Measuring inconsistency in meta-analyses (original) (raw)
Short abstract
Cochrane Reviews have recently started including the quantity _I_2 to help readers assess the consistency of the results of studies in meta-analyses. What does this new quantity mean, and why is assessment of heterogeneity so important to clinical practice?
Systematic reviews and meta-analyses can provide convincing and reliable evidence relevant to many aspects of medicine and health care.1 Their value is especially clear when the results of the studies they include show clinically important effects of similar magnitude. However, the conclusions are less clear when the included studies have differing results. In an attempt to establish whether studies are consistent, reports of meta-analyses commonly present a statistical test of heterogeneity. The test seeks to determine whether there are genuine differences underlying the results of the studies (heterogeneity), or whether the variation in findings is compatible with chance alone (homogeneity). However, the test is susceptible to the number of trials included in the meta-analysis. We have developed a new quantity, _I_2, which we believe gives a better measure of the consistency between trials in a meta-analysis.
Need for consistency
Assessment of the consistency of effects across studies is an essential part of meta-analysis. Unless we know how consistent the results of studies are, we cannot determine the generalisability of the findings of the meta-analysis. Indeed, several hierarchical systems for grading evidence state that the results of studies must be consistent or homogeneous to obtain the highest grading.2-4
Tests for heterogeneity are commonly used to decide on methods for combining studies and for concluding consistency or inconsistency of findings.5,6 But what does the test achieve in practice, and how should the resulting P values be interpreted?
Testing for heterogeneity
A test for heterogeneity examines the null hypothesis that all studies are evaluating the same effect. The usual test statistic (Cochran's Q) is computed by summing the squared deviations of each study's estimate from the overall meta-analytic estimate, weighting each study's contribution in the same manner as in the meta-analysis.7 P values are obtained by comparing the statistic with a χ2 distribution with _k_-1 degrees of freedom (where k is the number of studies).
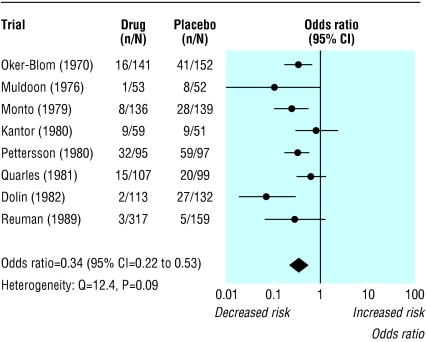
The test is known to be poor at detecting true heterogeneity among studies as significant. Meta-analyses often include small numbers of studies,6,8 and the power of the test in such circumstances is low.9,10 For example, consider the meta-analysis of randomised controlled trials of amantadine for preventing influenza (fig 1).11 The treatment effects in the eight trials seem inconsistent: the reduction in odds vary from 16% to 93%, with some of the confidence intervals not overlapping. But the test of heterogeneity yields a P value of 0.09, conventionally interpreted as being non-significant. Because the test is poor at detecting true heterogeneity, a non-significant result cannot be taken as evidence of homogeneity. Using a cut-off of 10% for significance12 ameliorates this problem but increases the risk of drawing a false positive conclusion (type I error).10
Fig 1.
Eight trials of amantadine for prevention of influenza.11 Outcome is cases of influenza. Summary odds ratios calculated with random effects method
Conversely, the test arguably has excessive power when there are many studies, especially when those studies are large. One of the largest meta-analyses in the Cochrane Database of Systematic Reviews is of clinical trials of tricyclic antidepressants and selective serotonin reuptake inhibitors for treatment of depression.13 Over 15 000 participants from 135 trials are included in the assessment of comparative drop-out rates, and the test for heterogeneity is significant (P = 0.005). However, this P value does not reasonably describe the extent of heterogeneity in the results of the trials. As we show later, a little inconsistency exists among these trials but it does not affect the conclusion of the review (that serotonin reuptake inhibitors have lower discontinuation rates than tricyclic antidepressants).
Since systematic reviews bring together studies that are diverse both clinically and methodologically, heterogeneity in their results is to be expected.6 For example, heterogeneity is likely to arise through diversity in doses, lengths of follow up, study quality, and inclusion criteria for participants. So there seems little point in simply testing for heterogeneity when what matters is the extent to which it affects the conclusions of the meta-analysis.
Quantifying heterogeneity: a better approach
We developed an alternative approach that quantifies the effect of heterogeneity, providing a measure of the degree of inconsistency in the studies' results.14 The quantity, which we call _I_2, describes the percentage of total variation across studies that is due to heterogeneity rather than chance. _I_2 can be readily calculated from basic results obtained from a typical meta-analysis as _I_2 = 100%×(Q - df)/Q, where Q is Cochran's heterogeneity statistic and df the degrees of freedom. Negative values of _I_2 are put equal to zero so that _I_2 lies between 0% and 100%. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity.
Examples of values of _I_2
The principal advantage of _I_2 is that it can be calculated and compared across meta-analyses of different sizes, of different types of study, and using different types of outcome data. Table 1 gives _I_2 values for six published meta-analyses along with 95% uncertainty intervals. The upper limits of these intervals show that conclusions of homogeneity in meta-analyses of small numbers of studies are often unjustified.11,13,15-19
Table 1.
Heterogeneity statistics for examples of meta-analyses from the literature. Meta-analyses were conducted using either meta or metan in STATA15
| Heterogeneity test | |||||||
|---|---|---|---|---|---|---|---|
| Topic | Outcome/analysis | Effect measure | No of studies | Q | df | P | I2(95% uncertainty interval)* |
| Tamoxifen for breast cancer16 | Mortality | Peto odds ratio | 55 | 55.9 | 54 | 0.40 | 3 (0 to 28) |
| Streptokinase after myocardial infarction17 | Mortality | Odds ratio | 33 | 39.5 | 32 | 0.17 | 19 (0 to 48) |
| Selective serotonin reuptake inhibitors for depression13 | Drop-out | Odds ratio | 135 | 179.9 | 134 | 0.005 | 26 (7 to 40) |
| Magnesium for acute myocardial infarction18 | Death | Odds ratio | 16 | 40.2 | 15 | 0.0004 | 63 (30 to 78) |
| Magnetic fields and leukaemia19 | All studies | Odds ratio | 6 | 15.9 | 5 | 0.007 | 69 (26 to 87) |
| Amantadine11 | Prevention of influenza | Odds ratio | 8 | 12.44 | 7 | 0.09 | 44 (0 to 75) |
The tamoxifen and streptokinase meta-analyses, in which all the included studies found similar effects,16,17 have _I_2 values of 3% and 19% respectively. These indicate little variability between studies that cannot be explained by chance. For the review comparing drop-outs on selective serotonin reuptake inhibitors with tricyclic antidepressants, _I_2 is 26%, indicating that although the heterogeneity is highly significant, it is a small effect.
The reviews of trials of magnesium after myocardial infarction (_I_2 = 63%) and case-control studies investigating the effects of electromagnetic radiation on leukaemia (69%) both included studies with diverse results. The high _I_2 values show that most of the variability across studies is due to heterogeneity rather than chance. Although no significant heterogeneity was detected in the review of amantadine,11 the inconsistency was moderately large (_I_2 = 44%).
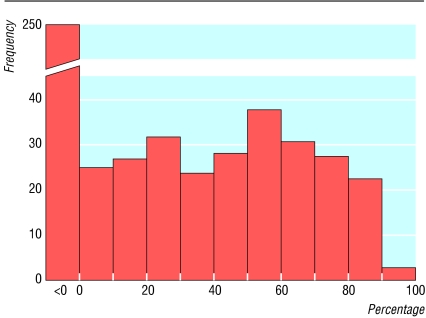
Figure 2 shows the observed values of _I_2 from 509 meta-analyses in the Cochrane Database of Systematic Reviews. Almost half of these meta-analyses (250) had no inconsistency (_I_2 = 0%). Among meta-analyses with some heterogeneity, the distribution of _I_2 is roughly flat.
Fig 2.
Distribution of observed values of _I_2 based on odds ratios from 509 meta-analyses of dichotomous outcomes in the Cochrane Database of Systematic Reviews. Data are from the first subgroup (if any) in the first meta-analysis (if any) in each review, if it involved a dichotomous outcome and at least two trials with events. Meta-analyses conducted with metan in STATA15
Further applications of _I_2
_I_2 can also be helpful in investigating the causes and type of heterogeneity, as in the three examples below.
Methodological subgroups
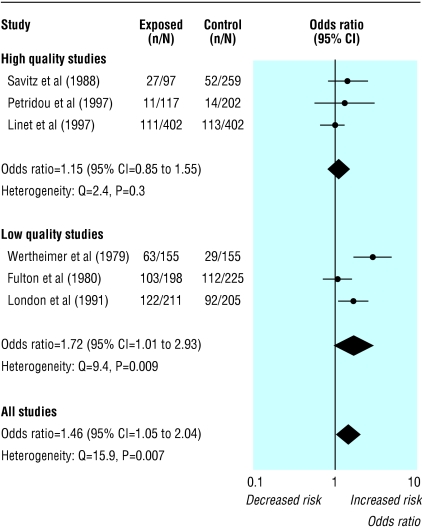
Figure 3 shows the six case-control studies of magnetic fields and leukaemia broken down into two subgroups based on assessment of their quality.19 If heterogeneity is identified in a meta-analysis a common option is to subgroup the studies. Because of loss of power, non-significant heterogeneity within a subgroup may be due not to homogeneity but to the smaller number of studies. Here, the P values for the heterogeneity test are higher for the two subgroups (P = 0.3 and P = 0.009) than for the complete data (P = 0.007), which suggests greater consistency within the subgroups. However, the values of _I_2 show that the three low quality studies are more inconsistent (_I_2 = 79%) than all six (_I_2 = 69%) (table 2). Substantially less inconsistency exists among the high quality studies (_I_2 = 15%), although uncertainty intervals for all of the _I_2 values are wide.
Fig 3.
Meta-analyses of six case-control studies relating residential exposure to electromagnetic fields to childhood leukaemia.19 Summary odds ratio calculated by random effects method
Table 2.
More advanced applications of _l_2 for assessing heterogeneity in three published meta-analyses. Meta-analyses were conducted with either meta or metan in STATA15
| Heterogeneity test | |||||||
|---|---|---|---|---|---|---|---|
| Topic | Outcome/analysis | Effect measure | No of studies | Q | df | P | _I_2 (95% uncertainty intervals)* |
| Magnetic fields and leukaemia19 | All studies | Odds ratio | 6 | 15.9 | 5 | 0.007 | 69 (26 to 87) |
| High quality | Odds ratio | 3 | 2.4 | 2 | 0.31 | 17 (0 to 91) | |
| Low quality | Odds ratio | 3 | 9.4 | 2 | 0.009 | 79 (32 to 94) | |
| Human albumin for critically ill20 | Death | Risk ratio | 24† | 15.3 | 23 | 0.88 | 0 (0 to 17) |
| Death | Risk difference | 30 | 36.7 | 29 | 0.15 | 21 (0 to 50) | |
| Tamoxifen to prevent recurrence of breast cancer17 | All studies | Peto odds ratio | 55 | 108.2 | 54 | 0.00002 | 50 (32 to 63) |
| Total within groups‡ | Peto odds ratio | — | 59.9 | 52 | 0.21 | 13 (0 to 39) | |
| Between groups‡ | Peto odds ratio | 3 groups | 48.3 | 2 | <0.00001 | 96 (91 to 98) |
Heterogeneity related to choice of effect measure
A systematic review of clinical trials of human albumin administration in critically ill patients concluded that albumin may increase mortality.20 These studies had no inconsistency in risk ratio estimates (_I_2 = 0%) and a narrow uncertainty interval. Table 2 shows the heterogeneity statistics for risk differences as well as for risk ratios. Six trials with no deaths in either treatment group do not contribute information on risk ratios, but they all provide estimates of risk differences. Using P values to decide which scale is more consistent with the data21 is inappropriate because of the differing numbers of studies. _I_2 values may validly be compared and show that the risk differences are less homogeneous, as is often the case.22
Clinically important subgroups
_I_2 can also be used to describe heterogeneity among subgroups. Table 2 includes results for the outcome of recurrence in the meta-analysis of trials of tamoxifen for women with early breast cancer. There was highly significant (P = 0.00002) and important heterogeneity (_I_2 = 50%) among the trials.16 However, a potentially important source of heterogeneity is the duration of treatment. The authors divided the trials into three duration categories and presented an overall heterogeneity test, a test comparing the three subgroups, and a test for heterogeneity within the subgroups. _I_2 values corresponding to each test show that 96% of the variability observed among the three subgroups cannot be explained by chance. This is not clear from the P values alone. The extreme inconsistency among all 55 trials in the odds ratios for recurrence (_I_2 = 50%) is substantially reduced (_I_2 = 13%) once differences in treatment duration are accounted for.
How much is too much heterogeneity?
A naive categorisation of values for _I_2 would not be appropriate for all circumstances, although we would tentatively assign adjectives of low, moderate, and high to _I_2 values of 25%, 50%, and 75%. Figure 2 shows that about a quarter of meta-analyses have _I_2 values over 50%. Quantification of heterogeneity is only one component of a wider investigation of variability across studies, the most important being diversity in clinical and methodological aspects. Meta-analysts must also consider the clinical implications of the observed degree of inconsistency across studies. For example, interpretation of a given degree of heterogeneity across several studies will differ according to whether the estimates show the same direction of effect.
Advantages of _I_2
- Focuses attention on the effect of any heterogeneity on the meta-analysis
- Interpretation is intuitive—the percentage of total variation across studies due to heterogeneity
- Can be accompanied by an uncertainty interval
- Simple to calculate and can usually be derived from published meta-analyses
- Does not inherently depend on the number of studies in the meta-analysis
- May be interpreted similarly irrespective of the type of outcome data (eg dichotomous, quantitative, or time to event) and choice of effect measure (eg odds ratio or hazard ratio)
- Wide range of applications
Summary points
Inconsistency of studies' results in a meta-analysis reduces the confidence of recommendations about treatment
Inconsistency is usually assessed with a test for heterogeneity, but problems of power can give misleading results
A new quantity _I_2, ranging from 0-100%, is described that measures the degree of inconsistency across studies in a meta-analysis
_I_2 can be directly compared between meta-analyses with different numbers of studies and different types of outcome data
_I_2 is preferable to a test for heterogeneity in judging consistency of evidence
An alternative quantification of heterogeneity in a meta-analysis is the among-study variance (often called τ2), calculated as part of a random effects meta-analysis. This is more useful for comparisons of heterogeneity among subgroups, but values depend on the treatment effect scale. We believe, _I_2 offers advantages over existing approaches to the assessment of heterogeneity (box). Focusing on the effect of heterogeneity also avoids the temptation to perform so called two stage analyses, in which the meta-analysis strategy (fixed or random effects method) is determined by the result of a statistical test. Such strategies have been found to be problematic.23,24 We therefore believe that _I_2 is preferable to the test of heterogeneity when assessing inconsistency across studies.
We thank Keith O'Rourke and Ian White for useful comments.
Contributors: The authors all work as statisticians and have extensive experience in methodological, empirical and applied research in meta-analysis. JH, JD, and DA are coconvenors of the Cochrane Statistical Methods Group. The views expressed in the paper are those of the authors. All authors contributed to the development of the methods described. JH and ST worked more closely on the development of _I_2. JH is guarantor.
Funding: This work was funded in part by MRC Project Grant G9815466.
Competing interests: None declared.
References
- 1.Egger M, Davey Smith G. Meta-analysis: potentials and promise. BMJ 1997;315: 1371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liberati A, Buzzetti R, Grilli R, Magrini N, Minozzi S. Which guidelines can we trust? West J Med 2001;174: 262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harbour R, Miller J for the Scottish Intercollegiate Guidelines Network Grading Review Group. A new system for grading recommendations in evidence based guidelines. BMJ 2001;323: 334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guyatt G, Sinclair J, Cook D, Jaeschke R, Schünemann H, Pauker S. Moving from evidence to action. In: Guyatt G, Rennie D, eds. Users' guides to the medical literature: a manual for evidence-based clinical practice. Chicago: American Medical Association, 2002: 599-608.
- 5.Petitti DB. Approaches to heterogeneity in meta-analysis. Stat Med 2001;20: 3625-33. [DOI] [PubMed] [Google Scholar]
- 6.Higgins J, Thompson S, Deeks J, Altman D. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy 2002;7: 51-61. [DOI] [PubMed] [Google Scholar]
- 7.Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10: 101-29. [Google Scholar]
- 8.Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 2001;54: 1046-55. [DOI] [PubMed] [Google Scholar]
- 9.Paul SR, Donner A. Small sample performance of tests of homogeneity of odds ratios in k 2×2 tables. Stat Med 1992;11: 159-65. [DOI] [PubMed] [Google Scholar]
- 10.Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med 1998;17: 841-56. [DOI] [PubMed] [Google Scholar]
- 11.Jefferson TO, Demicheli V, Deeks JJ, Rivetti D. Amantadine and rimantadine for preventing and treating influenza A in adults. Cochrane Database Syst Rev 2002;(4): CD001169. [DOI] [PubMed]
- 12.Dickersin K, Berlin JA. Meta-analysis: state-of-the-science. Epidemiol Rev 1992;14: 154-76. [DOI] [PubMed] [Google Scholar]
- 13.Barbui C, Hotopf M, Freemantle N, Boynton J, Churchill R, Eccles MP, Geddes JR, et al. Treatment discontinuation with selective serotonin reuptake inhibitors (SSRIs) versus tricyclic antidepressants (TCAs). Cochrane Database Syst Rev 2003;(3): CD002791. [DOI] [PubMed]
- 14.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21: 1539-58. [DOI] [PubMed] [Google Scholar]
- 15.Sterne JAC, Bradburn MJ, Egger M. Meta-analysis in STATA. In: Egger M, Davey Smith G, Altman DG, eds. Systematic reviews in health care: meta-analysis in context. 2nd ed. London: BMJ Publications, 2001: 347-69.
- 16.Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 1998;351: 1451-67. [PubMed] [Google Scholar]
- 17.Lau J, Antman EM, Jimenez-Silva J, Kupelink B, Mosteller SF, Chalmers TC. Cumulative meta-analysis of therapeutic trials for myocardial infarction. N Engl J Med 1992;327: 248-54. [DOI] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G. Misleading meta-analysis. BMJ 1995;310: 752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angelillo IF, Villari P. Residential exposure to electromagnetic fields and childhood leukaemia: a meta-analysis. Bull World Health Organ 1999;77: 906-15. [PMC free article] [PubMed] [Google Scholar]
- 20.Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ 1998;317: 235-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engels EA, Schmid CH, Terrin N, Olkin I, Lau J. Heterogeneity and statistical significance in meta-analysis: an empirical study of 125 meta-analyses. Stat Med 2000;19: 1707-28. [DOI] [PubMed] [Google Scholar]
- 22.Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med 2002;21: 1575-1600. [DOI] [PubMed] [Google Scholar]
- 23.Freeman PR. The performance of the two-stage analysis of two-treatment, two-period crossover trials. Stat Med 1989;8: 1421-32. [DOI] [PubMed] [Google Scholar]
- 24.Steyerberg EW, Eijkemans MJ, Habbema JD. Stepwise selection in small data sets: a simulation study of bias in logistic regression analysis. J Clin Epidemiol 1999;52: 935-42. [DOI] [PubMed] [Google Scholar]