Investigation of alpha nascent polypeptide-associated complex functions in a human CD8+ T cell ex vivo expansion model using antisense oligonucleotides (original) (raw)
Abstract
In order to determine molecules involved in the differentiation and proliferation of human CD8+ cells, two ex vivo expansion models were established: coculture of freshly purified human CD8+ cells with irradiated autologous feeders (AF) or stimulation with anti-CD3. Two different proliferation kinetics of CD8+ cells and expression patterns of CD57 were observed between these conditions. Differential display reverse transcriptase–polymerase chain reaction was applied to investigate the differential expression of mRNA species between CD8+ CD57+ and CD8+ CD57– populations. A differentially expressed RNA species called alpha nascent polypeptide associated complex (α NAC) was found at a higher level in CD8+ CD57– cells than in CD8+ CD57+ cells. In the presence of AF, the expression of α NAC was reduced on culturing whilst proliferation increased. Similarly, in cultures stimulated with anti-CD3, α NAC reverted to its inactive form and differentiation and proliferation increased. Using a phosphorothioate-modified oligodeoxynucleotide antisense directed specifically against α NAC mRNA, protein expression was inhibited and increased CD8+ cell proliferation and CD25 expression were observed irrespective of the culture conditions. This suggests that α NAC protein is antiproliferative molecule. This is the first description of the function of the α NAC protein in human CD8+ T cells.
Keywords: CD8+ T cell, α NAC, antisense oligonucleotides
Introduction
The molecular events that govern CD8+ cell proliferation and differentiations are poorly defined. The ability to regulate cells progressing from potent cytotoxic effectors to terminally differentiated regulatory cells may have impact an on the numbers and efficiency of antigen specific cells that can be generated for applications such as adaptive immunotherapy.
Following thymic maturation, CD8+ cells recirculate between the peripheral lymphoid tissues and blood until they encounter antigen. Naïve CD8+ cells encountering antigen differentiate into memory-type and effector-type cells as determined by phenotypic markers.1,2 Memory cells express CD27 and CD28 but not CD45RA and CD57. In contrast, effector cells are CD45RA+ CD57+ CD27– CD28–. CD57 is a sulphated glucuronyl carbohydrate moiety of 110 000 MW3 expressed on cell adhesion glycoproteins and encoded by a gene located on chromosome 11.4
A high expression pattern of CD57 antigen on CD8+ cells has been recognized in some pathological conditions5–7 and CD8high CD57+ cells are believed to represent an important regulatory subpopulation which have the capacity to control common lymphocyte functions.8–10 CD57+ cells are not a distinct subset or lineage but are derived directly from CD57– T cell by a process of differentiation which occurs late in the immune response.11 Therefore, CD8+ CD57+ cells are considered to be activated, terminally differentiated cells arising from CD8+CD57– cells after long-term proliferation and differentiation.12 We showed that in vitro culturing of purified CD8+ cells either with autologous feeders (AF) or with anti-CD3 results in different patterns of CD57 antigen expression.
We exploited two ex vivo models of human CD8+ T cell expansion, coculturing with AF or stimulation with anti-CD3, to investigate the molecular events which regulate differentiation and proliferation. Differential display reverse transcriptase–polymerase chain reaction (DDRT–PCR) was used to investigate the differential expression of RNA species between CD8+ populations expressing and lacking the terminal differentiation marker.
A differentially expressed band was present at higher level in CD57– than in CD57+ cells. BLAST searching revealed that the sequence of this band has 99·2% sequence homology with transcriptional coactivator gene alpha nascent polypeptide associated complex (α NAC). NAC is a stable heterodimeric protein consisting of α and β subunits.13 It binds nascent polypeptides lacking signal peptides as they emerge from the ribosome and prevents the translocation of these polypeptides to the endoplasmic reticulum.13α NAC functions as a developmentally regulated transcriptional coactivator in mice osteoblasts.14 It stabilizes the binding of AP-1 and TATA binding protein (TBP) with DNA leading to increase transcriptional activity.14 Using antisense technology, we have studied the function of α NAC protein since its role has not previously been investigated in human CD8+ cells. It was shown that α NAC protein functions as an antiproliferative molecule in this setting.
Materials and methods
All reagents were supplied by Sigma Chemicals, Poole, UK, unless otherwise specified.
Isolation of CD8+ T cells from human peripheral blood
CD8+ T cells were obtained by positive selection from mononuclear cells of four different healthy individuals as previously described.15 This was performed by positive selection using CD8-coated microbeads in accordance with the manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of CD8+ cells was assessed by flow cytometry and was routinely more than 98%. CD8+ cells were grown in RPMI-1640 medium, supplemented with 10% (v/v) human AB serum, 100 U/ml penicillin, 0·01% (w/v) streptomycin and 2 mm glutamine, at 37° in 5% CO2. These isolated CD8+ T cells were cultured under two different conditions. Cells were plated out into 96-well plates (Corning incorporated, Corning, NY) with each well containing 104 CD8+ cells supplemented with 5 × 104 AF for 21 days. Alternatively, 104 CD8+ cells/well were cultured in flat-bottomed microwell plates previously coated overnight with anti-CD3 (OKT3, 10 µg/ml, Cilag AG, Schaffhausen, Switzerland) for 10 days. All cultures were supplemented with 50 U/ml human recombinant interleukin-2 (IL-2) (R&D Systems Abingdon, UK). Cells were fed every 4 days with 0·5 volumes of tissue culture medium supplemented with IL-2. Flow cytometry was performed throughout the culture period for CD57 marker as previously described.15 In initial experiments, cells cultured with AF were analysed by fluorescence-activated cell sorting (FACS) into CD57+ and CD57– fractions for differential display analysis.
Cloning and probe labelling
The sequence of purified differentially expressed DNA band from DDRT–PCR was submitted for sequence homology searching using basic local alignment search tool16 and cloned using pGEM-T Easy, as described by the manufacturer (Promega, Southampton, UK). Cloned α NAC was used to generate [α-32P] dCTP (Amersham, Amersham, UK) labelled probes using a random DNA labelling system (Amersham) and was purified by NICK column (Amersham).
Northern blot
CD8+ T cells cultured with AF were used to conduct Northern blot as described in standard protocols.17 After day 14 of culture, isolated total RNA from FACS-sorted cells (CD8+ CD57+ and CD8+ CD57–) was resolved by 1% agarose/formaldehyde gel electrophoresis and transferred to nitrocellulose membrane (Amersham) overnight. The membrane was then baked for 2 hr at 80°. The filter was soaked for 4 h in prehybridization buffer and hybridized with 400 µl of labelled cloned probe of α NAC in the same hybridisation buffer for 18 h at 42°. The blot was washed in 2× standard sodium citrate (SSC), 0·1% sodium dodecyl sulphate (SDS, BDH, Poole, UK) at room temperature, twice for 15 min in 0·1 × SSC, 0·1% SDS at 45° and exposed to Kodak X-ray hyper films (Amersham) with intensifying screen at −70° overnight. To monitor the quality and the quantity of the RNAs loaded, the filter was re-hybridised with labelled cloned probe for glyceraldehyde-2-phosphate dehydrogenase (GAPDH).
Western blot
Western immunoblot was performed as previously described.18 Equal cell numbers (106 per lane) were boiled for 5 min in lysis buffer consisting 2% (w/v) SDS and 5% (v/v) mercaptoethanol and separated by a 10% SDS–polyacrylamide gel electrophoresis (PAGE) gel at 25 mA. Proteins were blotted onto immobilon membrane (Millipore, Molsheim, France) for 4 hr at 250 mA and probed overnight at 4° with rabbit polyclonal antisera against α NAC (final dilution, 1 : 1000, kind gift from Dr St. Arnuad-Canada). The membrane was incubated with a goat anti-rabbit immunoglobulin G peroxidase for 2 hr (final dilution, 1 : 1000). The blot was developed after 2 hr using chemiluminescent substrate (Kirlegaard and Perry, Gaithersburg, MD) and Kodak X-OMAT S film (Xograph, Tetbury, UK).
Oligonucleotide synthesis
Three partially modified phosphorothioate oligodeoxynucleotide (PS-ODN, 20-mer) were synthesized by a standard phosphoramidite method, using an ABI 394 DNA synthesizer (Sigma Genosys). Two oligonucleotides were used as negative controls; Sense PS-ODN has 100% similar sequence to α NAC mRNA at position 301–320 (T*A*G*A*G*TCACTATCCG*G*A*A*A*T) and Non-sense PS-ODN scrambled sequence (C*G*A*A*T*CTCGCGTCAA*T*G*G*C*A). Antisenses anti-cap (T*G*T*G*C*AGGGAACGCG*G*A*A*C*C) was designed to hybridize at position 23–4 to human α NAC mRNA (GenBank, X80909). BLAST searches confirmed the oligonucleotide sequences lacked sequence homology to any other known human genes.
Preparation of lipofectin–oligonucleotide mix
The optimal intracellular uptake of oligonucleotides has previously been observed to occur at a neutral or slightly positive (1·3 positive to negative molar ratio) net charge of lipofectin-oligonucleotide complexes.19 The oligonucleotide–lipofectin mix was prepared in a 10-fold dilution in 100 µl of distilled water as follows: 30 µl of lipofectin (30 µg/ml) and 1 µm oligonucleotide (final concentration). The solution was incubated for 30 min at room temperature before use.
3_H-thymidine incorporation assay_
Freshly purified CD8+ cells were cultured with AF or with anti-CD3 in the presence of antisense oligonucleotides. Cells were pulsed with 3H-thymidine (1 µCi/well, Amersham) over the last 18 hr of culture. Labelled cells were harvested on glass fibre filters and covered with a melt-on scintillator sheet. Incorporated thymidine was reported as triplicate counts per minute (c.p.m.) by scintillation counting (Wallac, Turku, Finland).
Results
Patterns of CD8+ growth with AF and anti-CD3 stimulation
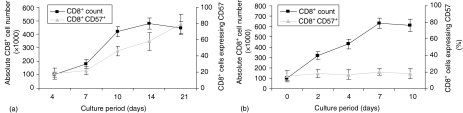
Two conditions for in vitro culturing of CD8+ cells were established: coculturing with AF and activation with anti-CD3. Cells grown under these conditions were shown to have different kinetics of proliferation and expression of CD57. The absolute CD8+ cell number and CD57 expression following either coculture with AF or stimulation with anti-CD3 (Fig. 1). The majority of cells (over 80%) cultured with AF expressed CD57 by day 14 following a period of expansion from day 4 to day 10 (Fig. 1a). In contrast, less than 20% of cells stimulated with anti-CD3 expressed CD57 (Fig. 1b). The maximum absolute cell number following AF coculture and anti-CD3 stimulation was 4·5 and 5·8 × 105/ml at day 14 and 7, respectively. Initially, cells cultured with AF were FACS sorted into CD57+ and CD57– fractions for differential display analysis. Flow cytometry confirmed that the purity of these cells was >95%.
Figure 1.
Expression pattern of CD57 antigen on CD8+ T cells.Purified human CD8+ T cells (1 × 105/ml, 98% purity) were expanded with IL-2 (50 U/ml) in the presence of either AF (5 × 105/ml, a) or in precoated wells with anti-CD3 (10 µg/ml, b) At each time point, 50 µl of tissue culture was collected and the absolute CD8+ cell number and the expression level of CD57 on CD8+ T cells was flow cytometrically determined. Data are means of 5 separate experiments and the vertical bars represent ±SD.
Analysis of α NAC mRNA expression
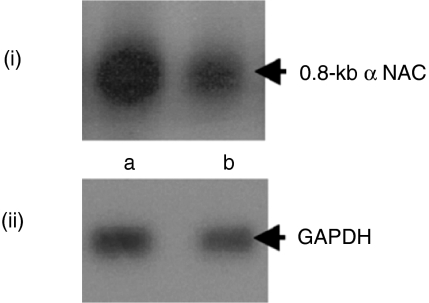
The differential expression pattern of mRNA from the DDRT-PCR was confirmed by Northern blot using a radiolabelled probe of cloned α NAC. It was shown that a band at 0·8-kb was expressed at a higher level in CD57– cells than in the CD57+ population (Fig. 2i) Re-probing the original filter with a radiolabelled probe of cloned GAPDH confirmed RNA integrity and quantity (Fig. 2ii).
Figure 2.
Analysis and confirmation of DDRT–PCR.Equal concentrations of the total RNA were prepared from FACS sorted CD8+ CD57– (lane a) and CD8+ CD57+ cells (lane b) after coculture with AF. The blot was probed with cloned α NAC (i) and reprobed with labelled cloned GAPDH to monitor the quantity and the integrity of loaded RNA (ii).
Expression pattern of α NAC protein analysis
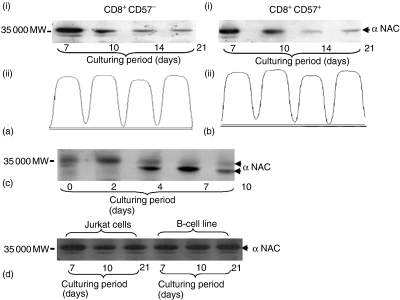
Western blotting analysis revealed a distinct difference in the pattern of α NAC protein expression, which was dependent on how the cells were cultured (Fig. 3ai, bi and c). Figure 3(ai and bi) shows that the expression level of α NAC protein diminishes over the culture period in both populations. Reprobing the membrane with α tubulin monoclonal antibody (mAb) and densitometric scanning showed that equivalent cell numbers were used and the reduction of α NAC protein expression throughout the culture was not caused by variation in cell numbers (Fig. 3aii and bii). In contrast, cells stimulated with anti-CD3 showed increased amounts of the phosphorylated form of α-NAC protein up to day 2, after which α NAC reverted to the unphosphorylated form (Fig. 3c). Treatment with alkaline phosphatase confirmed that the difference in molecular weight resulted from the phosphorylation process (data not shown). Further investigations were performed to confirm that the expression pattern of α NAC protein was specific for CD8+ T cells cultured with either condition. Immunoblotting assays using T-cell (Jurkat cells) and B-cell lines (transformed with Epstein–Barr virus) cultured for 21 days revealed that α NAC protein expression was at a steady level with no evidence for reversion to the unphosphorylated form after prolonged culturing (Fig. 3d).
Figure 3.
Expression patterns of α NAC protein. Expression of α NAC protein in CD8+ cells cultured either with AF (a, b) or with anti-CD3 (c) At the indicated time points, cells were either FACS sorted into CD8+ CD57+ and CD8+ CD57– cell populations (ai and bi) or harvested without sorting (c). Cell lysates were resolved on 10% SDS–PAGE. The membrane was probed with polyclonal anti-rabbit against α NAC protein. Densitometry scanning of α tubulin level in the FACS sorted CD8+ CD57+ and CD8+ CD57– cell (aii and bii). Expression of α NAC protein was also investigated in T- and B-cell lines(d).
Investigation of the biological functions of α-NAC protein in human CD8+ T cells using antisense technology
All the following experiments were performed using CD8+ T cells cultured with AF or with anti-CD3 for 14 and 7 days, respectively, in the presence of various PS-ODNs.
Inhibition of α NAC protein
Recently, we have optimized the conditions affecting the functional efficacy of PS-ODNs.20 In this study, anti-cap PS-ODN partially phosphorothioate modified antisense was used to investigate the biological functions of the 0·8-kb α NAC gene in human CD8+ T cells as it showed optimal inhibition of the α NAC protein.20 CD8+ T cells in each of the two culture conditions were treated with different PS-ODNs. The two blots were densiometrically scanned to quantify the percentage inhibition of α NAC protein expression. The expression level was standardized by comparison to cells treated with the nonsense PS-ODNs (100%). Figure 4(ai and bi) shows that the percentage of protein inhibition in the cells cultured with AF or with anti-CD3 was 75 and 70%, respectively. Reprobing the membranes with α tubulin mAb (Fig. 4aii and bii) confirmed that the reduction in protein level was the result of a specific inhibitory effect of anti-cap PS-ODN.
Figure 4.
Inhibition of α NAC protein in CD8+ T cells. Expression of α NAC protein in CD8+ T cells cultured with AF (a) or anti-CD3 (b) in the presence of various PS-ODNs at day 14 and 10, respectively. Western blotting was performed as detailed in Fig. 3
Quantification of cellular total protein in the CD8+ T cells treated with PS-ODNs
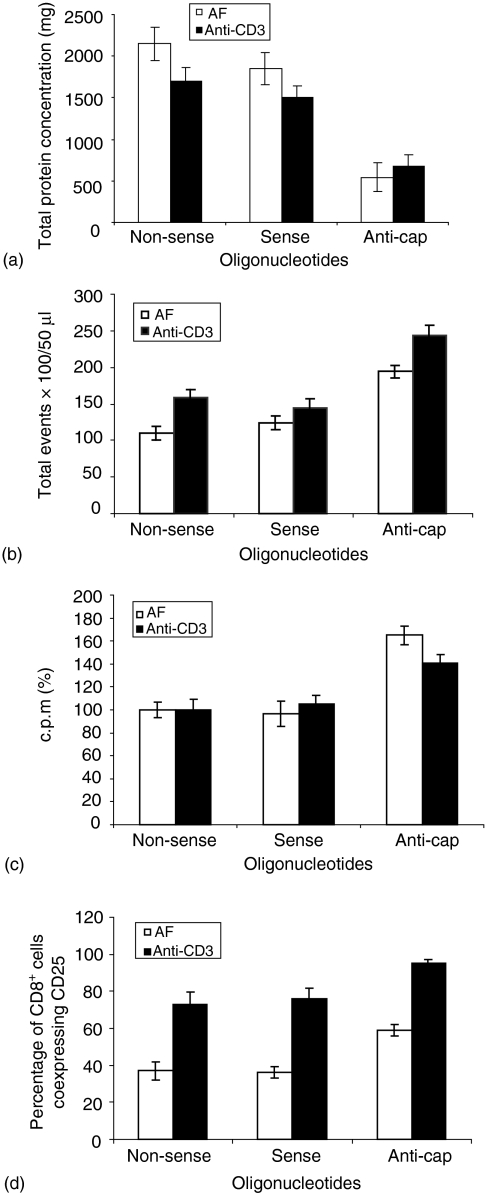
In order to examine whether the inhibition of α NAC protein affected the heterodimeric NAC protein function21,22 the total cellular protein concentration of the cells treated with different PS-ODN was quantified. Figure 5(a) shows a significant reduction (P < 0·05) in the total cellular protein concentrations in the presence of anti-cap PS-ODN in both culture conditions when compared with protein concentration in the cells treated with non-sense and sense PS-ODN. The total protein concentration was found to be reduced to more than 50% of the negative control levels, irrespective of culture condition (Fig. 5a).
Figure 5.
Effect of α NAC protein inhibition on CD8+ T-cell biology. Total cellular protein concentration (a) total cell count (b) the proliferative response (c) and CD8+ cells coexpressing CD25 (d) were determined following α NAC protein inhibition in the CD8+ T cells cultured under the conditions detailed in (Fig. 1a and b) and in the presence of various PS-ODNs. Data are the mean of three separate experiments ± SD in triplicate.
Effect of α NAC protein inhibition on proliferation of CD8+ T cells
The effect of α NAC protein inhibition on the proliferation of CD8+ cells was investigated following the observation that, as the proliferation rates increased the expression level of α NAC protein decreased or reverted into the inactive unphosphorylated form in both culture systems. In order to examine this, the absolute cell numbers were determined under each culture condition (Fig. 5b). At the indicated time points, the total cell count of 50 µl aliquots of the culture was established using flow cytometry. Absolute cell numbers increased by 76% and 52% (P < 0·05) in CD8+ cells cultured with AF or with anti-CD3, respectively, in the presence of anticap PS-ODNs (Fig. 5b). Cells treated with sense PS-ODN showed only marginal increases in cell number relative to the nonsense treated cells. This data was further confirmed by the thymidine incorporation assay, where results were expressed as a percentage of the mean of triplicate c.p.m. (Fig. 5c). The proliferative responses of the cells treated with non-sense PS-ODNs were considered to be a reference against which the remaining treated cells was compared. The proliferation of cells cultured with AF or with anti-CD3 in the presence of anti-cap PS-ODN was enhanced by 65 ± 8% and 41 ± 7% (P < 0·05), respectively. However, sense PS-ODNs did not show any significant effect in either culture systems (Fig. 5c).
Effect of α NAC protein inhibition on immunophenotype of CD8+ T cells
The expression level of the phosphorylated form of α NAC protein in CD8+ cells cocultured with AF was maximal at day 7 (differentiation and expansion phase) and gradually diminished over the culture time. In contrast, this protein reverted from the phosphorylated form into unphosphorylated form at day 4 (expansion phase) of culture time in CD8+ cells stimulated with anti-CD3. This prompted us to investigate the effect of α NAC protein inhibition on the CD8+ T-cell immunophenotype in both culture conditions. Inhibition of the α NAC protein had no discernible effect on the immunophenotypic profile of CD8+ T cells (CD45RA, CD45RO and CD57, data not shown). However, significant increases in the percentage of CD8+ cells coexpressing CD25 (P < 0·05) were observed irrespective of the culturing conditions (Fig. 5d). Cells treated with anti-cap PS-ODNs in the presence of AF showed significant increases in the percentage of cells expressing CD25 (59·3 ± 2·1%, P < 0·05). The percentage of positive cells incubated with non-sense and sense PS-ODNs were 37·3 ± 5·7% and 36 ± 3·1%. In CD8+ T cells cultured with anti-CD3, the percentage of positive cells bearing CD25 antigen in the presence of anti-cap PS-ODNs was 95·4 ± 2·3%. These were all significant increases (P < 0·05) compared to cells treated with non-sense PS-ODNs, which showed a lower percentage of CD25 positive cells (76 ± 5·6%).
Discussion
In the present study, two in vitro expansion models of human CD8+ T-cell differentiation and proliferation were established to enable us to determine novel genes involved in these events.
CD8+ T cells were expanded in the presence of AF and sorted into CD57+ and CD57– subsets. DDRT–PCR was then performed to compare gene expression in these subsets. The rationale for using this culture system was that the expression pattern of CD57 in cells stimulated with anti-CD3 was stable throughout the culture time whereas it dramatically changed in the population cultured with AF.
Interestingly, two different kinetics of proliferation of CD8+ T cells and expression pattern of CD57 were observed between these settings (Fig. 1a, b). It has been reported that the response of the T cell varies with the nature of stimulus used (signalling pathway) resulting in activation of distinct transcriptional factors.23–26 This may explain the variations in the expression pattern of CD57 and proliferation kinetics of CD8+ T cells between the two culturing systems. To confirm the DDRT–PCR results, Northern blot was performed at the same time point at which the DDRT–PCR was conducted where the maximum proliferation and differentiation was determined (day 14, Fig. 1a).
At the protein level, α NAC protein did not confirm the result of DDRT–PCR. However, its protein level was dramatically down regulated over the culture period in both cell populations (Fig. 3ai and bi). This result prompted a study of the expression pattern of α NAC protein in the second culture system (CD3 activation), because the two culture systems have different proliferation kinetics of CD8+ T cells and different expression patterns of CD57. Surprisingly, two different expression patterns of α NAC protein were obtained from the two culture conditions (Fig. 3ai, bi and c). In the presence of AF this was not possible, as we were unable to sort enough cells at day 0 and 4 to be used in a Western blot (because of limited cell numbers in the culture, Fig. 3a). The α NAC protein was maximally expressed at day 7 and then declined until day 21 in both populations (Fig. 3a). Thus, combining the proliferation and differentiation pattern of CD8+ T cells cultured with AF (Fig. 1a) with the expression pattern of α NAC protein (Fig. 3a), it was observed that once the cells start to differentiate and proliferate the expression level of this protein began to diminish. This may suggest that α NAC protein plays a role in CD8+ T-cell development. These observations were supported by the expression pattern of α NAC protein in CD8+ T cells cultured with anti-CD3. When the cells started to differentiate and to increase in number (Fig. 1b), this protein reverted from its active phosphorylated form to the inactive unphosphorylated form (Fig. 3c). The expression of α NAC protein was stable in tested cell lines and neither decreased nor reverted throughout the culture period (Fig. 3d), confirming the specific expression pattern of this protein in CD8+ T cells.
We hypothesized that the α NAC protein could play a role in CD8+ T cell differentiation and proliferation. To test this possibility, antisense oligos were used to block α NAC expression. By including the non-sense and sense PS-ODNs as negative controls and reprobing the two membranes with α tubulin mAB, it was demonstrated that antisense PS-ODNs specifically inhibit the expression of α NAC protein (Fig. 4aii and 4bii).
Previous studies have reported that the NAC heterodimeric complex protein (β NAC and α NAC) plays a crucial role in protein export from cells.21,22 In order to examine whether the inhibition of α NAC protein affected the heterodimeric NAC protein function, total cellular protein was investigated. Figure 5(a) shows a low level of the total protein concentration in the presence of anti-cap PS-ODN, irrespective of the culture conditions. This could be explained by the fact that inhibition of α NAC protein may block the NAC heterodimeric complex function. As a consequence, cellular proteins are secreted from cells, leading to a reduction in the total cellular protein concentration.
The inhibitory effect of α NAC protein on the proliferation of CD8+ T cells expanded under two culture conditions was then examined. Cells treated with anti-cap PS-ODNs showed increased proliferative responses compared to negative controls irrespective of the culture condition (Fig. 5c). The increased absolute cell number in the cells treated with antisense PS-ODNs in both culture systems (Fig. 5b) confirmed these results. This supports the suggestion that α NAC protein is an antiproliferative molecule.
Finally, the effect of α NAC protein inhibition on the immunophenotypic profile of CD8+ T cells was examined. It was shown that, with the exception of CD25, α NAC inhibition had no significant effect on the phenotype of CD8+ T cells. Cells treated with anti-cap PS-ODNs showed a significant increase in the percentage of cells expressing CD25 irrespective of the culture systems. This result is consistent with previous results in which high proliferative responses and cell numbers were observed in cultures treated with anti-cap PS-ODNs. Considering that CD25 is a component of the IL-2 receptor (IL-2Rα), which plays a central role in T-cell proliferation,27,28 it is suggested that inhibition of α NAC protein expression leading to up regulation of the expression of CD25 subsequently results in increased proliferation. Based on the function of α NAC protein as transcriptional coactivator, there are several hypotheses to explain the increased proliferation in response to α NAC protein suppression. One is that α NAC protein binds directly to an element within the CD25 enhancer and/or interacts with positive factors that bind to this element. A second hypothesis is α NAC repress the expression of a key positive regulator of CD25. A third scenario is α NAC protein induces the expression of repressor that in turn inhibits the IL-2Rα expression. Another possible mechanism for α NAC action is suggested by a recent report showing that the α NAC protein is a cellular inhibitor of the Fas-associated death-domain-containing (FADD) protein,29 which is required for T-cell proliferation in murine models.30
The differences in the suppression levels of α NAC protein expression (Fig. 4a, b) may explain the variations in the effect of α NAC inhibition on CD8+ T cells in our results.
In conclusion, our findings indicate that α NAC functions as an antiproliferative molecule in ex vivo expanded CD8+ T cells but has no discernible effect on their differentiation. However, future work will be necessary to determine the molecular mechanisms by which α NAC influences cell proliferation.
Acknowledgments
This work was funded by Karim Rida Said Foundation and the COGENT trust.
References
- 1.Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effecter human CD8+ T cells. J Exp Med. 1997;186:1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamann D, Roos ML, van Lier RA. Faces and phases of human CD8+ T-cell development. Immunol Today. 1999;20:177–81. doi: 10.1016/s0167-5699(99)01444-9. [DOI] [PubMed] [Google Scholar]
- 3.Kubagawa H, Abo T, Balch CM, Cooper MD. Biochemical analysis of antigenic determinants recognized by HNK-1 (leu-7) antibody. Fed Proc. 1983;42:1219–24. [Google Scholar]
- 4.Schroder J, Nikinmaa B, Kavathas P, Herzenberg LA. Fluorescence-activated cell sorting of mouse-human hybrid cells aids in locating the gene for the Leu 7 (HNK-1) antigen to human chromosome 11. Proc Natl Acad Sci U S A. 1983;80:3421–4. doi: 10.1073/pnas.80.11.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joly P, Guillon JM, Mayaud C, et al. Cell-mediated suppression of HIV-specific cytotoxic T lymphocytes. J Immunol. 1989;143:2193–201. [PubMed] [Google Scholar]
- 6.Reipert B, Scheuch C, Lukowsky A, et al. CD3+ CD57+ lymphocytes are not likely to be involved in antigen-specific rejection processes in long-term allograft recipients. Clin Exp Immunol. 1992;89:143–7. doi: 10.1111/j.1365-2249.1992.tb06893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang EC, Taylor-Wiedeman J, Perera P, et al. Subsets of CD8+,CD57+ cells in normal, healthy individuals: correlations with human cytomegalovirus (HCMV) carrier status, phenotypic and functional analysis. Clin Exp Immunol. 1993;94:297–305. doi: 10.1111/j.1365-2249.1993.tb03447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadat-Sowti B, Debre P, Idziorek T, et al. A lectin ibinding soluble factor relased by CD8+ CD57+ lymphocytes from AIDS patients inhibits T cell cytotoxicity. Eur J Immunol. 1991;21:737–41. doi: 10.1002/eji.1830210329. [DOI] [PubMed] [Google Scholar]
- 9.Autran B, Leblond V, Sadat-Sowti B, et al. A soluble factor released by CD8+ CD57+ lymphocytes from bone marrow transplanted patients inhibits cell-mediated cytolysis. Blood. 1991;77:2237–41. [PubMed] [Google Scholar]
- 10.Leroy E, Calvo CF, Divine M, et al. Persistence of T8+/HNK-1+ suppressor lymphocytes in the blood of long-term surviving patients after allogeneic bone marrow transplantation. J Immunol. 1986;137:2180–9. [PubMed] [Google Scholar]
- 11.d'Angeac AD, Monier S, Pilling D, et al. CD57+ T lymphocytes are derived from CD57+ precursor by differentiation occurring in late immune responses. J Immunol. 1994;24:1503–11. doi: 10.1002/eji.1830240707. [DOI] [PubMed] [Google Scholar]
- 12.Mollet L, Sadat-Sowti B, Duntze J, et al. CD8hi+CD57+ T lymphocytes are enriched in antigen-specific T cells capable of down-modulating cytotoxic activity. Int Immunol. 1998;10:311–23. doi: 10.1093/intimm/10.3.311. [DOI] [PubMed] [Google Scholar]
- 13.Wiedmann B, Sakai H, Davis TA, Wiedmann M. A protein complex required for signal-sequence-specific sorting and translocation. Nature. 1994;370:434–40. doi: 10.1038/370434a0. [DOI] [PubMed] [Google Scholar]
- 14.Yotov WV, Moreau A, St-Arnaud R. The alpha chain of the nascent polypeptide associated complex function as a transcriptional coactivator. Mol Cell Biol. 1998;18:1303–11. doi: 10.1128/mcb.18.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garland RJ, Kaneria SS, Hancock JP, Steward CG, Rowbottom AW. The use of Teflon cell culture bags to expand functionally active CD8+ cytotoxic T lymphocytes. J Immunol Methods. 1999;27:53–63. doi: 10.1016/s0022-1759(99)00068-x. [DOI] [PubMed] [Google Scholar]
- 16.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 18.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartmann G, Krug A, Bidlingmaier M, et al. Spontaneous and cationic lipid-mediated uptake of antisense oligonucleotides in human monocytes and lymphocytes. J Pharm Exp Ther. 1998;285:920–8. [PubMed] [Google Scholar]
- 20.Al-Shanti NA, Steward CG, Garland RJ. Optimization of functional efficacy of phosphothioate-modified oligonucleotides in a human CD8+ T-cell ex vivo expansion model. Scand J Immunol. 2003;58:462–70. doi: 10.1046/j.1365-3083.2003.01319.x. [DOI] [PubMed] [Google Scholar]
- 21.Wiedmann B, Prehn S. The nascent polypeptide associated complex (NAC) of yeast functions in the targeting process of ribosomes to the ER membrane. FEBS Lett. 1999;458:51–4. doi: 10.1016/s0014-5793(99)01118-7. [DOI] [PubMed] [Google Scholar]
- 22.Moller I, Jung M, Beatrix B, et al. A general mechanism for regulation of access to the translocon: competition for a membrane attachment site on ribosomes. Proc Natl Acad Sci USA. 1998;95:13425–30. doi: 10.1073/pnas.95.23.13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vollenweider I, Lazzarato M, Groscurth P. proliferation of IL-2 activated lymphocytes preferably occurs in aggregates by cells expressing the CD57 antigen. Scand J Immunol. 1995;42:381–6. doi: 10.1111/j.1365-3083.1995.tb03671.x. [DOI] [PubMed] [Google Scholar]
- 24.Cantrell D. The structure of the T cell antigen receptor. Annu Rev Immunol. 1996;14:259–74. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 25.Clements JL, Boerth NJ, Lee JR, Koretzky GA. Integration of T cell receptor-dependent signaling pathways by adaptor proteins. Annu Rev Immunol. 1999;17:89–108. doi: 10.1146/annurev.immunol.17.1.89. [DOI] [PubMed] [Google Scholar]
- 26.Germain RN, Stefanova I. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu Rev Immunol. 1999;17:467–522. doi: 10.1146/annurev.immunol.17.1.467. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura Y, Russell SM, Mess SA, Friedmann M, Erdos M, et al. Heterodimerization of the IL-2 receptor beta- and gamma-chain cytoplasmic domains is required for signalling. Nature. 1994;369:330–3. doi: 10.1038/369330a0. [DOI] [PubMed] [Google Scholar]
- 28.Nelson BH, Lord JD, Greenberg PD. Cytoplasmic domains of the interleukin-2 receptor beta and gamma chains mediate the signal for T-cell proliferation. Nature. 1994;369:333–6. doi: 10.1038/369333a0. [DOI] [PubMed] [Google Scholar]
- 29.Stilo R, Liguoro D, di Jeso B, Leonardi A, Vito P. The alpha-beta chain of the nascent polypeptide associated complex binds to and regulates FADD function. Biochem Biophys Res Commun. 2003;303:1034–41. doi: 10.1016/s0006-291x(03)00487-x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang G, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;19:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]