Histamine and its receptors (original) (raw)
Abstract
This article reviews the development of our knowledge of the actions of histamine which have taken place during the course of the 20th century. Histamine has been shown to have a key physiological role in the control of gastric acid secretion and a pathophysiological role in a range of allergic disorders. The synthesis of, and pharmacological studies on, selective agonists and antagonists has established the existence of four types of histamine receptor and histamine receptor antagonists have found very important therapeutic applications. Thus, in the 1940s, H1-receptor antagonists (‘the antihistamines') yielded and still provide valuable treatment for allergic conditions such as hay fever and rhinitis. In the late 1970s and 1980s, H2-receptor antagonists (in the discovery of which the two authors were personally involved) revolutionised the treatment of peptic ulcer and other gastric acid-related diseases. The H3-receptor antagonists, although available since 1987, have been slower to find a therapeutic role. However, the discovery of nonimidazole derivatives such as brain-penetrating H3 antagonists has provided drugs that are in early-phase clinical trials, possibly for application in obesity, and a variety of central nervous system disorders, such as memory, learning deficits and epilepsy. Finally, the most recently (1999) discovered H4 receptor promises the potential to provide drugs acting on the immunological system with possible applications in asthma and inflammation.
Keywords: Antihistamines, H2-receptor antagonists, H3-receptor antagonists, H4 receptors constitutive activity, gastric acid secretion, allergy, mepyramine, burimamide, cimetidine, thioperamide, JNJ 7777120
Introduction
It is now almost one hundred years since Sir Henry Dale (Figure 1) and his colleagues at the Wellcome Laboratories isolated histamine from the mould ergot. They then went on to carry out a ground-breaking series of experiments to explore its biological actions. They found that it had a stimulant effect on smooth muscle from the gut and respiratory tract, caused vasodepression, stimulated cardiac contractility and induced a shock-like syndrome when injected into animals (Dale & Laidlaw, 1910; 1919). Popielski (1920) demonstrated that histamine had a marked stimulant effect on the secretion of acid from the stomach of dogs. In 1924, Lewis described the classic ‘triple response' to histamine consisting of a red spot due to vasodilatation, a wheal which was the consequence of increased permeability and flare due to an axon reflex (Lewis & Grant, 1924).
Figure 1.
(a) Sir Henry Dale, (b) Professor Heinz Schild, (c) Sir James Black, (d) Professor Jean-Charles Schwartz.
However, it was not until 1927, when Best et al. (1927) isolated histamine from samples of liver and lungs, that this amine was found to be a natural constituent of the body, although some people thought that it had arisen simply due to the breakdown of histidine. Subsequently, the release of histamine in association with the anaphylactic reaction was demonstrated by showing the difference in histamine content of the lung before and after shock. Similarly, the marked increase in the histamine content of blood after anaphylaxis was sufficient to explain the shock.
The first antihistamines
The evidence that histamine had an active role in allergy and anaphylaxis was the stimulus for the search for compounds being able to counteract the pathological effects of histamine, which began at the Pasteur Institute in Paris, in the 1930s, where Bovet was working. He had access to Fourneau's bank of compounds and, as he stated in his Nobel lecture: “considering the number of features that histamine, acetylcholine, and epinephrine have in common, we looked for antagonism comparable to that exhibited by sympatholytic compounds toward epinephrine and by parasympatholytic compounds toward acetylcholine”. The first compound reported as an antihistamine by Ungar, Parrot and Bovet was the adrenolytic benzodioxan, piperoxan (933F), in 1937, which blocked the effect of histamine on the guinea-pig ileum. This was followed shortly afterwards by the report by Bovet & Staub (1937) of structurally related aryl ethers such as the thymol ether 929F, which protected the guinea-pig from the lethal effects of histamine-induced anaphylaxis. The latter compound proved to be too toxic for clinical development, but replacement of the ether oxygen by an amino group led to the discovery of aniline ethylene diamine derivatives. For his work on antihistamines and curare, Bovet was awarded the 1957 Nobel Prize for Physiology or Medicine.
The work on anilino compounds was followed up in France in collaboration with Rhone-Poulenc and, independently during the war years (1939–1945), by researchers in the U.S.A. The first antihistamine to be used in man in 1942 was Antergan™ (phenbenzamine, RP 2339), but this was subsequently replaced by Neoantergan™ (mepyramine, pyrilamine, RP 2786; Figure 2), which is still in use topically to counteract the unpleasant effects of histamine release in the skin. Many other antihistamines followed, such as diphenhydramine (Benadryl™), tripelennamine, chlorpheniramine and promethazine.
Figure 2.
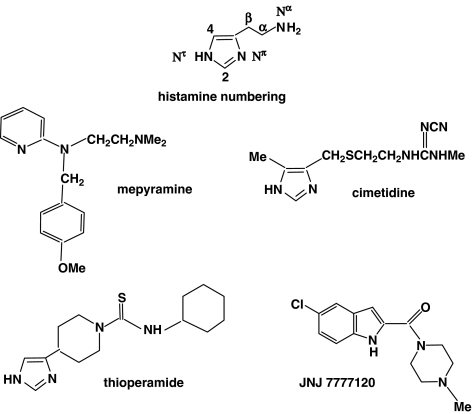
The structures of histamine and of key antagonist ligands.
After 1945, these antihistamines became widely used in the treatment of various allergic disorders such as hay fever, allergic rhinitis and urticaria. However, side effects were not uncommon and sedation was a drawback to their use (however, see below). Some side effects were put to good use; thus, antihistamines such as cyclizine (Marzine™) and diphenhydramine in the form of its 8-chlorotheophyllinate (Dramamine™) are used mostly as antiemetics for travel sickness.
Histamine receptors
The classical antihistamines antagonised the effects of histamine on a variety of smooth muscles, but it became apparent that some of the actions of histamine were refractory to inhibition by these drugs. In vitro studies showed that histamine's ability to increase heart rate and inhibit contraction of the rat uterus was not blocked by mepyramine and related drugs. Similarly, histamine-stimulated gastric secretion was shown to be unresponsive to three different antihistamines (Ashford et al., 1949). Folkow et al. (1948) found that the vasodilator response to lower doses of histamine in the anaesthetised cat could be antagonised by Benadryl but higher doses were completely refractory, and this led the authors in the summary of their article to suggest, for the first time, that ‘there are two types of receptors sensitive to histamine only one of which can be blocked by Benadryl and related compounds'.
One year prior to Folkow's work, a paper was published in the British Journal of Pharmacology by Schild (1947) (Figure 1), which has contributed considerably to the analysis of histamine receptor populations. It described the development of a new scale for the measurement of drug antagonism – the pA2 value (see also Rang, this issue). In the introduction to the paper, Schild stated that
when the activity of a new drug or drug antagonist has to be defined in terms of some other drug or of some of its own effects, the results are not equally reproducible since the apparent activity varies in successive experiments even though conditions are kept as constant as possible. The difficulty of making results of one laboratory available to another is aggravated by the multiplicity of methods used and frequently by the lack of information of their variability: this applies particularly to methods of expressing drug antagonism. It would obviously be of advantage if some common method of expressing drug antagonism could be agreed upon. In the present paper it is proposed to introduce a new measure of drug antagonism pA.
The introduction of the pA measurement strongly supported the concept of the existence of at least two distinct receptors for histamine, since the value for mepyramine in antagonising the positive chronotropic effect of histamine on the guinea-pig heart differed from that obtained against the contractile response of the isolated guinea-pig ileum.
Histamine storage and release
Although evidence for histamine receptor heterogeneity had been provided in the 1940s, much of the research activity on histamine at this time was devoted to the study of its storage, release and metabolism. This involved several of the doyens of British pharmacology, such as Blaschko, Feldberg, Gaddum, Mongar, Paton, Perry, Riley, Schild, Trendelenburg and West. Much of this work was brought together at a CIBA Foundation Symposium jointly with the Physiological Society and the British Pharmacological Society on Histamine in honour of Sir Henry Dale and published in 1956. Detailed discussion of the research in this period would be out of place here and readers are referred to the proceedings of this CIBA symposium (Schayer, 1956).
However, some of the highlights of histamine research in the 1950s and early 1960s will be considered. The storage of histamine in mast cells was established by Riley and West when they found that chemicals capable of inducing histamine release also disrupted mast cells and this was accompanied by a fall in tissue histamine content (Riley & West, 1952). Further, there was a strong positive correlation between histamine content and mast cell populations in a variety of tissues. Many of the studies on histamine release were performed using compound 48/80 and first described in detail by Paton in 1951 (Feldberg & Mongar, 1954). However, Riley and West were careful to point out that some tissues contained too few mast cells to account for their histamine content, and suggested that other cells may store histamine. Subsequently, it was shown that the blood basophil, blood platelets in some species and the enterochromaffin-like cell (ECL) in the stomach were additional sources of histamine. In mammalian species, histamine is now known to be present in all tissues in amounts ranging from less than 1 to over 100 _μ_g g−1 and, in general, the skin, connective tissue, lung and much of the gastrointestinal tract are rich in histamine.
Histamine metabolism
The origin and fate of histamine in the body was extensively studied during this period and Schayer (1956) showed that histamine is formed from 1-histidine by the action of the enzyme histidine decarboxylase and specific inhibitors of this enzyme such as _α_-fluoromethyl histamine have been described. The two major pathways for the catabolism of histamine were shown to be via diamine oxidase and histamine methyl transferase. The study of the former pathway was greatly facilitated by the availability of a potent and highly specific inhibitor of diamine oxidase, aminoguanidine. The half-life of pharmacologically active doses of histamine is less than 10 s in the rat and 20–30 s in the dog. In the early studies histamine levels were measured by bioassay, but subsequently fluorometric and radio-enzymatic techniques were employed.
Histamine H2 receptors
The pharmacological actions of histamine continued to be studied. In particular, its stimulant effect on gastric acid secretion was intensively studied by Code (1956) and colleagues, and they concluded that this action was not simply a pharmacological phenomenon but that histamine had a physiological role in controlling acid secretion, a suggestion to be confirmed some 16 years later, with the discovery of histamine H2-receptor antagonists.
Although histamine was confirmed as a potent vasodilator, its role in various vasodilator phenomena exhibited by the peripheral circulation, for example, immersion in cold water, was not established. This was, in part, because of negative results with the antihistamines available at the time and before the discovery of H2 receptors on the vasculature. The existence of histaminergic nerves was also proposed, but their functions would have to wait until the discovery of histamine H3 receptors and their agonists and antagonists.
The findings that the classical antihistaminic drugs failed to block all of the actions of histamine led to a research programme being commenced at the laboratories of SmithKline and French in Welwyn Garden City, U.K., under the direction of Dr James Black (Figure 1). The objective of the programme was to confirm histamine receptor heterogeneity and to discover an antagonist of the histamine receptor refractory to conventional antihistamines. It was argued that such an agent might be used to inhibit histamine-stimulated gastric acid secretion and thereby provide a potential therapy for acid-related diseases such as peptic ulcer and gastro-oesophageal reflux disease (GORD). This was a brave decision for a pharmaceutical company because, at this time (mid-1960s), the hormone gastrin had been isolated and sequenced at Liverpool University and this gastric secretagogue received the attention of most gastric physiologists and pharmaceutical companies.
In this search for an antagonist of the ‘other' histamine receptor, the chemical starting point was based on an analogy with the catecholamine field; _α_-adrenoceptor antagonists are structurally unrelated to the adrenergic transmitters, adrenaline and noradrenaline, whereas the early _β_-receptor antagonists resembled them quite closely chemically. Similarly, the classical antihistamines are bulky molecules structurally very different from the simple imidazole ring and ethylamine side chain of histamine, although, like histamine, they possess a basic amino group. Therefore, it was decided that the chemical starting point in the search for an antagonist of the hypothecated second class of histamine receptor would be the structure of histamine itself (Figure 2).
Five bioassays were established, two (guinea-pig ileum in vitro and the rat stomach in vivo) in which the responses to histamine were antagonised by drugs such as mepyramine and three (guinea-pig atrium and rat uterus in vitro and rat gastric secretion in vivo) where histamine responses were refractory to available antihistamines.
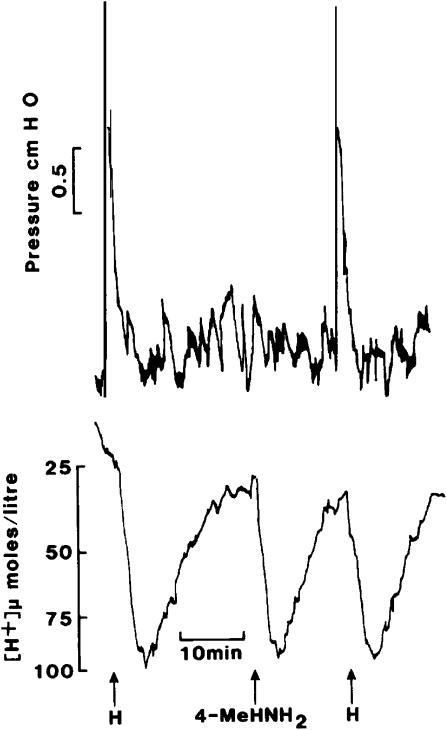
At a relatively early stage, these assays were used to study the agonist activity of a range of methylated histamines and some degree of selectivity for molecules having a methyl group on either the 2 or 4 position of the imidazole ring was established. The former had 17% of the potency of histamine of the ileum, but only 4% on the atrium. The difference was more dramatic for 4-methylhistamine, which had 40% the activity of histamine on the atrium but only 0.2% the potency in contracting the ileum. In vivo, this was confirmed by measuring gastric acid secretion and contraction of the stomach wall simultaneously in the anaesthetised rat. A dose of 4-methylhistamine which produced a secretory response comparable to that produced by 2 _μ_g kg−1 histamine by i.v. bolus injection failed to cause contraction of the stomach wall (Figure 3). These data provided further support for the concept of two histamine receptor populations. Similar studies were performed by Ash & Schild (1966) and they proposed the term H1 for the receptor blocked by the known antihistamines. However, to confirm the existence of a second class of histamine receptor, a selective antagonist was required.
Figure 3.
Effects of i.v. bolus injections of histamine (H, 2 _μ_g kg−1) and 4-methylhistamine (4MeHNH2, 5 _μ_g kg−1) upon secretion of gastric acid and stomach wall contraction in an anaesthetised rat.
H2-receptor antagonists
The breakthrough in the search for such a ligand came with the synthesis of N _α_-guanylhistamine, which was found to be a weak partial agonist on the heart, uterus and gastric secretion. In these days of combinatorial chemistry and robotic screening, it may seem strange that in the years between 1964 and 1972 only 700 compounds were synthesised and tested but success was achieved with the discovery of burimamide, which was 100 times more potent than N _α_-guanylhistamine and did not act as a partial agonist. Burimamide was shown to be a highly specific, competitive antagonist of the actions of histamine on non-H1 receptor-containing tissues and allowed the definition of these receptors as histamine H2 receptors and characterised burimamide as an H2-receptor antagonist (Black et al., 1972). The human pharmacology of burimamide was studied, but the compound lacked sufficient oral activity to explore its clinical potential.
A second, more potent compound with good oral bioavailability, was soon developed. Metiamide was taken into the clinic and proved to be of therapeutic value in duodenal ulcer disease. However, the occurrence of a reversible granulocytopenia in a small number of patients precluded its commercial exploitation. At first it was thought that this toxic effect might be a consequence of the pharmacology of metiamide, since H2 receptors had been identified on a range of white cells but, in fact, it was the chemistry of the molecule and, in particular, the thiourea group in the side chain that was the cause of the toxicity.
Subsequently, a molecule in which the thiourea group was replaced with a cyanoguanidine group was developed, which lacked the toxicity of metiamide. Cimetidine (Figure 2) was shown to be a competitive antagonist against histamine on the atrium and uterus and to produce a dose-related inhibition after oral and intravenous administration of histamine-stimulated gastric acid secretion (Brimblecombe et al., 1975). A large number of clinical trials demonstrated that cimetidine was effective in the treatment of acid-related diseases such as peptic ulcer and GORD and it was marketed under the trade name Tagamet. It should be noted that the structural similarity between the early histamine H2-receptor antagonists and histamine itself vindicated the original analogy with the _α_- and _β_-receptor antagonists in the catecholamine system. However, whereas the first _β_-receptor antagonist required a structural change to the ring of the agonist (isoprenaline) while retaining an unaltered side chain, for the first H2 antagonist, it was the ring that had to be retained while the side chain was modified. Although one works by analogy, Nature is always ready to fool the scientist!
Not only did the development of H2-receptor antagonists revolutionise the treatment of peptic ulcer and GORD, but it placed histamine in the forefront of the physiological control of gastric acid secretion. As early as 1938, it had been proposed that histamine in the gastric mucosa might be the local common mediator for stimulation of secretion, a proposition championed by Code and others throughout the 1950s and 1960s. Controversy raged during this period with many gastroenterologists placing the hormone gastrin, released from the antrum of the stomach, and acetylcholine, released from the vagal nerves, at the centre of secretory control. However, the fact that histamine H2-receptor antagonists could inhibit acid secretion stimulated not only by histamine but also that by gastrin and acetylcholine firmly placed histamine as the key transmitter. This was further supported by the ability of H2 antagonists to inhibit vagally stimulated secretion and that to the more physiological stimulus of food ingestion. It was the ability of H2 antagonists to inhibit nearly all forms of stimulated and basal secretion in man that led to their clinical efficacy.
After cimetidine, several other H2-receptor antagonists were developed (Cooper et al., 1990) and in particular ranitidine (Bradshaw et al., 1979). This compound was more potent than cimetidine and, unlike cimetidine, did not affect the cytochrome _P_450 enzymes in the liver. It therefore did not have the propensity for drug interactions, which, to a limited extent, had occurred with cimetidine. In excess of 20 H2 antagonists entered clinical development but only three in addition to cimetidine and ranitidine (famotidine, nizatidine and roxatidine) were marketed, reflecting the high attrition rate in drug development. Sir James Black shared the 1988 Nobel Prize for Physiology or Medicine for his work on _β_-receptor antagonists at ICI and on the histamine H2-receptor antagonists at SmithKline & French.
The search for other roles for histamine
The advent of the histamine H2-receptor antagonists led to an enormous revival of interest in the role of histamine in physiology and pathology. In the decade after the first publication on burimamide in 1972, over 1000 papers reported work on aspects of H2 receptors. Much of this work is brought together in a book entitled ‘Pharmacology of Histamine Receptors' published in 1982 (Ganellin & Parsons, 1982). At this time, investigation into the effects of histamine in a range of biological systems was assisted by the availability of relatively selective histamine H1- and H2-receptor agonists such as 2-thiazolylethylamine (H1) and dimaprit (H2). Subsequently, highly selective agonists were developed, such as 2-(3-trifluoromethylphenyl)histamine and histaprodifen for the H1 receptor (Pertz et al., 2004) and impromidine and arpromidine for the H2 receptor (Cooper et al., 1990).
A highlight during this period was the establishment of adenylate cyclase/cyclic AMP as the primary second messenger system for the action of histamine at H2 receptors (Hill et al., 1997). Perhaps surprisingly, it was not until the early 1990s that it was established that activation of the histamine H1 receptor led to either inositol phosphate accumulation or intracellular calcium mobilization (see also Nahorski, this issue). At both receptor subtypes histamine acts via G-proteins, but in the case of the H1 receptor this is pertussis toxin-insensitive and is probably related to the Gq/11 family, whereas histamine H2 receptors are generally accepted to act via the Gs family of G-proteins (see also Milligan & Kostenis, this issue).
Major advances were made in the understanding of the role of histamine and its receptors in the cardiovascular system. Unlike the stimulation of gastric secretion where solely H2 receptors are involved, both H1 and H2 receptors mediate the effects of histamine on the cardiovascular system. For example, systemic administration of histamine lowers blood pressure by interaction with H1 and H2 receptors. Further studies have indicated that the interaction between histamine and H1 and H2 receptors is, in part, time dependent. Infusions of histamine caused an immediate large fall in blood pressure, which persisted for the duration of the infusion. H1-receptor blockade with mepyramine significantly reduced the immediate fall in blood pressure with little effect on the sustained response, whereas H2-receptor blockade with cimetidine had little or no effect on the immediate response, but significantly reduced the sustained response. Combined H1 and H2 blockade abolished the histamine responses throughout the infusion. In the heart, the chronotropic response to histamine is H2 mediated, whereas the inotropic effects are predominantly through the H1 receptor.
In the lung, the application of selective H1- and H2-receptor agonists and antagonists established the concept that the two receptor populations may mediate opposing physiological and pharmacological effects in the pulmonary system. H1 receptors mediate actions such as bronchoconstriction, vasoconstriction and oedema formation, effects which may be deleterious in nature. Stimulation of pulmonary H2 receptors plays a modulatory role, causing bronchodilation and inhibiting mediator release. Histamine also plays a major role in immunologically mediated inflammation, having multiple pro- and anti-inflammatory effects involving the histamine-containing basophils.
The use of histamine H1 antagonists in the therapy of some allergic disorders had been established in the early 1950s and the advent of H2-receptor antagonists, some 20 years later, revolutionised the treatment of acid-related diseases. However, other potential therapeutic uses for H1 and H2 antagonists proved elusive. For example, a role for histamine in the aetiology of migraine and cluster headache had been suggested, but clinical studies with an H2 antagonist showed no clinical benefit. Similarly, evidence existed to suggest that combined treatment with an H1 and H2 antagonist might be beneficial in the treatment of inflammatory skin disorders such as pruritis and urticaria, but these conditions do not appear to be targets for combined antihistamine therapy. This presumably reflects the multiplicity of inflammatory mediators, in addition to histamine, that are involved in these disorders.
Histamine and the brain
The brain was one of the last organs in which histamine receptors were identified. This was partly because systemically applied histamine does not pass through the blood–brain barrier and it was believed that the function of histamine receptors in the brain was to respond to the action of endogenously released mediator. Subsequently, histaminergic neurones have been identified in a number of areas of the brain. Schwartz (Figure 1) and his colleagues used lesioning techniques to predict the existence of histaminergic neurones (Schwartz, 1975). Thus, unilateral lesions of the median forebrain bundle in the lateral hypothalamus resulted in the decrease of histidine decarboxylase (HDC) activity (used as a marker of histamine neurones) in various ipsilateral regions of the rat forebrain. The existence of histaminergic neurones was confirmed by immunohistochemistry using anti-HDC antibodies by Watanabe et al. (1984). Histaminergic neurones are localized in the tuberomammillary nucleus of the hypothalamus, project to all major areas of the brain and are involved in many functions including the regulation of sleep/wakefulness, feeding and memory processes. The existence of H1 receptors underlies the sedative effects of many of the classical H1 antagonists, even at therapeutic dosage. Most of the H1-receptor antihistamines are lipophilic compounds that readily penetrate into the brain; however, several drugs are now available that penetrate poorly, such as fexofenadine and cetirizine, which appear to be devoid of central depressant effects.
There are also H2 receptors in the brain, but their function has not been identified. One problem hindering their study is that most of the available H2-receptor antagonists are very polar substances which do not really penetrate the blood–brain barrier. However, one compound, zolantidine, was specifically designed to penetrate (Cooper et al., 1990), but, so far, it has only provided evidence for a possible involvement of H2 receptors in opioid antinociception.
Histamine H3 receptors
Schwartz (1975) and his colleagues also studied histamine release from cerebral neurones in rat cortex. They discovered that histamine could inhibit its own release, and they used H1 and H2 antagonists to characterise the receptor involved. This effect was competitively inhibited by burimamide at nanomolar concentrations (which is far below the concentration required for H2 receptor antagonism), but more potent H2 receptor antagonists were much less active; H1 antihistamines were ineffective. This led them to suggest, in 1983, the possibility of a H3 receptor. Their suggestion was confirmed and the receptor definitively characterised in 1987 by their discovery that (R)-_α_-methylhistamine was a potent agonist (chirally selective since the S isomer is much less potent) and that thioperamide (Figure 2) was a very specific competitive antagonist (Arrang et al., 1987). Interestingly, thioperamide can be considered as a homologue of burimamide in which the side chain has been cyclised into a piperidine ring and the thiourea-_N_-methyl group has been replaced by a thiourea-_N_-cyclohexyl group.
Histamine H3 receptors act as presynaptic autoreceptors that inhibit the synthesis and release of histamine in the histaminergic neurones in the central nervous system (CNS). They also occur as hetero-receptors on nonhistaminergic neurones, modulating the release of other neurotransmitters such as 5-hydroxytryptamine, dopamine, acetylcholine, noradrenaline and GABA in the CNS and periphery. Ligands for the H3 receptor have been reviewed (Stark et al., 2001; Leurs et al., 2005) and potential therapeutic applications, which have been proposed for an antagonist, include obesity and a variety of CNS disorders such as memory and learning deficits, Alzheimer's disease, epilepsy, schizophrenia and sleep disturbance.
Thioperamide has been widely used for investigating the involvement and role of H3 receptors in physiology. Although thioperamide is very potent in vitro (_K_i=4.3 nM), relatively high doses are required in vivo (e.g. ED50 values of about 2 mg kg−1 i.p. and about 5 mg kg−1 p.o. in rats) to enhance histamine release in the brain. This could be due to the pharmacokinetic properties of thioperamide, and also might suggest a possible low penetration of the blood–brain barrier. Unfortunately, thioperamide was not suitable for human studies because of potential liver toxicity. Many other potent and relatively selective H3-receptor agonists and antagonists have been developed by various academic and industrial laboratories (Stark et al., 2001; Leurs et al., 2005) and some have been taken into clinical studies. The first H3 agonist to be investigated in man was the prodrug of (R)-_α_-methylhistamine, BP2-94, from Schunack and colleagues, developed by Bioprojet. The first H3 antagonist to be taken into man was Perceptin™ (GT-2331, cipralisant) from Gliatch, which reached phase II studies for treatment of attention deficit disorders, but results have not been reported. Other H3 antagonists from other laboratories are under clinical investigation, but there is no information available about the possible indications or about the results.
H3-receptor antagonists without an imidazole group
Brain penetration is greatly reduced by the presence of polar hydrogen-bonding groups and this led to the search for a nonimidazole H3-receptor antagonist (Cowart et al., 2004). This proved to be quite difficult and for many years all the potent H3-receptor ligands were imidazole derivatives. Starting from first principles, Ganellin et al. (1998) devised a surprisingly simple series of compounds in which a cyclic aliphatic amine effectively replaced the imidazole ring. Thus, histamine was converted into a nonimidazole H3-receptor histamine antagonist by addition of a 4-phenylbutyl group on the side chain N, followed by removal of the imidazole ring. The resulting compound, _N_-ethyl-_N_-(4-phenylbutyl)amine, remarkably had a _K_i=1.3 _μ_M as an H3 antagonist. Using this as a lead compound, structure–activity studies furnished _N_-(5-_p_-cyanophenoxypentyl)pyrrolidine (_K_i=19 nM, ED50=1.0 mg kg−1 p.o. in the mouse). Further molecular modifications furnished UCL 2173, _N_-(3-_p_-acetylphenoxy-propyl)-3,5-dimethyl-piperidine (_K_i=1.8 nM, ED50=0.12 mg kg−1), which, in vivo, is considerably more potent than the reference drug, thioperamide. At least 8 pharmaceutical companies have since had patents published showing compounds containing this aminopropoxyphenyl pharmacophore.
Following the cloning of the human H3-receptor cDNA in 1999 by Lovenberg and colleagues (see below) and the resulting availability of the human recombinant receptor, many pharmaceutical companies set up high-throughput screens to seek other nonimidazole H3-receptor antagonists. Several such compounds have now entered the drug development process. Remarkably, it transpires that many of the imidazole compounds have much lower affinity for the human recombinant receptor than for the rat receptor, whereas the nonimidazole compounds all retain their potencies. Thus, UCL 2173 (vide supra) had a _K_i=1.0 nM at human H3 receptors, comparable to the value reported above (1.8 nM), whereas thioperamide was about 10 times less potent on the human H3 receptor than on the rat. Furthermore, thioperamide also possesses affinity for the H4 receptor, in contrast to many of the nonimidazole compounds which are devoid of H4-receptor activity. Thus, in general, the nonimidazoles are more potent at the human H3 receptor and more selective.
Receptor cloning
The influence of molecular biology on the histamine receptor field made its first impact in the early 1990s. Remarkably, molecular cloning of the cDNA and gene encoding for the bovine H1 and canine H2 receptors occurred in the same year, in 1991. It proved to be much more difficult, however, to find the H3 receptor, and this was not cloned until 1999 by Lovenberg et al. (1999) in Johnson and Johnson in San Diego, U.S.A. Phylogenetic and homology analysis of the H3 receptor showed it to be very different from the previously cloned H1 and H2 receptors (19–22 and 18–20%, respectively). Heterogeneity among H3 receptors had been suspected based on agonist kinetics and radioligand-binding characteristics, and this was confirmed by molecular studies showing that a single form of the H3 gene gave rise to multiple mRNA isoforms. The receptor variants are known to differ in the structure of their third cytoplasmic loops (Leurs et al., 2005). This led to a search for new proteins that might be related to the H3 receptor and resulted in the discovery of the histamine H4 receptor, which was cloned by at least 6 research laboratories and published during the period 2000–2001.
Histamine H4 receptors
The H4 receptor (Jablonowski et al., 2004) is preferentially expressed in various cells of the immune system and mast cells and induces the chemotaxis of, for example, eosinophils and mast cells. It has also been identified on lymphocyte T cells, dendritic cells and basophils. It is suggested that the H4 receptor is involved, along with the H2 receptor, in the control of IL-16 release from human lymphocytes, and it has been speculated that an H4-selective antagonist might be useful in helping to treat asthma. Antagonists, such as JNJ 7777120 (Figure 2), have also been reported to be effective in various models of inflammation. The H4 receptor shows considerable homology with the H3 receptor (58% for the transmembrane regions, but 34–35% overall) and many of the known H3 agonists and antagonists also bind to the H4 receptor. There appears to be considerable species variation in the receptor affinities, however. Across human, mouse, rat and guinea-pig receptors, the H4 receptors only have 65–70% homology, in contrast to the H3 receptor which retains more than 92% sequence homology.
Mutagenesis studies
Site-directed mutagenesis studies have investigated the molecular basis for binding of histamine and its antagonists. It is suggested (Shin et al., 2002) that, in the transmembrane region TM3, a conserved aspartic acid (amino acid position 107 in human H1, position 98 in human H2, position 114 in human H3 and position 94 in human H4) is essential for binding of both histamine and basic antagonists, presumably by providing a negative counter-ion for the protonated amine group of the ligand. Residues in another transmembrane region, TM5, have also been shown to be required for histamine binding. Asn207 of the H1 receptor hydrogen bonds to the N τ nitrogen atom of histamine, whereas Asp186 in the H2 receptor and Glu187 in the H4 receptor form an ion pair with the same N τ nitrogen atom. In addition, Thr190 in TM5 of the H2 receptor is thought to hydrogen bond to the N π nitrogen atom of histamine. Sequence analysis, molecular modelling and pharmacological analysis of human and rat H3 receptors suggest that key amino acids at positions 119 and 122 in TM3 play important roles in ligand recognition and are primarily responsible for the pharmacological differences between the rat and human receptors. Thus, mutant receptors in which these amino acids in the rat receptor are changed to those of the human receptor (119 Ala to Thr, 122 Val to Ala) exhibited changed ligand-binding potencies of the imidazole-containing antagonists, to levels similar to those of the human receptor (Ligneau et al., 2000).
Constitutive activity
The presently known histamine receptors (H1, H2, H3 and H4; Table 1) are all members of the G-protein-coupled receptor (GPCR) family (see also Hill, this issue) and they transduce extracellular signals via the G-proteins, Gq, Gs, Gi/o and Gi/o, respectively (Hough, 2001; see also Milligan & Kostenis, this issue). Whereas at the H2 receptor histamine is a potent stimulant of cAMP accumulation, at the H3 receptor activation leads to inhibition of cAMP production. The signalling mechanisms for the H4 receptor are still being explored, but they appear to involve increases in intracellular calcium levels. In common with other GPCRs, three recombinant histamine receptors (H1, H2 and H3) show constitutive activity, that is, spontaneous activity in the absence of agonist, and, under these circumstances, the antagonists may be reclassified as inverse agonists. It is noteworthy, however, that constitutive activity has been demonstrated to occur in vivo for native H3 receptors present in the rodent brain and that it controls histaminergic neuronal activity (Morisset et al., 2000).
Table 1. The activitiesa of histamine receptor ligands across four receptors _K_d/_K_i (nM).
| | Ileumb | Atriumb | Hc | Hc | | | -------------------------- | ---------------------- | ---------------------- | ----------------- | -------- | | | H1 | H2 | H3 | H4 | | | Mepyramine (pyrilamine) | 0.4 | 5,200 | >10,000 | >10,000 | | Diphenhydramine | 1.0 | >10,000 | >10,000 | >10,000 | | Cimetidine | >10,000 | 800 | >10,000 | >10,000 | | Ranitidine | >10,000 | 200 | >10,000 | >10,000 | | Dimaprit | >10,000 | 1,100 | 825 | 377 | | Impromidine | 3400 | 63 | 67 | 12 | | Thioperamide | >10,000 | >10,000 | 60d | 27 | | Clobenpropit | >10,000 | >10,000 | 0.6 | 12 | | UCL 2138e | | | 11f | >10,000 | | _R_-(α)-methylhistamine | | | 0.7 | 146 | | Imetit | | | 0.3 | 2.7 | | JNJ 7777120 | >10,000c | >10,000g | 5125 | 4.1 | | Histamine | | | 5.4 | 8.1 |
A discussion of the classification of histamine receptors (now out of date because it only deals with H1–H3) was published by the IUPHAR Committee on Receptor Nomenclature (Hill et al., 1997).
Future expectations
The past few years have seen a great flurry of activity from the pharmaceutical companies who have now become very interested in developing compounds as H3- or H4-receptor antagonist drugs. The stimulus for this has been the availability of the recombinant human receptors and the use of high-throughput screens. The companies have become particularly interested in nonimidazole compounds and patents claiming nonimidazole H3-receptor antagonists have appeared from at least 12 companies. The therapeutic uses for H3 antagonists are rather uncertain at present, but it is very likely that at least one of the following possibilities will become established.
One possibility is in the treatment of cognitive disorders: Claims have been made for improvement in cognitive dysfunction and in memory performance, and these could apply to treatments for attention deficit hyperactivity disorder (ADHD) or Alzheimer's disease. Other options are for treatments in sleep-related disorders such as narcolepsy, and in some forms of epilepsy. A substantial effort has also been put into investigating H3-receptor antagonists in various preclinical models of obesity and there have been claims made for a significant reduction in abdominal and subcutaneous fat in rodents. There appears to be a clear difference between the H3-receptor antagonists tested, and those that are active in anti-obesity models do not always work in models of cognition and vice versa. The reasons for the observed discrepancies have yet to be identified, but one suggestion has been that it may be related to the different isoforms of the receptor. A possible peripheral H3-receptor blockade to relieve nasal congestion, since H3 receptors inhibit noradrenaline release from sympathetic nerve terminals in the nasal mucosa, is being pursued in combination with an H1-receptor antihistamine, to improve the treatment of allergic rhinitis.
The H4 receptor is much more recent in its discovery, but the prospect of therapeutic application to inflammatory disease looks very promising. The possibility for a use in helping to treat asthma is very attractive and there is also a strong suggestion for a use in treating other common inflammatory conditions such as allergic rhinitis and rheumatoid arthritis. If these were to become reality, then there would be a host of grateful patients.
The big open-ended question is whether we have seen the last of the histamine receptors or are there more to come (beyond the four currently known). A few years ago, there was some discussion about the possible existence of intracellular histamine receptors, but this idea did not seem to get much support from noninvolved scientists. However, the past century has been an extremely exciting one for pharmacologists in general and histamine researchers in particular, with the recognition of the vital role for histamine as a mediator of numerous physiological and pathological responses. This has led to the development of histamine H1- and H2-receptor antagonists, which have proved to be valuable therapeutic agents for diseases such as allergy and peptic ulcer. Hopefully, therapeutic goals will be achieved with ligands directed at the H3 and H4 receptors, thus continuing the pharmacological and therapeutic success of the ‘histamine story'.
Glossary
CNS
central nervous system
ECL
enterochromaffin-like cell
GABA
gamma amino butyric acid
GORD
gastroesophageal reflux disease
GPCR
G-protein-coupled receptor
TM3/5
transmembrane region 3/5
References
- ARRANG J.-M., GARBARG M., LANCELOT J.-C., LECOMTE J.-M., POLLARD H., ROBBA M., SCHUNACK W., SCHWARTZ J.-C. Highly potent and selective ligands for histamine H3-receptors. Nature. 1987;327:117–123. doi: 10.1038/327117a0. [DOI] [PubMed] [Google Scholar]
- ASH A.S.F., SCHILD H.O. Receptors mediating some actions of histamine. Br. J. Pharmacol. 1966;27:427–439. doi: 10.1111/j.1476-5381.1966.tb01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHFORD C.A., HELLER H., SMART G.A. The action of histamine on hydrochloric acid and pepsin secretion in man. Br. J. Pharmacol. 1949;4:153–161. doi: 10.1111/j.1476-5381.1949.tb00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEST C.H., DALE H.H., DUDLEY H.W., THORPE W.V. The nature of the vaso-dilator constituents of certain tissues. J Physiol. 1927;62:397. doi: 10.1113/jphysiol.1927.sp002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK J.W., DUNCAN W.A.M., DURANT G.J., GANELLIN C.R., PARSONS M.E. Definition and antagonism of histamine H2-receptors. Nature. 1972;236:385–390. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- BOVET D., STAUB A.-M. Action protectrice des éthers phénoliques an cours de l'intoxication histaminique. C. R. Soc. Biol. (Paris) 1937;124:547–549. [Google Scholar]
- BRADSHAW J., BRITTAIN R.T., CLITHEROW J.W., DALY M.J., JACK D., PRICE B.J., STABLES R. Ranitidine (AM19065), a new potent, selective histamine H2-receptor antagonist. Br. J. Pharmacol. 1979;66:464. [PMC free article] [PubMed] [Google Scholar]
- BRIMBLECOMBE R.W., DUNCAN W.A.M., DURANT G.J., GANELLIN C.R., PARSONS M.E., BLACK J.W. The pharmacology of cimetidine, a new histamine H2-receptor antagonist. Br. J. Pharmacol. 1975;53:435–436. [PMC free article] [PubMed] [Google Scholar]
- CODE C.F.1956Histamine and gastric secretion Ciba Foundation Symposium on Histamineed. Wolstenholme, G.E.W. & O'Connor, C.M. pp. 189–220.London: J and A Churchill Ltd [Google Scholar]
- COOPER D.G., YOUNG R.C., DURANT G.J., GANELLIN C.R.1990Histamine receptors Comprehensive Medicinal Chemistryed. Emmett, J.C. Vol. 3, pp. 323–442.New York: Pergamon Press [Google Scholar]
- COWART M., ALTENBACH R., BLACK L., FAGHIH R., ZHOA C., HANCOCK A.A. Medicinal chemistry and biological properties of non-imidazole H3-antagonists. Mini-Rev. Med. Chem. 2004;4:979–992. doi: 10.2174/1389557043403215. [DOI] [PubMed] [Google Scholar]
- DALE H.H., LAIDLAW P.P. The physiological actions of β-iminazolethylamine. J. Physiol. 1910;41:318–344. doi: 10.1113/jphysiol.1910.sp001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALE H.H., LAIDLAW P.P. Histamine shock. J. Physiol. 1919;52:355–390. doi: 10.1113/jphysiol.1919.sp001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDBERG W., MONGAR J.L. Comparison of histamine release by compound 48/80 and octylamine in perfused tissues. Br. J. Pharmacol. 1954;9:197–201. doi: 10.1111/j.1476-5381.1954.tb00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLKOW B., HAEGER K., KAHLSON G. Observations on reactive hyperaemia as related to histamine on drugs antagonising vasodilatation induced by histamine and the vasodilator properties of adenosine triphosphate. Acta Physiol. Scand. 1948;15:244–278. [Google Scholar]
- GANELLIN C.R., LEURQUIN F., PIRIPITSI A., ARRANG J.-M., GARBARG M., LIGNEAU X., SCHUNACK W., SCHWARTZ J.-C. Synthesis of potent non-imidazole histamine H3-receptor antagonists. Arch. Pharm. Pharm. Med. Chem. 1998;331:395–404. doi: 10.1002/(sici)1521-4184(199812)331:12<395::aid-ardp395>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- GANELLIN C.R., PARSONS M.E. Pharmacology of Histamine Receptors. Bristol, London, Boston: Wright PSG; 1982. [Google Scholar]
- HILL S.J., GANELLIN C.R., TIMMERMAN H., SCHWARTZ J.-C., SHANKLEY N.P., YOUNG J.M., SCHUNACK W., LEVI R., HAAS H.L. International Union of Pharmacology, XIII, classification of histamine receptors. Pharm. Rev. 1997;49:253–278. [PubMed] [Google Scholar]
- HOUGH L.B. Genomics meets histamine receptors: new subtypes, new receptors. Mol. Pharm. 2001;59:415–419. [PubMed] [Google Scholar]
- JABLONOWSKI J.A., CARRUTHERS N.I., THURMOND R.L. The histamine H4 receptor and potential therapeutic uses for H4 ligands. Mini-Rev. Med. Chem. 2004;4:993–1000. doi: 10.2174/1389557043403152. [DOI] [PubMed] [Google Scholar]
- LEURS R., BAKKER R.A., TIMMERMAN H., DE ESCH I.J.P. The histamine H3 receptor: from gene cloning to H3 receptor drugs. Nat. Rev. Drug Discov. 2005;4:107–120. doi: 10.1038/nrd1631. [DOI] [PubMed] [Google Scholar]
- LEWIS T., GRANT R.T. Vascular reactions of the skin to injury. Part 11. The liberation of histamine-like substance in the injured skin, the underlying cause of factitious urticaria and of wheals produced by burning: and observations upon the nervous control of certain skin reactions. Heart. 1924;11:209–265. [Google Scholar]
- LIGNEAU X., MORRISET S., TARDIVEL-LACOMBE J., GBAHOU F., GANELLIN C.R., STARK H., SCHUNACK W., SCHWARTZ J.-C., ARRANG J.-M. Distinct pharmacology of the rat and human histamine H3 receptors. Br. J. Pharmacol. 2000;131:1247–1250. doi: 10.1038/sj.bjp.0703712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVENBERG T.W., ROLAND B.L., WILSON S.J., JIANG X., PYATI J., HUVAR A., JACKSON M.R., ERLANDER M.G. Cloning and functional expression of the human histamine H3 receptor. Mol. Pharmacol. 1999;55:1101–1107. [PubMed] [Google Scholar]
- MORISSET S., ROULEAU A., LIGNEAU X., GBAHOU F., TARDIVEL-LACOMBE J., STARK H., SCHUNACK W., GANELLIN C.R., SCHWARTZ J.-C., ARRANG J.-M. High constitutive activity of the native H3 receptor regulates histamine neurons in the brain. Nature. 2000;408:860–864. doi: 10.1038/35048583. [DOI] [PubMed] [Google Scholar]
- PERTZ H.H., ELZ S., SCHUNACK W. Structure activity relationships of histamine H1-receptor agonists. Mini Rev. Med. Chem. 2004;4:935–940. doi: 10.2174/1389557043403198. [DOI] [PubMed] [Google Scholar]
- POPIELSKI L. β-Imidazolylathylamin und die Organextrakte Erster Teil: β-Imidazolylathylamin als mechtiger Errezer der Magendrucken. Pfluegers Arch. 1920;178:214–236. [Google Scholar]
- RILEY J.F., WEST G.B. Histamine in tissue mast cells. J. Physiol. 1952;117:72. [PubMed] [Google Scholar]
- SCHAYER R.W.1956The origin and fate of histamine in the body Ciba Foundation Symposium on Histamineed. Wolstenholme, G.E.W. & O'Connor, C.M. pp. 183–188.London: J. and A. Churchill Ltd [Google Scholar]
- SCHILD H.O. pA, A new scale for the measurement of drug antagonism. Br. J. Pharmacol. 1947;2:189–206. doi: 10.1111/j.1476-5381.1947.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ J.-C. Histamine as a transmitter in the brain. Life Sciences. 1975;17:503–518. doi: 10.1016/0024-3205(75)90083-1. [DOI] [PubMed] [Google Scholar]
- SHIN N., COATES E., MURGOLO N.J., MORSE K.L., BAYNE M., STRADER C.D., MONSMA F.J., JR Molecular modelling and site-specific mutagenesis of the histamine-binding site of the histamine H4 receptor. Mol. Pharmacol. 2002;62:38–47. doi: 10.1124/mol.62.1.38. [DOI] [PubMed] [Google Scholar]
- STARK H., ARRANG J.-M., LIGNEAU X., GARBARG M., GANELLIN C.R., SCHWARTZ J.-C., SCHUNACK W. The histamine H3 receptor and its ligands. Progr. Med. Chem. 2001;38:279–308. doi: 10.1016/s0079-6468(08)70096-1. [DOI] [PubMed] [Google Scholar]
- WATANABE T., TAGUCHI Y., SHIOSAKA S., TANAKA J., KUBOTA H., TERANO Y., TOHYAMA M., WADA H. Distribution of the histaminergic neuron system in the central nervous system of rats; a fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res. 1984;295:13–25. doi: 10.1016/0006-8993(84)90811-4. [DOI] [PubMed] [Google Scholar]