Mechanisms of Arsenical and Diamidine Uptake and Resistance in Trypanosoma brucei (original) (raw)
Abstract
Sleeping sickness, caused by Trypanosoma brucei spp., has become resurgent in sub-Saharan Africa. Moreover, there is an alarming increase in treatment failures with melarsoprol, the principal agent used against late-stage sleeping sickness. In T. brucei, the uptake of melarsoprol as well as diamidines is thought to be mediated by the P2 aminopurine transporter, and loss of P2 function has been implicated in resistance to these agents. The trypanosomal gene TbAT1 has been found to encode a P2-type transporter when expressed in yeast. Here we investigate the role of TbAT1 in drug uptake and drug resistance in T. brucei by genetic knockout of _TbAT1. Tbat1_-null trypanosomes were deficient in P2-type adenosine transport and lacked adenosine-sensitive transport of pentamidine and melaminophenyl arsenicals. However, the null mutants were only slightly resistant to melaminophenyl arsenicals and pentamidine, while resistance to other diamidines such as diminazene was more pronounced. Nevertheless, the reduction in drug sensitivity might be of clinical significance, since mice infected with _tbat1_-null trypanosomes could not be cured with 2 mg of melarsoprol/kg of body weight for four consecutive days, whereas mice infected with the parental line were all cured by using this protocol. Two additional pentamidine transporters, HAPT1 and LAPT1, were still present in the null mutant, and evidence is presented that HAPT1 may be responsible for the residual uptake of melaminophenyl arsenicals. High-level arsenical resistance therefore appears to involve the loss of more than one transporter.
Sleeping sickness is caused by Trypanosoma brucei, and the disease is endemic throughout tropical Africa. Sleeping sickness is fatal if untreated, and vaccination is impossible due to antigenic variation of the parasites. The main chemotherapeutics for treatment of sleeping sickness are still the melaminophenyl arsenical melarsoprol and the diamidine pentamidine, drugs that were introduced more than 50 years ago. Cross-resistance between melamine-based arsenicals and diamidines has been repeatedly observed in trypanosomes with laboratory-induced drug resistance (14-16, 24, 26). Cellular uptake of both classes of trypanocide has been shown to occur via the P2 adenosine/adenine transport activity in T. brucei bloodstream forms, and loss of P2 has been implicated in drug resistance (5, 6). Subsequently, a trypanosome gene (TbAT1) that exhibited P2-like transport activity when expressed in yeast was cloned (19). The function of TbAT1 in trypanosomes, however, remained to be investigated.
No clear correlation was found between mutations in TbAT1 and relapses after melarsoprol treatment in sleeping sickness patients in Uganda (22), and T. brucei isolated from melarsoprol-refractory patients did not show high levels of in vitro arsenical resistance (4, 21). [3H]pentamidine transport in T. brucei was only partially blocked by adenosine (5, 7, 11), and adenosine transport in Saccharomyces cerevisiae expressing TbAT1 was not inhibited by pentamidine (19). The situation is further complicated by the fact that nucleoside transporters comprise a multigene family in T. brucei (28). Thus, a model whereby the loss of a single transporter (TbAT1) could mediate cross-resistance to all diamidines and melamine-based arsenicals appears overly simplistic.
Meanwhile, the problem of drug resistance in T. brucei appears to be increasing in the field. Sleeping sickness has recently become resurgent in sub-Saharan Africa, and the emergence of drug resistance is hindering efforts to control the disease. Melarsoprol treatment failures have reached alarming levels in several foci (4). In addition, resistance to the diamidine diminazene aceturate has also been reported from multiple foci (17, 23). Pentamidine resistance, in contrast, has so far not been reported from the field. The understanding of resistance mechanisms in bloodstream-form trypanosomes is crucial to circumventing existing resistance problems and avoiding the emergence of resistance to the next generation of drugs.
To investigate whether TbAT1 alone contributes to P2 activity in Trypanosoma brucei brucei, we have constructed a TbAT1 knockout clone by targeted gene replacement. We have further used this null mutant to resolve the role of TbAT1 in the uptake of, and resistance to, the diamidine and melaminophenyl arsenical classes of drugs.
MATERIALS AND METHODS
Propagation of trypanosomes.
Bloodstream-form T. brucei brucei strain 427 (s427; also known as MiTat 1.2/221 or BS 221) parasites were used to construct the _TbAT1_−/− line. They were cultured in minimal essential medium modified according to the work of Baltz et al. (1), supplemented with 5% fetal bovine serum. For transport experiments, adult female Wistar rats were infected by intraperitoneal injection. At peak parasitemia, blood was collected by cardiac puncture under terminal anesthesia. The parasites were isolated by using a DE52 (Whatman, Maidstone, United Kingdom) anion-exchange column (18) and washed twice in assay buffer (33 mM HEPES, 98 mM NaCl, 4.6 mM KCl, 0.55 mM CaCl2, 0.07 mM MgSO4, 5.8 mM NaH2PO4, 0.3 mM MgCl2, 23 mM NaHCO3, 14 mM glucose [pH 7.3]).
Constructs for the TbAT1 gene deletion.
TbAT1 alleles were replaced sequentially with resistance markers for the antibiotics neomycin and puromycin. Flanking sequences upstream (700 bp) and downstream (300 bp) of the TbAT1 open reading frame were amplified by PCR and cloned into plasmids so that they flanked NEO or PAC genes. The resulting deletion constructs were released from plasmid DNA by restriction digestion, purified by phenol extraction, and resuspended to 0.2 μg/μl in water.
Transfection of trypanosomes.
Bloodstream-form trypanosomes (s427) were cultured to a density of 2 × 106 ml−1. A total of 108 cells were washed in 15 ml of ZMG buffer (132 mM NaCl, 8 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, 0.775 mM magnesium acetate, 0.063 mM calcium acetate [pH 7.5]) and resuspended in 450 μl of ZMG and 50 μl of deletion construct DNA solution. The cell suspension was electroporated by using a Bio-Rad Genepulser at 1.5 kV, 25 μF, and 200 ΩF and was transferred to culture medium. Drug selection was applied after 24 h, and trypanosomes were cloned in vitro after 7 days of selection. Neomycin (1.5 μg/ml) was used for the first-round knockout, and neomycin plus puromycin (0.15 μg/ml) was used for the second-round knockout.
Southern blotting.
Genomic DNAs of the wild-type s427, two first-round knockout clones, and two second-round derivatives of each were digested with _Eco_RI and _Nde_I. Southern blotting was performed by standard protocols. TbAT1 and neomycin and puromycin resistance genes were labeled by using the DIG system (Roche Diagnostics, Basel, Switzerland).
Transport assays.
Transport assays for [3H]adenosine (NEN) and [3H]pentamidine (Amersham) were performed exactly as described previously (7, 31) by using a rapid oil stop protocol. Briefly, trypanosomes were harvested, washed twice with the assay buffer, and resuspended at 108 cells/ml. Cells were then incubated with the radioligand in the presence or absence of a competitive inhibitor and were spun through oil (for 30 s at 12,000 × g) after a predetermined time as indicated in Results. Radioactivity in the cell pellet was determined, after solubilization in 2% sodium dodecyl sulfate, by liquid scintillation counting.
In vitro drug sensitivity assays.
In vitro drug sensitivity was determined by using the Alamar Blue assay (25). Trypanosomes (104) were exposed to doubling dilutions of drugs for 2 days at 37°C before Alamar Blue reagent (Bio-Source, Camarillo, Calif.) was added. After a further 24 h of incubation, fluorescence was determined in a fluorimeter (Perkin-Elmer LS55B). Fifty percent inhibitory concentrations (IC50) were determined by nonlinear regression using the Prism (GraphPad) software package. For in vitro lysis assays (14), trypanosomes were exposed to 10 μM melarsen oxide or cymelarsan in the presence or absence of potential inhibitors of drug uptake. Lysis was monitored spectrophotometrically by determination of absorbance at 750 nm at 60-s intervals.
In vivo experiments.
Twenty-eight NMRI outbred mice (young adults, female, weighing around 25 g) were inoculated intraperitoneally with 105 trypanosomes. Seven groups of four mice each were infected as follows: groups A, D, and G received wild-type s427, groups B and E received knockout clone 12, and groups C and F received knockout clone 21. Three days postinfection, all mice showed strong parasitemia and were treated with melarsoprol at 2 mg kg of body weight−1 (groups A, B, and C) or 10 mg kg−1 (groups D, E, and F) for four consecutive days. Group G comprised untreated controls. Melarsoprol (3.6% solution in propylene glycol) was diluted with phosphate-buffered saline to 60 and 300 μg/100 μl, and mice were treated by intraperitoneal injection of 100 μl for the 2- and 10-mg kg−1 doses, respectively. The mice were monitored twice a week for parasitemia by examination of smears of tail blood.
Data analysis.
All experiments were performed in triplicate or more. Kinetic data, given as means and standard errors, were determined in at least three independent experiments and calculated by nonlinear regression using the Prism (GraphPad) software package from a minimum of 8 points over the relevant range. All uptake data are presented as “mediated uptake,” defined as total uptake minus diffusion. Diffusion is taken to be uptake in the presence of saturating concentrations of unlabeled permeant.
RESULTS
Construction of _tbat1_-null mutants.
The TbAT1 gene was replaced in two steps, by sequential transformation of T. b. brucei s427 bloodstream forms with constructs containing the neomycin and puromycin resistance markers. To investigate whether the constructs had correctly integrated and replaced TbAT1, genomic DNAs from several first- and second-round antibiotic-resistant transformant clones, as well as from the parental wild-type trypanosomes, were analyzed by Southern blotting. Hybridization with probes for TbAT1 or resistance marker genes revealed heterozygous and homozygous _tbat1_-null clones as expected (data not shown).
The _tbat1_−/− trypanosomes did not exhibit any obvious differences in morphology or motility from the wild-type parent strain s427. The population doubling time in culture was not significantly different, remaining at around 8 h for both strains. _tbat1_−/− trypanosomes remained fully infective to mice (data not shown). Thus, TbAT1 is not essential for cell survival, cell-cycle progression, or the course of infection in rodents, presumably because of the presence of additional purine transporters in bloodstream-form trypanosomes (6, 9, 10, 13).
Adenosine transport in _tbat1_-null mutants.
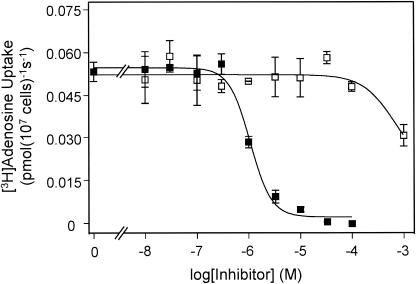
T. b. brucei bloodstream forms possess two adenosine transporter activities, P1 and P2, which are selectively inhibited by inosine and adenine, respectively (6, 10). The underlying genes encoding each activity have not been determined conclusively so far. In _tbat1_-null trypanosomes, uptake of 0.1 μM [3H]adenosine was completely inhibited by inosine (Fig. 1), with an IC50 of 0.74 ± 0.09 μM, very similar to values reported previously for the P1 activity (6, 10, 12). In contrast, as much as 100 μM adenine failed to inhibit [3H]adenosine transport (Fig. 1). This result indicates that the P2 adenosine transport activity has been completely deleted in the _tbat1_-null mutant, as adenosine transport mediated by P2 has been shown to be sensitive to adenine with a Ki of ∼0.2 μM (6, 10).
FIG. 1.
Uptake of [3H]adenosine by the _tbat1_-null mutant. Uptake of 20 nM [3H]adenosine was inhibited by inosine (filled squares) with an IC50 of 1.1 ± 0.1 μM. Adenine failed to inhibit [3H]adenosine at concentrations as high as 100 μM when it was added alone (open squares). The inhibition induced by 1 mM adenine is attributable to a low-affinity inhibition of P1.
Pentamidine transport in _tbat1_-null mutants.
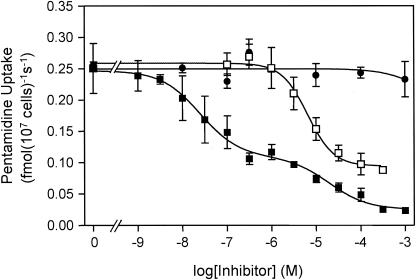
[3H]pentamidine transport in _tbat1_-null bloodstream forms was not inhibited by as much as 1 mM adenosine (Fig. 2). This is in marked contrast with transport in wild-type T. b. brucei, where an adenosine-sensitive pentamidine transporter (ASPT1) is responsible for about 50% of total pentamidine uptake (7, 11). The absence of an adenosine-sensitive pentamidine flux in the _tbat1_-null mutants clearly demonstrates that TbAT1 encodes the previously described ASPT1 activity (7, 11). The reported failure of pentamidine to inhibit adenosine uptake by TbAT1 expressed in yeast (19, 20) raises the question as to whether auxiliary factors, present in the trypanosome plasma membrane, are required to confer diamidine transport by TbAT1.
FIG. 2.
Uptake of [3H]pentamidine by the _tbat1_-null mutant. The uptake of 15 nM [3H]pentamidine was unaffected by as much as 1 mM adenosine (filled circles) but was inhibited to a maximum of 64% by propamidine (open squares), with an IC50 of 6.5 μM. When increasing amounts of unlabeled pentamidine (filled squares) were added, [3H]pentamidine uptake was inhibited in a biphasic manner (P < 0.0001 by the F test), with the high-affinity component (IC50 = 25.1 nM) contributing 62% of total [3H] pentamidine transport. The IC50 of the low-affinity component was 19.5 μM for this experiment.
Increasing concentrations of unlabeled pentamidine inhibited uptake of 15 nM [3H]pentamidine in a biphasic manner (Fig. 2), revealing the presence of a high-affinity and a low-affinity component of pentamidine transport, termed HAPT1 and LAPT1, respectively (7). At 15 nM, [3H]pentamidine transport by HAPT1 and LAPT1 in _tbat1_-null trypanosomes was inhibited by unlabeled pentamidine, with IC50 of 29 ± 8 nM and 50 ± 17 μM (n = 3), respectively, consistent with values reported previously for wild-type trypanosomes (7, 11). HAPT1 and LAPT1 can be further distinguished by the fact that only HAPT1 is inhibited by propamidine (7). Propamidine inhibited the high-affinity uptake of [3H]pentamidine in _tbat1_-null trypanosomes with an IC50 of 13 ± 3 μM (n = 4) (Fig. 2) but had no effect on the low-affinity uptake phase.
Drug resistance profiles of _tbat1_-null mutants.
In order to test the hypothesis that loss of TbAT1 causes drug resistance, _tbat1_-null and wild type trypanosomes were cultured in vitro in the presence of serial dilutions of trypanocidal diamidines and melaminophenyl arsenicals. The assays for both strains were performed in parallel in order to minimize the influence of interassay variation. As summarized in Table 1, _tbat1_-null mutants indeed showed decreased drug sensitivity. Resistance to melaminophenyl arsenicals (melarsoprol, melarsen oxide, cymelarsan), however, was consistently only two- to threefold relative to the s427 parent strain. Pentamidine resistance was in the same range. In contrast, _tbat1_-null mutants showed markedly higher levels of resistance to stilbamidine, propamidine, and in particular diminazene aceturate (Berenil).
TABLE 1.
Drug resistance phenotype of the _tbat1_-null mutant compared to the parental wild-type strain
| Drug | IC50a (ng/ml) | Resistance factor | |
|---|---|---|---|
| Wild type | _TbAT1_−/− mutant | ||
| Melarsoprol | 21 ± 3 | 49 ± 9 | 2.3 |
| Melarsen oxide | 3.7 ± 0.1 | 11 ± 0.1 | 3.0 |
| Cymelarsan | 6.1 ± 1 | 12 ± 3 | 2.0 |
| Pentamidine | 5.1 ± 2 | 12 ± 3 | 2.4 |
| Diminazene | 120 ± 30 | 2,300 ± 500 | 19 |
| Propamidine | 67 ± 7 | 750 ± 20 | 11 |
| Stilbamidine | 680 ± 70 | 5,000 ± 1,300 | 7.4 |
The low levels of resistance to melarsoprol in the null mutant were confirmed in vivo. Mice infected with s427 were all cured after treatment with 2 or 10 mg of melarsoprol/kg for four consecutive days. In contrast, all mice infected with one of the two knockout clones relapsed after treatment with 2 mg of melarsoprol/kg and died due to parasitemia by day 20 posttreatment. The 10-mg/kg dosage of melarsoprol still effectively cured mice infected with _tbat1_-null trypanosomes.
Additional pathways for arsenical uptake.
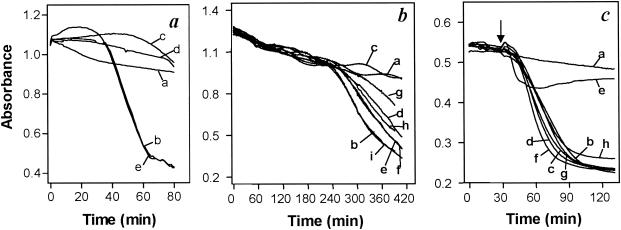
The comparably small reduction of melarsoprol sensitivity in _tbat1_-null trypanosomes suggests the presence of further, TbAT1-independent pathways for drug uptake. Transport of melaminophenyl arsenicals cannot be studied directly for lack of a radiotracer, but the lytic effect of these compounds on trypanosomes in vitro can serve as a bioassay for arsenical uptake (14, 29, 33). Treatment with 10 μM melarsen oxide or cymelarsan induced rapid lysis of wild-type s427 bloodstream forms, with onset typically occurring at 15 to 30 min and complete lysis within 1 h (Fig. 3a). Simultaneous incubation with adenosine, adenine, or pentamidine prevented lysis, while inosine, guanosine or hypoxanthine had no effect (Fig. 3a and data not shown). For the _tbat1_-null mutant, however, the onset of melaminophenyl arsenical-induced lysis was significantly delayed (typically to 4 h) and lysis was much slower (Fig. 3b). This is consistent with TbAT1 representing the transporter responsible for the fast component of melaminophenyl arsenical uptake.
FIG. 3.
In vitro sensitivities of bloodstream-form trypanosomes to arsenical trypanocides. (a) Wild-type T. b. brucei s427 parasites were incubated with or without 10 μM melarsen oxide in the presence of potential inhibitors. Traces: a, control (no arsenical); b, melarsen oxide only; c, melarsen oxide plus 4 mM adenosine; d, melarsen oxide plus 1 mM pentamidine; e, melarsen oxide plus 4 mM hypoxanthine. (b) Cells of the _tbat1_-null mutant were incubated with or without 10 μM cymelarsan. Traces: a, control (no arsenical); b, cymelarsan only; c, cymelarsan plus 0.1 μM pentamidine; d, cymelarsan plus 0.03 μM pentamidine; e, cymelarsan plus 0.01 μM pentamidine; f, cymelarsan plus 10 μM stilbamidine; g, cymelarsan plus 1 μM propamidine; h, cymelarsan plus 0.3 μM propamidine; i, cymelarsan plus 0.1 μM propamidine. (c) Cells of the _tbat1_-null mutant were incubated with or without 0.5 μM phenylarsine oxide. Traces: a, control (no arsenical); b, phenylarsine oxide only; c, phenylarsine oxide plus 10 mM adenosine; d, phenylarsine oxide plus 4 mM hypoxanthine; e, phenylarsine oxide plus 1 mM pentamidine; f, phenylarsine oxide plus 100 μM pentamidine; g, phenylarsine oxide plus 100 μM propamidine; h, phenylarsine oxide plus 100 μM stilbamidine. Arrow indicates time of phenylarsine oxide addition.
Adenosine and other purines had no effect on the slow lysis of _tbat1_-null cells by melaminophenyl arsenicals (data not shown), but lysis was fully prevented by diamidines, most potently by pentamidine. Fifty percent effective concentrations were 30 nM for pentamidine, 300 nM for propamidine, and approximately 10 μM for stilbamidine (Fig. 3b). To investigate whether this counteracting effect of diamidines was caused by competition for a common transporter or occurred at the site of arsenical action, phenylarsine oxide was used as an arsenical trypanocide that diffuses rapidly across the cell membrane and therefore does not rely on transporters for its trypanocidal action (6, 29). Treatment of _tbat1_-null mutants with 0.5 μM phenylarsine oxide caused rapid lysis (Fig. 3c, trace b). Neither adenosine (10 mM), hypoxanthine (4 mM), pentamidine, stilbamidine, nor propamidine (the last three at 100 μM) prevented lysis; only a very high concentration of pentamidine (1 mM) did have some effect (Fig. 3c). The most likely explanation for the counteracting effects of diamidines on the slow cymelarsan-induced lysis of _tbat1_-null trypanosomes is therefore the inhibition of a transport mechanism. Furthermore, this transporter is likely to be HAPT1, as half-maximal diamidine concentrations for inhibition of cymelarsan-induced lysis correlate well with Ki values of the respective diamidines for HAPT1 (7). The observation that melarsen oxide exhibits low affinity for HAPT1 (IC50, ∼250 μM [data not shown]) is consistent with this hypothesis.
DISCUSSION
Since Carter and Fairlamb showed that adenosine and adenine protect T. b. brucei from melarsen-induced lysis in vitro (6), several studies have pointed to a role for the P2 transport activity in the resistance of African trypanosomes to both melaminophenyl arsenicals and diamidines (2, 5, 27, 29, 30). A T. b. brucei gene, TbAT1, was found to encode an adenine-sensitive adenosine transporter when expressed in yeast (19), but formal proof that TbAT1 encodes the P2 activity in trypanosomes has awaited the gene deletion study presented here. The total absence of P2-type transport in _tbat1_-null bloodstream-form trypanosomes proves that TbAT1 is the P2 transporter. We found that loss of TbAT1 did indeed reduce the sensitivity of trypanosomes to melaminophenyl arsenicals, but only by factors of 2 to 3 (Table 1). However, even such a limited loss of sensitivity could be significant in a clinical setting, since the drug levels in the cerebrospinal fluid of melarsoprol-treated patients are at the threshold of those needed to kill trypanosomes (reviewed in reference (8)). A small reduction in drug sensitivity could therefore lead to survival of some parasites in the central nervous system or other extravascular sites and cause clinical relapse in an increased percentage of patients. This model is in agreement with the lack of high-level arsenical resistance in clinical isolates from melarsoprol-refractory patients (4, 21).
The P2 transporter, when analyzed in trypanosomes, displays high affinity for several trypanocidal diamidines, including pentamidine, as judged by their ability to inhibit adenosine uptake (2, 5, 7, 10). [3H]pentamidine transport, however, was only partially blocked by adenosine in T. brucei (5). Here we show that adenosine-sensitive pentamidine transport (ASPT1) is completely lost in _tbat1_-null trypanosomes. Nevertheless, _tbat1_-null trypanosomes are only about twofold more resistant to pentamidine than wild-type trypanosomes, while resistance to other diamidines such as diminazene is much more pronounced. This can be explained by the presence of two additional, adenosine-insensitive transporters specific for pentamidine: the high-affinity pentamidine transporter HAPT1 and the low-affinity pentamidine transporter LAPT1 (7). We show here that both activities are retained in the _tbat1_-null mutants. The veterinary trypanocide diminazene, though structurally related to pentamidine, is not an effective substrate for HAPT1 or LAPT1 (7, 11) and may not enter trypanosomes, to an appreciable extent, by routes other than TbAT1. This may explain why trypanosomes have not developed resistance to pentamidine in the field while resistance to diminazene is much more common (17, 23), although both drugs have been used extensively over several decades (3, 32).
Some laboratory strains of T. brucei, selected for arsenical resistance by prolonged exposure to subcurative doses, have been shown to be deficient in P2 activity, but, unlike the _tbat1_-null trypanosomes, exhibit high levels of resistance to melaminophenyl arsenicals (14, 29). This indicates that besides loss of TbAT1, further events are required for high-level arsenical resistance. At least part of this is likely to occur at the level of plasma membrane transport, as the in vitro lysis assays showed that the _tbat1_-null trypanosomes remained fully sensitive to phenylarsine oxide, which is lipophilic and diffuses across membranes without the need for transporters (6, 29). This arsenical contains the same trivalent toxophore as melarsen oxide, differing only in the presence of an extra melamine haptophore in the latter arsenical at the para position of the phenyl ring. It is therefore probable that the cause for high-level melaminophenyl arsenical resistance, like that for low-level resistance, is associated with the uptake of the drug over the plasma membrane. The in vitro lysis assays with _tbat1_-null trypanosomes also revealed a slow, adenosine-insensitive uptake of melaminophenyl arsenicals. Inhibitor analysis indicated that this transporter could be HAPT1. These data need to be interpreted with some caution, since the possibility that these diamidines inhibit an additional transport mechanism with affinities similar to those they exhibit for HAPT1 cannot be discounted. In this scenario, pentamidine might well be an inhibitor rather than a permeant for any such putative transport protein for melaminophenyl arsenicals, as there is currently no evidence of pentamidine transporters in addition to HAPT1, LAPT1, and TbAT1 (7, 11). However, if HAPT1 and TbAT1 do both mediate cellular uptake of melaminophenyl arsenicals, loss of both may be necessary for high-level drug resistance.
In conclusion, genetic knockout of TbAT1 in T. b. brucei bloodstream forms revealed a major role for TbAT1 in the uptake of clinically important trypanocides. Loss of TbAT1 activity appears to cause loss of sensitivity to melaminophenyl arsenicals and diamidines and is an obligatory component of high-level resistance. _Tbat1_-null trypanosomes further allowed the characterization and possible identification of additional drug transport activities. The exploitation of alternative pathways for drug uptake will be crucial for the development of new-generation trypanocides that do not share cross-resistance with P2 substrates.
Acknowledgments
This work was supported by grant 3100-058927.99 from the Swiss National Science Foundation, grant C00.0042 from COST program B16, the BBSRC (17/C13486), grant 970391 from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, and the Wellcome Trust. E.M. was the recipient of WHO Training Grant ID 990028, and P.M. is the holder of a Human Frontiers fellowship.
REFERENCES
- 1.Baltz, T., D. Baltz, C. Giroud, and J. Crockett. 1985. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 4**:**1273-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett, M. P., Z. Q. Zhang, H. Denise, C. Giroud, and T. Baltz. 1995. A diamidine-resistant Trypanosoma equiperdum clone contains a P2 purine transporter with reduced substrate affinity. Mol. Biochem. Parasitol. 73**:**223-229. [DOI] [PubMed] [Google Scholar]
- 3.Bray, P. G., M. P. Barett, S. A. Ward, and H. P. de Koning. 2003. Pentamidine uptake and resistance in pathogenic protozoa: past, present and future. Trends Parasitol. 19**:**232-239. [DOI] [PubMed] [Google Scholar]
- 4.Brun, R., R. Schumacher, C. Schmid, C. Kunz, and C. Burri. 2001. The phenomenon of treatment failures in human African trypanosomiasis. Trop. Med. Int. Health 6**:**906-914. [DOI] [PubMed] [Google Scholar]
- 5.Carter, N. S., B. J. Berger, and A. H. Fairlamb. 1995. Uptake of diamidine drugs by the P2 nucleoside transporter in melarsen-sensitive and -resistant Trypanosoma brucei brucei. J. Biol. Chem. 270**:**28153-28157. [DOI] [PubMed] [Google Scholar]
- 6.Carter, N. S., and A. H. Fairlamb. 1993. Arsenical-resistant trypanosomes lack an unusual adenosine transporter. Nature 361**:**173-175. [DOI] [PubMed] [Google Scholar]
- 7.De Koning, H. P. 2001. Uptake of pentamidine in Trypanosoma brucei is mediated by three distinct transporters: implications for cross-resistance with arsenicals. Mol. Pharmacol. 59**:**586-592. [DOI] [PubMed] [Google Scholar]
- 8.De Koning, H. P. 2001. Transporters in African trypanosomes: role in drug action and resistance. Int. J. Parasitol. 31**:**512-522. [DOI] [PubMed] [Google Scholar]
- 9.De Koning, H. P., and S. M. Jarvis. 1997. Purine nucleobase transport in bloodstream forms of Trypanosoma brucei brucei is mediated by two novel transporters. Mol. Biochem. Parasitol. 89**:**245-258. [DOI] [PubMed] [Google Scholar]
- 10.De Koning, H. P., and S. M. Jarvis. 1999. Adenosine transporters in bloodstream forms of Trypanosoma brucei brucei: substrate recognition motifs and affinity for trypanocidal drugs. Mol. Pharmacol. 56**:**1162-1170. [DOI] [PubMed] [Google Scholar]
- 11.De Koning, H. P., and S. M. Jarvis. 2001. Uptake of pentamidine in Trypanosoma brucei brucei is mediated by the P2 adenosine transporter and at least one novel, unrelated transporter. Acta Trop. 80**:**245-250. [DOI] [PubMed] [Google Scholar]
- 12.De Koning, H. P., C. J. Watson, and S. M. Jarvis. 1998. Characterization of a nucleoside/proton symporter in procyclic Trypanosoma brucei brucei. J. Biol. Chem. 273**:**9486-9494. [DOI] [PubMed] [Google Scholar]
- 13.De Koning, H. P., C. J. Watson, L. Sutcliffe, and S. M. Jarvis. 2000. Differential regulation of nucleoside and nucleobase transporters in Crithidia fasciculata and Trypanosoma brucei brucei. Mol. Biochem. Parasitol. 106**:**93-107. [DOI] [PubMed] [Google Scholar]
- 14.Fairlamb, A. H., N. S. Carter, M. Cunningham, and K. Smith. 1992. Characterisation of melarsen-resistant Trypanosoma brucei brucei with respect to cross-resistance to other drugs and trypanothione metabolism. Mol. Biochem. Parasitol. 53**:**213-222. [DOI] [PubMed] [Google Scholar]
- 15.Frommel, T. O., and A. E. Balber. 1987. Flow cytofluorimetric analysis of drug accumulation by multidrug-resistant Trypanosoma brucei brucei and T. b. rhodesiense. Mol. Biochem. Parasitol. 26**:**183-192. [DOI] [PubMed] [Google Scholar]
- 16.Fulton, J. D., and P. T. Grant. 1955. The preparation of a strain of Trypanosoma rhodesiense resistant to stilbamidine and some observations on its nature. Exp. Parasitol. 4**:**377-388. [DOI] [PubMed] [Google Scholar]
- 17.Geerts, S., P. H. Holmes, O. Diall, and M. C. Eisler. 2001. African bovine trypanosomiasis: the problem of drug resistance. Trends Parasitol. 17**:**25-28. [DOI] [PubMed] [Google Scholar]
- 18.Lanham, S. M. 1968. Separation of trypanosomes from the blood of infected rats and mice by anion-exchangers. Nature 218**:**1273-1274. [DOI] [PubMed] [Google Scholar]
- 19.Mäser, P., C. Sütterlin, A. Kralli, and R. Kaminsky. 1999. A nucleoside transporter from Trypanosoma brucei involved in drug resistance. Science 285**:**242-244. [DOI] [PubMed] [Google Scholar]
- 20.Mäser, P., D. Vogel, C. Schmid, B. Räz, and R. Kaminsky. 2001. Identification and characterization of trypanocides by functional expression of an adenosine transporter from Trypanosoma brucei in yeast. J. Mol. Med. 97**:**121-127. [DOI] [PubMed] [Google Scholar]
- 21.Matovu, E., J. C. Enyaru, D. Legros, C. Schmid, T. Seebeck, and R. Kaminsky. 2001. Melarsoprol refractory T. b. gambiense from Omugo, north-western Uganda. Trop. Med. Int. Health 6**:**407-411. [DOI] [PubMed] [Google Scholar]
- 22.Matovu, E., F. Geiser, V. Schneider, P. Mäser, J. C. K. Enyaru, R. Kaminsky, S. Gallati, and T. Seebeck. 2001. Genetic variants of the TbAT1 adenosine transporter from African trypanosomes in relapse infections following melarsoprol therapy. Mol. Biochem. Parasitol. 117**:**73-81. [DOI] [PubMed] [Google Scholar]
- 23.Matovu, E., T. Seebeck, J. C. Enyaru, and R. Kaminsky. 2001. Drug resistance in Trypanosoma brucei spp., the causative agents of sleeping sickness in man and nagana in cattle. Microbes Infect. 3**:**763-770. [DOI] [PubMed] [Google Scholar]
- 24.Pospichal, H., R. Brun, R. Kaminsky, and L. Jenni. 1994. Induction of resistance to melarsenoxide cysteamine (Mel Cy) in Trypanosoma brucei brucei. Acta Trop. 58**:**187-197. [DOI] [PubMed] [Google Scholar]
- 25.Räz, B., M. Iten, Y. Grether-Bühler, R. Kaminsky, and R. Brun. 1997. The Alamar Blue assay to determine drug sensitivity of African trypanosomes in vitro. Acta Trop. 68**:**139-147. [DOI] [PubMed] [Google Scholar]
- 26.Rollo, I. M., and J. Williamson. 1951. Acquired resistance to ‘melarsen’, tryparsamide and amidines in pathogenic trypanosomes after treatment with ′melarsen' alone. Nature 167**:**147-148. [DOI] [PubMed] [Google Scholar]
- 27.Ross, C. A., and A. M. Barns. 1996. Alteration to one of three adenosine transporters is associated with resistance to Cymelarsan in Trypanosoma evansi. Parasitol. Res. 82**:**183-188. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez, M., R. Tryon, J. Green, I. Boor, and S. Landfear. 2002. Six related nucleoside/nucleobase transporters from Trypanosoma brucei exhibit distinct biochemical functions. J. Biol. Chem. 277**:**21499-21504. [DOI] [PubMed] [Google Scholar]
- 29.Scott, A. G., A. Tait, and C. M. R. Turner. 1997. Trypanosoma brucei: lack of cross-resistance to melarsoprol in vitro by cymelarsan-resistant parasites. Exp. Parasitol. 86**:**181-190. [DOI] [PubMed] [Google Scholar]
- 30.Suswam, E. A., D. W. Taylor, C. A. Ross, and R. J. Martin. 2001. Changes in properties of adenosine transporters in Trypanosoma evansi and modes of selection of resistance to the melaminophenyl arsenical drug, Mel Cy. Vet. Parasitol. 102**:**193-208. [DOI] [PubMed] [Google Scholar]
- 31.Wallace, L. J. M., D. Candlish, and H. P. de Koning. 2002. Different substrate recognition motifs of human and trypanosome nucleobase transporters: selective uptake of purine antimetabolites. J. Biol. Chem. 277**:**26149-26156. [DOI] [PubMed] [Google Scholar]
- 32.Williamson, J. 1970. Review of chemotherapeutic and chemoprophylactic agents, p. 125-221. In H. W. Mulligan (ed.), The African trypanosomiases. George Allen and Unwin Ltd., London, United Kingdom.
- 33.Yarlett, N., B. Goldberg, H. C. Nathan, J. Garofalo, and C. J. Bacchi. 1991. Differential sensitivity of Trypanosoma brucei rhodesiense isolates to in vitro lysis by arsenicals. Exp. Parasitol. 72**:**205-215. [DOI] [PubMed] [Google Scholar]