Bone Morphogenetic Protein Signaling Is Required for Maintenance of Differentiated Phenotype, Control of Proliferation, and Hypertrophy in Chondrocytes (original) (raw)
Abstract
To examine the role of bone morphogenetic protein (BMP) signaling in chondrocytes during endochondral ossification, the dominant negative (DN) forms of BMP receptors were introduced into immature and mature chondrocytes isolated from lower and upper portions of chick embryo sternum, respectively. We found that control sternal chondrocyte populations expressed type IA, IB, and II BMP receptors as well as BMP-4 and -7. Expression of a DN-type II BMP receptor (termed DN-BMPR-II) in immature lower sternal (LS) chondrocytes led to a loss of differentiated functions; compared with control cells, the DN-BMPR- II–expressing LS chondrocytes proliferated more rapidly, acquired a fibroblastic morphology, showed little expression of type II collagen and aggrecan genes, and upregulated type I collagen gene expression. Expression of DN-BMPR-II in mature hypertrophic upper sternal (US) chondrocytes caused similar effects. In addition, the DN-BMPR-II–expressing US cells exhibited little alkaline phosphatase activity and type X collagen gene expression, while the control US cells produced both alkaline phosphatase and type X collagen. Both DN-BMPR-II–expressing US and LS chondrocytes failed to respond to treatment with BMP-2 . When we examined the effects of DN forms of types IA and IB BMP receptors, we found that DN-BMPR-IA had little effect, while DN-BMPR-IB had similar but weaker effects compared with those of DN-BMPR-II. We conclude that BMP signaling, particularly that mediated by the type II BMP receptor, is required for maintenance of the differentiated phenotype, control of cell proliferation, and expression of hypertrophic phenotype.
Bone morphogenetic proteins (BMPs)1 were originally identified as factors able to induce ectopic endochondral ossification (Reddi, 1992). It has been suggested that BMPs play a number of important roles in skeletal formation. For example, in the developing limb, BMPs appear to mediate the action of Sonic hedgehog during anterior–posterior axis determination (Francis et al., 1994), to act as competitors for fibroblast growth factors during proliferation of mesenchymal cells in the progress zone (Niswander and Martin, 1993), and to be inducers of programmed cell death in interdigital mesenchymal cells (Yokouchi et al., 1996; Zou and Niswander, 1996). In addition, BMPs are possible regulators of proliferation and differentiation of chondrocytes. BMP-2 and -4 are able to induce differentiation of mesenchymal cells into chondrocytes (Chen et al., 1991; Duprez et al., 1996_a_ ), and BMP-2, -3, and/or -4 stimulates and maintains proteoglycan synthesis and enhances alkaline phosphatase (APase) activity in differentiated chondrocytes in vitro (Hiraki et al., 1991; Luyten et al., 1992, 1994). Although BMPs have various effects on chondrocytes, it remains unclear whether and how they regulate the development and function of chondrocytes at cellular level.
BMPs exert their biological function by interactions with cell surface receptors. The BMP receptors consist of heterodimers or heterotetramers of type I and type II receptors (Koenig et al., 1994; Liu et al., 1995; Nohno et al., 1995). Both types of receptors contain intracellular serine/ threonine kinase domains, which are required for signaling, and heteromeric complexes of two types of receptors are necessary for high-affinity ligand binding and signaling (Liu et al., 1995; Nohno et al., 1995). We recently cloned chicken BMP receptors, i.e., BRK-1, -2, and -3, which correspond to mammalian type IA, IB, and II receptors, respectively (Kawakami et al., 1996). To examine the role of BMP signaling during chick limb chondrogenesis, we constructed viral vectors expressing dominant negative (DN) forms of these receptors lacking the intracellular kinase domain; these DN receptors are expressed at the cell surface and bind BMPs, but they do not transmit signals (Ishikawa et al., 1995; Nohno et al., 1995). We found that expression of a DN type IB receptor (termed DN-BMPR-IB) caused suppression of chondrogenesis in vitro, and that coexpression of DN type IB (DN-BMPR-IB) and type II (DN-BMPR-II) receptors resulted in limb truncation, suggesting that BMP signaling has crucial roles in chondrogenesis and skeletal patterning in the developing limb (Kawakami et al., 1996).
In the present study, we used similar strategies to determine the role of BMPs in chondrocytes undergoing endochondral ossification. During this process, immature small-sized chondrocytes first proliferate rapidly, then withdraw from mitotic activity and increase production of typical cartilage matrix components such as type II collagen and aggrecan, and finally mature into hypertrophic chondrocytes that produce abundant type X collagen and APase (Horton, 1993). Accordingly, we isolated immature and mature chondrocytes from the lower and upper portions of chick embryo sternum (termed LS and US chondrocytes, respectively) (Leboy et al., 1989; Iwamoto et al., 1993_a_ ). US chondrocytes mature to display terminal differentiation phenotype characterized by synthesis of APase and type X collagen, and LS chondrocytes are immature chondrocytes that do not proceed toward a terminal differentiation stage. We found that introduction of DN-BMPR-II into LS and US cells inhibited type II collagen and aggrecan gene expressions, altered cell morphology, and markedly changed the proliferative characteristics of these cells. Furthermore, DN-BMPR-II severely inhibited type X collagen gene expression and APase activity in US cells. The results indicate that BMP signaling is required for maintenance of the differentiated phenotype, control of proliferation, and terminal differentiation in chondrocytes.
Materials and Methods
Cell Cultures
Chicken embryo fibroblasts were obtained from the torso of virus-free White Leghorn 11-day-old embryos (line M) (Nisseiken, Yamanashi, Japan) and cultured in medium 199 containing 10% FBS. Upper sterna (US) and lower sterna (LS) chondrocytes were isolated from the cephalic portion and one-third caudal portion, respectively, of 17-day-old embryo (line M) sternum and cultured in high-glucose DME containing 10% FBS as described previously (Leboy et al., 1989; Pacifici et al., 1991). In serum-free cultures, the cells were grown in DME containing 10−8 M hydrocortisone (Sigma Chemical Co., St. Louis, MO), 30 μg protein/ml egg yolk lipoprotein (Cosmo Bio Co., LTD, Tokyo, Japan), 30 μg/ml transferrin (Sigma Chemical Co.), 10 μg/ml insulin (Sigma Chemical Co.), 10−8 M thyroxine (Sigma Chemical Co.), and 10 μg/ml of ascorbic acid. The media were changed every other day. Recombinant human BMP-2 (rhBMP-2) was provided from Yamanouchi Pharmaceutical Co. Ltd. (Tokyo, Japan) and was added starting on day 1 of culture.
Construction and Expression of Kinase Domain–deficient BMP receptors (DN-BMPR-IA, DN-BMPR-IB, and DN-BMPR-II)
Amino-terminal coding sequences of chicken type IA and type IB BMP receptors, BMPR-IA and BMPR-IB, respectively (Kawakami et al., 1996), were subcloned into a modified pBluescript vector that contains a myc epitope (MEQKLISEEDLN) and a stop codon. DN-BMPR-IA and -IB clones contain amino-terminal 203 and 178 amino acids, respectively. DN-BMPR-II construct was derived from the human type II BMP receptor (Ishikawa et al., 1995). To construct the truncated form of BMPR-II, a myc epitope and a stop codon were inserted after Ile540 in the carboxyl terminus by PCR. The DN-BMPR cDNAs were subcloned into the RCAS(A) retroviral vector (Hughes et al., 1987) and transfected into chicken embryo fibroblasts by the calcium phosphate precipitation method (Iwamoto et al., 1993_b_ ). Recombinant virus in the medium was concentrated by centrifugation and used to infect freshly isolated US and LS chondrocytes.
Northern Hybridization
Whole cellular RNAs isolated by the guanidium isothiocyanate method (Smale and Sasse, 1992) were denatured by glyoxalation, electrophoresed on agarose gels, transferred to Hybond-N membrane (Amersham Life Science, Tokyo, Japan) by capillary blotting, and hybridized to 32P-labeled DNA probes. Plasmids used were chick type X collagen cDNA pDLr10 (Leboy et al., 1988), a 197-bp subclone of pYN3116 (Ninomiya et al., 1986); pCs2, a plasmid containing a 1.2-kb cDNA representing a portion of the 3′ end of chicken α1(II) collagen (Young et al., 1984); pCPG.1, a plasmid containing a 1.6-kb cDNA coding for a portion of the COOH-terminal end of the chicken aggrecan (Oettinger and Pacifici, 1990); and pCol3, a plasmid containing an 800-bp cDNA of the chicken α1(I) collagen (Yamamoto et al., 1980).
Reverse Transcriptase PCR for BMP-2, BMP-4, and BMP-7, and BMPR-IA, BMPR-IB, and BMPR-II
1 μg of whole cellular RNA was reverse transcribed by Superscript reverse transcriptase (GIBCO BRL, Gaithersburg, MD) and random hexamers. Subsequent amplification was carried out with Elongase (GIBCO BRL) and gene-specific primers for 30 cycles under the following conditions: 95°C for 10 s and 60°C for 1 min. Primers for amplification were as follows: 5′-CAG CTT CCA CCA CGA AGA AG-3′ and 5′-TAG GTT GTC CGT GTG CAA TC-3′ for chick BMP-2 (Francis et al., 1994), generating a 341-bp fragment; 5′-CAC CAG GCA CAG ACT CAT CA-3′ and 5′-AGG TAG AGC ATG GAG ATG GC-3′ for chick BMP-4 (Francis et al., 1994), generating a 425-bp fragment; 5′-ACC TTG GCC TGC AGC TAT CT-3′ and 5′-CTC TGG AGC GAT GAT CCA GT-3′ for chick BMP-7 (Houston et al., 1994), generating a 332-bp fragment; 5′-AGC GAT TGC TTG GAG CCT ATC T-3′ and 5′-AGC TGG CTT CTT CTG TGG TGA A-3′ for chick BMPR-IA (Kawakami et al., 1996); 5′-ATG CTG CAC AGG CCA AGA TTA C-3′ and 5′-CCA AGA GCC TGT GCC TTT AAT G-3′ for chick BMPR-IB (Kawakami et al., 1996); and 5′-GGT CGA TAC GGA GCA GTG TAC A-3′ and 5′-CTG CTC CTT CAA GCA CTT CTG G-3′ for chick BMPR-II (Kawakami et al., 1996).
Measurement of DNA Content and Sulfated Glycosaminoglycan Content
Cultures were harvested in saline solution containing 0.2% Triton X-100 and 0.02 N NaOH and sonicated. Cell lysates were centrifuged, and the supernatant was used for determination of DNA and sulfated glycosaminoglycan (GAG) contents by the fluorometric procedure of Johnson-Wint and Wellis (1982) and direct spectrophotometric microassay of Frandale et al. (1982), respectively.
Measurement of APase Activity
APase activity associated with the cell layer was measured by a modification of Bessey et al. (1946) using _p_-nitrophenyl phosphate as a substrate as described previously (Pacifici et al., 1991).
Histochemistry
Cultures were washed with PBS twice, fixed with 3.7% neutralized formaldehyde solution, and stained with 0.5% crystal violet in 20% methanol (Zhang et al., 1993). To detect cartilage matrix accumulation, cultures were fixed with 3% acetic acid (adjusted to pH 1.0 with HCl) for 5 min and stained with 0.1% Alcian blue in 3% acetic acid, pH 1.0 (Lev and Spicer, 1964). To detect APase activity, cultures were washed with saline twice, incubated with 50 mM Tris-HCl, pH 9.5, containing 0.5 mg/ml of naphthol AS-BI phosphate and 1 mg/ml of Fast Red trisodium salts (Sigma Chemical Co.) for 15 min at 37°C (Leboy et al., 1989), and then fixed with 3.7% neutralized formaldehyde solution.
For determination of efficiency of virus infection, cultures were harvested by trypsinization and replated on poly-l-lysine–coated dishes at the density of 2.0 × 106 cells/ml. After 15 min, cultures were washed with PBS twice, fixed with 3.7% neutralized formaldehyde solution, and permeabilized by incubation with PBS containing 0.5% Triton X-100 for 15 min. Cultures were then incubated with hybridoma supernatant containing AMV-3C2 (Developmental Studies Hybridoma Bank, Baltimore, MD), a mouse monoclonal antibody against the gag protein of avian myeloblastosis virus, recognizing cells harboring Rous sarcoma or avian leukemia virus. For determination of expression of the constructed genes, parallel cultures were incubated with ascites fluid containing 9E10 (Berkeley Antibody Company, Richmond, CA), a mouse monoclonal antibody that recognizes the amino acid sequence EQKLISEEDL, a specific portion of the human c-myc gene. Localization of the antibody was visualized by ABC methods using Histofine kits (Nichirei, Tokyo, Japan).
Results
Expression of BMP Receptors and BMPs in Chondrocytes
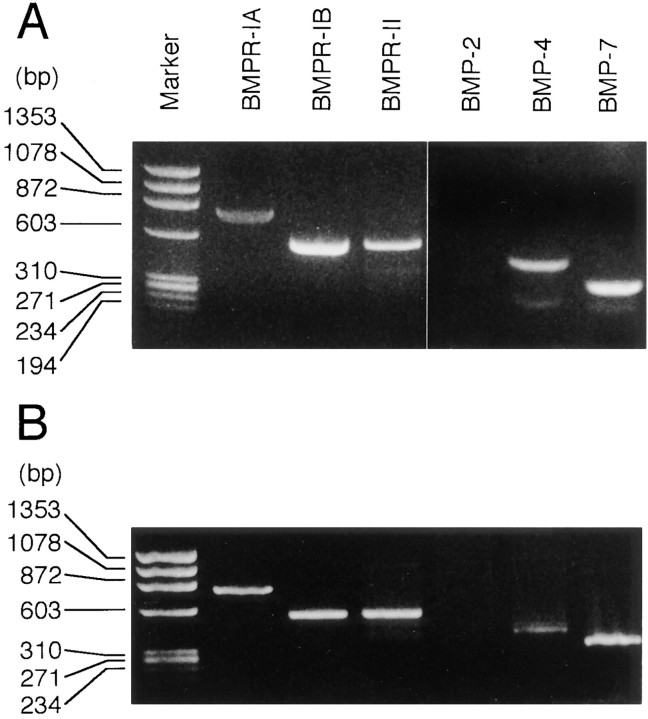
In the first series of experiments, we examined whether chick sternal chondrocytes express BMP receptors and BMPs. Total RNAs were extracted from sterna of 17-day-old chicken embryos and subjected to reverse transcriptase PCR (RT-PCR) (Fig. 1 A). After 30 cycles of RT-PCR amplification, BMPR-IA, -IB, and -II were detectable (Fig. 1 A). A similar 30-cycle amplification revealed that the sternal cartilage contained transcripts encoding BMP-4 and -7, whereas only trace amounts of BMP-2 transcripts were visible after 40 cycles. When RNAs were isolated from cultured chondrocytes and examined by RT-PCR, similar results were obtained (Fig. 1 B). These findings indicate that chondrocytes expressed BMPR-IA, BMPR-IB, BMPR-II, BMP-4, and BMP-7, while BMP-2 expression was undetectable.
Figure 1.
Expression of BMP receptors and BMPs in chondrocytes. Total RNA was isolated from (A) day 17 chicken embryo sterna and (B) upper sternal chondrocytes cultured for 3 d. RT-PCR for the indicated gene was performed as described in Materials and Methods. Amplified products were analyzed by 2% agarose gel electrophoresis.
Infection of Chondrocytes with Viral Particles Encoding DN-BMPR-IA, BMPR-IB, and BMPR-II
Before analyzing the phenotypic effects of DN-BMPRs on chondrocytes, it was important to establish that most cells became rapidly infected by the recombinant viral particles and expressed the recombinant protein. Accordingly, primary populations of immature and mature chondrocytes isolated from the LS and US portions of day 17 chick embryo sternum, respectively, were infected with viruses encoding DN-BMPR-IA, -IB or -II and harvested by trypsinization 1 wk after infection. Efficiency of viral infection was determined by immunohistochemical analysis using an antibody against viral gag protein. We found that more than 95% of both US and LS chondrocytes had become infected (Figs. 2, H–J, and 3 D); a similar efficiency was displayed by control viruses (Figs. 2 G and 3 B). Uninfected cultures were negative (Fig. 2 F).
Figure 2.
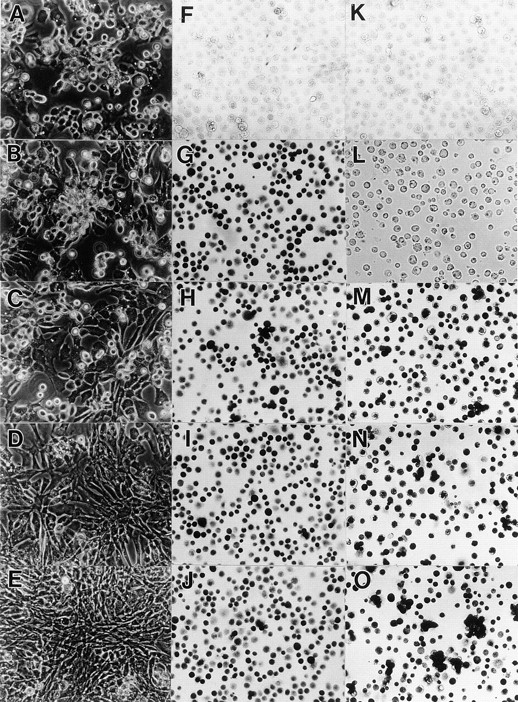
Efficiency of infection in upper sternal chondrocyte cultures by DN-BMPR–encoding viruses. Freshly isolated US chondrocytes were infected with RCAS viruses encoding DN-BMPR-IA (C, H, and M), DN-BMPR-IB (D, I, and N), DN-BMPR-II (E, J, and O), or vector alone (B, G, K, and L) for 3 d in medium 199 containing 10% FBS. The medium was then changed to high glucose DME containing 10% FBS, and cells were cultured for an additional 4 d. On day 7, the cells were harvested and replated in 35-mm dishes at the density of 1.5 × 105 cells/dish, and the microscopic photographs of live cells were taken after 5 d (A–E). Aliquots of the harvested cells were plated onto poly- l-lysine–coated dishes at the density of 2.0 × 106/ml, fixed with 3.7% formaldehyde after 15 min from plating, and stained with antibodies to the RCAS virus gag protein (F–J) or c-myc peptide (L–O) as described in Materials and Methods. A and F represent uninfected cultures. K is a culture incubated with control nonimmune ascites.
Figure 3.
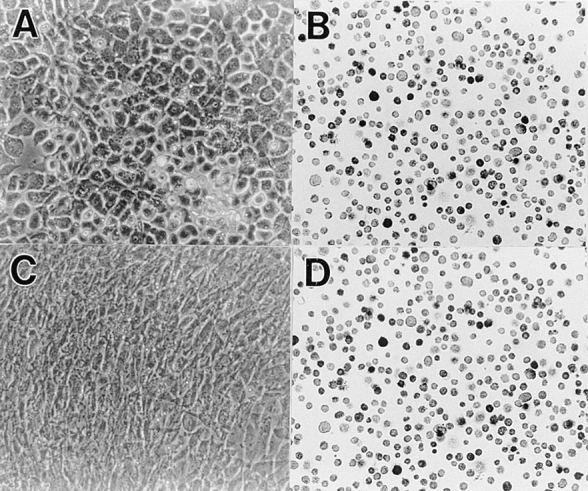
Efficiency of infection in lower sternal chondrocyte cultures by DN-BMPR-II–encoding virus. Freshly isolated LS chondrocytes were infected with RCAS virus encoding DN-BMPR-II (C and D) or vector alone (A and B) in medium 199 containing 10% FBS for 3 d and were then processed as cells in Fig. 2. (A) Control cells; (B) control cells stained with viral gag antibody; (C) BMPR-II–expressing cells; (D) BMPR-II–expressing cells stained with viral gag antibody.
To determine whether the infected cultures produced recombinant protein, cultures were stained with an antibody to the c-myc tag (Fig. 2, K–O). Cells infected with viruses encoding DN-BMPR-IA (Fig. 2 M), DN-BMPR-IB (Fig. 2 N), or BMPR-II (Fig. 2 O) were nearly all positive, while uninfected cells (not shown) and control-virus infected cells (Fig. 2 L) were weakly positive, and their positive signal was limited to the nucleus, probably reflecting endogenous c-myc.
The data show that chondrocytes are rapidly infected by the recombinant viruses used and readily express virally encoded recombinant protein.
Morphological Changes Induced by Expression of DN-BMPRs
Primary uninfected and infected US cultures described above were subcultured and grown for an additional 6 d in secondary cultures. In the uninfected cultures (Fig. 2 A) and the cultures infected with the control virus (Fig. 2 B), the US chondrocytes displayed hypertrophic phenotype; the cells were large in size, polygonal to round in shape, and surrounded by a refractile matrix, and their growth rates were very slow. The cultures infected with DN-BMPR-IA virus (Fig. 2 C) were morphologically indistinguishable from the control cultures. In contrast, the cultures infected with DN-BMPR-IB virus contained fibroblastic-shaped cells, and the growth rate of these cultures was higher than that of the controls (Fig. 2 D). The effect of DN-BMPR-II–encoding virus was more remarkable; all of the cells in these cultures became fibroblastic (Fig. 2 E), actively proliferated, and reached confluency in 5 d. Similar effects were obtained with LS cultures. The control immature LS chondrocytes and control virus–infected chondrocytes exhibited the expected smaller size and a polygonal morphology (Fig. 3 A), while the DN-BMPR-II–expressing (Fig. 3 C) or DN-BMPR-IB–expressing (not shown) LS chondrocytes displayed a fibroblastic shape and high proliferative activity (Fig. 3 C). As seen in US cultures, the effects of DN-BMPR-IA were weak in LS cultures.
To determine whether expression of DN-BMPRs blocked the ability of chondrocytes to respond to BMPs, the above US and LS cultures were treated with 200 ng/ml of rhBMP-2. Uninfected cells and the cells infected with control viruses readily responded to this BMP-2 treatment, as indicated by a change from a polygonal to round cell morphology and increased pericellular refractile matrix (Fig. 4, B and F) compared with their respective untreated controls (Fig. 4, A and E). In contrast, DN-BMPR-II–expressing cells did not appear to respond to BMP-2 treatment (Fig. 4, D and H) and closely resembled their untreated counterparts (Fig. 4, C and G).
Figure 4.
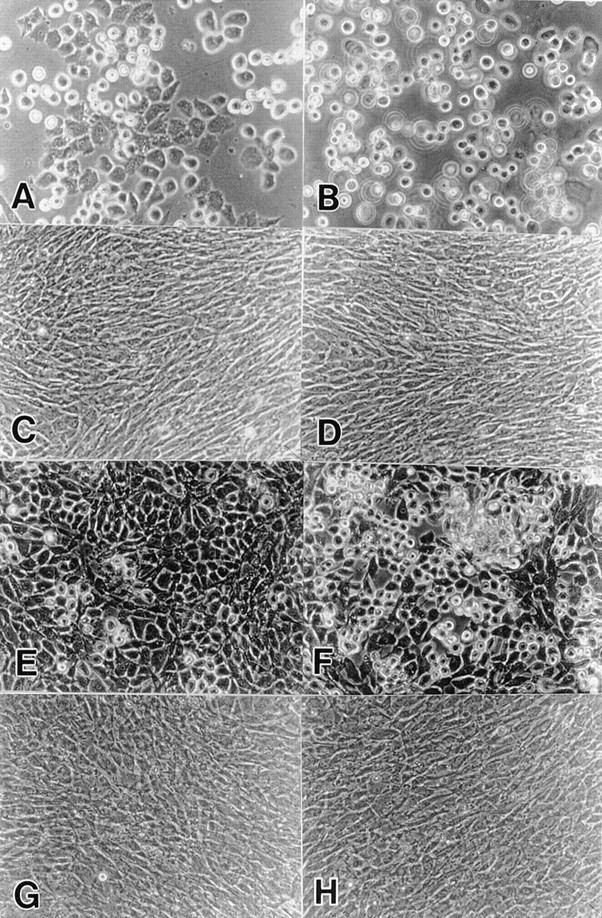
Effects of the exogenous BMP-2 on the cell morphology. Freshly isolated US (A–D) and LS (E–H) chondrocytes were infected with RCAS virus encoding DN-BMPR-II (C, D, G, and H) or vector alone (A, B, E, and F) in medium 199 containing 10% FBS for 3 d. The medium was then changed to high glucose DME containing 10% FBS. On day 7 (US) or 5 (LS), the cells were harvested and replated in 35-mm dishes at the density of 1.5 × 105 cells/dish. 200 ng/ml of rhBMP-2 was added on the next day (B, D, F, and H). Photographs was taken after 5 d.
Stimulation of Cell Proliferation by DN-BMPRs
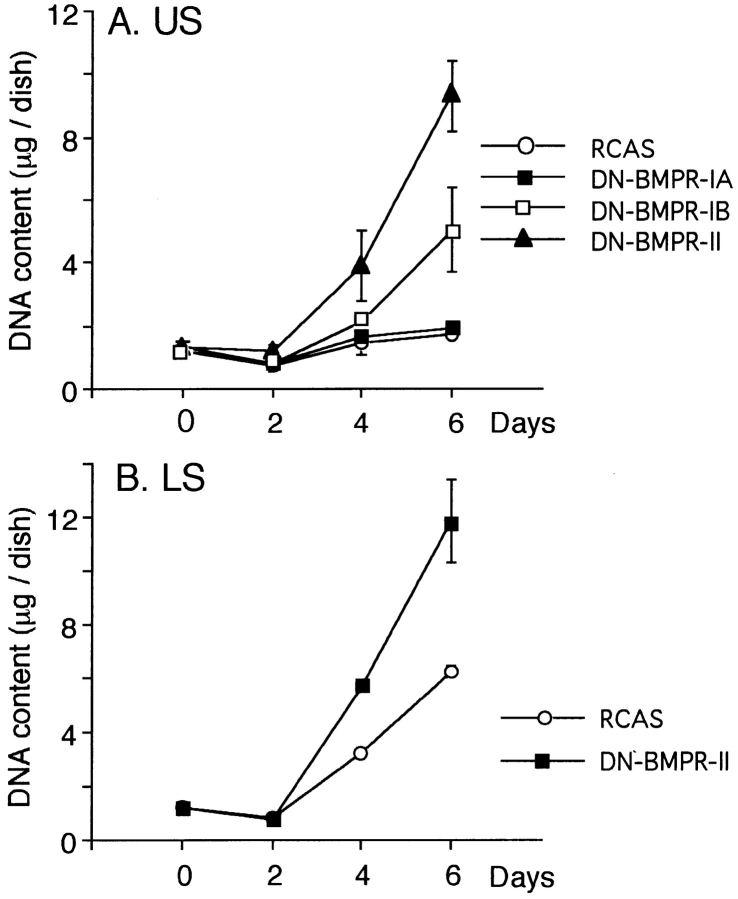
To obtain quantitative data on the effects of DN-BMPRs on proliferation of chondrocytes, a DNA content analysis was performed. In secondary cultures of US chondrocytes, most of the control cells (RCAS) and DN-BMPR-IA–expressing cells became postmitotic, while DN-BMPR-II– or DN-BMPR-IB–expressing cells exhibited strong proliferative activity and accumulated about 5.0- and 2.5-fold more DNA than controls on day 6.
In LS chondrocyte cultures, the control cells (RCAS) actively proliferated. DN-BMPR-II–expressing LS cultures proliferated even more rapidly, and the DNA content was twofold compared with the control value (Fig. 5 B).
Figure 5.
Effects of DN-BMPRs on cell proliferation. US (A) and LS (B) chondrocytes were infected with DN-BMPR viruses, harvested by trypsinization on day 7 and 5, respectively, and then replated in 35-mm dishes at the density of 1.5 × 105 cells/dish. DNA contents were determined at the indicated times as described in Materials and Methods. Values represent average ± SD for three cultures, and similar results were obtained in three independent experiments.
Changes in Proteoglycan and Collagen Expression Produced by DN-BMPRs
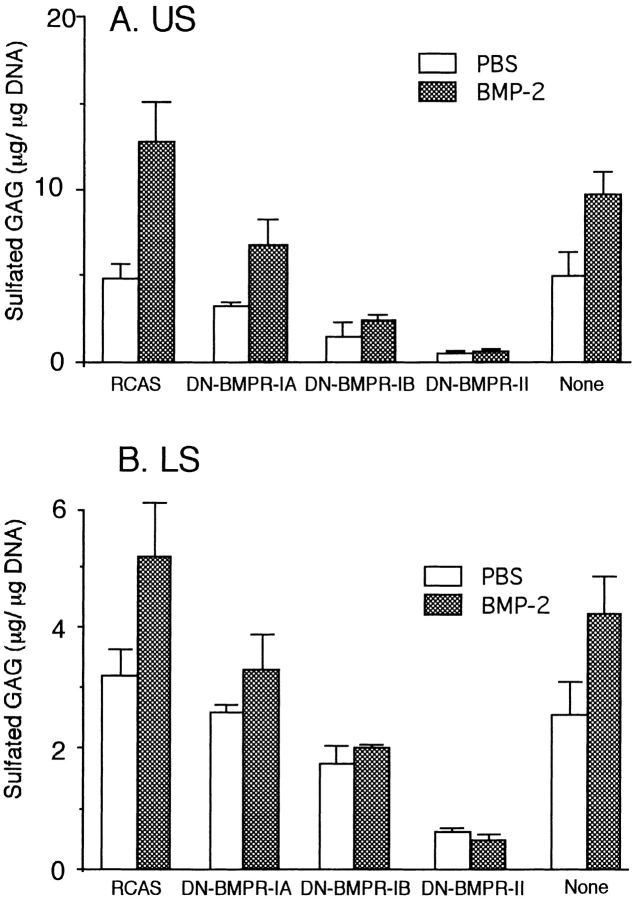
We next investigated the effects of DN-BMPRs on proteoglycan synthesis and accumulation, as measured by biochemical quantification of sulfated GAG content and histochemical staining. Proteoglycan synthesis and accumulation were severely inhibited by DN-BMPR-II, moderately by DN-BMPR-IB, and weakly by DN-BMPR-IA (Figs. 6 A and 7). The uninfected cultures and the control cultures infected with RCAS virus carrying vector alone responded to exogenous rhBMP-2, and their GAG synthesis was enhanced 1.5- to 2.0-fold by the addition of the protein, as reported before (Hiraki et al., 1991). In contrast, DN-BMPR-II–expressing cultures did not show an enhancement of GAG synthesis in response to BMP-2 treatment, and DN-BMPR-IA–expressing cultures and DN-BMPR-IB–expressing cultures showed weaker responses to BMP-2 (Figs. 6 A and 7). Similar results were obtained in cultures of immature LS chondrocytes (Fig. 6 B).
Figure 6.
Effects of DN-BMPRs on GAG synthesis in US and LS chondrocyte cultures. US (A) and LS (B) chondrocytes were infected with DN-BMPR viruses, harvested by trypsinization on day 7 (US) or day 5 (LS), and then replated in 24-well plates at the density of 5.0 × 104 cells/dish. Sulfated GAG and DNA contents were determined on day 5 as described in Materials and Methods. rhBMP-2 was added from day 1 at the concentration of 200 ng/ml. Data represent average ± SD for three cultures, and similar results were obtained in two independent experiments.
Figure 7.
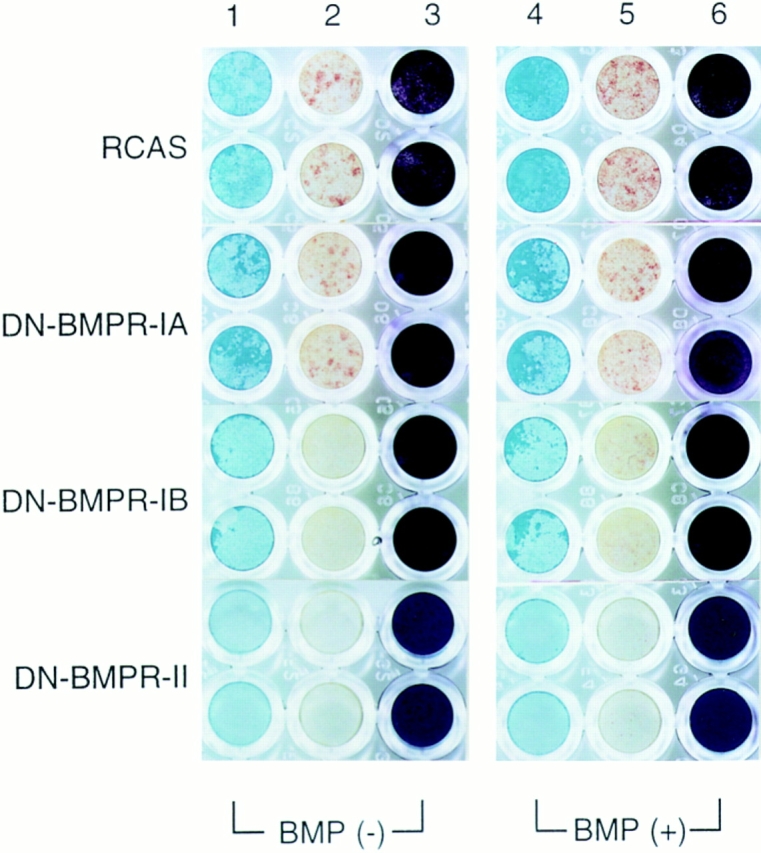
Histochemical analysis of proteoglycan accumulation and APase activity in US chondrocytes infected by DN-BMPR viruses. US chondrocytes were infected with RCAS virus encoding DN-BMPRs or vector alone (RCAS) and replated on type I collagen–coated 96-well plates at the density of 2.0 × 104 cells/ dish on day 7. Cells were cultured in the presence of 10 μg/ml of ascorbic acid with (BMP (+)) or without (BMP (−)) rhBMP-2 (200 ng/ml) for 7 d. Cultures were then fixed with 3.7% formaldehyde and stained with Alcian blue (lanes 1 and 4) or crystal violet (lanes 3 and 6) as described in Materials and Methods. Lanes 2 and 5 show the results of histochemical detection of APase.
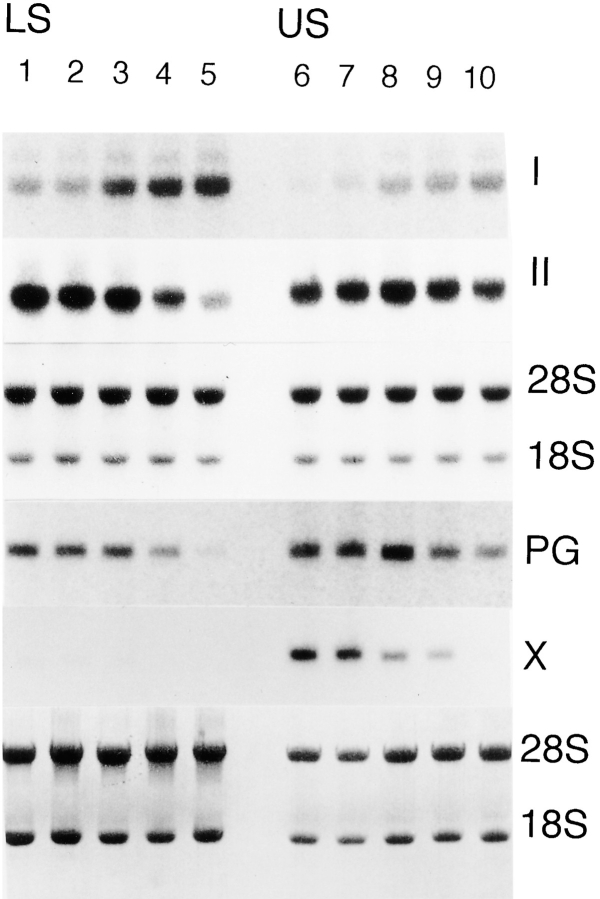
The inhibitory effects of DN-BMPR-IB and -II on proteoglycan synthesis were also detectable by Northern blot analysis. As shown in Fig. 8, the expression of the aggrecan gene (PG) was decreased by DN-BMPR-IB and -II in both LS and US cultures, and the expression of the type II collagen gene (II) was also inhibited by these mutants in both US and LS cultures. In contrast, an induction of type I collagen gene expression (I) was observed in both DN-BMPR–expressing LS and US cultures (Fig. 8).
Figure 8.
Changes in expression of extracellular matrix genes induced by DN-BMPRs. LS (lanes 1–5) and US (lanes 6–10) chondrocytes were infected with RCAS virus encoding DN-BMPR-IA (lanes 3 and 8), DN-BMPR-IB (lanes 4 and 9), DN-BMPR-II (lanes 5 and 10), or vector alone (lanes 2 and 7), harvested by trypsinization on day 7 for US and day 5 for LS, and then replated in 100-mm dishes at the density of 1.0 × 106 cells/plate. 7 d after plating, whole cellular RNAs were extracted, separated on agarose gels, and blotted onto nylon membranes. Membranes were hybridized to 32P-labeled probes encoding type I collagen (I) and type X collagen (X) and then reblotted with the 32P-labeled probes encoding type II collagen (II ) and aggrecan (PG), respectively. Blots were prestained with methylene blue to mark the 28S and 18S rRNA subunits. Lanes 1 and 6 represent uninfected cultures.
It may be argued that the above downregulation of type II collagen and aggrecan gene expression and the concomitant increase in type I collagen gene expression do not reflect specific effects of DN-BMPRs on the phenotype of differentiated chondrocytes but are rather parts of an overall generalized process of dedifferentiation, in which the cells become morphologically and biochemically fibroblast-like cells. To address this point, we investigated whether the DN-BMPR–expressing chondrocytes would reexpress their differentiated phenotype once they were switched from monolayer to suspension. The rationale behind this experiment is that dedifferentiated chondrocytes in monolayer have been shown to reexpress differentiated traits when they were switched to suspension culture (Benya and Shaffer, 1982). Accordingly, control virus–infected and DN-BMPR-II–expressing LS cultures were switched from monolayer to suspension culture, and DNA and proteoglycan contents were measured 7 d later. We found that the DNA content was the same in both cultures, suggesting that the stimulation of proliferation by DN-BMPR-II is anchorage dependent. However, the proteoglycan content of DN-BMPR-II–expressing cultures was ∼30% of that of the control cultures, indicating that DN-BMPR-II inhibited proteoglycan synthesis even in suspension cultures and that the cells had not reacquired the ability to express differentiated functions in suspension.
Inhibition of Terminal Differentiation by DN-BMPRs
Having shown that DN-BMPRs can inhibit expression of chondrocyte-characteristic products, we next examined whether they can also inhibit expression of products typical of mature hypertrophic US chondrocytes, such as type X collagen and APase. Northern blot analyses revealed that DN-BMPR-II completely inhibited expression of type X collagen (Fig. 8) and APase activity (Fig. 7) compared with control cultures. DN-BMPR-IB had similar but more moderate effects, while DN-BMPR-IA had marginal effects (Figs. 7 and 8).
Treatment with rhBMP-2 caused a marked stimulation of APase activity in control cultures (Fig. 7). In contrast, rhBMP-2 elicited a small response in cultures expressing DN-BMPR-IA or -IB and no response in cultures expressing DN-BMPR-II (Fig. 7).
Effects of DN-BMPRs in Serum-free Cultures
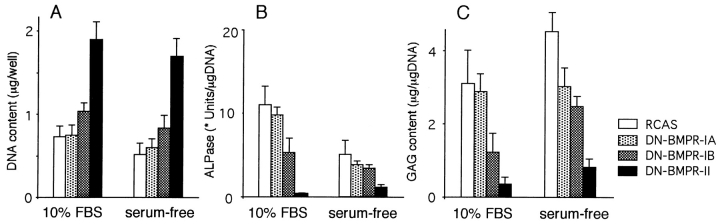
In a final set of experiments, we asked whether the effects caused by expression of DN-BMPRs in chondrocytes were affected by or dependent on the serum present in the culture medium. Thus, primary US cultures expressing DN-BMPR-II were replated and maintained in serum-free medium for 7 d. As shown in Fig. 9, in this chemically defined medium the DN-BMPR-II–expressing cells contained over twofold more DNA than control cultures, as seen in standard serum-containing cultures described above. Likewise, APase activity and proteoglycan content were significantly lower in DN-BMPR-II cultures than in the control cultures (Fig. 9). The effects of DN-BMPR-IB were weaker than those of DN-BMPR-II, and the effects of DN-BMPR-IA were marginal. DN-BMPRs thus inhibited expression of differentiated functions in chondrocytes in both serum-free and serum-containing cultures.
Figure 9.
Effects of DN- BMPRs on US chondrocytes in serum-free cultures. US chondrocytes were infected with RCAS virus encoding DN-BMPRs or vector alone (RCAS) and replated in type I collagen–coated 96-well plates at the density of 1.0 × 104 cells/dish on day 7. Cells were then cultured in chemically defined medium as described in Materials and Methods for 7 d. The DNA content (A), sulfated GAG content (B), and APase activity (C) were measured as described in Materials and Methods. The data represent average ± SD for three cultures. * unit: One unit of APase activity corresponds to the hydrolysis of 1 nmol per min.
Discussion
Roles of BMP Signaling during Chondrocyte Maturation
The results of our study show that an interference with BMP signaling by expression of DN-BMPR-II or -IB in both immature and mature chondrocytes alters the proliferative characteristics of the cells and inhibits expression of differentiated and maturation-related traits, including types II and X collagen, aggrecan, and APase. At the same time, the cells acquire a fibroblastic morphology and upregulate expression of type I collagen. These phenotypic alterations do not simply reflect a generalized dedifferentiation process in chondrocytes caused by the DN-BMPRs. When DN-BMPR-II–expressing cells were shifted from monolayer to suspension, they were not able to restore normal proteoglycan production, even though a similar shift has been shown to restore function in chondrocytes dedifferentiated by protracted growth in monolayer (Benya and Shaffer, 1982). In addition, the downregulation of type II collagen gene expression and the upregulation of type I collagen gene expression were more severe in DN-BMPR-II–expressing LS than in the US chondrocytes, indicating that the effects of DN-BMPR-II are not generalized but are the results of specific interactions with the ongoing maturation programs of LS and US chondrocytes. It is suggested then that BMP signaling has a crucial role at multiple steps during chondrocyte development in endochondral bone formation and is essential for maintenance of the differentiated chondrocyte phenotype, modulation of proliferative activity normally occurring during chondrocyte maturation, and expression of terminal mature functions by hypertrophic chondrocytes. Each step of the maturation process is important for normal skeletogenesis, and disturbances result in skeletal deformities (Horton and Hecht, 1993); it follows that any alteration of BMP signaling may lead to destabilization of the chondrocyte maturation process and may affect skeletogenesis at any given step. Our results and conclusions correlate well with and extend our previous studies on the roles of BMP signaling during differentiation of mesenchymal cells into chondrocytes (Kawakami et al., 1996). Interestingly, we found that DN-BMPR-IB inhibited mesenchymal cell differentiation, whereas DN-BMPR-II had little effect. Thus, the type IB BMP receptor may interact with other receptors such as the activin type II receptor (Stern et al., 1995; Yamashita et al., 1995) and affect mesenchymal cell differentiation, while the type II BMP receptor may be more critical in differentiated chondrocytes undergoing maturation and endochondral ossification.
The withdrawal from mitotic activity is an essential step in the normal progression of terminal differentiation of chondrocytes during endochondral bone formation (Kato et al., 1988). We showed previously that when immature chondrocytes are forced to remain proliferative, for example by overexpression of c-myc, they are unable to mature into hypertrophic chondrocytes (Iwamoto et al., 1993). Our present data show that interference with BMP signaling also forces immature LS chondrocytes into a more active proliferative behavior and reinitiates mitotic activity in mature US chondrocytes. We have recently observed that overexpression of BMP-2 or -4 in cultured sternal chondrocytes leads to a decrease in proliferative activity as well (our unpublished observations). Thus, BMP signaling appears to be an important part of mechanisms by which proliferation is tightly regulated during chondrocyte maturation. We should acknowledge here seemingly contrasting data reported by others, in which treatment with BMP-2, -3 or -4 was found to cause increases in thymidine incorporation into cultured chondrocytes (Hiraki et al., 1991; Luyten et al., 1994). Because the stimulatory effect is not strong as compared with other mitogens, and since there are no reports that BMPs increase cell number, the stimulation of thymidine incorporation by BMPs might not be directly linked to cell division.
Ligands and Receptors as Components of BMP Signaling in Chondrocytes
Our RT-PCR data show that chondrocytes contain transcripts encoding type IA, IB, and II BMP receptors. In the developing limb, the type IB and II receptors are expressed in precartilaginous regions, while the type IA receptor is expressed at lower levels and more broadly (Dewulf et al., 1995; Kawakami et al., 1996). The expression of type IA receptor is upregulated specifically in prehypertrophic chondrocytes (Zou et al., 1997). In the present study, the DN-BMPR-II and -IB were strong and moderate suppressors, respectively, of phenotypic expression in chondrocytes, while DN-BMPR-IA showed only weak effects. Based on the identical expression of the c-myc tag attached to each of the DN-BMPRs that we observed, it appears that the different effectiveness of the DN-BMPRs in phenotypic inhibition is not due simply to differences in expression levels; the c-myc tag was equally evident after expression of every DN form in chondrocytes. Rather, it appears that the BMP receptors most functionally relevant in chondrocytes are the type IB and type II BMP receptors. Zou et al. (1997) have reported that expression of a constitutively activated form of type IA BMP receptor in developing limb delays maturation of cartilage, whereas the same form of type IB receptor does not. Their findings and our results may be two sides of one coin. The expression of the dominant negative form of type IB receptor may not only interrupt the BMP signaling through type IB receptor but may also activate the signaling pathway through the other type I receptor, type IA receptor. Conversely, activation of type IA receptor may inactivate the BMP signaling through type IB receptor. However, further investigation should be required before any conclusions are made as to how and which type I BMP receptor regulates chondrocyte function.
A number of BMPs have been shown to be expressed in prechondrogenic cells and chondrocytes. For example, BMP-2 and -4 transcripts are present in the limb interdigital zones and the mesenchyme surrounding the chondrogenic regions (Francis et al., 1994). BMP-7 proteins are detected in hypertrophic chondrocytes of vertebrae of human embryos by immunohistochemical methods (Vukicevic et al., 1994), and BMP-6 transcripts are detectable in freshly isolated growth plate chondrocytes (Carey and Liu, 1995). Growth and differentiation factor 5 (GDF5), a member of the BMP superfamily that binds to BMPR-IB and -II and activin receptor-II and -IIB, is expressed in cartilage (Nishitoh et al., 1996). Our data show that transcripts encoding BMP-4 and -7 are present in sternal chondrocytes. It is possible that BMP-4, BMP-7, or heterodimers of these BMPs are components of BMP signaling operating in chondrocytes undergoing endochondral ossification. These BMPs may be important endogenously produced regulators of chondrocyte activity, given our observation that in serum-free cultures, interference with BMP signaling pathway led to inhibition of proteoglycan synthesis and APase activity. However, since we observed higher APase activity in serum-containing versus serum-free cultures, it is also possible that serum components, including serum-derived BMPs, may have a favorable effect on phenotypic expression in chondrocytes. In summary, combinations of autocrine and paracrine BMP signaling mechanisms are likely to be operative in chondrocytes undergoing endochondral ossification.
The involvement of BMPs in chondrocyte metabolism and function was previously suggested by the findings that BMP treatment stimulates proteoglycan synthesis and APase activity and inhibits proteoglycan degradation in cultured chondrocytes (Hiraki et al., 1991; Luyten et al., 1992, 1994; Erickson et al., 1997). Likewise, ectopic expression of BMP-2, -4, or -7 was shown to cause increased cartilage growth and deformities in vivo (Duprez et al., 1996_b_ ; Monsoro-Burq et al., 1996). However, these studies did not provide information on the essential role of BMP signaling in chondrocytes. BMP or BMPR gene knockout studies, which could potentially provide such information, resulted in embryonic lethality at very early stages (Mishina et al., 1995; Zhang and Bradley, 1996) and thus could not provide information on the roles of BMPs in skeletal development. In conclusion, our study of the effects of BMP signaling interference provides the first clear demonstration that BMP signaling is essential in chondrocytes and exerts key functions in the regulation of endochondral bone formation, including maintenance of the differentiated chondrocyte phenotype, control of proliferative activity, and expression of the mature hypertrophic phenotype.
Acknowledgments
We thank Dr. Maurizio Pacifici (University of Pennsylvania, School of Dental Medicine, Philadelphia, PA) for critical reading and helpful comments.
This work was supported by grants-in-aid for scientific research from the Ministry of Education, Science, and Culture of Japan.
Abbreviations used in this paper
APase
alkaline phosphatase
BMP
bone morphogenetic protein
BMPR
BMP receptor
DN
dominant negative
GAG
glycosaminoglycan
RT-PCR
reverse transcriptase PCR
US and LS
upper and lower sterna
Footnotes
Address all correspondence to Masahiro Iwamoto, Department of Oral Anatomy and Developmental Biology, Osaka University Faculty of Dentistry, 1-8 Yamadaoka, Suita, Osaka 565, Japan. Tel.: 81-6-879-2872. Fax: 81-6-879-2875. E-mail: mal@dent.osaka-u.ac.jp
References
- Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bessey OA, Lowry OH, Brock MJ. A method for the rapid determination of alkaline phosphatase with five cubic millimeters of serum. J Biol Chem. 1946;164:321–329. [PubMed] [Google Scholar]
- Carey DE, Liu X. Expression of bone morphogenetic protein-6 messenger RNA in bovine growth plate chondrocytes of different size. J Bone Miner Res. 1995;10:401–405. doi: 10.1002/jbmr.5650100310. [DOI] [PubMed] [Google Scholar]
- Chen P, Carrington JL, Hammonds RG, Reddi AH. Stimulation of chondrogenesis in limb bud mesoderm cells by recombinant human bone morphogenetic protein 2B (BMP-2B) and modulation by transforming growth factor β1 and β2. Exp Cell Res. 1991;195:509–515. doi: 10.1016/0014-4827(91)90403-h. [DOI] [PubMed] [Google Scholar]
- Dewulf N, Verschueren K, Lonnoy O, Moren A, Grimsby S, Vande-Spiegle K, Miyazono K, Huylebroeck D, Ten-Dijke P. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2653. doi: 10.1210/endo.136.6.7750489. [DOI] [PubMed] [Google Scholar]
- Duprez DM, Coltey M, Amthor H, Brickell PM, Tickle C. Bone morphogenetic protein-2 (BMP-2) inhibits muscle development and promotes cartilage formation in chick limb bud cultures. Dev Biol . 1996a;174:448–452. doi: 10.1006/dbio.1996.0087. [DOI] [PubMed] [Google Scholar]
- Duprez D, Bell EJDH, Richardson MK, Archer CW, Wolpert L, Brickell PM, Francis-West PH. Overexpression of BMP-2 and BMP-4 alters the size and shape of developing skeletal elements in the chick limb. Mech Dev. 1996b;57:145–157. doi: 10.1016/0925-4773(96)00540-0. [DOI] [PubMed] [Google Scholar]
- Erickson DM, Harris SE, Dean DD, Harris MA, Wozney JM, Boyan BD, Schwartz Z. Recombinant bone morphogenetic protein (BMP)-2 regulates costochondral growth plate chondrocytes and induces expression of BMP-2 and BMP-4 in a cell maturation-dependent manner. J Orthop Res. 1997;15:371–380. doi: 10.1002/jor.1100150309. [DOI] [PubMed] [Google Scholar]
- Francis PH, Richardson MK, Brickell PM, Tickle C. Bone morphogenetic proteins and a signaling pathway that controls patterning in the developing chick limb. Development (Camb) 1994;120:209–218. doi: 10.1242/dev.120.1.209. [DOI] [PubMed] [Google Scholar]
- Frandale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Hiraki Y, Inoue H, Shigeno C, Sanma Y, Bentz H, Rosen DM, Asada A, Suzuki F. Bone morphogenetic proteins (BMP-2 and BMP-3) promote growth and expression of the differentiated phenotype of rabbit chondrocytes and osteoblastic MC3T3-E1 cells in vitro. J Bone Miner Res. 1991;6:1373–1385. doi: 10.1002/jbmr.5650061215. [DOI] [PubMed] [Google Scholar]
- Horton, W.A. 1993. Morphology of connective tissue: cartilage. In Connective Tissue and Its Heritable Disorders. P.M. Royce and B. Steinmanns, editors. Wiley-Liss, Inc., New York. 73–84.
- Horton, W.A., and J.T. Hecht. 1993. The chondrodysplasias. In Connective Tissue and Its Heritable Disorders. P.M. Royce and B. Steinmanns, editors. Wiley-Liss, New York. 641–675.
- Houston B, Thorp BH, Burt DW. Molecular cloning and expression of bone morphogenetic protein-7 in the chick epiphyseal growth plate. J Mol Endocrinol. 1994;13:289–301. doi: 10.1677/jme.0.0130289. [DOI] [PubMed] [Google Scholar]
- Hughes SH, Greenhouse JJ, Petropoulos CJ, Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987;61:3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Yoshioka H, Ohuchi H, Noji S, Nohno T. Truncated type II receptor for BMP-4 induces secondary axial structures in Xenopusembryos. Biochem Biophys Res Commun. 1995;216:26–33. doi: 10.1006/bbrc.1995.2587. [DOI] [PubMed] [Google Scholar]
- Iwamoto M, Golden EB, Adams SL, Noji S, Pacifici M. Responsiveness to retinoic acid changes during chondrocyte maturation. Exp Cell Res. 1993a;205:213–224. doi: 10.1006/excr.1993.1079. [DOI] [PubMed] [Google Scholar]
- Iwamoto M, Yagami K, Lu VP, Olsen BR, Petropoulos CJ, Ewert DL, Pacifici M. Expression and role of c-myc in chondrocytes undergoing endochondral ossification. J Biol Chem. 1993b;268:9645–9652. [PubMed] [Google Scholar]
- Johnson-Wint B, Wellis S. A rapid in situ deoxyribonucleic acid assay for determining cell number in culture and tissue. Anal Biochem. 1982;122:338–344. doi: 10.1016/0003-2697(82)90292-5. [DOI] [PubMed] [Google Scholar]
- Kato Y, Iwamoto M, Koike T, Suzuki F, Takano Y. Terminal differentiation and calcification in rabbit chondrocyte cultures grown in centrifuge tubes: regulation by transforming growth factor β and serum factors. Proc Natl Acad Sci USA. 1988;85:9552–9556. doi: 10.1073/pnas.85.24.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Ishikawa T, Shimabara M, Tanda N, Enomoto-Iwamoto M, Iwamoto M, Kuwana T, Ueki A, Noji S, Nohno T. BMP signaling during bone pattern determination in the developing limb. Development (Camb) 1996;122:3557–3566. doi: 10.1242/dev.122.11.3557. [DOI] [PubMed] [Google Scholar]
- Koenig BB, Cook JS, Wosing DH, Ting J, Tiesman JP, Correa PE, Olson CA, Pecquet AL, Ventura F, Grant RA, et al. Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Mol Cell Biol. 1994;14:5961–5974. doi: 10.1128/mcb.14.9.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboy PS, Shapiro IM, Uschmann BD, Oshima O, Lin D. Gene expression in mineralizing chick epiphyseal cartilage. J Biol Chem. 1988;263:8515–8520. [PubMed] [Google Scholar]
- Leboy PS, Vaias L, Uschmann B, Golub E, Adams SL, Pacifici M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J Biol Chem. 1989;264:17281–17286. [PubMed] [Google Scholar]
- Lev R, Spicer SS. Specific staining of sulfate groups with Alcian blue at low pH. J Histochem Cytochem. 1964;12:309. doi: 10.1177/12.4.309. [DOI] [PubMed] [Google Scholar]
- Liu F, Ventura F, Doody J, Massague J. Human type II receptor for bone morphogenetic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol Cell Biol. 1995;15:3479–3486. doi: 10.1128/mcb.15.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten FP, Yu YM, Yanagishita M, Vukicevic S, Hammonds RG, Reddi AH. Natural bovine osteogenin and recombinant human bone morphogenetic protein-2B are equipotent in the maintenance of proteoglycans in bovine articular cartilage explant cultures. J Biol Chem. 1992;267:3691–3695. [PubMed] [Google Scholar]
- Luyten FP, Chen P, Paralkar V, Reddi H. Recombinant bone morphogenetic protein-4, transforming growth factor-β1, and activin A enhance the cartilage phenotype of articular chondrocytes in vitro. Exp Cell Res. 1994;210:224–229. doi: 10.1006/excr.1994.1033. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq A-H, Duprez D, Watanabe Y, Bontoux M, Vincent C, Brickell P, Douarin NL. The role of bone morphogenetic proteins in vertebral development. Development (Camb) 1996;122:3607–3616. doi: 10.1242/dev.122.11.3607. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Gordon M d.R.M. van, T. Schmid, T. Linsenmayer, and B.R. Olsen. The developmentally regulated type X collagen gene contains a long open reading frame without introns. J Biol Chem. 1986;261:5041–5050. [PubMed] [Google Scholar]
- Nishitoh H, Ichijo H, Kimura M, Matsumoto T, Makishima F, Yamaguchi A, Yamashita H, Enomoto S, Miyazono K. Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J Biol Chem. 1996;271:21345–21352. doi: 10.1074/jbc.271.35.21345. [DOI] [PubMed] [Google Scholar]
- Niswander L, Martin GR. FGF-4 and BMP-2 have opposite effects on limb growth. Nature. 1993;361:68–71. doi: 10.1038/361068a0. [DOI] [PubMed] [Google Scholar]
- Nohno T, Ishikawa T, Saito T, Hosokawa K, Noji S, Wolsing DH, Rosenbaum JS. Identification of a human type II receptor for bone morphogenetic protein-4 that forms differential heterodimeric complexes with bone morphogenetic protein type I receptors. J Biol Chem. 1995;270:22522–22526. doi: 10.1074/jbc.270.38.22522. [DOI] [PubMed] [Google Scholar]
- Oettinger HF, Pacifici M. Type X collagen gene expression is transiently up-regulated by retinoic acid treatment in chick chondrocyte cultures. Exp Cell Res. 1990;191:292–298. doi: 10.1016/0014-4827(90)90017-5. [DOI] [PubMed] [Google Scholar]
- Pacifici M, Golden EB, Adams SL, Shapiro IM. Cell hypertrophy and type X collagen synthesis in cultured articular chondrocytes. Exp Cell Res. 1991;192:266–270. doi: 10.1016/0014-4827(91)90185-w. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Regulation of cartilage and bone differentiation by bone morphogenetic proteins. Curr Opin Cell Biol. 1992;4:850–855. doi: 10.1016/0955-0674(92)90110-x. [DOI] [PubMed] [Google Scholar]
- Smale G, Sasse J. RNA isolation from cartilage using density gradient centrifugation in cesium trifluoroacetate: an RNA preparation technique effective in the presence of high proteoglycan content. Anal Biochem. 1992;203:352–356. doi: 10.1016/0003-2697(92)90324-z. [DOI] [PubMed] [Google Scholar]
- Stern CD, Yu RT, Kakizuka A, Kintner CR, Mathews LS, Vale WW, Evans RM, Umesono K. Activin and its receptors during gastrulation and the later phases of mesoderm development in the chick embryo. Dev Biol. 1995;172:192–205. doi: 10.1006/dbio.1995.0015. [DOI] [PubMed] [Google Scholar]
- Vukicevic S, Latin V, Chen P, Batorsky A, Reddi AH, Sampath K. Localization of osteogenic protein-1 (bone morphogenetic protein-7) during human embryonic development: high affinity binding to basement membranes. Biochem Biophys Res Commun. 1994;198:693–700. doi: 10.1006/bbrc.1994.1100. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sobel ME, Adams SL, Avvediment VE, DiLauro R, Crombrugghe B, Showalter A, Pesciotta D, Fietzek P, Olsen B. Construction of a recombinant bacterial plasmid containing pro-α1(I) collagen DNA sequences. J Biol Chem. 1980;255:2612–2615. [PubMed] [Google Scholar]
- Yamashita, H., P. ten-Dijke, D. Huylebroeck, T.K. Sampath, M. Andries, J.C. Smith, C.H. Heldin, and K. Miyazono. 1995. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J. Cell Biol. 130:217–226. [DOI] [PMC free article] [PubMed]
- Yokouchi Y, Sakiyama J, Kameda T, Iba H, Suzuki A, Ueno N, Kuroiwa A. BMP-2/-4 mediate programmed cell death in chicken limb buds. Development (Camb) 1996;122:3725–3734. doi: 10.1242/dev.122.12.3725. [DOI] [PubMed] [Google Scholar]
- Young MF, Vogeli G, Nunex AM, Fernandez MP, Sullian M, Sobel ME. Isolation of cDNA and genomic DNA clones encoding type II collagen. Nucleic Acid Res. 1984;12:4207–4228. doi: 10.1093/nar/12.10.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development (Camb) 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Morla AO, Vuori K, Bauer JS, Juiano RL, Ruoslahti E. The αvβ1 integrin functions as a fibronectin receptor but does not support fibronectin matrix assembly and cell migration on fibronectin. J Cell Biol. 1993;122:235–242. doi: 10.1083/jcb.122.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Niswander L. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science. 1996;272:738–741. doi: 10.1126/science.272.5262.738. [DOI] [PubMed] [Google Scholar]
- Zou H, Wieser R, Massague J, Niswander L. Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev. 1997;11:2191–2203. doi: 10.1101/gad.11.17.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]