Intravascular Location of Breast Cancer Cells after Spontaneous Metastasis to the Lung (original) (raw)
Abstract
In this study, we examined the hypothesis that early pulmonary metastases form within the vasculature. We introduced primary tumors in immunocompromised mice by subcutaneous injection of murine breast carcinoma cells (4T1) expressing green fluorescent protein. Isolated ventilated and perfused lungs from these mice were examined at various times after tumor formation by fluorescent microscopy. The vasculature was visualized by counterstaining with 1,1-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI)-acetylated low-density lipoprotein. These experiments showed that metastatic cells derived by spontaneous metastases were intravascular, and that early colony formation was intravascular. The location of the tumor cells was confirmed by deconvolution analysis. This work extends our previous study 1 that sarcoma cells injected intravenously form intravascular colonies to spontaneous metastasis and to a carcinoma model system. Many of the tumor cells seen were single implying that tumor cells may travel as single cells. These results support a model for pulmonary metastasis in mice in which 1) tumor cells can attach to lung endothelium soon after arrival; 2) surviving tumor cells proliferate intravascularly in this model; and 3) extravasation of the tumor occurs when intravascular micrometastatic foci outgrow the vessels they are in.
We recently reported direct observations of the sequence of events in pulmonary metastasis after injection of fluorescent tumor cells into the venous circulation using fluorescent microscopy of isolated perfused lungs. 1 Because endothelium has receptors for oxidized or acetylated low-density lipoprotein (LDL), the pulmonary endothelium can be precisely visualized in these preparations through infusion of DiI-acetylated LDL. Because the lung is translucent, these methods allow high-resolution imaging of the microvasculature of the lungs at up to 100-μm depth beneath the pleural surface and enable the precise localization of the tumor cells. We used these methods to show in a mouse experimental metastasis model that tumor cells attach to the pulmonary endothelium and proliferate intravascularly.
We have also demonstrated that differences in apoptosis of tumor cells in vivo correlated to differences in metastatic potential. 1-3 Based on these observations, we proposed a new model for pulmonary metastasis in which endothelium-attached tumor cells that survived the initial apoptotic stimuli proliferate intravascularly. A principal tenet of this new model is that extravasation of tumor cells is not a prerequisite for metastatic foci formation. 1-3
Because the initial experiments were based on intravenous injection of tumor cells, it was possible that the results were an artifact of this mode of introduction of the tumor cells or of the relatively large numbers of cells infused simultaneously. To determine whether our observations in an experimental model of metastasis were valid for cells that metastasize spontaneously from a primary tumor, we have tested our hypothesis using a spontaneous metastasis model. Our previous results were obtained using fibrosarcoma cells, so we sought to extend this hypothesis to carcinoma cells by using the breast carcinoma cell line 4T1. 4 The results indeed indicate that extravasation is also rare after spontaneous metastasis. These observations confirmed that metastasizing tumor cells proliferate intravascularly within the lung. This suggests that drug discovery efforts could be directed to blocking the metastatic cascade at the steps of the initial attachment of tumor cells to lung endothelium and intravascular proliferation.
Materials and Methods
Cells and Cell Culture
4T1 cells were derived from a single spontaneously arising mammary tumor from a BALB/cfC3H mouse. Primary 4T1 tumor cells injected into mammary fatpad of mice spontaneously metastasize to both the lung and liver. 4 Cells were cultured at 37°C in 5% CO2 in Dulbecco’s modified Eagle’s medium (Life Technologies, Inc., Grand Island, NY) supplemented with penicillin-streptomycin (Life Technologies, Inc.) and 10% fetal bovine serum (Hyclone Laboratories, Inc., Logan, UT).
Spontaneous Metastasis Assay
Female NCR-nu/nu mice (4 to 6 weeks old) were obtained from Taconic Farms (Germantown, NY) and stored in the rodent facility of the Institute for Human Gene Therapy at the University of Pennsylvania under appropriate conditions. Cells used for injection were grown to subconfluence, subjected to brief trypsin treatment, washed, and resuspended in serum-free Dulbecco’s modified Eagle’s medium. Mice were injected subcutaneously in the right flank with 5 × 105 cells in single cell suspension in 100-μL of serum-free Dulbecco’s modified Eagle’s medium. Tumors were measured using Vernier calipers for calculation of tumor size. After 1, 2, or 3 weeks, the mice were sacrificed and in situ fluorescent microscopy was performed on their lungs.
Isolated Lung in Situ Fluorescent Microscopy
This assay was performed as described. 1 Briefly, mice were sacrificed by an intraperitoneal sodium pentobarbital-induced anesthesia followed by exsanguination. A cannula connected to a rodent ventilator dispensing a mixture of 5% CO2 in air was attached to the trachea of the mouse. The chest was opened and pulmonary circulation was cleared of blood by gravity flow of perfusate through a cannula inserted in the main pulmonary artery, exiting from the transected left ventricle. The perfusate was Krebs-Ringer bicarbonate solution (118.45 mmol/L NaCl, 4.74 mmol/L KCl, 1.17 mmol/L MgSO4.7H2O, 1.27 mmol/L CaCl2.2H2O, 1.18 mmol/L KH2PO4, 24.87 mmol/L NaHCO3, pH 7.4, 10 mmol/L glucose, 5% dextran). To visualize lung vasculature, the lungs were infused with DiI-conjugated, acetylated LDL (Molecular Probes, Inc; Eugene, OR) for 5 minutes. The lungs were removed and examined under an inverted fluorescence microscope. Images were analyzed, and three-dimensional reconstruction of tumor colonies were created using MetaMorph Imaging software (Universal Imaging, West Chester, PA) and Openlab software (Improvision, Coventry, UK).
Results
Tumor Cells Spontaneously Metastasize to the Lungs as Single Cells
To determine whether spontaneously occurring tumor cells colonize at intravascular locations in the lung, we implanted 5 × 105 4T1 mouse breast carcinoma cells subcutaneously into the flanks of female nu/nu mice. After 1, 2, or 3 weeks, the entire surface of each lung from 13 mice with subcutaneous tumors was scanned by epifluorescence microscopy for green fluorescent protein-expressing tumor cells using the isolated lung preparation (Table 1) ▶ . At 1 week after subcutaneous injection (primary tumor diameter, 4 to 6 mm), no tumor cells were detected in subpleural vessels in the four animals studied. At 2 weeks (primary tumor diameter, 6 to 8 mm; four animals), one to three solitary tumor cells were found inside the subpleural vessels in each lung. At 3 weeks (primary tumor diameter, 8 to 15 mm; five animals), 3 to 10 solitary tumor cells were found attached intravascularly in the lung endothelium in each lung with the exception of one mouse in which 38 individual cells were found. These solitary tumor cells were attached to the vasculature and every one was observed to be intravascular. The cells were found in both larger, precapillary or postcapillary vessels (Figure 1A) ▶ and in capillaries (Figure 1B) ▶ . This suggests that 4T1 tumor cells mainly detach from the primary tumor as single cells, rather than as an aggregate of several cells.
Table 1.
Spontaneous Metastasis of 4T1 Tumor Cells to the Lung
| Age of primary tumor | Mouse no. | Number of single cells | Number of large colonies (100 to 200 cells) |
|---|---|---|---|
| 1 week | 1 | 0 | 0 |
| 2 | 0 | 0 | |
| 3 | 0 | 0 | |
| 4 | 0 | 0 | |
| 2 weeks | 1 | 2 | 0 |
| 2 | 3 | 0 | |
| 3 | 1 | 0 | |
| 4 | 1 | 0 | |
| 3 weeks | 1 | 38 | 1 |
| 2 | 4 | 2 | |
| 3 | 7 | 0 | |
| 4 | 3 | 1 | |
| 5 | 4 | 2 |
Figure 1.
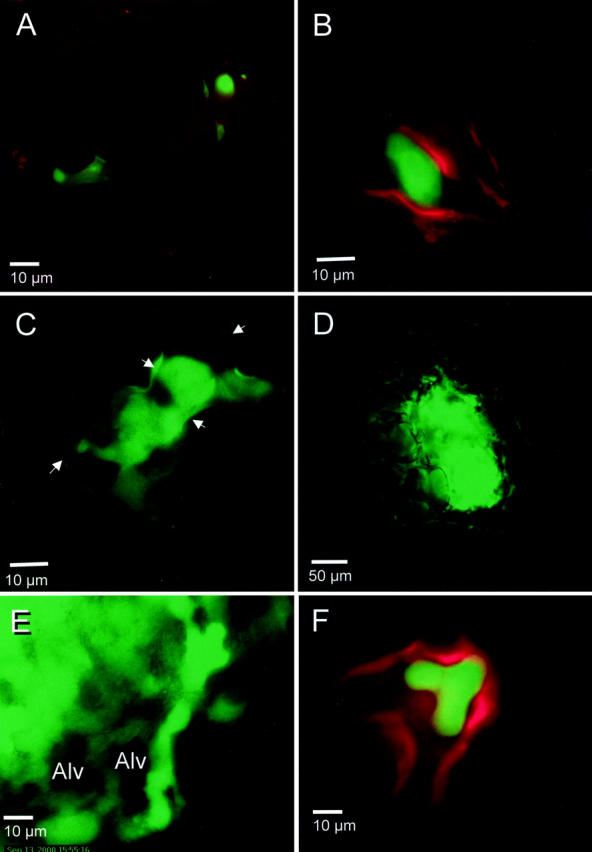
Intravascular location of spontaneous lung metastases. 4T1 tumor cells that spontaneously metastasized into the lung were visualized by high-resolution digital video microscopy as described in Materials and Methods. In A, B, and F, the lung endothelium has been labeled with a red fluorescent dye, DiI-acetylated LDL. A: Solitary tumor cells (green) attached within the precapillary arterioles. B: Solitary tumor cell attached in a capillary. C: Colony of ∼10 cells within a blood vessel. The endothelial margins, indicated by arrows, are visible because of reflection and scattering of green fluorescent protein fluorescence from tumor cells. D: Large colony of tumor cells within the lung vasculature. E: Another large colony that probably has outgrown the vessel of initial attachment continues to exhibit intravascular growth along capillaries around alveoli (Alv). Note how the outline of the alveolar wall is formed by strings of green fluorescent protein-expressing tumor cells. F: Front projection of a three dimensional-reconstructed image of a small colony shows intravascular location. The endothelium was labeled with DiI-acetylated LDL (red). Fifty planes of a stack of images taken separately in the green and red fluorescence channels taken along the z axis spanning a depth of 50 μm were overlaid.
Presence of Multicell Colonies in the Lung
In addition to individual tumor cells, colonies of varying sizes were also found at 3 weeks of primary tumor age but not earlier (Table 1) ▶ . Small colonies, ranging from those with only 5 to 10 cells (Figure 1C) ▶ , to large colonies, ∼100 to 200 cells could be seen (Figure 1D) ▶ . Lungs from each mouse contained one to two large colonies (>100 cells). These large colonies exhibited vascular boundary destruction, and at the periphery, linear growth inside capillaries (Figure 1E) ▶ . At 2 weeks, lungs from two animals contained one small colony each (∼10 cells). This is similar to our observation in an experimental model for metastasis that intravascular colonies increased in size throughout time. 1
Intravascular Location of Tumor Cells
In addition to direct visual observation, the intravascular position of the single cells and the small colonies was ascertained by three-dimensional reconstruction of stacks of images acquired at various z axis positions. Figure 1F ▶ shows a reconstruction that reveals a small, intravascular colony of cells located entirely within in a precapillary arteriole.
Discussion
The presence of tumor cells circulating in the vasculature has long been documented in human patients. 5,6 Many of these cells become nonviable and will not be able to complete the metastatic cascade and develop into a secondary tumor. 6,7 However, a small number of circulating cells complete the metastatic process. Our evidence suggests that the initial proliferation takes place within the blood vessels. The ability of tumor cells to proliferate intravascularly has also been reported by other investigators. Crissman and colleagues 8 observed the formation of thrombi aggregates within 2 minutes of tumor cell adhesion to the lung endothelium, and that proliferation of tumor cells occurred within 24 hours, before extravasation of the intravascular colony after 48 to 72 hours. Although extravasation of tumor cells has been demonstrated in the sinusoids of the liver and indeed of all cells observed in a chorioallantoic assay for metastasis, 9 there have also been observations that early tumor growth occurred within the vasculature in the liver in the presinusoidal portal vein branches. 10,11 It may certainly be the case that some tumor cells may lie dormant in the lungs or other organs. 12-14
Is the lung vasculature an attractive environment for metastatic tumor growth? There have been reports that the organ microenvironment can influence metastasis. 15-17 It is generally accepted that angiogenesis and neovascularization are required for tumor growth. Although our metastasis assay is not orthotopic, the intravascular location of the tumors in the lung, in a region with easy access to oxygen and other blood-borne nutrients, raises the possibility that tumor-host interactions resulted in the proliferation of attached tumor cells instead of extravasation from the blood vessels. This idea can be supported by a recent study showing that aggressive melanoma cells express the endothelial-specific gene VE-cadherin, resulting in formation of vasculogenic networks in culture. 18 Another study showed that colon carcinoma cells implanted in mice induced formation of new blood vessels consisting of both tumor and endothelial cells. 19 The ability of the tumor cells to form colonies within the lung vasculature suggests another mechanism for the tumor to ensure that it is vascularized. Because the dye DiI-aceylated LDL could be seen both proximal and distal to small colonies of tumor cells, we can conclude that the presence of these intravascular colonies does not entirely obstruct blood flow. As the colonies become large however, the vascular boundaries are no longer intact as evidenced by seepage of the endothelial marker into the surrounding tissues and alveoli. Because the proliferation of tumor cells does not prevent blood flow, this suggests that as the colony grows in size, it might be able to recruit existing blood vessels and incorporate them into the tumor. The ability to co-opt existing blood vessels by glioma and mammary carcinoma cells in the rat brain and Lewis lung carcinoma cells in the mouse lung has been described. 20,21 Although the co-opted vessels regressed, the accompanying expression of Ang-2 and vascular endothelial growth factor led to renewed angiogenic response. 20
The results described herein are consistent with our observations using experimental metastasis assays. 1-3 Our evidence suggests that the initial proliferation takes place within blood vessels, at least in this model system of spontaneous metastasis assay. This provides additional support for a pulmonary metastasis model in which 1) tumor cells can attach to lung endothelium soon after arrival; 2) the majority of tumor cells undergo apoptosis; 3) surviving tumor cells begin to proliferate, expanding intravascularly; and 4) extravasation of tumor occurs.
Acknowledgments
We thank Dr. Eric Bernhard for critical reading of the manuscript and helpful discussions.
Footnotes
Address reprint requests to Abu-Bakr Al-Mehdi, M.D., Ph.D., University of South Alabama, 307 N. University Blvd., MSB 3370, Mobile, AL 36688. E-mail: aalmehdi@jaguar1.usouthal.edu.
Supported by the Susan G. Komen Breast Cancer Foundation (grant no. 99-003201 to A. A.) and the National Cancer Institute (grants RO1 CA-46830 and RO1 CA 89188 to R. J. M.).
References
- 1.Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ: Intravascular origin of metastasis from proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med 2000, 6:100-102 [DOI] [PubMed] [Google Scholar]
- 2.Wong CW, Lee A, Shientag L, Yu J, Dong Y, Kao G, Al-Mehdi AB, Bernhard EJ, Muschel RJ: Apoptosis: an early event in metastatic inefficiency. Cancer Res 2001, 61:333-338 [PubMed] [Google Scholar]
- 3.Ito S, Nakanishi H, Ikehara Y, Kato T, Kasai Y, Ito K, Akiyama S, Nakao A, Tatematsu M: Real time observation of micrometastasis formation in the living mouse liver using a green fluorescent protein gene-tagged rat tongue carcinoma cell line. Int J Cancer , 93:212-217 [DOI] [PubMed] [Google Scholar]
- 4.Aslakson CJ, Miller FR: Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res 1992, 52:1399-1405 [PubMed] [Google Scholar]
- 5.Iwasaki T: Histological and experimental observations on the destruction of tumour cells in the blood vessels. J Pathol Bacteriol 1915, 20:85-105 [Google Scholar]
- 6.Glaves D, Huben RP, Weiss L: Haematogenous dissemination of cells from human renal adenocarcinomas. Br J Cancer 1988, 57:32-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehes G, Witt A, Kubista E, Ambros PF: Circulating breast cancer cells are frequently apoptotic. Am J Pathol 2001, 159:17-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crissman JD, Hatfield JS, Menter DG, Sloane B, Honn KV: Morphological study of the interaction of intravascular tumor cells with endothelial cells and subendothelial matrix. Cancer Res 1988, 48:4065-4072 [PubMed] [Google Scholar]
- 9.Koop S, Schmidt EE, MacDonald IC, Morris VL, Khokha R, Grattan M, Leone J, Chambers AF, Groom AC: Independence of metastatic ability and extravasation: metastatic ras-transformed and control fibroblasts extravasate equally well. Proc Natl Acad Sci USA 1996, 93:11080-11084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang HH, McIntosh AR, Hasinoff BB, Rector ES, Ahmed N, Nance DM, Orr FW: B16 melanoma cell arrest in the mouse liver induces nitric oxide release and sinusoidal cytotoxicity: a natural hepatic defense against metastasis. Cancer Res 2000, 60:5862-5869 [PubMed] [Google Scholar]
- 11.Scherbarth S, Orr FW: Intravital videomicroscopic evidence for regulation of metastasis by the hepatic microvasculature: effects of interleukin-1 alpha on metastasis and the location of B16F1 melanoma cell arrest. Cancer Res 1997, 57:4105-4110 [PubMed] [Google Scholar]
- 12.Aguirre-Ghiso JA, Liu D, Mignatti A, Kovalski K, Ossowski L: Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol Biol Cell 2001, 12:863-879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguirre Ghiso JA, Kovalski K, Ossowski L: Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol 1999, 147:89-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron MD, Schmidt EE, Kerkvliet N, Nadkarni KV, Morris VL, Groom AC, Chambers AF, MacDonald IC: Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res 2000, 60:2541-2546 [PubMed] [Google Scholar]
- 15.Fidler IJ: Modulation of the organ microenvironment for treatment of cancer metastasis. J Natl Cancer Inst 1995, 87:1588-1592 [DOI] [PubMed] [Google Scholar]
- 16.Sternlicht MD, Bissell MJ, Werb Z: The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene 2000, 19:1102-1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howlett AR, Petersen OW, Steeg PS, Bissell MJ: A novel function for the nm23–H1 gene: overexpression in human breast carcinoma cells leads to the formation of basement membrane and growth arrest. J Natl Cancer Inst 1994, 86:1838-1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA, Schatteman GC, Seftor RE: Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci USA 2001, 98:8018-8023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL: Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci USA 2000, 97:14608-14613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ: Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 1999, 284:1994-1998 [DOI] [PubMed] [Google Scholar]
- 21.Holash J, Wiegand SJ, Yancopoulos GD: New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene 1999, 18:5356-5362 [DOI] [PubMed] [Google Scholar]