In Polarized MDCK Cells Basolateral Vesicles Arise from Clathrin-γ-adaptin–coated Domains on Endosomal Tubules (original) (raw)
Abstract
Human transferrin receptors (TR) and receptors for polymeric immunoglobulins (pIgR) expressed in polarized MDCK cells maintain steady-state, asymmetric distributions on the separate basolateral and apical surfaces even though they are trafficking continuously into and across these cells. The intracellular mechanisms required to maintain these asymmetric distributions have not been located. Here we show that TR and pIgR internalize from both surfaces to a common interconnected endosome compartment that includes tubules with buds coated with clathrin lattices. These buds generate vesicles that carry TR to the basolateral border. The lattices contain γ-adaptin and are dispersed by treatment with brefeldin A (BFA). Since BFA treatment abrogates the vectorial trafficking of TR in polarized MDCK cells, we propose that the clathrin-coated domains of the endosome tubules contain the polarized sorting mechanism responsible for their preferential basolateral distribution.
Trafficking membrane proteins traverse the intracellular pathways of polarized epithelial cells in a strongly vectorial manner. For integral membrane proteins, this transcellular routing is largely determined by signals carried in their cytoplasmic domains (Trowbridge et al., 1993; Aroeti et al., 1994; Matter and Mellman, 1994). By analogy with the clathrin-coated pits of plasma membranes (Pearse and Robinson, 1990), it is thought that intracellular sorting mechanisms able to recognize these signals divert the protein into the appropriate apical or basolateral pathways. However, although polarized sorting mechanisms for integral membrane proteins are thought to be located on the biosynthetic and the transcytotic pathways of polarized epithelial cells, none have thus far been identified.
Coated domains containing clathrin are known to be distributed in the biosynthetic pathway in the TGN (Pearse and Robinson, 1990; Robinson, 1990), and it is generally believed that in MDCK cells, the most widely used epithelial system for studies on polarized sorting (Simons and Wandinger-Ness, 1990; Mostov et al., 1992; Rodriguez-Boulan and Powell, 1992), this compartment contains mechanisms that can sort proteins directly to the apical and basolateral surfaces (Simons and Wandinger-Ness, 1990; Matter and Mellman, 1994; Ikonen et al., 1995). To date, however, unequivocal evidence showing that the TGN contains sorting mechanisms able to discriminate between proteins traveling to apical and basolateral surfaces has not been obtained.
Coated domains that have been shown to recruit trafficking proteins are also known to be present in the earlier stages of the biosynthetic pathway (Orci et al., 1986; Kreis and Pepperkok, 1994; Rothman and Wieland, 1996; Schekman and Orci, 1996), and components of one of these cytoplasmic coats, the COP1 complex, have been identified on endosomes (Whitney et al., 1995; Aniento et al., 1996), the compartment which represents the first step on the transcytotic pathway in polarized MDCK cells (Apodaca et al., 1994; Barroso and Sztul, 1994). Studies of a temperature-sensitive CHO cell mutant defective in the ε-COP component of COP1 (Guo et al., 1994; Hobbie et al., 1994) indicate a role of these components in sorting of recycling and lysosomally directed proteins within the endosome (Daro et al., 1997; Gu et al., 1997). However, there is no evidence yet that this kind of coat has a role to play in the polarized trafficking of epithelial cells.
Recently, clathrin-coated buds have also been shown to be present on endosomal tubules containing internalized transferrin receptors (TR)1 (Stoorvogel et al., 1996), and in a recent study of the trafficking of TR within polarized MDCK cells, we have shown that these cells contain a common endosome compartment that receives internalized TR from both apical and basolateral surfaces but returns them preferentially to the basolateral border (Odorizzi et al., 1996). Since many basolateral trafficking signals are similar to the tyrosine-based sequences of signals that promote internalization in the clathrin-coated pits of the plasma membrane (Trowbridge et al., 1993; Matter and Mellman, 1994), it is likely that a clathrin-containing, polarized sorting mechanism exists in the endosome compartment of MDCK cells. This question has been evaluated experimentally in the work presented in this paper, and we have obtained evidence to suggest that a mechanism that sorts internalized TR in a polarized fashion in MDCK is contained within the 60-nm-diam tubules of the endosome compartment. Similar tubules have been previously shown to participate in the polarized traffic of endocytosed TR in hepatocytes (Hemery et al., 1996), and we show here that these tubules can give rise to the 60-nm-diam vesicles that carry receptors recycling back to the basolateral border as well as receptors transcytosing to the basolateral border from the apical surface. These vesicles arise from clathrin-coated domains that contain γ-adaptin and are thus similar to the coated buds on the TGN (Pearse and Robinson, 1990; Robinson, 1990), the compartment on the biosynthetic pathway that routes receptors to the endosome compartment (Ludwig et al., 1991; Futter et al., 1995). Our evidence suggests, nevertheless, that the TGN and the TR-containing endosome in MDCK cells are discrete and not in continuity.
Materials and Methods
Reagents
Dimeric IgA (IgA) was generously supplied by Robert Drew (Department of Immunology, Birmingham University, Birmingham, UK), and IgA tracers and all other reagents and radiolabels were prepared by the same procedures as described previously (Futter et al., 1996). Colloidal gold sols were made as described by Slot and Geuze (1985).
Monoclonal and polyclonal anticlathrin antibodies (X22 and Pansy) were generous gifts from F. Brodsky (Stanford University, Stanford, CA) and M. Robinson (Cambridge, UK), respectively. Anti–γ-adaptin antibody (100/3) and anti–β′-COP antibody (23c) (Harrison-Lavoie et al., 1993) were from Sigma Chemical Co. (Poole, UK) and the Institute of Cancer Research (Sutton, UK), respectively. Monoclonal antibodies to the lumenal (B3/25) and cytoplasmic (H68.4) domains of TR were raised in the laboratory of I.S. Trowbridge.
Cells, Cell Culture, and Incubation Conditions
MDCK cells expressing pIgR and TR were generated by infecting MDCK strain II cells expressing the receptor for RSV(A) (Bates et al., 1993) and expressing human TR (Odorizzi et al., 1996) with BH-RCAS(A)-pIgR. The recombinant retrovirus vector was made from a plasmid containing the cDNA for rabbit pIgR (kindly provided by Keith Mostov, University of California, San Francisco, CA) digested with Bg1II to excise the 2.4-kb insert. Cla1 linkers were added to this fragment, which was then ligated into the Cla1 site of the replication competent vector BH-RCAS(A) (Odorizzi and Trowbridge, 1994). MDCK cells expressing tailless TR were prepared as described (Odorizzi et al., 1996). MDCK cells stably expressing sialyl transferase-HRP (ST-HRP) (Stinchcombe et al., 1995) were prepared as described in Connolly et al. (1994).
Cells were maintained and grown as polarized monolayers. Receptor expression was increased by sodium butyrate treatment, and the cells were incubated with tracers, all as described (Odorizzi et al., 1996). To determine the effect of brefeldin A (BFA) on polarized sorting, polarized monolayers were incubated with 125I-TF in the basolateral chamber for 2 h at 37°C in the absence of BFA, surface stripped as described (Odorizzi et al., 1996), and then chased at 37°C in medium containing 100 μg/ml unlabeled TF with or without 5 μg/ml BFA.
DAB cross-linking was performed as described (Odorizzi et al., 1996).
Electron Microscopy
For conventional electron microscopy, cells were fixed, processed, treated with tannic acid, and embedded in Epon, as described previously (Stinchcombe et al., 1995).
To show the distribution of coat proteins in polarized cells grown on filters, HRP-containing compartments were first preserved by incubation of cells in Tris-buffered saline containing 300–750 μg/ml DAB and 0.02% H2O2 for 30 min at 4°C in the dark. Cells were then permeabilized by incubation at 4°C for 10–20 min with digitonin in either the basolateral (40 μg/ml) or apical (250–400 μg/ml) chambers in permeabilization buffer (PB) (38 mM potassium aspartate, 38 mM potassium gluconate, 38 mM potassium glutamate, 2.5 mM MgCl2, 1.5 mM EGTA, 25 mM Hepes, pH 7.2). Cells were then washed in PB, followed by two 5-min washes in PB containing 2% BSA. In some cases, cells were incubated for 15 min at 4°C in 0.5 M Tris to remove coat proteins. In some cases, to remove membranes and non–HRP-containing compartments, cells were incubated in 1% Triton X-100 in PBS containing 0.02% azide for 10 min at 4°C. Cells were fixed in 2% paraformaldehyde, quenched in 15 mM glycine in PB, incubated with primary antibody in PB containing 1% BSA for 90 min at room temperature, washed, and then incubated with 5-nm gold-labeled secondary antibodies in the presence of 2% BSA and 2% FCS for 1–2 h at room temperature. For γ-adaptin labeling, an intermediate rabbit anti–mouse antibody was used between the primary and gold-conjugated secondary antibody incubations to enhance the signal. After thorough washing, cells were fixed and processed for conventional electron microscopy.
To quantitate the relative distribution of TR on endosomal tubules and basolateral vesicles, polarized cells grown on filters were loaded basolaterally with TF-HRP, the DAB reaction performed as described above except that 50 mM ascorbic acid was included in the apical medium to prevent DAB reaction product forming on the apical surface (Stoorvogel et al., 1996). The cells were then permeabilized from the apical surface and labeled as described above but using H68.4, an antibody specific for the cytoplasmic domain of the TR (White et al., 1992), conjugated directly to 5-nm colloidal gold. The membrane surface area of TF-HRP–containing compartments was measured as described by Weibel (1979), and the density of H68.4–gold particles per unit membrane on uncoated basolateral transport vesicles (defined by TF-HRP content and a size of <80 nm) was compared with that of the remaining TF-HRP–containing compartments. A total of 6,269 gold particles were counted on 48 negatives, taken at a magnification of 22,000.
Immunogold labeling of cryosectioned cells was performed essentially as described (Futter et al., 1996), but for γ-adaptin labeling, cells were fixed in 0.1% glutaraldehyde, and an intermediate rabbit anti–mouse antibody was used between the primary and gold-conjugated secondary antibody incubations.
Light Microscopy
After incubation with FITC-labeled tracers, cells were fixed with 4% paraformaldehyde, quenched with 15 mM glycine, and permeabilized with 0.05% saponin, all in PBS. Subsequent antibody incubations were performed in PBS containing 1% BSA, 0.01% saponin. For triple labeling, secondary antibodies conjugated to FITC, rhodamine, and Cy5, all raised in donkey, were used (Stratech Scientific Ltd., Luton, UK). Cells were examined in a laser scanning confocal imaging system (model MRC-1045; Bio-Rad Laboratories, Hertfordshire, UK) with an argon/krypton mixed gas laser. Final images were merged using Photoshop (Adobe, San Jose, CA) and photographed using a Sapphire Slide Recorder (Management Graphics, Minneapolis, MN).
Results
pIgR and TR Internalized from Apical and Basolateral Plasma Membranes Enter an Interconnected Endosome Compartment Where They Undergo Polarized Sorting
We have used monolayers of MDCK cells expressing pIgR and human TR so that internalized IgA and TF tracers can be used to identify polarized trafficking pathways. While the majority of surface pIgR and TR are on the basolateral surface in polarized monolayers, there are sufficient numbers on the apical surface for the cells to be loaded with ligand from both surfaces. Electron microscopy shows that apically and basolaterally introduced tracers become rapidly codistributed throughout a pleiomorphic endosome compartment that consists of irregularly shaped 0.3–0.5-μm-diam vacuoles and associated tubules (Fig. 1). The endosomal tubules are of at least two types: a larger (100– 150-nm-diam) form, which is often orientated in the apical basolateral axis and can be seen to extend for up to 2.0 μm within a single section plane, and a smaller (60-nm-diam) form, which is more convoluted, branches frequently, and has coated buds at its free ends (see below).
Figure 1.
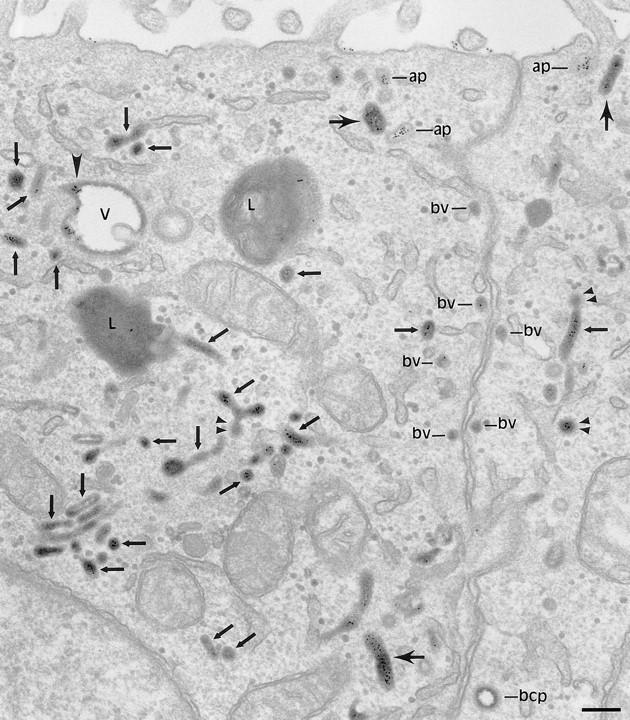
Codistribution of TF and IgA tracers applied from basolateral and apical surfaces of polarized cells. Polarized MDCK expressing TR and pIgR were incubated basolaterally with TF-HRP (60 min at 37°C) and apically with IgA-gold (30 min at 37°C). Endosomal elements labeled with both tracers are distributed throughout the cytoplasm and include 0.3-μm-diam vacuoles (V; with a tubular extension, arrowhead), narrower branching tubules (small arrows), larger 100– 150-nm-diam tubules (large arrows) and 60-nm-diam basolateral vesicles (bv). Basolaterally applied TF-HRP is not present on the apical membrane, and the coated pits and vesicles derived from this membrane (ap) are identifiable because they have only been reached by apical tracer. bcp indicates a coated vesicle derived from basolateral surface containing only TF-HRP and has, typically, a peripheral distribution of DAB reaction product. Double arrowheads indicate coated buds on 60-nm tubules. L, lysosome. Bar, 0.1 μm.
To quantitate the extent of colocalization within this interconnected endosome system, monolayers were loaded with iodinated and HRP-labeled ligands at 20°C (which allows internalization but reduces the rate of transcytosis) and cross-linked with DAB-H2O2. As shown in Fig. 2, up to 60% of iodinated IgA can be cross-linked by TF-HRP when both tracers are introduced basolaterally, and similar levels of cross-linking are seen if both tracers are introduced apically or if they are loaded from opposite sides of the monolayer.
Figure 2.
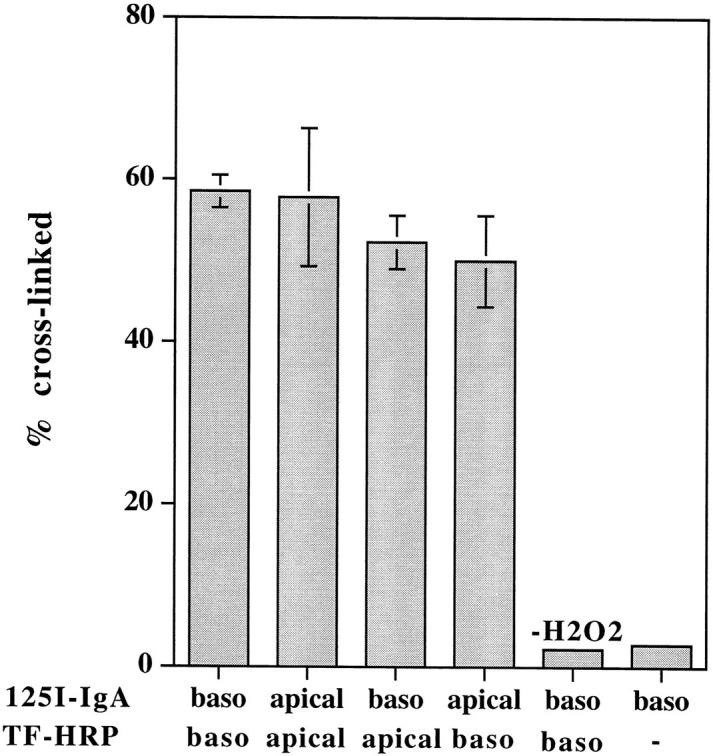
Cross-linking of TF and IgA tracers internalized from the same and from opposite sides of the monolayer at low temperature. Polarized MDCK cells expressing TR and pIgR were incubated with radio-labeled or HRP-conjugated tracers from either the same or opposite sides of the monolayer, as indicated, for 2 h at 20°C. DAB cross-linking was then performed, as described in Materials and Methods. Results are means ± SD of three or four observations.
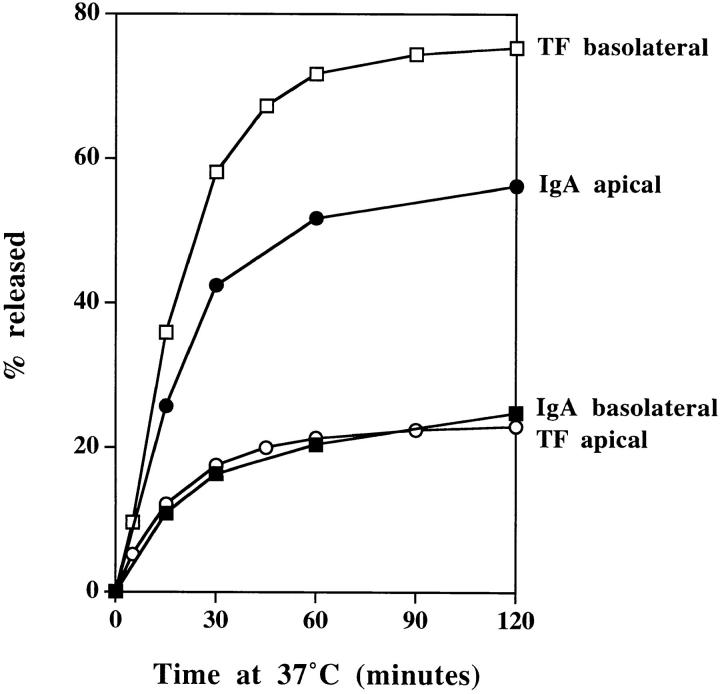
To follow the polarized sorting of these tracers, they were loaded basolaterally at 20°C, and then the incubation temperature was shifted to 37°C. As shown in Fig. 3, the internalized TF and IgA are released in a polarized fashion with basolateral/apical ratios of 4:1 and 1:3, respectively. Correlating these results with the electron microscopy thus suggests that TR and pIgR internalized from apical and basolateral surfaces of polarized MDCK cells are processed within a common, interconnected endosome compartment, and from this compartment, TR are directed preferentially to the basolateral surface and pIgR to the apical surface.
Figure 3.
Polarized sorting of TF and IgA at 37°C. Polarized MDCK cells expressing TR and pIgR were preloaded basolaterally for 2 h at 20°C with 125I-IgA (filled symbols) or 125I-TF (open symbols) and chased at 37°C, and apical (circles) and basolateral (squares) media were collected. Results are mean ± SD of three to six observations. SD are smaller than the symbols.
60-nm-diam Basolateral Transport Vesicles Arise from Coated Buds on Endosome Tubules
In a previous study, we introduced TF-HRP from the basolateral surface and anti-TR (B3/25) gold tracer from the apical surface and showed that, with time, a population of 60-nm-diam vesicles formed that delivered apically labeled TR to the basolateral surface (Odorizzi et al., 1996). The colocalization of the apical and basolateral tracers showed that the vesicles contained receptors that were recycling back to the basolateral border as well as receptors being transcytosed from the apical surface. In the present study, using MDCK-expressing pIgR as well as TR, this approach was again used, TF-HRP being preloaded from the basolateral surface to steady state (60 min at 37°C) and gold tracer (B3/25-gold or IgA-gold) being introduced at 37°C from the apical surface. During the first 10 min of incubation with the apical tracer, the gold label gains access to coated pits on the apical membrane (Fig. 4 a) and to 100–150-nm-diam vesicles immediately below this membrane and begins to appear in the endosomal vacuoles and tubules loaded with the basolaterally applied TF-HRP (Fig. 4, b–d). From 10 min onwards, gold label begins to appear in the 60-nm-diam vesicles distributed along the basolateral border (Fig. 4 e), and after 30 min, gold label can be found on the basolateral surface. Some of the 60-nm-diam vesicles have a clathrin coat (see below), but they can be discriminated from the clathrin-coated vesicles that arise from the basolateral membrane because they are smaller (50–60- vs 100–150-nm-diam) and because the HRP reaction product they contain is smoothly homogeneous and completely fills the lumen (Fig. 4, c and e). These vesicles contain up to 10 5-nm particles of apical tracer, and in appropriate section planes, they are seen to be grouped centrally, separated from the inner surface of the vesicle membrane by a 10–12-nm perimeter zone (Fig. 4 c, but see also Fig. 5 b). With incubations of less than 10 min, apically derived gold tracer is not seen at the basolateral border. Tubules and vesicles processing HRP tracer derived from the basolateral plasma membrane are identifiable because they lack apical gold tracer. They can be discriminated from basolateral vesicles traveling to the basolateral border because they are of larger diameter and display a distinctive peripheral distribution of DAB reaction product that leaves a clear electron lucent central lumen (Figs. 1 and 4, b and d).
Figure 4.
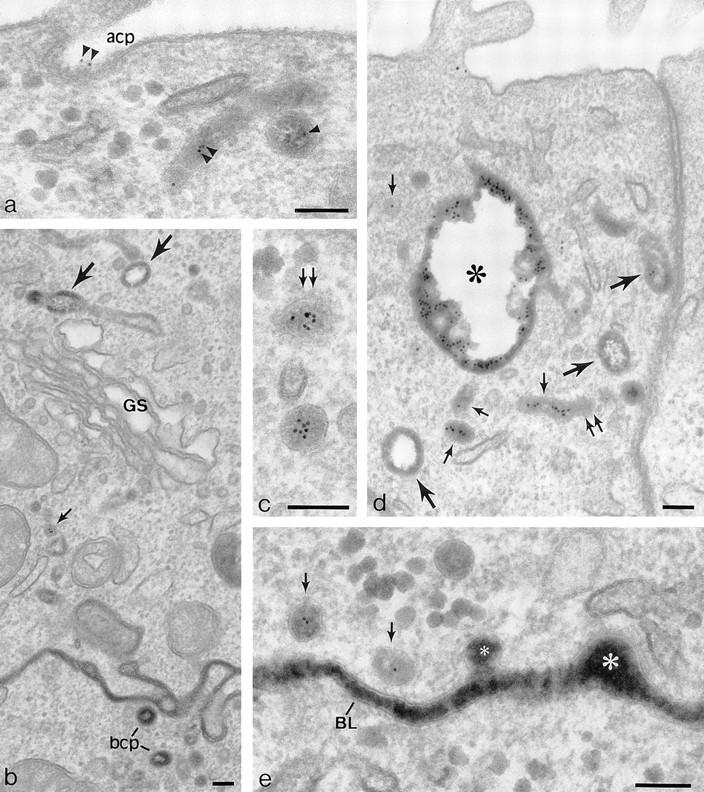
Penetration of gold-labeled TR from the apical surface into transcytotic elements preloaded with TF-HRP from the basolateral surface. (a) At the apical border, apically applied gold-labeled TR are present in coated pits (acp) and have gained access to endosomal elements (small arrowheads) containing TF-HRP that has been loaded from the basolateral surface. (b) At the basolateral border, TF-HRP is distributed in the basolateral space and in coated pits/vesicles (bcp). Deeper in the cell, the TF-HRP is present in tubules and vacuoles of variable diameter (arrows) and in small (60-nm-diam) basolateral vesicles (small arrow) that also contain apically derived gold-labeled TR. (c) Two 60-nm-diam vesicles (one coated, double arrows) showing (1) the homogeneous distribution of DAB reaction product due to TF-HRP derived from the basolateral border and (2) the central cluster of gold-labeled TR (internalized from the apical surface) that characterize the content of basolateral vesicles. (d) Endosomal vacuole (*) surrounded by tubules and vesicles containing apically and basolaterally derived tracer. Large arrows indicate larger tubules with a peripheral distribution of DAB reaction product similar to the vacuole. Small arrows indicate 60-nm-diam tubules and vesicles filled with DAB reaction product. In cross-sections of these narrow tubules, the gold particles occupy a central position, which in longitudinal sections are often seen in single file. Double arrows indicate a clathrin-coated bud. (e) Basolateral border (BL) showing 60-nm-diam basolateral tubules/vesicles (arrows) containing apically derived gold TR tracer and a smooth surfaced invagination (small asterisk) where an exocytotic vesicle has fused. The density of the DAB reaction product within the exocytotic invagination is similar to that given by the TF-HRP in the lateral space (and much stronger than in the unfused vesicles in the peripheral cytoplasm), suggesting that there is a rapid equilibration of intra- and extracellular tracer when vesicles fuse. The large asterisk indicates an endocytotic invagination identifiable because it is larger (∼100-nm-diam) and is clathrin-coated. Bars, 0.1 μm.
Figure 5.
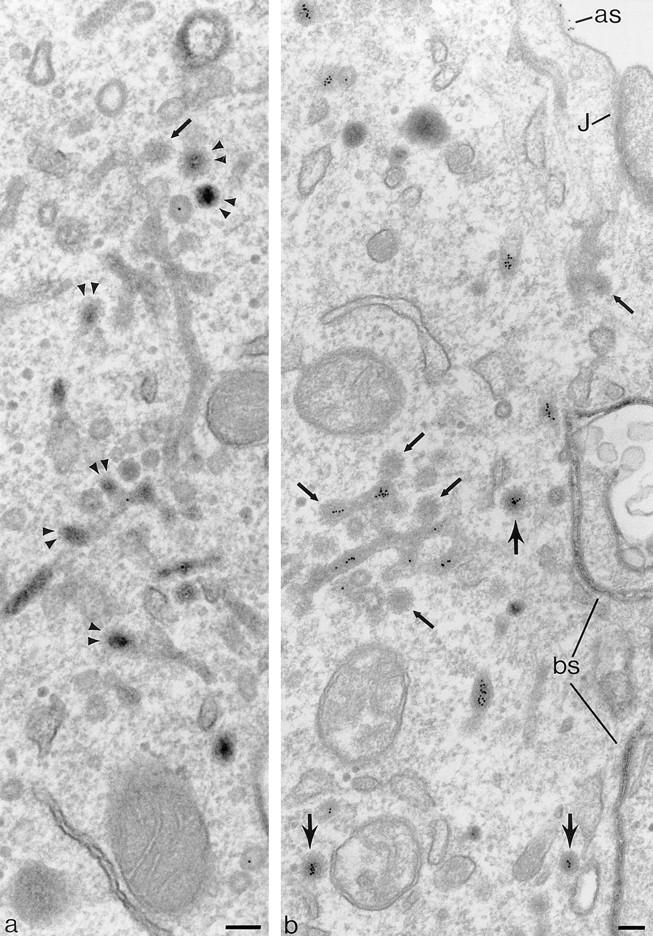
Coated buds and 60-nm vesicles in polarized cells containing basolaterally directed tracers. Endosomal tubules containing basolaterally applied TF-HRP and apically applied IgA-gold. The TF-HRP serves to identify endosomal elements accessible from the basolateral surface, and the IgA-gold demonstrates that these elements also process apically derived tracer. Fig. 3 shows that TR are routed preferentially to the basolateral border, and significant amounts of the IgA are also routed basolaterally from the endosome. (a) Double arrowheads indicate coated buds containing concentrated TF-HRP reaction product. The small arrow indicates clathrin lattice. (b) Cytoplasmic coats are seen on the tubules (small arrows) and on apparently free 60-nm-diam vesicles (larger arrows) that contain concentrated TF-HRP and IgA-gold tracer. as, IgA-gold on apical surface; J, junctional complex; bs, basolateral surface with TF-HRP within intercellular space. Bars, 0.1 μm.
The morphology of the 60-nm-diam basolateral vesicles is very similar to the 60-nm-diam coated buds distributed over endosomal tubules that contain internalized tracers (Fig. 5, a and b). The distinction between free vesicles and the buds on the tubules is not always clear in conventional thin sections, but their content of DAB reaction product allows them to be identified in sections 200–250 nm thick, and in these sections vesicles and tubules can be discriminated with confidence. In these thick sections, the tubules can be seen to branch and to be in continuity with both 100-nm-diam tubules and 0.2–0.5-μm-diam endosomal vacuoles, which also contain TF-HRP. Thick sections do not reveal continuities between TF-HRP–containing endosome tubules and _trans_-Golgi elements (discussed further below). Where the 60-nm-diam tubules are straight, they often contain a single line of gold particles, but at their ends, where they expand into coated buds, they contain groups of particles similar to those seen in free 60-nm-diam vesicles (Fig. 5 b but see also distribution of internalized gold in Fig. 6 e). The morphological similarity of the coated buds and the coated 60-nm-diam vesicles clearly suggests that the vesicles arise by budding from the tubules.
Figure 6.
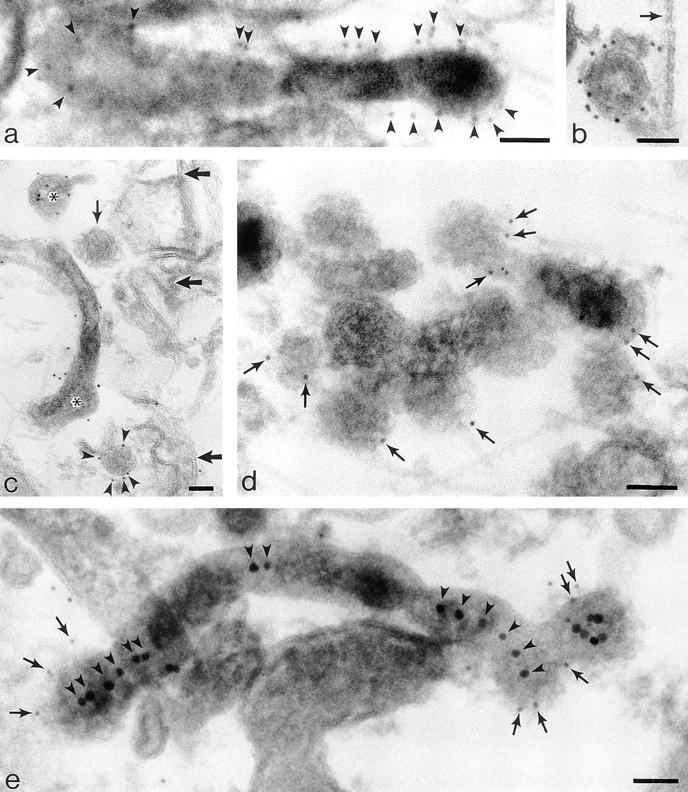
Labeling cytoplasmic domains of TR and coat proteins on 60-nm-diam tubules and vesicles loaded basolaterally with TF-HRP in polarized monolayers permeabilized with digitonin. (a) Thick section showing distribution of H68.4-labeled TR in a 100-nm-diam form of tubule that extends into a 60-nm-diam form with a terminal bud. Permeabilized cell treated with Tris and Triton X-100 to remove clathrin lattices. TF-HRP reaction product and the gold labeling (arrowheads) indicates some concentration as the tubule narrows. (b) 60-nm-diam vesicle containing internalized TF-HRP. The cytoplasmic domains of the TR in this free vesicle are labeled with 5-nm H68.4 antibody. Arrow indicates the plasma membrane. (c) Thin section showing distribution of H68.4–gold on TF-HRP–containing endosomal elements (asterisks) and basolateral vesicles. One basolateral vesicle is coated and unlabeled with gold (small arrow) and the other is labeled (arrowheads). Large arrows indicate basolateral plasma membrane. (d) Thick section showing 60-nm-diam endosomal tubules labeled for clathrin (small arrows). (e) Thick section of 60-nm-diam endosomal tubules labeled for γ-adaptin with 5-nm-gold (small arrows). The tubules contain apically derived TR gold tracer in linear array (arrowheads) and the coated bud contains a central cluster of six particles. Bars, 0.05 μm.
The intensity of DAB staining generated by the internalized HRP tracer within 60-nm-diam tubules and vesicles is variable because their full width is not always included within the plane of the section but, in general, the contents of the coated buds and the adjacent vesicles stain more intensely than the tubules (Fig. 5 a). We have not demonstrated quantitatively that gold-labeled TR become concentrated in coated buds on endosomal tubules because this would require the endosomal system to be loaded close to saturation by, for instance, loading at reduced temperature, when gold particles tend to accumulate in vacuoles, rather than tubules.
To demonstrate the native distribution of TR trafficking through the endosome, a localization method was used in which the endosome was loaded with TF-HRP and fixed before TR were labeled using an antibody (H68.4)–gold complex specific for the cytoplasmic domain of the TR. These complexes were applied to permeabilized monolayers in which the native distribution of the TR in the endosome had been preserved by DAB cross-linking, induced by activating the TF-HRP contained within the tubule lumen. Activation was achieved by loading monolayers with TF-HRP and then incubating at 5°C with DAB-H2O2 in the presence of ascorbic acid to prevent the formation of DAB reaction product on the cell surface (Stoorvogel et al., 1996). Once the contents of the TF-HRP–containing tubules have been cross-linked, the monolayer can be permeabilized with detergents, and H68.4–gold complexes can be applied. In these preparations, the 60-nm-diam endosomal tubules and the free, uncoated 60-nm-diam vesicles are readily identified by their content of DAB reaction product (Fig. 6, a–c). The H68.4–gold shows the TR to be distributed on the plasma membranes (data not shown) and throughout the endosomal elements containing internalized TR tracer (in some experiments both TF-HRP and B3/25 gold were used). In these preparations clathrin-coated domains on the plasma membrane and on endosomal tubules remain unlabeled, presumably because the coat prevents access to the antibody. Attempts to remove the coats using Tris buffer (Chang et al., 1993; Stoorvogel et al., 1996) were only partially successful because remnants of coats could still be seen, even when the visible membrane boundary had been removed with Triton X-100 (see Materials and Methods). However, a strong, specific label for TR was displayed on the surfaces of all other TF-HRP–containing elements.
To assess the degree of concentration of TR that takes place during their packaging into 60-nm basolateral vesicles, the density of labeled TR on the TF-HRP–containing tubules was compared with their density on DAB-positive vesicles in preparations in which these membrane boundaries were measured morphometrically (see Materials and Methods). In monolayers loaded to steady state at 37°C with TF-HRP, the results showed that H68.4–gold particles were 2.09 times as dense on the membranes of uncoated basolateral vesicles as on uncoated TF-HRP–containing endosomal membranes. A similar analysis (our unpublished data) in which the density of internalized pIgR (labeled on their cytoplasmic domains) were also located on these vesicles showed the ratio of TR to pIgR on basolateral vesicles to be 7:3. The complexities of the recycling and transcytotic pathways operating in the endosome compartment and the different affinities of TR and pIgR ligands make it difficult to be precise about the relative densities of TR and pIgR expected to be in basolateral vesicles. Nevertheless, the increased numbers of TR on basolateral vesicles relative to the number on endosomal elements and relative to pIgR, as indicated by these morphometric data, clearly suggest that TR become concentrated as they are packaged into basolateral vesicles.
Coated Buds on Endosomal Tubules Contain Clathrin and γ-Adaptin
The coats on endosomal tubule buds were characterized by loading polarized, filter-grown cells with TF-HRP from the basolateral surface, cross-linking with DAB and H2O2 as described above, permeabilizing the cells with digitonin, and then using immunogold procedures to localize coat proteins. As shown in Fig. 6, d and e, the coats on tubules, identifiable by their content of internalized TF-HRP as endosomes, are positive for both clathrin and γ-adaptin.
Clathrin and γ-adaptin–coated endosomal buds containing internalized tracers could also be identified in MDCK cells grown on solid substrata. These cells, which can be used to advantage for confocal microscopy (see below), can be readily prepared for cryoimmunoelectron microscopy and show coated buds containing internalized tracer that label strongly with both clathrin and γ-adaptin antibodies (Fig. 7). In these preparations, endosomal tubules were identified by their content of IgA-gold to avoid cross-reaction of gold-conjugated secondary antibodies with the internalized anti–TR-gold.
Figure 7.
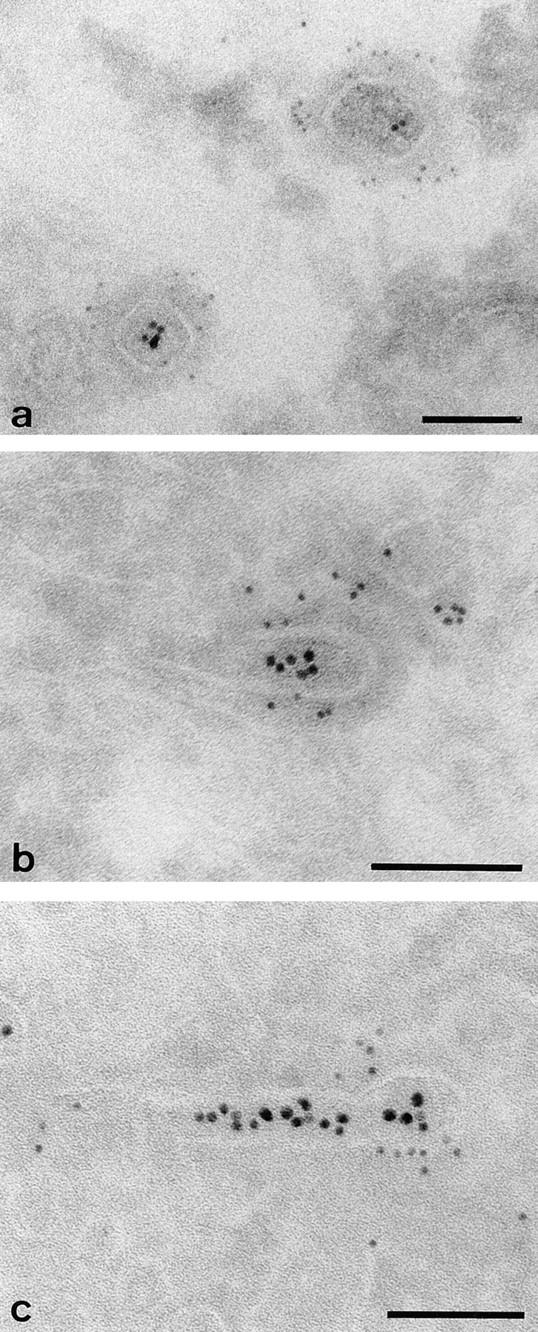
Clathrin and γ-adaptin on endosomal tubules in cells grown on solid substrata. Cells grown on solid substrata loaded with TF-HRP and IgA-gold (12 nm), prepared for cryosections and (a) clathrin (Pansy) and (b and c) γ-adaptin localized with immunogold. The endocytosed 12-nm gold tracer identifies the tubules as endosomal, and the 5-nm particles show that the coats contain clathrin and γ-adaptin. Bars, 0.1 μm.
The Relationship between the Coated Buds on Endosomal Tubules and γ-Adaptin–coated Domains on the TGN
The demonstration that buds on endosomal tubules contain γ-adaptin raised the question of whether these clathrin-coated buds belong to the same population of clathrin-γ-adaptin–containing domains as those known to be present in the TGN. To study the relationship in more detail, we identified the components of the _trans_-Golgi area by expressing ST-HRP, a chimaeric reporter enzyme that we have previously shown by electron microscopy to be distributed within the TGN and _trans_-most cisternae of the Golgi stack (Stinchcombe et al., 1995), and we transiently expressed human TR in the same cells so that the endosome could be loaded with TF-FITC. The distribution of the ST-HRP–containing elements can be most clearly defined by confocal microscopy of cells grown flat on a solid substratum (e.g., glass coverslips), where immunofluorescence shows that γ-adaptin is distributed throughout the peripheral cytoplasm as well as being concentrated on the ST-containing elements of the _trans_-Golgi area (Fig. 8). In the peripheral cytoplasm, the γ-adaptin is closely associated with the TF-containing vacuoles, and at high magnification, it often appears to form caps on the tubular extensions of these vacuoles (Fig. 8, inset). Overlap between TF-FITC–containing endosomal elements and ST-HRP– loaded _trans_-Golgi elements is negligible, although it is clear that γ-adaptin is present on both of these compartments (Fig. 8 b). There are some peripheral γ-adaptin– positive punctae that are not apparently associated with TF-FITC–labeled structures. This is likely to be because many TR-containing tubules are below the level of detection by immunofluorescence, although we cannot exclude the possibility that an additional population of peripheral γ-adaptin–positive vesicles not involved in TR recycling also exists.
Figure 8.
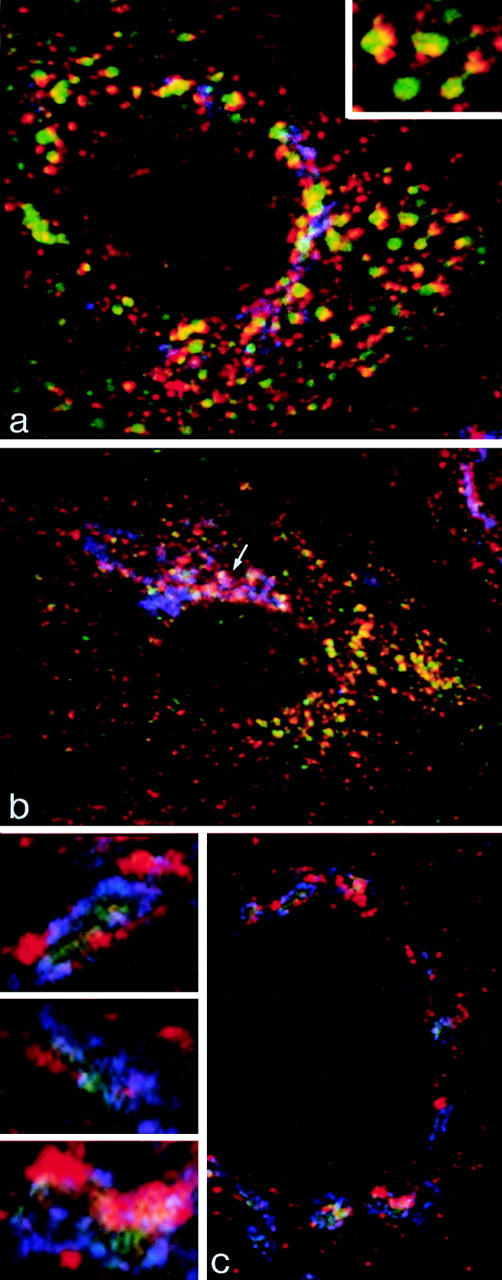
Immunofluorescent localization of γ-adaptin in cells grown on solid substrata. (a and b) MDCK cells stably expressing ST-HRP were transiently transfected with human TR and were incubated for 1 h at 37°C with TF-FITC. They were triple labeled for γ-adaptin (red), TF-FITC (green), and ST-HRP (blue). (a) In the peripheral cytoplasm, γ-adaptin is closely associated with endocytosed TF-FITC. In the enlarged structures shown in the inset, γ-adaptin appears to cap the tubules extending from the TF-FITC–containing vacuoles. (b) In the Golgi region, the γ-adaptin is closely associated with ST-HRP (arrow), but overlap between TF-FITC and the ST-HRP is negligible. (c) Triple label for γ-adaptin (red), ST-HRP (green), and β′-COP (blue). In addition to labeling the punctate structures in the peripheral cytoplasm, γ-adaptin is closely associated with β′-COP label in the ST-HRP– containing Golgi elements. Seen at high magnification (insets), it is clear that the Golgi elements contain discrete domains in which either γ-adaptin or COP1 are concentrated.
The Differential Effect of BFA on γ-Adaptin and COP1 Coats
The distribution of γ-adaptin on the ST-HRP–containing _trans_-Golgi and on TR-containing endosomal elements was also examined in cells treated with the fungal metabolite brefeldin A (BFA). Previous published studies have shown that BFA causes the tubulation and redistribution of Golgi elements into the ER (Lippincott-Schwartz et al., 1990) and extensive tubulation of the TGN and endosomal elements (Lippincott-Schwartz et al., 1991; Wood et al., 1991; Stoorvogel et al., 1996). In MDCK cells, BFA treatment causes the TGN to tubulate (Wagner et al., 1994) but does not cause the Golgi stack to redistribute (Hunziker et al., 1991; Wagner et al., 1994). The effects of BFA are thought to be mediated through coat proteins as, in many cell types, BFA treatment causes the release of γ-adaptin from the TGN (Robinson and Kreis, 1992; Wong and Brodsky, 1992), COP1 components from the Golgi (Donaldson et al., 1990; Wong and Brodsky, 1992), and clathrin coats from endosomes (Stoorvogel et al., 1996). However, in MDCK cells, while BFA causes γ-adaptin coats to disperse (Wagner et al., 1994), the COP1 coats on Golgi elements are, like the Golgi itself, unaffected (Hunziker et al., 1991; Wagner et al., 1994).
In cells expressing ST-HRP and loaded with TF-FITC, both γ-adaptin and COP1, as identified by the distribution of β′-COP, are seen to be distributed on the _trans_-Golgi elements containing ST-HRP. Immunofluorescence shows that while the two coat proteins are closely associated in the Golgi, they have separate, discrete distributions (Fig. 8 c). In the peripheral cytoplasm, where COP1 and γ-adaptin both have punctate distributions, there is also negligible overlap. Electron microscopy (data not shown) shows that the peripheral β′-COP staining is associated with RER exit sites (vesiculo-tubular clusters) (Pind et al., 1994), rather than endosomal elements.
The most dramatic effect of BFA addition is to rapidly disperse (within 2 min) the γ-adaptin staining on both the ST-HRP–containing _trans_-Golgi and the TR-containing endosomes (Fig. 9 c). This treatment leaves the distribution of the Golgi-associated COP1 unchanged (Fig. 9 d). With longer incubations, BFA treatment also causes extensive tubulation of the TR-containing endosome compartment (Fig. 9 a) and some tubulation of the TGN (Fig. 9 b), but the Golgi stack remains largely intact.
Figure 9.
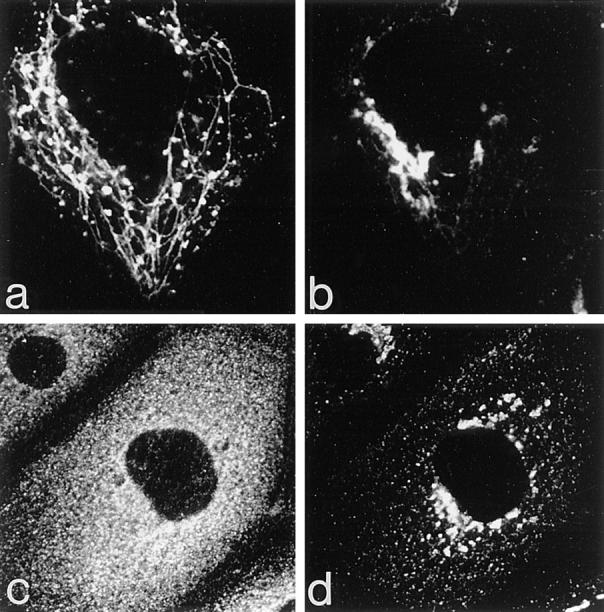
The effects of BFA on the Golgi, endosomes, and their associated coat proteins. Cells expressing ST-HRP and transiently transfected with human TR were incubated with TF-FITC for 1 h at 37°C and then treated with 5 μg/ml BFA for 10 min at 37°C. (a) TF-FITC–containing endosomes show dramatic tubulation while (b) ST-HRP–containing _trans_-Golgi elements develop relatively few tubules. (c) BFA causes γ-adaptin from both Golgi and peripheral locations to become dispersed while (d) β′-COP remains concentrated in the Golgi area.
BFA Treatment Abolishes Polarized Sorting of TR
To examine the effects of BFA treatment on polarized sorting from the endosome, polarized monolayers expressing either wild-type or tailless TR were loaded with 125I-TF at 37°C from the basolateral border, and the release of the tracer was assayed at 37°C. A previous study by Wan et al. (1992) has shown that BFA enhances TF transcytosis, but to determine the effect on polarized sorting it is necessary to compare apical and basolateral release. In our previous work, we were able to show that the volumes of traffic to apical and basolateral surfaces of polarized MDCK cells are approximately equal since cells expressing mutant TR lacking a cytoplasmic domain (and thus a basolateral targeting signal) release endocytosed TF to apical and basolateral surfaces in a 50:50 ratio (Odorizzi et al., 1996). In the presence of BFA, cells expressing wild-type TR showed enhanced apical delivery of TF and a corresponding reduction in basolateral delivery (Fig. 10 a), and as a consequence, the preloaded tracer was released to basolateral and apical borders in approximately equal amounts. Monolayers expressing tailless TR showed no change in the polarized pattern of release in response to BFA (Fig. 10 b). In view of the extensive tubulation of endosomes caused by BFA, it is of interest that BFA treatment does not affect the kinetics with which TF is released. From these observations we conclude that (a) a polarized sorting mechanism within the endosome is able to recognize basolateral targeting information in the cytoplasmic domain of the TR, (b) this mechanism is responsible for increasing the proportion of TR returning to the basolateral and apical surfaces from 1:1 to 4:1, and (c) BFA abrogates the effect of this mechanism.
Figure 10.
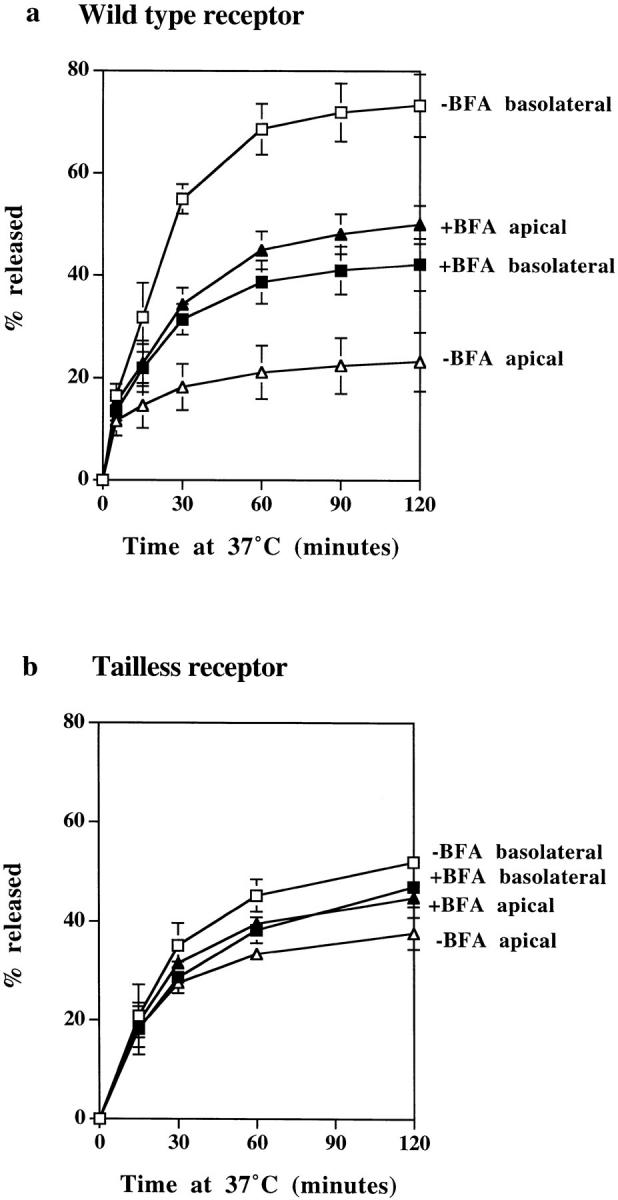
The effects of BFA on polarized sorting of TF. Polarized monolayers preloaded basolaterally at 37°C for 2 h with 125I-TF were stripped of surface label and then incubated at 37°C with (filled symbols) or without (open symbols) BFA, and apical (triangles) and basolateral (squares) media were collected. (a) In cells expressing wild-type TR, TF is preferentially released to the basolateral surface, but in the presence of BFA, release to apical and basolateral surfaces is approximately equal. (b) In cells expressing tailless TR, TF release to the separate surfaces is approximately the same with and without BFA treatment. All results are mean ± SD of three to six observations.
Discussion
Clathrin-coated Buds on Endosomal Tubules Contain γ-Adaptin
We have identified clathrin-coated buds on endosome tubules that contain γ-adaptin, suggesting that the distributions of clathrin/γ-adaptin coats on membrane boundaries in polarized cells are more widespread than previously thought. Clathrin-coated buds containing γ-adaptin have previously been described as being localized mainly on the TGN (Robinson, 1990) but have also been found associated with endosomes (Le Borgne et al., 1996). Increasing the expression of a protein trafficking through the TGN, TGN38, has been reported to increase the distribution of γ-adaptin on endosomal membranes (Reaves and Banting, 1994). However, in unpublished studies we have found the distribution of γ-adaptin in MDCK cells to be unaffected by increasing the level of exogenous TR expression, and we believe, therefore, that the clathrin localization we have observed on endosomal tubules is unlikely to be caused by a transfection-induced overexpression. Clathrin-coated buds on endosomal tubules have been previously described, but most of these buds did not contain detectable γ-adaptin (Stoorvogel et al., 1996). It is possible, therefore, that there is more than one class of clathrin-coated bud on endosome tubules and that there is a relatively higher frequency of γ-adaptin–containing buds in polarized cells.
The Relationship between γ-Adaptin–coated Buds on Endosomal Tubules and Those on the TGN
There is, as yet, no evidence that γ-adaptin associates with any coat proteins other than those of the AP1 adaptor protein complex, and the composition of the γ-adaptin–containing coats on the endosome is thus probably the same as those on the TGN. Internalized TR and low-density lipoprotein receptors (LDL-R) have been reported to cycle through the TGN (Snider and Rogers, 1985; Stoorvogel et al., 1988; Green and Kelly, 1992), raising the possibility that the basolateral sorting of transcytotic traffic we have observed in polarized MDCK cells could be taking place in the TGN and that the peripheral distribution of γ-adaptin–coated buds we have identified simply shows that the TGN extends throughout the apical cytoplasm of MDCK cells. There is also a long-standing observation that endocytosed TR can become resialylated by recycling through the TGN (Snider and Rogers, 1985), although the efficiency of resialylation is low. We have been unable to detect TR within the sialyl transferase-HRP–containing TGN in any of our studies on MDCK cells. It is also clear that BFA has very different effects on TR-containing and ST-HRP–containing compartments in these cells and this, in agreement with a recent study by Volz et el. (1995), argues against the view that the TR-containing endosome and the TGN comprise a single compartment in MDCK cells. Analyses of trafficking signals within TR and TGN38 also show, at least in unpolarized cells (Humphrey et al., 1993; Wong and Hong, 1993; Ponnambalam et al., 1994), that TGN38 contains information that allows it to traffic to locations beyond those normally accessible to TR. This implies that a signal-dependent step probably separates TR-containing endosome compartments from the TGN. Taken together, these considerations suggest that the clathrin-coated endosome tubules we have identified in the transcytotic pathway of MDCK cells are unlikely to be in continuity with the clathrin-coated tubules of the _trans_-Golgi.
The Role of Clathrin Lattices in Vesiculation and in the Concentration of TR in Endosomal Tubules
The role of cytoplasmic coat proteins in vesiculation and/or in cargo selection is the subject of much current debate (Schekman and Orci, 1995; Aridor and Balch, 1996). In a previous study on clathrin-coated vesicle formation on the plasma membrane, we showed that an increased expression of TR increases the amount of lattice formed on the membrane without a corresponding increase in membrane vesiculation (Miller et al., 1991). These studies also showed that the lattice formation induced by the increased expression of TR probably depends upon the internalization signal contained in their cytoplasmic domain since wild-type TR internalize six times more efficiently than tailless TR (Jing et al., 1990; Miller et al., 1991). Together, these studies suggested that clathrin coats are involved in the recruitment of TR but do not promote vesicle formation. The observations that show that the removal of the clathrin by BFA does not affect the kinetics of TF release, made on the clathrin-coated buds of endosomal tubules in the present study, can be similarly interpreted since they suggest that in the absence of the γ-adaptin/clathrin coat, vesiculation and the production of basolateral vesicles continues, and only the ability to sort selectively is lost.
An unequivocal demonstration that the clathrin coats on endosomal tubules concentrate TR has been difficult to achieve for technical reasons. Nevertheless, our micrographs clearly show that the basolateral vesicles that arise from these coated buds contain concentrated TR. Since the removal of the coats from these buds by BFA results in the loss of polarized delivery of TR to the basolateral surface, we propose that γ-adaptin–containing buds on endosomal tubules are responsible for concentrating the TR being packaged into basolateral vesicles.
The Relationship between Basolateral Targeting Signals and Clathrin-based Sorting Mechanisms
Many of the signals required for basolateral targeting are tyrosine-based, and some, but not all, overlap with tyrosine-based internalization motifs (Trowbridge et al., 1993; Matter and Mellman, 1994). The tyrosine-based signal required for concentration of TR in clathrin-coated pits at the plasma membrane has been well characterized (Jing et al., 1990) and shown to mediate high-affinity binding to the medium chain of the AP2 or plasma membrane adaptor protein complex (Ohno et al., 1996). This signal does not appear to mediate high-affinity binding to the medium chain of AP1 (i.e., the γ-adaptin–containing coat) complex (Ohno et al., 1996), suggesting additional requirements for binding of this protein to the AP1 adaptor. These data are consistent with a previous study showing that deletion of the internalization signal of the TR had only a small effect on basolateral targeting (Dargemont et al., 1993), and recent work showing that the basolateral targeting signal and internalization signals of TR are distinct (Odorizzi and Trowbridge, 1997).
The Clathrin-based Sorting in the Endosome Can Explain the Polarized Distribution of TR on the MDCK Cell Surface
The available evidence implicating tyrosine-containing motifs in basolateral targeting, taken together with the data presented in the current study, suggests that a relatively simple regulatory system probably underlies the basolaterally biased, steady-state distribution of constitutively trafficking proteins on the surfaces of polarized MDCK cells. This idea follows from the observation that proteins like TR internalize from both apical and basolateral surfaces of MDCK cells into a single interconnected endosome system (Odorizzi et al., 1996) since it suggests that a single polarized sorting mechanism within such a system should be sufficient to maintain a steady-state, asymmetric distribution on the separate surfaces. Thus, we suggest that the clathrin-coated domains on 60-nm-diam endosomal tubules selectively divert continuously recycling receptors like TR into vesicles that are basolateral because these same domains also package the appropriate basolateral SNARE machinery into them (Sollner et al., 1993; Low et al., 1996). TR-containing vesicles thus fuse preferentially with the basolateral border.
The Role of a Polarized Endosomal Sorting Mechanism in Biosynthetic Pathways
The signals in the cytoplasmic domain of the TR recognized by the intracellular sorting mechanisms on the biosynthetic pathway remain to be fully characterized. For LDL-R (Matter et al., 1993) and pIgR (Aroeti and Mostov, 1994), the same amino acids have been shown to be required for basolateral sorting in both the TGN and the endosome, suggesting that both locations contain similar recognition mechanisms. However, subtle differences in the structural requirements for basolateral sorting of TR on the biosynthetic and endocytic pathways have been detected (Odorizzi et al., 1997). At least two basolaterally sorted proteins, TR (Futter et al., 1995) and LDL-R (Leitinger et al., 1995), can travel directly from the TGN to the endosome when newly synthesized. This raises the possibility that all basolaterally directed proteins could follow a common route to the cell surface via the endosome and could be processed by a single polarized sorting mechanism located in this compartment. Alternative arrangements using more than one basolateral pathway and using the same clathrin-based, polarized sorting mechanisms on parallel routes can also be envisioned, but it then also becomes necessary to duplicate the targeting mechanisms (i.e., the v-SNAREs) to ensure that both populations of vesicles are delivered to the same basolateral destination.
Acknowledgments
This work was supported by a program grant from the Medical Research Council to C.R. Hopkins and in part by a National Institute of Allergy and Infectious Diseases grant to I.S. Trowbridge.
Abbreviations used in this paper
IgA
dimeric IgA
BFA
brefeldin A
LDL-R
low-density lipoprotein receptor
PB
permeabilization buffer
pIgR
polymeric immunoglobulin receptor
ST-HRP
sialyl transferase-HRP
TF
transferrin
TR
transferrin receptor(s)
Footnotes
Address all correspondence to Colin R. Hopkins, Medical Research Council Laboratory for Molecular Cell Biology, University College London, Gordon St., London WC1E 6BT, England. Tel.: 0171-380-7806. Fax: 0171-380-7804.
References
- Aniento F, Gu F, Parton RG, Gruenberg J. An endosomal COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol. 1996;133:29–42. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G, Katz LA, Mostov KE. Receptor mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Balch WE. Principles of selective transport: coat complexes hold the key. Trends Cell Biol. 1996;6:315–320. doi: 10.1016/0962-8924(96)10027-1. [DOI] [PubMed] [Google Scholar]
- Aroeti B, Mostov KE. Polarized sorting of the polymeric immunoglobulin receptor in the exocytotic and endocytotic pathways is controlled by the same amino acids. EMBO (Eur Mol Biol Organ) J. 1994;13:2297–2304. doi: 10.1002/j.1460-2075.1994.tb06513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso M, Sztul ES. Basolateral to apical transcytosis in polarized cells is indirect and involves BFA and trimeric G protein sensitive passage through the apical endosome. J Cell Biol. 1994;124:83–100. doi: 10.1083/jcb.124.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates P, Young JAT, Varmus HE. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993;74:1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- Chang MP, Mallet WG, Mostov KE, Brodsky FM. Adaptor self-aggregation, adaptor-receptor recognition and binding of the α-subunit to the plasma membrane contribute to recruitment of adaptor (AP2) components of clathrin-coated pits. EMBO (Eur Mol Biol Organ) J. 1993;12:2169–2180. doi: 10.1002/j.1460-2075.1993.tb05865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly C, Futter CE, Hopkins CR, Cutler DF. Transport into and out of the Golgi complex studied by transfecting cells with cDNAs encoding horseradish peroxidase. J Cell Biol. 1994;127:641–652. doi: 10.1083/jcb.127.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargemont C, LeBivic A, Rothenburger S, Iacopetta B, Kuhn L. The internalisation signal and the phosphorylation site of transferrin receptor are distinct from the main basolateral sorting information. EMBO (Eur Mol Biol Organ) J. 1993;12:1713–1721. doi: 10.1002/j.1460-2075.1993.tb05816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Lippincott-Schwartz J, Bloom GS, Kreis TE, Klausner RD. Dissociation of a 110-kD peripheral membrane protein from the Golgi apparatus is an early event in brefeldin A action. J Cell Biol. 1990;111:2295–2306. doi: 10.1083/jcb.111.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daro E, Sheff D, Gomez M, Kreis T, Mellman I. Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component ε-COP. J Cell Biol. 1997;139:1747–1759. doi: 10.1083/jcb.139.7.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter CE, Connolly CN, Cutler DF, Hopkins CR. Newly synthesised transferrin receptors can be detected in the endosome before they appear on the cell surface. J Biol Chem. 1995;270:10999–11003. doi: 10.1074/jbc.270.18.10999. [DOI] [PubMed] [Google Scholar]
- Futter CE, Pearse A, Hewlett L, Hopkins CR. Multivesicular endosomes containing internalized EGF–EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Kelly RB. Low density lipoprotein receptor and cation-independent mannose 6-phosphate receptor are transported from the cell surface to the Golgi apparatus at equal rates in PC-12 cells. J Cell Biol. 1992;117:47–55. doi: 10.1083/jcb.117.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Aniento F, Parton R, Gruenberg J. Functional dissection of COP-I subunits in the biogenesis of multivesicular endosomes. J Cell Biol. 1997;139:1183–1195. doi: 10.1083/jcb.139.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Vasile E, Krieger M. Disruptions in Golgi structure and membrane traffic in a conditional lethal mammalian cell mutant are corrected by ε-COP. J Cell Biol. 1994;125:1213–1224. doi: 10.1083/jcb.125.6.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison-Lavoie KJ, Lewis VA, Hynes GM, Collison KS, Nutland E, Willison KR. A 102kDa subunit of a Golgi-associated particle has homology to β subunits of trimeric G proteins. EMBO (Eur Mol Biol Organ) J. 1993;12:2847–2853. doi: 10.1002/j.1460-2075.1993.tb05946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemery I, Durand-Schneider A-M, Feldmann G, Vaerman J-P, Maurice M. The transcytotic pathway of an apical membrane protein (B10) in hepatocytes is similar to that of IgA and occurs via a tubular pericentriolar compartment. J Cell Sci. 1996;109:1215–1227. doi: 10.1242/jcs.109.6.1215. [DOI] [PubMed] [Google Scholar]
- Hobbie L, Fisher AS, Lee S, Flint A, Krieger M. Isolation of three classes of conditional lethal Chinese hamster ovary cell mutants with temperature-dependent defects in low density lipoprotein receptor stability and intracellular membrane transport. J Biol Chem. 1994;269:20958–20970. [PubMed] [Google Scholar]
- Humphrey JS, Peters PJ, Yuan LC, Bonafacino JS. Localization of TGN38 to the trans-Golgi network: involvement of a cytoplasmic tyrosine-containing sequence. J Cell Biol. 1993;120:1123–1135. doi: 10.1083/jcb.120.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Whitney JA, Mellman I. Selective inhibition of transcytosis by brefeldin A in MDCK cells. Cell. 1991;67:617–628. doi: 10.1016/0092-8674(91)90535-7. [DOI] [PubMed] [Google Scholar]
- Ikonen E, Tagaya M, Ullrich O, Montecucco C, Simons K. Different requirements for NSF, SNAP, and Rab proteins in apical and basolateral transport in MDCK cells. Cell. 1995;81:571–580. doi: 10.1016/0092-8674(95)90078-0. [DOI] [PubMed] [Google Scholar]
- Jing S, Spencer T, Miller K, Hopkins C, Trowbridge IS. Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J Cell Biol. 1990;110:283–294. doi: 10.1083/jcb.110.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis TE, Pepperkok R. Coat proteins in intracellular membrane transport. Curr Opin Cell Biol. 1994;6:533–537. doi: 10.1016/0955-0674(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Griffiths G, Hoflack B. Mannose 6-phosphate receptors and ADP-ribosylation factors cooperate for high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J Biol Chem. 1996;271:2162–2170. doi: 10.1074/jbc.271.4.2162. [DOI] [PubMed] [Google Scholar]
- Leitinger B, Hille-Rehfeld A, Spiess M. Biosynthetic transport of the asialoglycoprotein receptor H1 to the cell surface occurs via endosomes. Proc Natl Acad Sci USA. 1995;92:10109–10113. doi: 10.1073/pnas.92.22.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Donaldson JG, Schweizer A, Berger EG, Hauri HP, Yuan LC, Klausner RD. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner RD. Brefeldin A's effects on endosomes, lysosomes and the trans Golgi network suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Low S-H, Chapin SJ, Weimbs T, Komuves LG, Bennett MK, Mostov KE. Differential localisation of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1996;7:2007–2018. doi: 10.1091/mbc.7.12.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig T, Griffiths G, Hoflack B. Distribution of newly synthesised lysosomal enzymes on the endocytic pathway of normal rat kidney cells. J Cell Biol. 1991;115:1561–1572. doi: 10.1083/jcb.115.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Whitney JA, Yamamoto EM, Mellman I. Common signals control low density lipoprotein receptor sorting in endosomes and the Golgi complex of MDCK cells. Cell. 1993;74:1053–1064. doi: 10.1016/0092-8674(93)90727-8. [DOI] [PubMed] [Google Scholar]
- Matter K, Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Miller K, Shipman M, Trowbridge IS, Hopkins C R. Transferrin receptors promote the formation of clathrin lattices. Cell. 1991;65:621–632. doi: 10.1016/0092-8674(91)90094-f. [DOI] [PubMed] [Google Scholar]
- Mostov KE, Apodaca G, Aroeti B, Okamoto C. Plasma membrane protein sorting in polarized epithelial cells. J Cell Biol. 1992;116:577–583. doi: 10.1083/jcb.116.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Trowbridge IS. Recombinant Rous sarcoma virus vectors for avian cells. Methods Cell Biol. 1994;43:79–98. doi: 10.1016/s0091-679x(08)60599-3. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Trowbridge IS. Structural requirements for basolateral sorting on the human transferrin receptor in the biosynthetic and endocytic pathways of Madin-Darby canine kidney cells. J Cell Biol. 1997;137:1255–1264. doi: 10.1083/jcb.137.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Trowbridge IS, Hopkins CR. Apical and basolateral endosomes of MDCK cells are interconnected and contain a polarized sorting mechanism. J Cell Biol. 1996;135:139–152. doi: 10.1083/jcb.135.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Fournier MC, Poy G, Bonifacino JS. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J Biol Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- Orci L, Glick BS, Rothman JE. A new type of coated vesicular carrier that appears not to contain clathrin: its possible role in protein transport within the Golgi stack. Cell. 1986;46:171–184. doi: 10.1016/0092-8674(86)90734-8. [DOI] [PubMed] [Google Scholar]
- Pearce BMF, Robinson MS. Clathrin, adaptors and sorting. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- Pind SN, Nuoffer C, McCaffery JM, Plutner H, Davidson HW, Farquhar MG, Balch WE. Rab1 and Ca2+are required for the fusion of carrier vesicles mediating endoplasmic reticulum to Golgi transport. J Cell Biol. 1994;125:239–252. doi: 10.1083/jcb.125.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnambalam S, Rabouille C, Luzio JP, Nilsson T, Warren G. The TGN38 glycoprotein contains two nonoverlapping signals that mediate localization to the trans-Golgi network. J Cell Biol. 1994;125:253–268. doi: 10.1083/jcb.125.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves B, Banting G. Overexpression of TGN38/41 leads to mislocalisation of gamma-adaptin. FEBS Lett. 1994;351:448–456. doi: 10.1016/0014-5793(94)00813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. Cloning and expression of γ adaptin, a component of clathrin-coated vesicles associated with the Golgi apparatus. J Cell Biol. 1990;111:2319–2326. doi: 10.1083/jcb.111.6.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS, Kreis TE. Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cells: effects of brefeldin A and G protein activators. Cell. 1992;69:129–138. doi: 10.1016/0092-8674(92)90124-u. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Powell SK. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein transport by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Simons K, Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990;62:207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ. A new method for preparing gold probes for multiple labeling cytochemistry. Eur J Cell Biol. 1985;38:87–93. [PubMed] [Google Scholar]
- Snider MD, Rogers OC. Intracellular movement of cell surface receptors after endocytosis: resialylation of asialo-transferrin receptor in human erythroleukemia cells. J Cell Biol. 1985;100:826–834. doi: 10.1083/jcb.100.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Stinchcombe J, Cutler D, Hopkins CR. Anterograde and retrograde traffic between the rough endoplasmic reticulum and the Golgi complex. J Cell Biol. 1995;131:1387–1401. doi: 10.1083/jcb.131.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W, Geuze HJ, Griffith JM, Strous GJ. The pathways of endocytosed transferrin and secretory protein are connected in the trans-Golgi reticulum. J Cell Biol. 1988;106:1821–1829. doi: 10.1083/jcb.106.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W, Oorschot V, Geuze HJ. A novel class of clathrin-coated vesicles budding from endosomes. J Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge IS, Collawn JF, Hopkins CR. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Volz B, Orberger G, Porwoll S, Hauri H-P, Tauber R. Selective reentry of recycling cell surface glycoproteins to the biosynthetic pathway in human hepatocarcinoma HepG2 cells. J Cell Biol. 1995;130:537–552. doi: 10.1083/jcb.130.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Rajasekaran AK, Hanzel DK, Mayor S, Rodriguez-Boulan E. Brefeldin A causes structural and functional alterations of the trans-Golgi network of MDCK cells. J Cell Sci. 1994;107:933–943. doi: 10.1242/jcs.107.4.933. [DOI] [PubMed] [Google Scholar]
- Wan J, Taub ME, Shah D, Shen W-C. Brefeldin A enhances receptor-mediated transcytosis of transferrin in filter–grown Madin-Darby canine kidney cells. J Biol Chem. 1992;267:13446–13450. [PubMed] [Google Scholar]
- Weibel, E.R. 1979. Stereological Methods. 1. Practical Methods for Biological Morphometry. Academic Press, Inc., New York. 371 pp.
- White S, Miller K, Hopkins C, Trowbridge IS. Monoclonal antibodies against defined epitopes of the human transferrin receptor cytoplasmic tail. Biochim Biophys Acta. 1992;1136:28–34. doi: 10.1016/0167-4889(92)90081-l. [DOI] [PubMed] [Google Scholar]
- Whitney JA, Gomez M, Sheff D, Kreis TE, Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Wong DH, Brodsky FM. 100-kD proteins of the Golgi and trans-Golgi network–associated coated vesicles have related but distinct membrane binding properties. J Cell Biol. 1992;117:1171–1179. doi: 10.1083/jcb.117.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Hong W. The SXYQRL sequence in the cytoplasmic domain of TGN38 plays a major role in trans-Golgi network localisation. J Biol Chem. 1993;268:22853–22862. [PubMed] [Google Scholar]
- Wood SA, Park JE, Brown WJ. Brefeldin A causes a microtubule-mediated fusion of the trans-Golgi network and early endosomes. Cell. 1991;67:591–600. doi: 10.1016/0092-8674(91)90533-5. [DOI] [PubMed] [Google Scholar]