 g/kg of body weight) or AICAR (0.5 g/kg) by intraperitoneal injection. Blood was collected from the tail, and the glucose concentration was determined with a One Touch II glucose monitor (Lifescan). Muscle glucose uptake in vivo was determined as described previously (47). Blood samples (25 μl) for the measurement of glucose and specific activities were obtained from the tail vein at 0, 20, 40, 60, 90, and 120 min. Accumulation of 3H-2-deoxyglucose-6-P in muscle was measured to determine glucose uptake (11). To determine d-[14C(U)]glucose incorporation into glycogen, an aliquot of the muscle digestate was processed as described previously (8). To determine basal and insulin-stimulated rates of glucose disposal, 5-mU/kg/min hyperinsulinemic-euglycemic clamps were performed as described previously (32).
Statistical analysis.
Data are means ± standard errors of the means (SEM) All data were compared using Student's t test or one-way ANOVA. The differences between groups were considered significant when P < 0.05.
RESULTS
Generation of MLKB1KO mice.
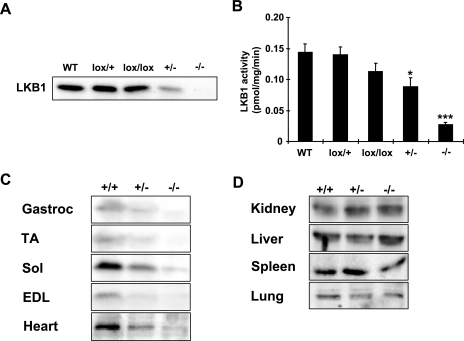
Muscle-specific inactivation of LKB1 was achieved by breeding mice carrying an LKB1 allele in which exons 2 to 6 were flanked by loxP sequences (4) with MCK-Cre transgenic mice (6). While whole-body LKB1 knockout mice are embryonic lethal (52), MLKB1KO mice were fertile and were born in the expected Mendelian ratio. Throughout our characterization, we did not observe any significant phenotypic or metabolic differences between wild-type mice and mice harboring the MCK-Cre transgene or mice homo- or heterozygous for the LKB1-lox allele, including LKB1 protein levels and kinase activity (Fig. 1A and B).
FIG. 1.
High-efficiency ablation of the LKB1 protein selectively in skeletal muscle. (A) LKB1 expression was not significantly altered for wild-type (WT) mice compared to that for mice harboring one (lox/+) or two (lox/lox) alleles in gastrocnemius muscle lysates. Heterozygotes (+/−) showed approximately 50% of control LKB1 expression, while MLKB1KO (−/−) mice showed nearly complete loss of LKB1 expression. (B) LKB1 activity was assessed in an in vitro kinase activity assay using LKB1tide as the substrate. Consistent with protein data, +/− and −/− animals showed reduced LKB1 activity compared to controls. (C and D) Muscles from MLKB1KO animals (−/−) show nearly complete depletion of the LKB1 protein (C), while expression is not affected in nonmuscle tissue (D). Gastroc, gastrocnemius; TA, tibialis anterior; Sol, soleus. Data are means ± SEM; n = 3 to 6/group. *, P < 0.05; ***, P < 0.001 versus control results (+/+).
To validate muscle-specific knockout of LKB1, we immunoblotted lysates of multiple tissues from 10-week-old control mice, heterozygotes, and MLKB1KO mice. Compared with controls, LKB1 expression was reduced by 50% and 95% in multiple skeletal muscles from heterozygotes and MLKB1KO mice, respectively (Fig. 1C). LKB1 expression was also reduced by ∼70% in heart but unaltered in nonmuscle tissues (Fig. 1D), indicating that muscle-specific knockout of LKB1 was both successful and specific to muscle. Protein levels of MO25, an LKB1 regulatory protein, were not different among the genotypes (not shown).
Effects of LKB1 disruption on AMPK and MARK activities.
In skeletal muscle, both the α1 and α2 catalytic isoforms of AMPK are expressed, with α2 expression and activity considered predominant (42). For MLKB1KO mice, basal AMPKα2 but not α1 activity was reduced to extremely low levels in multiple skeletal muscle tissues (see Fig. S1A and B in the supplemental material). AICAR- and contraction-stimulated AMPKα2, but not α1, activity was fully blunted for the MLKB1KO mice and was not significantly decreased for the heterozygotes (see Fig. S1C to F in the supplemental material). AICAR and contraction increased AMPKα1 activity in all groups, but the increase in AMPKα1 activity in response to contraction was significantly lower for MLKB1KO mice (see Fig. S1F in the supplemental material). Alterations of AMPKα1 activity with contraction in soleus were similar to results in EDL muscle (not shown). Furthermore, for MLKB1KO mice, AMPK Thr172 phosphorylation was abolished in the basal state and with AICAR or contraction stimulation, with no significant change in heterozygotes (see Fig. S2A and C in the supplemental material). For MLKB1KO mice, AICAR and contraction increased Ser79 phosphorylation of the AMPK substrate ACC, but this level of phosphorylation was reduced by greater than 85% and 75%, respectively, compared to results with controls (see Fig. S2B and D in the supplemental material). These data indicate that LKB1 is the major upstream kinase for the AMPKα2 catalytic subunit under both basal and stimulated conditions and that disruption of LKB1 dramatically suppresses ACC phosphorylation.
We next immunoprecipitated AMPKα1 and measured Thr172 phosphorylation from contraction- and AICAR-stimulated muscle lysates. We detected Thr172 phosphorylation in the AMPKα1 immunoprecipitates, but levels in the MLKB1KO muscles were lower than those for controls in the basal, AICAR, and contraction-stimulated states (see Fig. S2E and F in the supplemental material), suggesting that AMPKα1 activity is maintained despite reductions in Thr172 phosphorylation.
Previous reports have shown that changes in AMPK activity can influence the relative expression of AMPK subunits (23). In gastrocnemius muscle from MLKB1KO mice, AMPKα1 expression was not significantly altered (see Fig. S3 in the supplemental material), consistent with unaltered basal AMPK α1 activity (see Fig. S1B in the supplemental material). However, the AMPKα2, -β2, and -γ3 proteins were significantly increased for MLKB1KO mice (see Fig. S3 in the supplemental material).
MARK proteins have been identified as members of the AMPK-related family of kinases and are substrates for LKB1 in cultured cell systems. MARK2/3 activity was not reduced for heterozygote or MLKB1KO mice (see Fig. S4A in the supplemental material). In contrast, MARK4 activity was significantly reduced by 50% for the MLKB1KO mice (see Fig. S4B in the supplemental material).
Physiological effects of muscle-specific LKB1 disruption.
Body weights for male MLKB1KO and heterozygote mice were not significantly different except at week 9, where weights of MLKB1KO and heterozygotes were lower than those of controls (Fig. 2A). Body weights of female mice were unaltered between MLKB1KO mice and heterozygotes (not shown). Weights of multiple nonskeletal muscle tissues were not different, and dual-energy X-ray absorbometry scans revealed no difference in body fat percentages and body lengths (not shown).
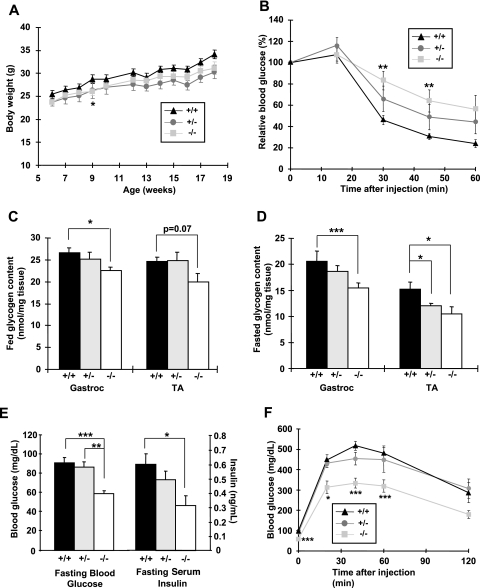
FIG. 2.
MLKB1KO mice are AICAR intolerant and have lower skeletal muscle glycogen content and improved whole-body glucose homeostasis. (A) Body weights of male mice (n = 5 to 10/group) were tracked for 12 weeks, beginning at 6 weeks of age. (B) Mice were injected i.p. with AICAR (0.5 g/kg), and blood from the tail vein was used to measure glucose. (C and D) Gastrocnemius (gastroc) and tibialis anterior (TA) muscles were dissected from fed (C) and 14-h-fasted (D) mice and assayed for glycogen. (E) Fasting blood glucose and serum insulin concentrations were lower in MLKB1KO. (F) Mice were injected i.p. with glucose (2 g/kg), and blood from the tail vein used to measure glucose. For panels B to F, data are means ± SEM; n = 5 to 12/group. , P < 0.05; **, P < 0.01; ***, P < 0.001 versus results with corresponding +/+ mice.
AICAR increases glucose transport in skeletal muscle, an effect that is mediated by the α2 AMPK catalytic isoform (23, 46). Since MLKB1KO mice have ablated AICAR-stimulated AMPKα2 activity (see Fig. S1C in the supplemental material), we determined if LKB1 is necessary for AICAR-stimulated whole-body glucose disposal in vivo. While AICAR significantly reduced blood glucose concentrations for controls and heterozygotes, the hypoglycemic effects of AICAR were blunted for MLKB1KO mice (Fig. 2B), demonstrating whole-body AICAR resistance.
Since LKB1 and AMPK have been implicated in the regulation of glycogen metabolism (2, 49), we next assessed glycogen concentrations in multiple skeletal muscles. Under fed conditions, glycogen concentrations in the gastrocnemius and tibialis anterior muscles were lower for MLKB1KO mice than for controls (Fig. 2C), and these effects were more pronounced with fasting (Fig. 2D). There was no difference in the liver glycogen concentration among the genotypes (not shown). Given the critical role that ACC plays in fatty acid oxidation and our findings of significantly decreased ACC phosphorylation in MLKB1KO muscle, we also assessed tissue triglyceride content. Intramuscular triglyceride content for MLKB1KO mice was higher than that for controls (13.8 ± 3.6 versus 6.1 ± 1.3 μg/mg tissue; n = 5; P < 0.05), while triglycerides in liver and adipose tissue were not altered (not shown). Expression of GLUT1 and GLUT4, the major glucose transporter isoforms expressed in skeletal muscle, was not altered (not shown).
In the fed or fasted state, serum concentrations of leptin, free fatty acids, adiponectin, triglycerides, and cholesterol were not significantly different among the genotypes (see Table S1 in the supplemental material). Fed-state serum glucose and insulin concentrations were also not altered for MLKB1KO mice or heterozygotes (not shown). However, under fasting conditions, both glucose and insulin concentrations were significantly decreased for MLKB1KO animals but not for heterozygotes (Fig. 2E). A standard GTT in the fasted state showed an accelerated glucose disposal for MLKB1KO mice, with no effect in heterozygotes (Fig. 2F). Thus, LKB1 depletion in skeletal muscle results in increased insulin sensitivity and improved control of glucose homeostasis.
Insulin-stimulated glucose uptake is enhanced in MLKB1KO mice.
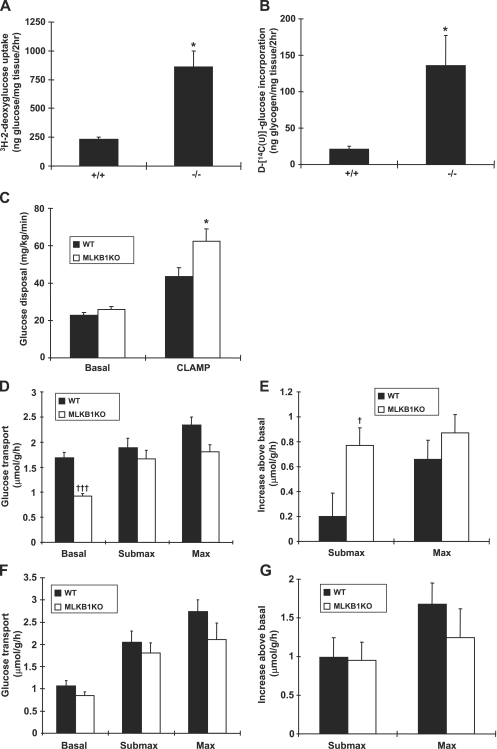
To determine if the improved glucose tolerance is due to increased glucose disposal in skeletal muscle, we measured skeletal muscle glucose uptake and glycogen incorporation in vivo 2 h following an intraperitoneal (i.p.) injection of radiolabeled glucose at the same dose administered during the GTT. Thirty minutes after glucose administration, MLKB1KO mice showed significantly lower serum insulin levels than control mice (1.31 ± 0.07 versus 1.59 ± 0.15 ng/ml; n = 6; P < 0.05). Consistent with the improved glucose tolerance, muscle glucose uptake was 2.7-fold higher for MLKB1KO mice (Fig. 3A), and incorporation of d-[14C(U)]glucose into glycogen was 4.6-fold higher for MLKB1KO mice (Fig. 3B). We also assessed whole-body glucose homeostasis by hyperinsulinemic-euglycemic clamp. Basal rates of glucose utilization for MLKB1KO mice were not significantly different from those for controls (25.7 ± 1.6 versus 22.6 ± 1.6 mg/kg/min for MKLB1KO and controls, respectively; n = 5 to 7). The rate of glucose utilization during physiological hyperinsulinemia was 43% higher for MLKB1KO mice than for controls (Fig. 3C). At this insulin infusion rate, hepatic glucose production was completely suppressed (not shown). Taken together, these data suggest that MLKB1KO mice have increased insulin sensitivity and improved glucose homeostasis, presumably by increased glucose uptake into muscle.
FIG. 3.
MLKB1KO mice have improved skeletal muscle glucose uptake in vivo and in vitro. (A and B) Mice were injected i.p. with glucose (2 g/kg) containing 3H-2-deoxyglucose and d-[14C(U)]glucose as radioactive tracers and followed for 2 h. Tibialis anterior muscles were processed and assayed for glucose uptake (A) and glycogen incorporation (B). (C) Glucose disposal rate before and during the hyperinsulinemic-euglycemic clamp (5 mU/kg/min). EDL (D and E) or soleus (F and G) muscles were dissected and incubated in the absence of insulin or with submaximal (450 μU/ml) (Submax) or maximal (50 mU/ml) (Max) insulin. (D) Basal glucose transport was decreased and insulin-stimulated glucose transport was normal in the EDL. (E) Expressed as an increase above the basal, level, submaximal insulin-stimulated transport was significantly greater in EDL muscle from MLKB1KO mice. (F and G) There were no differences between the genotypes in glucose transport in soleus. Data are means ± SEM; n = 6 to 12/group. *, P < 0.05 versus basal level of same genotype. †, P < 0.05; †††, P < 0.001 versus results with corresponding +/+ mice.
We next isolated EDL and soleus muscles and measured glucose transport in vitro. Basal rates of glucose transport for MLKB1KO mice were decreased by 45% in EDL muscles (Fig. 3D). Low basal rates of glucose transport may result from impaired hypoxia-stimulated glucose transport in the absence of AMPKα2 activity (30), since muscle incubation can cause a slight hypoxic stress. Insulin-stimulated glucose transport, in response to both submaximal and maximal insulin concentrations, was not significantly altered for MLKB1KO mice. Expressed as an increase above the basal level, increases in glucose transport for MLKB1KO mice were significantly greater with submaximal insulin (Fig. 3E). Glucose transport in isolated soleus muscles was not significantly decreased in the basal state, with normal increases in submaximal and maximally insulin-stimulated glucose transport (Fig. 3F and G).
Insulin-stimulated signaling in MLKB1KO mice.
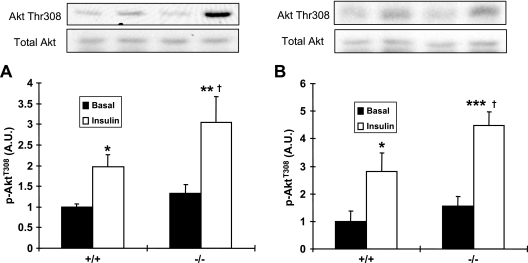
There is considerable evidence that Akt is necessary for insulin-stimulated glucose uptake (7, 14, 34). Therefore, we incubated muscles in the absence or presence of submaximal insulin to determine the effects of LKB1 knockout on phosphorylation of the regulatory Thr308 site of Akt. A submaximal dose was used to mimic the physiological changes in insulin that occur with glucose tolerance tests and measurement of glucose uptake and disposal in vivo. In EDL muscle, basal levels of Akt Thr308 phosphorylation were not significantly different. Insulin increased Akt Thr308 phosphorylation for both control and MLKB1KO mice, but the increase was significantly greater for the MLKB1KO mice (Fig. 4A). Similar results were observed in soleus muscle, with significantly greater increases in insulin-stimulated Akt Thr308 phosphorylation in MLKB1KO mice (Fig. 4B). These changes occurred in the absence of significant increases in the Akt protein. Akt phosphorylation was not altered in liver from MLKB1KO mice (not shown).
FIG. 4.
Submaximal insulin stimulation of Akt Thr308 phosphorylation. EDL and soleus muscles were dissected and incubated with submaximal insulin (450 μU/ml). (A and B) Insulin-stimulated Akt Thr308 phosphorylation in EDL and soleus muscle was greater in MLKB1KO mice than in controls. Data are means ± SEM; n = 5 to 6/group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus basal level of same genotype. †, P < 0.05 versus results with corresponding +/+ mice.
LKB1 regulates PGC1α, PPARα, and TRB3 expression.
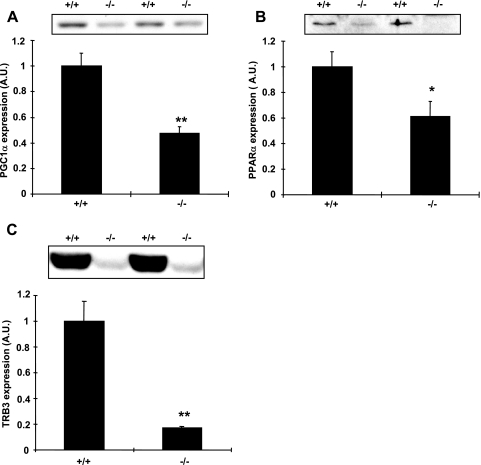
We next tested the hypothesis that loss of LKB1 in skeletal muscle would result in decreases in PGC1α and PPARα expression, which in turn could potentially mediate decreases in TRB3 expression. The PGC1α protein in tibialis anterior muscle from MLKB1KO mice was decreased by 53% compared to levels for controls (Fig. 5A), and PPARα was decreased by 39% (Fig. 5B). We next assessed the protein content of TRB3 for MLKB1KO mice and found that TRB3 expression was reduced by 83% compared to that for controls (Fig. 5C). Similar results were found in gastrocnemius muscle (not shown). TRB3 expression was not altered for heterozygotes compared to that for controls (not shown).
FIG. 5.
Protein expression of PGC1α, PPARα, and TRB3 is decreased in MLKB1KO mice. PGC1α (A), PPARα (B), or TRB3 (C) protein was assessed by immunoblotting with muscle lysates obtained from tibialis anterior muscle. There were significant decreases in PGC1α, PPARα, and TRB3 protein in muscles from MLKB1KO mice. Data are means ± SEM, and the blots shown are representative of six mice per group. *, P < 0.05; **, P < 0.001 versus results with control (+/+) mice; A.U., arbitary units.
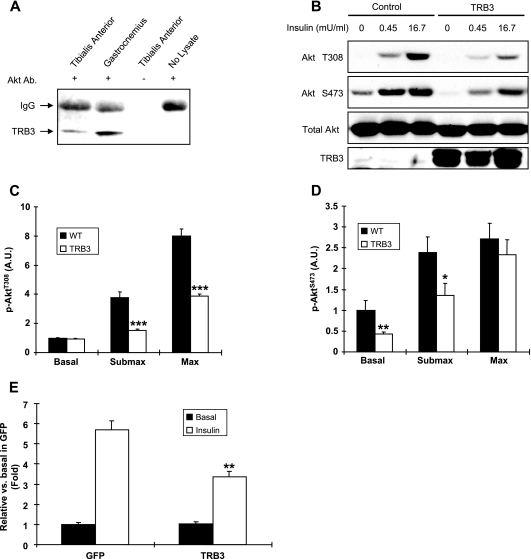
To determine if TRB3 acts as a negative regulator of Akt signaling in skeletal muscle, we first determined if TRB3 associates with Akt in skeletal muscle. Immunoprecipitation of lysates from gastrocnemius and tibialis anterior muscles from control mice with Akt antibody revealed a band of the appropriate molecular weight that was recognized by TRB3 immunoblotting (Fig. 6A). No TRB3 band was detected in immunoprecipitates prepared in the absence of muscle lysates or Akt antibody. To determine whether TRB3 modulates Akt activity in skeletal muscle, we measured Akt phosphorylation in response to insulin activation. Mouse myoblast C2C12 cells were infected with adenoviruses containing GFP or TRB3 and stimulated with insulin for 10 min. Treatment of C2C12 cells with insulin induced significantly less Akt phosphorylation at Thr308 in response to both submaximal and maximal insulin and at Ser473 in response to submaximal insulin in TRB3-infected cells compared to results with control cells infected with GFP (Fig. 6B to D). The effect of TRB3 on insulin-stimulated Akt signaling was dose dependent (see Fig. S5 in the supplemental material), and the effects of TRB3 on insulin-stimulated Akt phosphorylation were comparable with Akt activity (Fig. 6E). These data suggest that TRB3 directly associates with Akt and inhibits Akt phosphorylation in response to insulin in skeletal muscle and that reduced muscle TRB3 expression in MLKB1KO mice may underlie the observed enhancements in insulin sensitivity and increases in insulin-stimulated Akt phosphorylation.
FIG. 6.
TRB3 binds Akt and inhibits Akt phosphorylation in response to insulin. (A) Western blot analysis of TRB3 in immunoprecipitates prepared from gastrocnemius and tibialis anterior muscles in the absence or presence of Akt antibody. A band of the appropriate molecular weight for TRB3 was recovered from muscle lysates, but no band was detected in immunoprecipitates prepared in the absence of muscle lysates or Akt antibody. (B to D) Overexpression of TRB3 blunted insulin-stimulated Akt Thr308 (C) or Ser473 (D) phosphorylation without altering the Akt protein level in C2C12 cells. (E) Akt activity was assayed by in vitro immune complex assay as described in Materials and Methods. Data are means ± SEM, and the blots shown are representative of three per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus control results. Submax, submaximal insulin; Max, maximal insulin; A.U., arbitrary units.
DISCUSSION
LKB1 has been implicated in a diverse array of roles, many of which impact on human health and disease. In addition to functioning as a tumor suppressor and the causative agent of Peutz-Jeghers syndrome (21), for example, LKB1 has been shown to control glucose homeostasis in the liver (41). Since cancer and diabetes constitute the second and sixth leading causes of death among Americans (Health, United States, 2005; http://www.cdc.gov/nchs/hus.htm), continuing studies of the function of LKB1 are likely to provide novel insights into diseases that affect the health of many worldwide. In this study, we show that LKB1 plays a prominent role in the negative regulation of glucose homeostasis in skeletal muscle. Specifically, MLKB1KO mice displayed improved whole-body glucose homeostasis, enhanced insulin sensitivity in skeletal muscle, and increased insulin-stimulated Akt signaling in skeletal muscle. Interestingly, our preliminary studies show that the improved glucose tolerance is maintained in MLKB1KO mice following 5 weeks on a high-fat diet (N. Fujii, H. J. Koh, and L. J. Goodyear, unpublished observations).
Given the putative links between AMPK activation and improvements in insulin sensitivity (5, 19, 29), as well as the role of LKB1 as a positive regulator of glucose homeostasis in the liver (41), our finding of improved insulin sensitivity and glucose homeostasis in MLKB1KO mice is surprising. The improved whole-body glucose homeostasis is associated with increased skeletal muscle insulin-stimulated glucose uptake in vivo, and enhanced Akt phosphorylation could be an important mechanism for this effect (7, 14, 34). Given the role of Akt in cell proliferation and tumor genesis, increased signaling through Akt in the absence of LKB1 is consistent with the well-established role of LKB1 as a tumor suppressor.
We believe that the enhancements in Akt signaling and ultimately in whole-body glucose homeostasis in the MLBK1KO mice are due, at least in part, to downregulation of TRB3 expression in skeletal muscle. Consistent with this hypothesis is our finding that heterozygous mice, which do not have enhanced glucose tolerance or decreases in fasting blood glucose or insulin concentrations, also do not have decreases in muscle TRB3 expression. In recent studies, TRB3 has been found to inhibit Akt activation in liver by physically binding to Akt and masking the Thr308 activation site (10, 25). In this study, we show that TRB3 binds Akt in skeletal muscle and that increases in TRB3 expression in C2C12 cells result in decreased insulin-stimulated Akt phosphorylation. TRB3 thus appears to downregulate Akt signaling in skeletal muscle, as it does in liver. Since reduced TRB3 protein levels in both liver (10, 25) and skeletal muscle (current study) are associated with enhanced glucose tolerance in vivo, the development of TRB3 inhibitors could be extremely valuable in the treatment of type 2 diabetes.
An important implication of the current study is that LKB1 regulates TRB3 expression, providing a novel link between this tumor suppressor protein and a key signaling molecule mediating cell metabolism. In the liver, PGC1α and PPARα have been shown to regulate TRB3 expression (25). Here, our data suggest that LKB1 regulation of TRB3 expression occurs through the PGC1α/PPARα pathway in skeletal muscle. Previous reports on skeletal muscle have shown that increases in LKB1 protein with chronic exercise training (45) and activation of AMPK via AICAR (24, 26, 43) correlate with increases in PGC1α expression in skeletal muscle. Given that AMPKα2 is required for the AICAR effect in skeletal muscle (24), it seems plausible that the reductions in AMPKα2 activity in MLKB1KO muscle may partially mediate the decreases in PGC1α and PPARα expression. However, compared to MLKB1KO mice, transgenic mice expressing dominant-negative AMPKα2 (13) do not have decreases in PGC1α or PPARα and have only slight decreases in TRB3 (N. Fujii, H. J. Koh, and L. J. Goodyear, unpublished observations), suggesting that other LKB1-regulated kinases play a role in the regulation of these proteins in muscle.
While we believe that increased Akt signaling mediated by downregulation of TRB3 is likely the primary cause of the enhanced glucose tolerance and insulin signaling in the MLKB1KO mice, we cannot entirely rule out other possibilities that may contribute to the phenotype of these mice. One alternative potential mechanism is that the lack of AMPKα2 activity and marked decrease in ACC phosphorylation result in a severe reduction in muscle fatty acid oxidation. This hypothesis is supported by the increased intramuscular triglycerides that we observed in the MLKB1KO mice. In response to a reduction in fatty acid oxidation, MLKB1KO mice may preferentially utilize glucose over fatty acids for ATP generation, leading to the reductions in muscle glycogen content that we observed. Lower muscle glycogen content and increased glycogen synthase activity are a combination that is well known to increase insulin-stimulated glucose uptake in skeletal muscle (22). However, this hypothesis is not fully supported by previous data for whole-body AMPK knockout mice. Similarly to MLKB1KO mice, AMPKα2 knockout mice have decreased ACC phosphorylation and lower muscle glycogen content, yet the AMPKα2 knockout mice have impaired glucose tolerance, which could be due to nonmuscle effects (23). In addition, there is no significant phenotype in the AMPKα1 knockout mice (46). Thus, the available data suggest that the enhanced glucose homeostasis in the MLKB1KO mice is due to upregulation of Akt signaling, stemming from decreased TRB3 expression caused by the loss of LKB1 activity.
LKB1 has been termed a “master kinase” that can increase not only AMPK activity but also the activities of at least 13 AMPK-related kinases (20, 27). Of these additional 13 kinases, only MARK2/3, MARK4, QSK, and QIK/SIK2 are detectable in skeletal muscle, and none are regulated by AICAR, contraction, or phenformin (36). In the current investigation we found that MARK4 but not MARK2/3 activities were reduced for MLKB1KO mice. Whether downregulation of skeletal muscle MARK4 is a mechanism for improved glucose homeostasis and alterations in TRB3 expression will be an important area of future investigation, and those studies are ongoing in our laboratory.
Recently, Sakamoto et al. reported generation of LKB1 knockout mice that have an approximately 90% reduction in LKB1 in all tissues and ablation of LKB1 activity in skeletal muscle (38). While our model and theirs show similar effects in regard to AMPKα2 activity, there appear to be very significant differences in the metabolic phenotypes. While we show a pronounced improvement in glucose homeostasis and insulin sensitivity for MLKB1KO mice, Sakamoto et al. reported normal blood glucose concentrations up to 10 weeks of age and reported little additional physiological data. The major differences between the studies may be due to the 90% hypomorphic phenotype of their LKB1flox/flox mice; our double-floxed mice did not have significant decreases in LKB1 protein or enzyme activity (Fig. 1A and B). The significance of this difference is highlighted by a recent report that hepatic LKB1 is essential for the regulation of gluconeogenesis and normal glucose tolerance (41). Given that loss of hepatic LKB1 leads to glucose intolerance and fasting hyperglycemia and our data showing that loss of skeletal muscle LKB1 leads to improvements in glucose tolerance, it seems plausible that for the hypomorphic animals described by Sakamoto, the beneficial effects of a reduction in skeletal muscle LKB1 expression on glucose homeostasis are masked by the detrimental effects of a reduction in hepatic LKB1 expression. Interestingly, loss of LKB1 in liver leads to increases in PGC1α (41), whereas we clearly show decreases in PGC1α in skeletal muscle (Fig. 5A), which likely explains the opposite effects of liver and muscle LKB1 deficiency on glucose homeostasis. Recently, a decrease in TORC2 phosphorylation has been proposed to be a mechanism by which LKB1 regulates PGC1α in liver. However, we found that TORC2 was barely detectable in mouse skeletal muscles compared to levels in liver, and we found no evidence for alterations in TORC2 phosphorylation in MLKB1KO mice (H. J. Koh and L. J. Goodyear, unpublished observation), implying that LKB1 regulates PGC1α and TRB3 through a distinct mechanism in skeletal muscle.
While LKB1 has been reported to regulate both AMPKα1 and α2 activities in vitro and in cell culture systems (15, 51), our results suggest that LKB1 is necessary for the activation of AMPKα2 but not AMPKα1. This finding is consistent with recent studies performed with cardiac muscle, in which LKB1 was found to be necessary for hypoxia-induced activation of AMPKα2 but not α1 (39). We are unable to determine whether this is due to isoform-specific AMPKα2 activation by LKB1 or to the presence of an alternative AMPKα1 kinase. Another possible explanation for the divergence between AMPKα1 and AMPKα2 activities in the MLKB1KO mice is that the majority of AMPKα1 activity present in muscle tissue is not of skeletal muscle origin. AMPKα1 is highly expressed in vascular endothelial and smooth muscle cells, which are embedded in skeletal muscle tissue (35, 50). Therefore, it is possible that AMPKα1 is regulated by LKB1 but is expressed only at low levels in skeletal muscle and that most of the AMPKα1 activity detected may be from nonmuscle cells which would have normal LKB1 expression in MLKB1KO animals.
In summary, in addition to serving as the major upstream kinase for the regulation of AMPKα2 activity in skeletal muscle, skeletal muscle LKB1 is a negative regulator of glucose homeostasis and insulin sensitivity. Disruption of LKB1 in skeletal muscle results in enhanced glucose tolerance and upregulation of insulin sensitivity for glucose uptake and signaling in skeletal muscle; this affect appears to be mediated, at least in part, through PGC1α- and PPARα-mediated downregulation of TRB3 expression, resulting in enhanced insulin-stimulated Akt signaling. Given that whole-body AMPKα2 knockout mice have impaired glucose homeostasis (46), it is possible that LKB1 itself or non-AMPK substrates, such as the MARK proteins, are responsible for the beneficial adaptations to muscle insulin sensitivity in MLKB1KO mice. The concept of enhanced insulin or growth factor signaling in the absence of LKB1, including upregulation of Akt, is consistent with the well-established role of LKB1 as a tumor suppressor and provides additional evidence for the dual function of LKB1 in both cell metabolism and cancer biology.
Supplementary Material
[Supplemental material]
Acknowledgments
We thank R. A. DePinho and N. M. Bardeesy (Dana Farber Cancer Institute) for providing the floxed LKB1 mice, W. W. Winder (Brigham Young University) for the MO25 antibody, and N. Mukai for assistance with experiments.
This work was supported by NIH grants to L. J. Goodyear (AR45670 and DK68626) and the Joslin Diabetes Center DERC (DK36836). H. J. Koh is supported by a Mary K. Iacocca Fellowship, R. C. Ho by NIH F32AR04662, and N. Jessen by a grant from the University of Aarhus, Aarhus, Denmark.
Footnotes
▿
Published ahead of print on 11 September 2006.
REFERENCES
- 1.Alessi, D. R., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15**:**6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Aschenbach, W. G., M. F. Hirshman, N. Fujii, K. Sakamoto, K. F. Howlett, and L. J. Goodyear. 2002. Effect of AICAR treatment on glycogen metabolism in skeletal muscle. Diabetes 51**:**567-573. [DOI] [PubMed] [Google Scholar]
- 3.Baas, A. F., J. Kuipers, N. N. van der Wel, E. Batlle, H. K. Koerten, P. J. Peters, and H. C. Clevers. 2004. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 116**:**457-466. [DOI] [PubMed] [Google Scholar]
- 4.Bardeesy, N., M. Sinha, A. F. Hezel, S. Signoretti, N. A. Hathaway, N. E. Sharpless, M. Loda, D. R. Carrasco, and R. A. DePinho. 2002. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature 419**:**162-167. [DOI] [PubMed] [Google Scholar]
- 5.Bergeron, R., R. R. Russell III, L. H. Young, J. M. Ren, M. Marcucci, A. Lee, and G. I. Shulman. 1999. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am. J. Physiol. 276**:**E938-E944. [DOI] [PubMed] [Google Scholar]
- 6.Bruning, J. C., M. D. Michael, J. N. Winnay, T. Hayashi, D. Horsch, D. Accili, L. J. Goodyear, and C. R. Kahn. 1998. A muscle-specific insulin receptor knockout challenges the current concepts of glucose disposal and NIDDM pathogenesis. Mol. Cell 2**:**559-569. [DOI] [PubMed] [Google Scholar]
- 7.Cho, H., J. Mu, J. K. Kim, J. L. Thorvaldsen, Q. Chu, E. B. Crenshaw III, K. H. Kaestner, M. S. Bartolomei, G. I. Shulman, and M. J. Birnbaum. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292**:**1728-1731. [DOI] [PubMed] [Google Scholar]
- 8.Crettaz, M., E. S. Horton, L. J. Wardzala, E. D. Horton, and B. Jeanrenaud. 1983. Physical training of Zucker rats: lack of alleviation of muscle insulin resistance. Am. J. Physiol. 244**:**E414-E420. [DOI] [PubMed] [Google Scholar]
- 9.Drewes, G., A. Ebneth, U. Preuss, E. M. Mandelkow, and E. Mandelkow. 1997. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell 89**:**297-308. [DOI] [PubMed] [Google Scholar]
- 10.Du, K., S. Herzig, R. N. Kulkarni, and M. Montminy. 2003. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 300**:**1574-1577. [DOI] [PubMed] [Google Scholar]
- 11.Ferre, P., A. Leturque, A.-F. Burnol, L. Penicaud, and J. Girard. 1985. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem. J. 228**:**103-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii, N., M. D. Boppart, S. D. Dufresne, P. F. Crowley, A. C. Jozsi, K. Sakamoto, H. Yu, W. G. Aschenbach, S. Kim, H. Miyazaki, L. Rui, M. F. White, M. F. Hirshman, and L. J. Goodyear. 2004. Overexpression or ablation of JNK in skeletal muscle has no effect on glycogen synthase activity. Am. J. Physiol. Cell. Physiol. 287**:**C200-C208. [DOI] [PubMed] [Google Scholar]
- 13.Fujii, N., M. F. Hirshman, E. M. Kane, R. C. Ho, L. E. Peter, M. M. Seifert, and L. J. Goodyear. 2005. AMP-activated protein kinase (alpha)2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J. Biol. Chem. 280**:**39033-39041. [DOI] [PubMed] [Google Scholar]
- 14.Garofalo, R. S., S. J. Orena, K. Rafidi, A. J. Torchia, J. L. Stock, A. L. Hildebrandt, T. Coskran, S. C. Black, D. J. Brees, J. R. Wicks, J. D. McNeish, and K. G. Coleman. 2003. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J. Clin. Investig. 112**:**197-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawley, S. A., J. Boudeau, J. L. Reid, K. J. Mustard, L. Udd, T. P. Makela, D. R. Alessi, and D. G. Hardie. 2003. Complexes between the LKB1 tumor suppressor, STRADalpha/beta and MO25alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2**:**28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi, T., M. F. Hirshman, E. J. Kurth, W. W. Winder, and L. J. Goodyear. 1998. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 47**:**1369-1373. [DOI] [PubMed] [Google Scholar]
- 17.Hemminki, A., D. Markie, I. Tomlinson, E. Avizienyte, S. Roth, A. Loukola, G. Bignell, W. Warren, M. Aminoff, P. Hoglund, H. Jarvinen, P. Kristo, K. Pelin, M. Ridanpaa, R. Salovaara, T. Toro, W. Bodmer, S. Olschwang, A. S. Olsen, M. R. Stratton, A. de la Chapelle, and L. A. Aaltonen. 1998. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 391**:**184-187. [DOI] [PubMed] [Google Scholar]
- 18.Hutber, C. A., D. G. Hardie, and W. W. Winder. 1997. Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase. Am. J. Physiol. 272**:**E262-E266. [DOI] [PubMed] [Google Scholar]
- 19.Jakobsen, S. N., D. G. Hardie, N. Morrice, and H. E. Tornqvist. 2001. 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J. Biol. Chem. 276**:**46912-46916. [DOI] [PubMed] [Google Scholar]
- 20.Jaleel, M., A. McBride, J. M. Lizcano, M. Deak, R. Toth, N. A. Morrice, and D. R. Alessi. 2005. Identification of the sucrose non-fermenting related kinase SNRK, as a novel LKB1 substrate. FEBS Lett. 579**:**1417-1423. [DOI] [PubMed] [Google Scholar]
- 21.Jenne, D. E., H. Reimann, J. Nezu, W. Friedel, S. Loff, R. Jeschke, O. Muller, W. Back, and M. Zimmer. 1998. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet. 18**:**38-43. [DOI] [PubMed] [Google Scholar]
- 22.Jensen, J., R. Aslesen, J. L. Ivy, and O. Brors. 1997. Role of glycogen concentration and epinephrine on glucose uptake in rat epitrochlearis muscle. Am. J. Physiol. 272**:**E649-E655. [DOI] [PubMed] [Google Scholar]
- 23.Jorgensen, S. B., B. Viollet, F. Andreelli, C. Frosig, J. B. Birk, P. Schjerling, S. Vaulont, E. A. Richter, and J. F. Wojtaszewski. 2004. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside but not contraction-induced glucose uptake in skeletal muscle. J. Biol. Chem. 279**:**1070-1079. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen, S. B., J. F. Wojtaszewski, B. Viollet, F. Andreelli, J. B. Birk, Y. Hellsten, P. Schjerling, S. Vaulont, P. D. Neufer, E. A. Richter, and H. Pilegaard. 2005. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 19**:**1146-1148. [DOI] [PubMed] [Google Scholar]
- 25.Koo, S. H., H. Satoh, S. Herzig, C. H. Lee, S. Hedrick, R. Kulkarni, R. M. Evans, J. Olefsky, and M. Montminy. 2004. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat. Med. 10**:**530-534. [DOI] [PubMed] [Google Scholar]
- 26.Lee, W. J., M. Kim, H. S. Park, H. S. Kim, M. J. Jeon, K. S. Oh, E. H. Koh, J. C. Won, M. S. Kim, G. T. Oh, M. Yoon, K. U. Lee, and J. Y. Park. 2006. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem. Biophys. Res. Commun. 340**:**291-295. [DOI] [PubMed] [Google Scholar]
- 27.Lizcano, J. M., O. Goransson, R. Toth, M. Deak, N. A. Morrice, J. Boudeau, S. A. Hawley, L. Udd, T. P. Makela, D. G. Hardie, and D. R. Alessi. 2004. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23**:**833-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin, S. G., and D. St Johnston. 2003. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature 421**:**379-384. [DOI] [PubMed] [Google Scholar]
- 29.Merrill, G. F., E. J. Kurth, D. G. Hardie, and W. W. Winder. 1997. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. 273**:**E1107-E1112. [DOI] [PubMed] [Google Scholar]
- 30.Mu, J., J. T. Brozinick, Jr., O. Valladares, M. Bucan, and M. J. Birnbaum. 2001. A role for AMP-activated protein kinase in contraction-and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell 7**:**1085-1094. [DOI] [PubMed] [Google Scholar]
- 31.Musi, N., N. Fujii, M. F. Hirshman, I. Ekberg, S. Froberg, O. Ljungqvist, A. Thorell, and L. J. Goodyear. 2001. AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes 50**:**921-927. [DOI] [PubMed] [Google Scholar]
- 32.Norris, A. W., L. Chen, S. J. Fisher, I. Szanto, M. Ristow, A. C. Jozsi, M. F. Hirshman, E. D. Rosen, L. J. Goodyear, F. J. Gonzalez, B. M. Spiegelman, and C. R. Kahn. 2003. Muscle-specific PPARgamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J. Clin. Investig. 112**:**608-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ossipova, O., N. Bardeesy, R. A. DePinho, and J. B. Green. 2003. LKB1 (XEEK1) regulates Wnt signalling in vertebrate development. Nat. Cell Biol. 5**:**889-894. [DOI] [PubMed] [Google Scholar]
- 34.Peng, X. D., P. Z. Xu, M. L. Chen, A. Hahn-Windgassen, J. Skeen, J. Jacobs, D. Sundararajan, W. S. Chen, S. E. Crawford, K. G. Coleman, and N. Hay. 2003. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 17**:**1352-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubin, L. J., L. Magliola, X. Feng, A. W. Jones, and C. C. Hale. 2005. Metabolic activation of AMP kinase in vascular smooth muscle. J. Appl. Physiol. 98**:**296-306. [DOI] [PubMed] [Google Scholar]
- 36.Sakamoto, K., O. Goransson, D. G. Hardie, and D. R. Alessi. 2004. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am. J. Physiol. Endocrinol. Metab. 287**:**E310-E317. [DOI] [PubMed] [Google Scholar]
- 37.Sakamoto, K., M. F. Hirshman, W. G. Aschenbach, and L. J. Goodyear. 2002. Contraction regulation of Akt in rat skeletal muscle. J. Biol. Chem. 277**:**11910-11917. [DOI] [PubMed] [Google Scholar]
- 38.Sakamoto, K., A. McCarthy, D. Smith, K. A. Green, H. D. Grahame, A. Ashworth, and D. R. Alessi. 2005. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 24**:**1810-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakamoto, K., E. Zarrinpashneh, G. R. Budas, A. C. Pouleur, A. Dutta, A. R. Prescott, J. L. Vanoverschelde, A. Ashworth, A. Jovanovic, D. R. Alessi, and L. Bertrand. 2006. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPK(alpha)2 but not AMPK(alpha)1. Am. J. Physiol. Endocrinol. Metab. 290**:**E780-E788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw, R. J., M. Kosmatka, N. Bardeesy, R. L. Hurley, L. A. Witters, R. A. DePinho, and L. C. Cantley. 2004. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 101**:**3329-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw, R. J., K. A. Lamia, D. Vasquez, S. H. Koo, N. Bardeesy, R. A. DePinho, M. Montminy, and L. C. Cantley. 2005. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310**:**1642-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stapleton, D., K. I. Mitchelhill, G. Gao, J. Widmer, B. J. Michell, T. Teh, C. M. House, C. S. Fernandez, T. Cox, L. A. Witters, and B. E. Kemp. 1996. Mammalian AMP-activated protein kinase subfamily. J. Biol. Chem. 271**:**611-614. [DOI] [PubMed] [Google Scholar]
- 43.Suwa, M., H. Nakano, and S. Kumagai. 2003. Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles. J. Appl. Physiol. 95**:**960-968. [DOI] [PubMed] [Google Scholar]
- 44.Taniguchi, C. M., B. Emanuelli, and C. R. Kahn. 2006. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell. Biol. 7**:**85-96. [DOI] [PubMed] [Google Scholar]
- 45.Taylor, E. B., J. D. Lamb, R. W. Hurst, D. G. Chesser, W. J. Ellingson, L. J. Greenwood, B. B. Porter, S. T. Herway, and W. W. Winder. 2005. Endurance training increases skeletal muscle LKB1 and PGC-1alpha protein abundance: effects of time and intensity. Am. J. Physiol. Endocrinol. Metab. 289**:**E960-E968. [DOI] [PubMed] [Google Scholar]
- 46.Viollet, B., F. Andreelli, S. B. Jorgensen, C. Perrin, A. Geloen, D. Flamez, J. Mu, C. Lenzner, O. Baud, M. Bennoun, E. Gomas, G. Nicolas, J. F. Wojtaszewski, A. Kahn, D. Carling, F. C. Schuit, M. J. Birnbaum, E. A. Richter, R. Burcelin, and S. Vaulont. 2003. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J. Clin. Investig. 111**:**91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Virkamaki, A., E. Rissanen, S. Hamalainen, T. Utriainen, and H. Yki-Jarvinen. 1997. Incorporation of [3-3H]glucose and 2-[1-14C]deoxyglucose into glycogen in heart and skeletal muscle in vivo: implications for the quantitation of tissue glucose uptake. Diabetes 46**:**1106-1110. [DOI] [PubMed] [Google Scholar]
- 48.Watts, J. L., D. G. Morton, J. Bestman, and K. J. Kemphues. 2000. The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development 127**:**1467-1475. [DOI] [PubMed] [Google Scholar]
- 49.Wojtaszewski, J. F., S. B. Jorgensen, Y. Hellsten, D. G. Hardie, and E. A. Richter. 2002. Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)- riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes 51**:**284-292. [DOI] [PubMed] [Google Scholar]
- 50.Woods, A., D. Azzout-Marniche, M. Foretz, S. C. Stein, P. Lemarchand, P. Ferre, F. Foufelle, and D. Carling. 2000. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol. Cell. Biol. 20**:**6704-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woods, A., S. R. Johnstone, K. Dickerson, F. C. Leiper, L. G. Fryer, D. Neumann, U. Schlattner, T. Wallimann, M. Carlson, and D. Carling. 2003. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 13**:**2004-2008. [DOI] [PubMed] [Google Scholar]
- 52.Ylikorkala, A., D. J. Rossi, N. Korsisaari, K. Luukko, K. Alitalo, M. Henkemeyer, and T. P. Makela. 2001. Vascular abnormalities and deregulation of VEGF in Lkb1-deficient mice. Science 293**:**1323-1326. [DOI] [PubMed] [Google Scholar]
- 53.Yu, H., N. Fujii, M. F. Hirshman, J. M. Pomerleau, and L. J. Goodyear. 2004. Cloning and characterization of mouse 5′-AMP-activated protein kinase (gamma)3 subunit. Am. J. Physiol. Cell. Physiol. 286**:**C283-C292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental material]