Liquid secretion properties of airway submucosal glands (original) (raw)
Abstract
The tracheobronchial submucosal glands secrete liquid that is important for hydrating airway surfaces, supporting mucociliary transport, and serving as a fluid matrix for numerous secreted macromolecules including the gel-forming mucins. This review details the essential structural elements of airway glands and summarizes what is currently known regarding the ion transport processes responsible for producing the liquid component of gland secretion. Liquid secretion most likely arises from serous cells and is principally under neural control with muscarinic agonists, substance P, and vasoactive intestinal peptide (VIP) functioning as effective secretogogues. Liquid secretion is driven by the active transepithelial secretion of both Cl− and HCO3− and at least a portion of this process is mediated by the cystic fibrosis transmembrane conductance regulator (CFTR), which is highly expressed in glands. The potential role of submucosal glands in cystic fibrosis lung disease is discussed.
Introduction
The submucosal glands of the tracheobronchial airways secrete liquid that is essential for flushing the macromolecular component of gland secretion from the gland ducts and for augmenting airway surface liquid (ASL) volume for the support of mucociliary transport. In this review, we provide an analysis of the current literature regarding the mechanisms of ion and liquid secretion by the tracheobronchial glands. Because the arrangement of glandular structural elements is important to their secretory function, when possible we emphasize studies performed with intact airways, where the complex architecture of glandular and surface epithelium is maintained. Because the cystic fibrosis transmembrane conductance regulator (CFTR) is known to mediate at least a portion of gland liquid secretion, we include a discussion of the potential role of submucosal glands in cystic fibrosis (CF) lung disease. Due to space constraints, however, we will not review the macromolecular component of gland secretion, about which a considerable literature exists owing to its importance in the aetiology of obstructive airway diseases. The reader is referred to several excellent reviews that provide more in-depth discussions of gland structure as well as fluid and macromolecular secretion (Tos 1966; Rogers, 1993; Shimura et al. 1994; Rogers 2000).
Gland morphology
Submucosal glands populate the trachea and bronchial airways of higher mammals including humans, monkeys, sheep, pigs, goats, oxen, opossums, cats and dogs (Goco et al. 1963; Sorkin, 1965; Choi et al. 2000). In adult humans, sheep, oxen, dogs and pigs, gland density is approximately 1mm−2 (Tos, 1976; Choi et al. 2000). In man, glands are well-expressed throughout the cartilaginous airways (Bloom & Fawcett, 1975), a pattern that is likely to hold for most higher mammals as well. Bronchioles, the compliant thin-walled distal airways that contain little cartilage, are aglandular; consequently, there is an abrupt transition in gland expression at the bronchial–bronchiolar junction, which occurs at about 1mm airway diameter (Ballard et al. 1995). Rats, mice, guinea-pigs and hamsters express submucosal glands only in the most cranial portion of the trachea (Borthwick et al. 1999; Widdicombe et al. 2001). Rabbit airways are devoid of submucosal glands, but they do exhibit numerous shallow pits or depressions in the airway surface in which goblet cells are thought to cluster (Widdicombe et al. 2001).
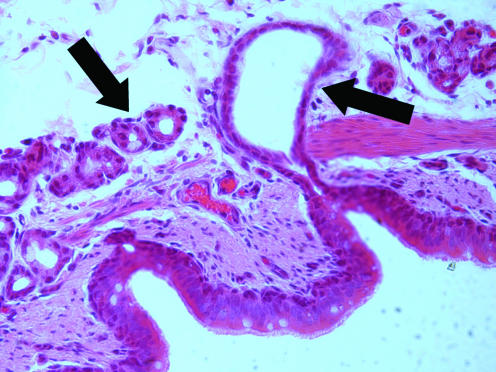
An individual airway gland typically consists of a primary (collecting) gland duct, lateral ducts and numerous secretory tubules (Tos, 1966). The primary gland duct passes from the surface epithelium through the lamina propria and smooth muscle layers into the submucosal space. The proximal segment of the primary duct (i.e. portion closer to the duct opening) is lined by ciliated cells whose morphology resembles that of the surface epithelium (Meyrick et al. 1969). The submucosal portions of the primary duct may form ‘antra’, i.e. distended duct regions whose diameters are 3- to 4-fold greater than the primary ducts (Meyrick et al. 1969; Inglis et al. 1997_a_) (Fig. 1). The functional significance of these distended duct regions, beyond simple conduction of glandular secretion products, is unclear. Many primary ducts do not form these antra, though their morphology may vary greatly from long straight segments to convoluted structures (Tos, 1966; Inglis et al. 1997_a_). The primary ducts serve as collectors for a pair of lateral ducts from which numerous secretory tubules arise (Tos, 1966; Meyrick et al. 1969). These secretory tubules are categorized as either mucous or serous depending on the relative predominance of these respective cell types (Meyrick et al. 1969). The mucous tubules may bifurcate once or more into other mucous tubules, but they always terminate in serous tubules.
Figure 1. Slide section of submucosal gland from porcine bronchus.
The right arrow identifies dilated segment, or antrum, of the primary (collecting) duct in the submucosa. The left arrow shows numerous secretory tubules.
The principal exocrine cells of the airway glands are the mucous and serous cells. Mucous cells closely resemble the goblet cells, which are found in the surface epithelium, in that their apices are packed with large mucin-containing granules that compress the nucleus and cytoplasm into the basal portions of the cells. The serous cells are pyramidal in shape and the nucleus is also basally located (Basbaum et al. 1990). The apices of the serous cells are filled with numerous electron-dense secretory granules that are 100–1800nm in diameter. When stimulated with glandular secretogogues, serous cells undergo morphological changes that parallel the magnitude of fluid secretion (Quinton, 1981); consequently, serous cells are thought to be the principal mediators of fluid secretion in submucosal glands. Thus, because the serous tubules always lie distal to the mucous tubules, they are logically orientated to flush the mucin glycoprotein secretions of the mucous cells out of the ducts. Indeed, when fluid secretion is inhibited pharmacologically, the gland ducts become impacted with mucin glycoproteins (Inglis et al. 1997_b_) demonstrating the importance of this structural arrangement. In adult human airway glands, serous cells outnumber mucous cells by about 50% (Takizawa & Thurlbeck, 1971). Much of the basal surfaces of the glandular epithelial cells, including the mucous and serous tubules and portions of the gland ducts, are surrounded by myoepithelial cells. These cells are contractile and, when activated, facilitate emptying of the luminal contents (Shimura et al. 1986).
Techniques for measuring liquid secretion from glands
Because glands are small and most of their mass is embedded in the submucosal space, study of the their exocrine function is problematic. Several experimental approaches have been employed. One approach is to cover the mucosal surface of the airways with a thin coating of tantalum power (Nadel & Davis, 1978). When glands are stimulated to secrete, the fluid that emerges from the ducts pushes the powder aside forming ‘hillocks’ at the duct openings. By modelling the hillock dimensions as liquid droplets, this technique permits useful estimates of short-term volume secretion rates (Hejal et al. 1995; Phillips et al. 2002_a_). Another technique, pioneered by Quinton (1979), involves drying the airway surface with a stream of gas and then layering the surface with water-equilibrated oil. As gland fluid emerges from the duct openings, it forms visible pools of liquid underneath the oil layer. This liquid can be collected with micropipettes for determination of volume and composition (Quinton, 1979; Ueki et al. 1980) or the volumes can be determined optically in situ from droplet dimensions (Joo et al. 2001). Others have isolated the glandular contribution to fluid secretion in intact airways by abrasively removing the surface epithelium whilst leaving the submucosal structures intact (Ballard et al. 1999; Trout et al. 2001). Fluorescence imaging techniques have been employed to measure acid/base transport in the acinar cells of isolated glands (Hug & Bridges, 2001) or to measure pH and ion compositions of the gland fluid as it emanates from the gland duct openings (Jayaraman et al. 2001). Though technically challenging, it is feasible to dissect individual submucosal glands from the tracheobronchial airways. We are unaware of studies to date that achieve quantitative collection of secreted fluid from isolated glands, but the rate of 22Na efflux from isolated glands (apparently across the basolateral membrane of gland cells via the Na+,K+-ATPase) has proven to be a useful correlate of secretory ion transport activity (Sasaki et al. 1990). Individual submucosal glands have even been attached to myographs for measurement of the contractile responses of myoepithelial cells (Shimura et al. 1986).
Useful information, particularly about electrolyte secretion, has also been obtained from studies of cultured gland cells. However, caution must be employed when extrapolating the results of these studies to overall glandular function since important structure–function relationships are lost. In addition, cells removed from their native environments and grown in complex media containing growth factors for prolonged periods may not accurately reflect in vivo behaviour. Indeed, isolated serous and mucous cells tend to dedifferentiate in primary culture expressing both serous and mucous cell proteins (Sommerhoff & Finkbeiner, 1990). The Calu-3 cell line, derived from a human lung adenocarcinoma, expresses many characteristics of submucosal gland serous cells including expression of CFTR (Shen et al. 1994). This cell line has proven to be a convenient model for evaluating serous cell function; but, since these cells are aneuploid, lacking chromosomes 1, 13, 15, and 17 (ATCC, Manassas VA, USA), studies of their function should be interpreted with caution.
Regulation of ion and liquid secretion
In the absence of secretogogues or neural stimulation, glands produce small quantities of liquid (Quinton, 1979; Joo et al. 2001; Ueki et al. 1980). However, vagal stimulation, either direct or through activation of sensory nerves, induces copious secretion of fluid from tracheal glands (Davis et al. 1982; Haxhiu et al. 1990). Direct application of acetylcholine (ACh) or other muscarinic agonists to excised airways mirrors this response demonstrating that fluid secretion from glands is under cholinergic control (Quinton, 1979; Ballard et al. 1999; Joo et al. 2002_b_). The response to these agonists is due to activation of M3 muscarinic receptors (Ishihara et al. 1992) and perhaps M1 receptors as well (Yang et al. 1988). Substance P, which is normally released from the terminals of sensory nerves, also induces vigorous fluid secretion from glands both in vivo (Haxhiu et al. 1990) and in vitro (Trout et al. 2001; Phillips et al. 2003). The secretion response to substance P is mediated predominantly through NK1 receptors on gland cells though prejunctional NK3 receptors are thought to reinforce secretion by inducing secondary release of ACh (Khawaja et al. 1999; Phillips et al. 2003). Calcitonin gene-related peptide (CGRP) and neurokinin A (NK-A) also stimulate gland secretion (Webber, 1989; Webber et al. 1991). Adrenergic control of fluid secretion appears to be less straightforward. α-Adrenergic receptor agonists are efficacious gland secretogogues in feline and ferret tracheas (Quinton, 1979; Borson et al. 1980; Ueki et al. 1980; Joo et al. 2001) but have little or no effect on volume secretion in pig, sheep, or human glands (Joo et al. 2001; Trout et al. 2001). β-Adrenergic agonists are ineffective fluid secretogogues for human, feline and pig glands (Quinton, 1979; Trout et al. 2001; Joo et al. 2001). Vasoactive intestinal peptide (VIP) induces liquid secretion from human and porcine glands though the rate of secretion is comparatively less than that seen with the muscarinic agonists (Joo et al. 2002_a_, b). The response to VIP is likely to be mediated through VPAC2 receptors (formally VIP2 receptors), which have been localized to acinar and ductal gland cells (Groneberg et al. 2001). In human airways, VIP-containing neurones are often coincident with cholinergic neurones, but this is not the case in all species (Fischer et al. 1996).
Recent studies suggest that autocrine and/or paracrine mechanisms play a role in the regulation of gland secretion. Stimuli such as hyposmotic and flow-induced stress induce the release of ATP from Calu-3 cells (Guyot & Hanrahan, 2002). Extracellular ATP, as well as UTP, is capable of directly stimulating a number of P2Y (formally P2U) receptor subtypes which in turn evoke a rise in intracellular Ca2+ and induce anion secretion in cultured human gland cells (Yamaya et al. 1996). In cultures of Calu-3 cells, ATP can also be broken down by ectonucleotidases to adenosine, which stimulates anion secretion through A2B receptors via both protein kinase A and phospholipase A2-dependent pathways (Huang et al. 2001; Cobb et al. 2002). The relative importance of these pathways in the moment-to-moment regulation of gland fluid secretion is not fully understood at this time. Platelet-activating factor (PAF) is also a potent stimulant of gland fluid secretion (Steiger et al. 1987). Human neutrophil elastase, a powerful inducer of mucus macromolecule secretion, is a likely stimulant of gland fluid secretion as well (Schuster et al. 1992).
Mechanism of gland liquid secretion
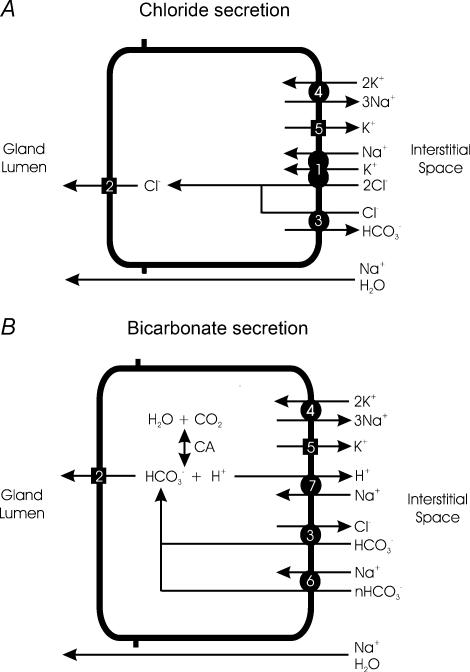
Substantial information has been gathered on the cellular mechanism of ion and fluid secretion using a variety of preparations and species. A summary of the transport processes that are likely to be involved in this process, as discussed below, is shown in Fig. 2.
Figure 2. Summary of proposed cellular mechanisms for Cl− and HCO3− secretion by serous submucosal gland cells.
Though represented separately for clarity, it is likely that both anion secretion mechanisms exist in the same cell. See text for details. A, Cl− secretion. Cl− enters across the basolateral membrane by NKCC (1) and exits across the apical membrane through CFTR and possibly alternative anion channels (2). While NKCC is the major entry route for Cl−, AE2 (3) could theoretically support Cl− entry across the basolateral membrane if the electrochemical driving forces are appropriate. Na+,K+-ATPase (4) is localized to the basolateral membrane. Agonist stimulation is likely to result in activation of apical membrane anion channel(s) (2) and a population of K+ channels on the basolateral membrane (5). B, HCO3− secretion. HCO3− is either transported across the basolateral membrane through electrogenic NBC (6) or AE2 (3), or it is generated intracellularly through the actions of carbonic anhydrase (CA). Intracellular generation of HCO3− also produces H+, which is removed from the cell interior by NHE (7). HCO3− then exits across the apical membrane through the anion channels (2). Agonist stimulation similarly controls secretion by activating apical membrane anion channels (2) and basolateral membrane K+ channels (5). Transepithelial secretion of either anion establishes a voltage gradient for cations, principally Na+, to follow through the paracellular pathway. H2O flows across the barrier, either paracellularly (as shown) or transcellularly, in response to the resultant osmotic gradient.
The secretion response to muscarinic agonists or substance P is vigorous and driven in large part by bumetanide-sensitive Cl− secretion (Trout et al. 1998_a_, 2001; Joo et al. 2002_b_) indicating that this process is dependent upon Cl− entry across the basolateral membrane by Na+–K+–2Cl− cotransport (NKCC) (Fig. 2_A_). Chloride exits the cell across the apical membrane through anion channels whose identity remains controversial. CFTR is a likely candidate for this role because antibodies to this protein localize to the apical membrane of gland serous cells in situ (Engelhardt et al. 1992; Jacquot et al. 1993) and bioelectric evidence for this channel has been found both in Calu-3 cells (Haws et al. 1994) and primary cultures of gland cells (Becq et al. 1993). However, the actions of ACh and substance P are likely to be transduced through inositol 1,4,5-triphosphate (IP3), protein kinase C, and Ca2+ (Shimura et al. 1993; Nagaki et al. 1994; Sasaki et al. 1994) rather than the cAMP–protein kinase A pathway which is known to predominantly regulate CFTR (Tabcharani et al. 1991). This quandary has three possible explanations. First, CFTR in gland cells may be constitutively active even in the absence of cAMP-elevating agonists. Thus, anion efflux through the CFTR could be controlled chiefly by changes in cell membrane potential induced by alteration of basolateral K+ conductance, a notion which has been proposed to explain the anion secretion responses to elevation of intracellular Ca2+ in both Calu-3 cells (Moon et al. 1997) and primary cultures of gland cells (Yamaya et al. 1993). Second, it is possible that CFTR-activating pathways lie downstream of Ca2+ and protein kinase C in the muscarinic and substance P signal transduction cascade. Third, it is possible that alternative Cl− channels such as Ca2+-activated Cl− channels (CaCC) coexist with CFTR in the apical membrane and are activated by ACh and substance P through elevation of intracellular Ca2+ and/or activation of protein kinase C. Although functional studies have suggested that CFTR is the principal anion conductance pathway in the apical membrane of Calu-3 cells (Haws et al. 1994), muscarinic agonists do stimulate fluid secretion from glands in freshly excised CF airways, which should not express functional CFTR (Joo et al. 2002_a_). While myoepithelial cell contraction, which occurs following cholinergic stimulation (Shimura et al. 1987), could mimic at least the initial secretion response of CF glands by compressing the glands and forcing the extrusion of resident mucus liquid from the lumen of the ducts, this effect should last only a few seconds and therefore minimally contribute to the observed volume secretion.
The electrical driving force for sustaining Cl− efflux across the apical membrane of secretory epithelia is likely to be derived from increases in basolateral membrane K+ conductance, which should hyperpolarize the cells (Smith & Frizzell, 1984). The identity of the specific population(s) of K+ channels involved in the secretion responses to endogenous gland secretogogues, however, remains poorly defined. In Calu-3 cells, elevation of intracellular Ca2+ with thapsigargan activates both clofilium- and clotrimazole-sensitive conductances, which are likely to represent KvLQT and intermediate-conductance Ca2+-activated K+ channels, respectively (Cowley & Linsdell, 2002). 1-EBIO activates intermediate-conductance Ca2+-activated K+ channels in Calu-3 cells that are clotrimazole- and charybdotoxin-sensitive (Devor et al. 1999), but this agonist has no measurable effect on fluid secretion by porcine airway glands (S. T. Ballard, unpublished observations).
In addition to Cl− secretion, muscarinic agonists and substance P also stimulate HCO3− secretion from airway glands (Fig. 2_B_). In the presence of either ACh or substance P, HCO3− secretion appears to be primarily dependent upon intracellular generation of this anion since inhibitors of Na+/H+ exchange (NHE), such as dimethylamiloride, block this process (Trout et al. 1998_a_, 2001). The presence of carbonic anhydrase in gland cells suggests that these cells are capable of intracellular generation of HCO3− (Spicer et al. 1982). When intracellular HCO3− exceeds its electrochemical equilibrium, it can exit across the apical membrane through the CFTR (Poulsen et al. 1994) and perhaps through alternative anion channels as well. Trout et al. (1998_a_) report that the magnitude of HCO3− secretion relative to Cl− secretion is low following application of these agonists as evidenced by the weak inhibitory effect of dimethylamiloride alone on ACh-induced liquid secretion (Trout et al. 1998_a_) and the relatively neutral pH of gland fluid (Jayaraman et al. 2001). Joo et al. (2002_b_) find that HCO3− may play a larger role in gland secretion since removal of HCO3− and CO2 from the submucosal bath inhibits about half of the carbachol-induced gland liquid secretion in pig airways. In any event, ACh-induced secretion of HCO3− is sufficient to induce measurable alkalinization of an airway perfusate (Inglis et al. 2003). When Cl− secretion is inhibited with bumetanide in the presence of ACh or substance P, HCO3− secretion is probably increased, evidenced by the nearly threefold increase in HCO3− concentration in secreted fluid, approximate doubling of the inhibitory effect of dimethylamiloride on the volume of secreted fluid (Trout et al. 1998_a_, 2001), and significantly increased alkalinization of luminal fluid (Inglis et al. 2003).
VIP is also an effective gland liquid secretogogue though sustained secretion rates induced with this agonist appear to be about 40% of that produced with cholinergic stimulation (Joo et al. 2002_b_). This peptide most likely induces secretion through cAMP and protein kinase A pathways because its effects are reproduced by forskolin, a direct activator of adenylyl cyclase. The anion channel which mediates the VIP response is certainly CFTR based on studies by Joo et al. (2002_a_) who showed that the VIP-sensitive fraction of human gland fluid secretion is absent in CF airways expressing severe CFTR mutations. Comparatively less is known about the mechanism of VIP-induced anion and liquid secretion in glands. The liquid secretion response of porcine glands to forskolin, as with ACh and substance P, is sensitive to bumetanide and removal of HCO3− from the bath, indicating that these airways secrete both Cl− and HCO3− when stimulated with this agonist (Joo et al. 2002_b_). Several studies have investigated the actions of forskolin on anion secretion in the Calu-3 cell line. Devor et al. (1999) report that Calu-3 cells secrete HCO3−, rather than Cl−, when exposed to forskolin and provide evidence that HCO3− transport is principally transcellular and dependent on Na+ entry across the basolateral membrane via an electrogenic Na+–HCO3− cotransporter (NBC). They reason that HCO3− is principally secreted when cell membrane potentials are near resting values. But, at hyperpolarizing membrane potentials that result from activating populations of basolateral membrane K+ channels, HCO3− entry via NBC is inhibited, and a switch to Cl− secretion is induced. In support of this notion, these investigators found that forskolin alone, which they expected to elevate cAMP and activate the CFTR, did not increase basolateral membrane K+ conductance. However, this model may not fully represent the mechanisms by which these cells secrete anions since others report evidence that KVLQT channels are activated by forskolin in Calu-3 cells (Cowley & Linsdell, 2002). The anion exchanger AE2 is also present in Calu-3 cells and reportedly capable of supporting HCO3−-dependent Cl− secretion (Loffing et al. 2000; Cuthbert et al. 2003) and regulation of intracellular pH (Inglis et al. 2002) in this cell line. Given the appropriate driving forces, AE2 could potentially support Cl−-dependent HCO3− secretion as well.
Active secretion of Cl− (and to a lesser extent HCO3−) across the glandular epithelial cells creates an electrical gradient for cations to passively follow through the paracellular pathway. Because of its abundance in extracellular liquid, Na+ is the principal cation to move by this route and therefore is the major cation represented in gland secretions (Quinton, 1979; Jayaraman et al. 2001). The K+ concentrations in gland liquid also resemble those of extracellular fluid suggesting that little, if any, active secretion of this cation occurs in submucosal glands (Quinton, 1979).
The osmotic gradient generated by ion secretion creates the driving force for water movement across the glandular epithelia. To support high rates of liquid secretion, the serous cells of glands must have a high permeability to water. Freeze-fracture studies show that the tight junctions which form between serous cells contain significantly fewer strands than junctions between mucous cells or the cuboidal epithelial cells that line the gland ducts (Schneeberger & McCormack, 1984). The tight junctions between serous cells are permeable to colloidal lanthanum whereas junctions between these other cell types are not (Schneeberger & McCormack, 1984). Because this marker solute penetrates spaces larger than 20 Å (Revel & Karnovsky, 1967), it is likely that the tubules lined by serous cells have a relatively high paracellular permeability to water and small solutes. Indeed, even though the model depicted in Fig. 2 is consistent with electrogenic anion secretion, induction of gland secretion is associated with little or no change in the transepithelial PD or short-circuit current of intact airways (Boucher & Gatzy, 1982; Trout et al. 1998_a_), consistent with the presence of ‘leaky’ paracellular junctions that typify secretory epithelia. Transcellular water movement across glandular epithelium is probably facilitated by expression of aquaporin 5 (AQP5), a mercury-sensitive water channel, in the apical membrane of submucosal gland cells (Kreda et al. 2001; Song & Verkman, 2001).
Currently, it is unclear whether the secreted acinar fluid is modified as it passes through the gland ducts to the airway surface. mRNA for both α and β subunits of epithelial Na+ channels (ENaC) are expressed in human gland ducts, and it has been suggested that these channels might be involved in the absorption of salt and water (Burch et al. 1995). This notion is supported by observations that amiloride, an ENaC inhibitor, increases the rate of ACh-induced fluid secretion in porcine tracheal glands by 60% (Phillips et al. 2002_b_).
Role of submucosal glands in cystic fibrosis lung disease
The earliest histological indication of CF lung disease is seen in the third trimester of fetal life when the submucosal gland ducts become impacted with mucin (Ornoy et al. 1987). At birth, the lungs of CF neonates exhibit no overt signs of disease. However, infants afflicted with CF soon begin to express the clinical signs of the disease which include cough, production of a thick, inspissated mucus, impaired mucociliary transport, and unusually high susceptibility to microbial colonization. Hyperplasia of the submucosal glands and mucin occlusion of gland ducts are histological hallmarks of this disease (Zuelzer & Newton, 1949; Oppenheimer & Esterly, 1975). Because of the changes in gland morphology and the prominence of abnormal mucus in CF airways, a defect in submucosal gland secretion has been suspected as the root cause of this disease as far back as the 1940s (Zuelzer & Newton, 1949). For the decades that followed, the search for the primary defect in this disease focused largely on the physical and chemical properties of CF mucus which led to much confusion and little consensus about the mechanism of pathogenesis (Quinton, 1999). The discovery that CF was associated with a defect in epithelial Cl− permeability (Quinton, 1983) dramatically shifted the emphasis of CF research away from mucus secretion and toward epithelial anion and fluid transport because of the lack of an obvious relationship between these processes. In 1989, the defective gene which causes CF was identified and found to normally code for the CFTR (Riordan et al. 1989), which was subsequently demonstrated to be a cAMP-activated anion channel (Anderson et al. 1991). Once antibodies and mRNA probes for this protein were available, CFTR was found to be highly expressed in the serous cells of the submucosal glands where it was speculated to mediate anion and liquid secretion (Engelhardt et al. 1992). Consequently, research into CF pulmonary pathogenesis has been gradually returning to the submucosal glands.
In the tracheobronchial airways of domestic pigs, pharmacological inhibition of ACh-induced Cl− and HCO3− secretion from submucosal glands produces important correlates to CF pathology including occlusion of gland ducts with mucin (Inglis et al. 1997_b_), secretion of thick dehydrated mucus (Trout et al. 1998_b_), impairment of mucociliary transport (Ballard et al. 2002), and depletion of periciliary fluid with consequent flattening of cilia at the airway surface (Trout et al. 2003). However, because of the unavailability of highly selective CFTR inhibitors, abolition of anion secretion inhibition in these studies required inhibition of basolateral membrane transporters or use of channel blockers that potentially target non-CFTR anion channels as well. Consequently, these studies could not conclude with certainty that CFTR is the sole channel that mediates anion and liquid secretion from glands. Indeed, as noted earlier, Joo et al. (2002_a_) report that the VIP/forskolin component of gland fluid secretion is absent in CF airways but that the muscarinic component remains at least partially intact. Because maximum liquid secretion rate with VIP is only about 40% of that produced with muscarinic agonists (Joo et al. 2002_a_), one must conclude that submucosal glands in CF airways retain the capacity, albeit reduced, to secrete liquid.
These observations establish important groundwork for future study into the role that submucosal glands play in the development of CF airway disease. For instance, how do the neuronal pathways that release VIP, ACh and substance P interact to control gland liquid and glycoprotein secretion? Under what conditions are each of these neurotransmitters released? Can loss of only the VIP component lead to the airway complications associated with CF? Do anion channels other than CFTR contribute significantly to gland secretion? If CF airway glands maintain the capability to secrete liquid to VIP-independent agonists, could these pathways be selectively manipulated to increase the delivery of low-viscosity fluid to the airway surface to rescue mucociliary transport in these patients? We are hopeful that the answers to these questions will lead to a better understanding of the mechanisms of submucosal gland secretion and CF pathogenesis.
Acknowledgments
S.T.B. is supported by NIH grant HL-63302. S.K.I. is supported by a Wellcome Research Career Development Award.
References
- Anderson MP, Gregory RJ, Thompson S, Sousa DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Ballard ST, Fountain JD, Inglis SK, Corboz MR, Taylor AE. Chloride secretion across distal airway epithelium: relationship to submucosal gland distribution. Am J Physiol. 1995;268:L526–L531. doi: 10.1152/ajplung.1995.268.3.L526. [DOI] [PubMed] [Google Scholar]
- Ballard ST, Trout L, Bebök Z, Sorscher EJ, Crews A. CFTR involvement in chloride, bicarbonate and liquid secretion by airway submucosal glands. Am J Physiol. 1999;277:L694–L699. doi: 10.1152/ajplung.1999.277.4.L694. [DOI] [PubMed] [Google Scholar]
- Ballard ST, Trout L, Mehta A, Inglis SK. Liquid secretion inhibitors reduce mucociliary transport in glandular airways. Am J Physiol. 2002;283:L329–L335. doi: 10.1152/ajplung.00277.2001. [DOI] [PubMed] [Google Scholar]
- Basbaum CB, Jany B, Finkbeiner WE. The serous cell. Annu Rev Physiol. 1990;52:97–113. doi: 10.1146/annurev.ph.52.030190.000525. [DOI] [PubMed] [Google Scholar]
- Becq F, Merten MD, Voelckel MA, Gola M, Figarella C. Characterization of cAMP dependent CFTR-chloride channels in human tracheal gland cells. FEBS Lett. 1993;321:73–78. doi: 10.1016/0014-5793(93)80624-4. [DOI] [PubMed] [Google Scholar]
- Bloom W, Fawcett DW. A Textbook of Histology. 10. Philadelphia: W.B. Saunders; 1975. [Google Scholar]
- Borson DB, Chin RA, Davis B, Nadel JA. Adrenergic and cholinergic nerves mediate fluid secretion from tracheal glands of ferrets. J Appl Physiol. 1980;49:1027–1031. doi: 10.1152/jappl.1980.49.6.1027. [DOI] [PubMed] [Google Scholar]
- Borthwick DW, West JD, Keighren MA, Flockhart JH, Innes BA, Dorin JR. Murine submucosal glands are clonally derived and show a cystic fibrosis gene-dependent distribution pattern. Am J Respir Cell Mol Biol. 1999;20:1181–1189. doi: 10.1165/ajrcmb.20.6.3475. [DOI] [PubMed] [Google Scholar]
- Boucher RC, Gatzy JT. Regional effects of autonomic agents in ion transport across excised canine airways. J Appl Physiol. 1982;52:893–901. doi: 10.1152/jappl.1982.52.4.893. [DOI] [PubMed] [Google Scholar]
- Burch LH, Talbot CR, Knowles MR, Canessa CM, Rossier BC, Boucher RC. Relative expression of the human epithelial Na+ channel subunits in normal and cystic fibrosis airways. Am J Physiol. 1995;269:C511–C518. doi: 10.1152/ajpcell.1995.269.2.C511. [DOI] [PubMed] [Google Scholar]
- Choi HK, Finkbeiner WE, Widdicombe JH. A comparative study of mammalian tracheal mucous glands. J Anat. 2000;197:361–372. doi: 10.1046/j.1469-7580.2000.19730361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb BR, Ruiz F, King CM, Fortenberry J, Greer H, Kovacs T, Sorscher EJ, Clancy JP. A2 adenosine receptors regulate CFTR through PKA and PLA2. Am J Physiol. 2002;282:L12–L25. doi: 10.1152/ajplung.2002.282.1.L12. [DOI] [PubMed] [Google Scholar]
- Cowley EA, Linsdell P. Characterization of basolateral K+ channels underlying anion secretion in the human airway cell line Calu-3. J Physiol. 2002;538:747–757. doi: 10.1113/jphysiol.2001.013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert AW, Supuran CT, MacVinish LJ. Bicarbonate-dependent chloride secretion in Calu-3 epithelia in response to 7,8-benzoquinoline. J Physiol. 2003;551:79–92. doi: 10.1113/jphysiol.2003.046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B, Roberts AM, Coleridge HM, Coleridge JCG. Reflex tracheal gland secretion evoked by stimulation of bronchial C-fibers in dogs. J Appl Physiol. 1982;53:985–991. doi: 10.1152/jappl.1982.53.4.985. [DOI] [PubMed] [Google Scholar]
- Devor DC, Singh AK, Lambert LC, DeLuca A, Frizzell RA, Bridges RJ. Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J General Physiol. 1999;133:743–760. doi: 10.1085/jgp.113.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt JF, Yankaskas JR, Ernst ST, Yang Y, Marino CR, Boucher RC, Cohn JA, Wilson JM. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet. 1992;2:240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- Fischer A, Canning BJ, Kummer W. Correlation of vasoactive intestinal peptide and nitric oxide synthase with choline acetyltransferase in the airway innervation. Ann N Y Acad Sci. 1996;805:717–722. doi: 10.1111/j.1749-6632.1996.tb17547.x. [DOI] [PubMed] [Google Scholar]
- Goco RV, Kress MB, Brantigan OC. Comparison of mucus glands in the tracheobronchial tree of man and animals. Ann NY Acad Sci. 1963;106:555–571. doi: 10.1111/j.1749-6632.1963.tb16665.x. [DOI] [PubMed] [Google Scholar]
- Groneberg DA, Hartmann P, Dihn QT, Fischer A. Expression and distribution of vasoactive intestinal polypeptide receptor VPAC2 mRNA in human airways. Laboratory Invest. 2001;81:749–755. doi: 10.1038/labinvest.3780283. [DOI] [PubMed] [Google Scholar]
- Guyot A, Hanrahan JW. ATP release from human airway epithelial cells studied using a capillary culture system. J Physiol. 2002;545:199–206. doi: 10.1113/jphysiol.2002.030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haws C, Finkbeiner WE, Widdicombe JH, Wine JJ. CFTR in Calu-3 human airway cells: channel properties and role in cAMP-activated Cl− conductance. Am J Physiol. 1994;266:L502–L512. doi: 10.1152/ajplung.1994.266.5.L502. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Haxhiu-Poskurica B, Moracic V, Carlo WA, Martin RJ. Reflex and chemical responses of trachea; submucosal glands in piglets. Respir Physiol. 1990;82:267–278. doi: 10.1016/0034-5687(90)90097-i. [DOI] [PubMed] [Google Scholar]
- Hejal R, Strohl KP, Erokwu B, Cherniack NS, Haxhiu MA. Effect of hypoxia on reflex responses of tracheal submucosal glands. J Appl Physiol. 1995;78:1651–1656. doi: 10.1152/jappl.1995.78.5.1651. [DOI] [PubMed] [Google Scholar]
- Huang P, Lazarowski ER, Tarran R, Milgram SL, Boucher RC, Stutts MJ. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc Natl Acad Sci U S A. 2001;98:14120–14125. doi: 10.1073/pnas.241318498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug MJ, Bridges RJ. pH regulation and bicarbonate transport of isolated porcine submucosal glands. J Pancreas (Online) 2001;2:274–279. [PubMed] [Google Scholar]
- Inglis SK, Corboz MR, Taylor AE, Ballard ST. In situ visualization of bronchial submucosal glands and their secretory response to acetylcholine. Am J Physiol. 1997a;272:L203–L210. doi: 10.1152/ajplung.1997.272.2.L203. [DOI] [PubMed] [Google Scholar]
- Inglis SK, Corboz MR, Taylor AE, Ballard ST. Effect of anion transport inhibition on mucus secretion by airway submucosal glands. Am J Physiol. 1997b;272:L372–L377. doi: 10.1152/ajplung.1997.272.2.L372. [DOI] [PubMed] [Google Scholar]
- Inglis SK, Finlay L, Ramminger SJ, Richard K, Ward MR, Wilson SM, Olver RE. Regulation of intracellular pH in Calu-3 human airway cells. J Physiol. 2002;538:527–539. doi: 10.1113/jphysiol.2001.012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis SK, Wilson SM, Olver RE. Secretion of acid and base equivalents by intact distal airways. Am J Physiol. 2003;284:L855–L862. doi: 10.1152/ajplung.00348.2002. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Shimura S, Satoh M, Masuda T, Nonaka H, Kase H, Sasaki T, Takishima T, Tamura K. Muscarinic receptor subtypes in feline tracheal submucosal gland secretion. Am J Physiol. 1992;262:L223–L228. doi: 10.1152/ajplung.1992.262.2.L223. [DOI] [PubMed] [Google Scholar]
- Jacquot JE, Puchelle E, Hinnrasky J, Fuchey C, Bettinger C, Spilmont C, Bonnet N, Dieterle A, Dreyer D, Pavirani A, Dalemans W. Localization of the cystic fibrosis transmembrane conductance regulator in airway secretory glands. Eur Respir. 1993;J 6:169–176. [PubMed] [Google Scholar]
- Jayaraman S, Joo NS, Reitz B, Wine JJ, Verkman AS. Submucosal gland secretions in airways from cystic fibrosis patients have normal [Na+] and pH but elevated viscosity. Proc Natl Acad Sci U S A. 2001;98:8119–8123. doi: 10.1073/pnas.131087598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo NS, Irokawa T, Wu JV, Robbins RC, Whyte RI, Wine JJ. Absent secretion to vasoactive intestinal peptide in cystic fibrosis airway glands. J Biol Chem. 2002a;277:50710–50715. doi: 10.1074/jbc.M208826200. [DOI] [PubMed] [Google Scholar]
- Joo NS, Saenz Y, Krouse ME, Wine JJ. Mucus secretion from single submucosal glands of pig. J Biol Chem. 2002b;277:28167–28175. doi: 10.1074/jbc.M202712200. [DOI] [PubMed] [Google Scholar]
- Joo NS, Wu JV, Krouse ME, Saenz Y, Wine WW. Optical method for quantifying rates of mucus secretion from single submucosal glands. Am J Physiol. 2001;281:L458–L468. doi: 10.1152/ajplung.2001.281.2.L458. [DOI] [PubMed] [Google Scholar]
- Khawaja AM, Liu Y-C, Rogers DF. Effect of non-peptide tachykinin NK1 receptor antagonists on non-adrenergic, non-cholinergic neurogenic mucus secretion in ferret trachea. Eur J Pharmacol. 1999;384:173–181. doi: 10.1016/s0014-2999(99)00694-9. [DOI] [PubMed] [Google Scholar]
- Kreda SM, Gynn MC, Fenstermacher DA, Boucher RC, Gabriel SE. Expression and localization of epithelial aquaporins in the adult human lung. Am J Respir Cell Mol Biol. 2001;24:224–234. doi: 10.1165/ajrcmb.24.3.4367. [DOI] [PubMed] [Google Scholar]
- Loffing J, Moyer BD, Reynolds D, Shmukler BE, Alper SL, Stanton BA. Functional and molecular characterization of an anion exchanger in airway serous epithelial cells. Am J Physiol. 2000;279:C1016–C1023. doi: 10.1152/ajpcell.2000.279.4.C1016. [DOI] [PubMed] [Google Scholar]
- Meyrick B, Sturgess JM, Reid L. A reconstruction of the duct system and secretory tubules of the human bronchial submucosal gland. Thorax. 1969;24:729–736. doi: 10.1136/thx.24.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S, Singh M, Krouse ME, Wine JJ. Calcium-stimulated Cl− secretion in Calu-3 human airway cells requires CFTR. Am J Physiol. 1997;273:L1208–L1219. doi: 10.1152/ajplung.1997.273.6.L1208. [DOI] [PubMed] [Google Scholar]
- Nadel JA, Davis B. Regulation of Na+ and Cl− transport and mucous gland secretion in airway epithelium. Ciba Foundation Symp. 1978;54:133–147. doi: 10.1002/9780470720356.ch7. [DOI] [PubMed] [Google Scholar]
- Nagaki M, Ishihara H, Shimura S, Sasaki T, Takishima T, Shirato K. Tachykinins induce a [Ca2+]i rise in the acinar cells of feline tracheal submucosal gland. Respir Physiol. 1994;98:111–120. doi: 10.1016/0034-5687(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Oppenheimer EH, Esterly JR. Pathology of cystic fibrosis: review of the literature and comparison with 146 autopsied cases. In: Dosenberg HS, Bolarde RP, editors. Perspectives in Pediatric Pathology. Vol. 2. Chicago: Yearbook Medical Publications; 1975. pp. 421–278. [PubMed] [Google Scholar]
- Ornoy A, Arnon J, Katznelson D, Granat M, Caspi B, Chemke J. Pathological confirmation of cystic fibrosis in the fetus following prenatal diagnosis. Am J Med General. 1987;28:935–947. doi: 10.1002/ajmg.1320280420. [DOI] [PubMed] [Google Scholar]
- Phillips JE, Hey JA, Corboz MR. Computer assisted measurement of airway gland secretions by the hillocks technique. Comp Meth Prog Biomed. 2002a;68:215–222. doi: 10.1016/s0169-2607(01)00170-5. [DOI] [PubMed] [Google Scholar]
- Phillips JE, Hey JA, Corboz MR. Effects of ion transport inhibitors on MCh-mediated secretion from porcine airway submucosal glands. J Appl Physiol. 2002b;93:873–881. doi: 10.1152/japplphysiol.00174.2002. [DOI] [PubMed] [Google Scholar]
- Phillips JE, Hey JA, Corboz MR. Tachykinin NK3 and NK1 receptor activation elicits secretion from porcine airway submucosal glands. Br J Pharmacol. 2003;138:254–260. doi: 10.1038/sj.bjp.0705029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton PM. Composition and control of secretions from tracheal bronchial submucosal glands. Nature. 1979;279:551–552. doi: 10.1038/279551a0. [DOI] [PubMed] [Google Scholar]
- Quinton PM. Possible mechanisms of stimulus-induced vacuolation in serous cells of tracheal secretory glands. Am J Physiol. 1981;241:C25–C32. doi: 10.1152/ajpcell.1981.241.1.C25. [DOI] [PubMed] [Google Scholar]
- Quinton PM. Chloride impermeability in cystic fibrosis. Nature. 1983;301:421–422. doi: 10.1038/301421a0. [DOI] [PubMed] [Google Scholar]
- Quinton PM. Physiological basis of cystic fibrosis: A historical perspective. Physiol Rev. 1999;79:S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- Revel JP, Karnovsky MJ. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967;33:C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B-S, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou J-L, Drumm ML, Iannuzzi MC, Collins FS, Tsui L-C. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rogers DF. Airway submucosal gland and goblet cell secretion. In: Chung KF, Barnes PJ, editors. In Pharmacology of the Respiratory Tract. Experimental and Clinical Research. New York: Marcel Dekker; 1993. pp. 583–620. [Google Scholar]
- Rogers DF. Motor control of airway goblet cells and glands. Respir Physiol. 2000;125:129–144. doi: 10.1016/s0034-5687(00)00209-7. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Shimura S, Ikeda K, Sasaki H, Takishima T. Sodium efflux from isolated submucosal gland in feline trachea. Am J Physiol. 1990;258:L112–L117. doi: 10.1152/ajplung.1990.258.2.L112. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Shimura S, Wakui M, Ohkawara Y, Takishima T, Mikoshiba K. Apically localized IP3 receptors control chloride current in airway gland acinar cells. Am J Physiol. 1994;267:L152–L158. doi: 10.1152/ajplung.1994.267.2.L152. [DOI] [PubMed] [Google Scholar]
- Schneeberger EE, McCormack JM. Intracellular junctions in upper airway submucosal glands of the rat: a tracer and freeze fracture study. Anat Rec. 1984;210:421–433. doi: 10.1002/ar.1092100303. [DOI] [PubMed] [Google Scholar]
- Schuster A, Ueki I, Nadel JA. Neutrophil elastase stimulates tracheal submucosal gland secretion that is inhibited by ICI 200,355. Am J Physiol. 1992;262:L86–L91. doi: 10.1152/ajplung.1992.262.1.L86. [DOI] [PubMed] [Google Scholar]
- Shen BQ, Finkbeiner WE, Wine JJ, Mrsny RJ, Widdicombe JH. Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl− secretion. Am J Physiol. 1994;266:L493–L501. doi: 10.1152/ajplung.1994.266.5.L493. [DOI] [PubMed] [Google Scholar]
- Shimura S, Ishihara H, Nagaki M, Sasaki H, Takishima T. A stimulatory role of protein kinase C in feline tracheal submucosal gland secretion. Respir Physiol. 1993;93:239–247. doi: 10.1016/0034-5687(93)90008-x. [DOI] [PubMed] [Google Scholar]
- Shimura S, Sasaki T, Okayama H, Sasaki H, Takishima T. Neural control of contraction in isolated submucosal gland from feline trachea. J Appl Physiol. 1987;62:2404–2409. doi: 10.1152/jappl.1987.62.6.2404. [DOI] [PubMed] [Google Scholar]
- Shimura S, Sasaki T, Sasaki H, Takishima T. Contractility of isolated single submucosal gland from trachea. J Appl Physiol. 1986;60:1237–1247. doi: 10.1152/jappl.1986.60.4.1237. [DOI] [PubMed] [Google Scholar]
- Shimura S, Takishima T. Airway submucosal gland secretion. In: Takishima T, Shimura S, editors. Airway Secretion: Physiological Bases for the Control of Mucous Hypersecretion. New York: Marcel Dekker; 1994. pp. 325–398. [Google Scholar]
- Smith PL, Frizzell RA. Chloride secretion by canine tracheal epithelium: IV. Basolateral membrane K permeability parallels secretion rate. J Membr Biol. 1984;77:187–199. doi: 10.1007/BF01870568. [DOI] [PubMed] [Google Scholar]
- Sommerhoff CP, Finkbeiner WE. Human tracheobronchial submucosal gland cells in culture. Am J Respir Cell Mol Biol. 1990;2:41–50. doi: 10.1165/ajrcmb/2.1.41. [DOI] [PubMed] [Google Scholar]
- Song Y, Verkman AS. Aquaporin-5 dependent fluid secretion in airway submucosal glands. J Biol Chem. 2001;276:41288–41292. doi: 10.1074/jbc.M107257200. [DOI] [PubMed] [Google Scholar]
- Sorkin SP. On the cytology and cytochemistry of the opossum bronchial glands. Am J Anat. 1965;117:311–338. doi: 10.1002/aja.1001170302. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Sens MA, Tashian RE. Immunocytochemical demonstration of carbonic anhydrase in human epithelial cells. J Histochem Cytochem. 1982;30:864–873. doi: 10.1177/30.9.6813372. [DOI] [PubMed] [Google Scholar]
- Steiger J, Bray MA, Subramanian N. Platelet-activating factor (PAF) is a potent stimulator of porcine tracheal fluid secretion in vitro. Eur J Pharmacol. 1987;142:367–372. doi: 10.1016/0014-2999(87)90075-6. [DOI] [PubMed] [Google Scholar]
- Tabcharani JA, Chang X-B, Riordan JR, Hanrahan JW. Phosphorylation-regulated Cl− channel in CHO cells stably expressing the cystic fibrosis gene. Nature. 1991;352:628–631. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- Takizawa T, Thurlbeck WM. A comparative study of four methods of assessing the morphologic changes in chronic bronchitis. Am Rev Respir Dis. 1971;103:774–783. doi: 10.1164/arrd.1971.103.6.774. [DOI] [PubMed] [Google Scholar]
- Tos M. Development of the tracheal glands in man. Acta Pathol Microbiol Scand supplement. 1966;185:1–130. [PubMed] [Google Scholar]
- Tos M. Mucous elements in the airways. Acta Otolaryngol. 1976;82:249–251. doi: 10.3109/00016487609120896. [DOI] [PubMed] [Google Scholar]
- Trout L, Corboz MR, Ballard ST. Mechanism of substance P-induced liquid secretion across porcine bronchial epithelium. Am J Physiol. 2001;281:L639–L645. doi: 10.1152/ajplung.2001.281.3.L639. [DOI] [PubMed] [Google Scholar]
- Trout L, Gatzy JT, Ballard ST. Acetylcholine-induced liquid secretion by bronchial epithelium: role of Cl− and HCO3− transport. Am J Physiol. 1998a;275:L1095–L1099. doi: 10.1152/ajplung.1998.275.6.L1095. [DOI] [PubMed] [Google Scholar]
- Trout L, King M, Feng W, Inglis SK, Ballard ST. Inhibition of airway liquid secretion and its effects on the physical properties of airway mucus. Am J Physiol. 1998b;274:L258–L263. doi: 10.1152/ajplung.1998.274.2.L258. [DOI] [PubMed] [Google Scholar]
- Trout L, Townsley MI, Bowden A, Ballard ST. Disruptive effects of anion secretion inhibitors on airway mucus morphology in isolated perfused pig lungs. J Physiol. 2003;549:845–853. doi: 10.1113/jphysiol.2002.035923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki I, German VF, Nadel JA. Micropipette measurement of airway submucosal gland secretion. Autonomic effects. Am Rev Respir Dis. 1980;121:351–357. doi: 10.1164/arrd.1980.121.2.351. [DOI] [PubMed] [Google Scholar]
- Webber SE. Receptors mediating the effects of substance P and neurokinin A on mucus secretion and smooth muscle tone of the ferret trachea: potentiation by an enkephalinase inhibitor. Br J Pharmacol. 1989;98:1197–1206. doi: 10.1111/j.1476-5381.1989.tb12665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber SE, Lim JCS, Widdicombe JG. The effects of calcitonin gene-related peptide on submucosal gland secretion and epithelial albumin transport in the ferret trachea in vitro. Br J Pharmacol. 1991;102:79–84. doi: 10.1111/j.1476-5381.1991.tb12135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe JH, Chen LL, Sporer H, Choi HK, Pecson IS, Bastacky SJ. Distribution of tracheal and laryngeal mucous glands in some rodents and the rabbit. J Anat. 2001;198:207–221. doi: 10.1046/j.1469-7580.2001.19820207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaya M, Ourui T, Finkbeiner WE, Widdicombe JH. Calcium-dependent chloride secretion across cultures of human tracheal surface epithelium and glands. Am J Physiol. 1993;265:L170–L177. doi: 10.1152/ajplung.1993.265.2.L170. [DOI] [PubMed] [Google Scholar]
- Yamaya M, Sekizawa K, Kakuta Y, Ohrui T, Sawai T, Sasaki H. P2U-purinoceptor regulation of chloride secretion in cultured human tracheal submucosal glands. Am J Physiol. 1996;270:L979–L984. doi: 10.1152/ajplung.1996.270.6.L979. [DOI] [PubMed] [Google Scholar]
- Yang CM, Farley JM, Dwyer TM. Acetylcholine-stimulated chloride flux in tracheal submucosal gland cells. J Appl Physiol. 1988;65:1891–1894. doi: 10.1152/jappl.1988.65.4.1891. [DOI] [PubMed] [Google Scholar]
- Zuelzer WW, Newton WA. The pathogenesis of fibrocystic disease of the pancreas. Pediatrics. 1949;4:53–69. [PubMed] [Google Scholar]