An Immunologically Privileged Retinal Antigen Elicits Tolerance: Major Role for Central Selection Mechanisms (original) (raw)
Abstract
Immunologically privileged retinal antigens can serve as targets of experimental autoimmune uveitis (EAU), a model for human uveitis. The tolerance status of susceptible strains, whose target antigen is not expressed in the thymus at detectable levels, is unclear. Here, we address this issue directly by analyzing the consequences of genetic deficiency versus sufficiency of a uveitogenic retinal antigen, interphotoreceptor retinoid-binding protein (IRBP). IRBP-knockout (KO) and wild-type (WT) mice on a highly EAU-susceptible background were challenged with IRBP. The KO mice had greatly elevated responses to IRBP, an altered recognition of IRBP epitopes, and their primed T cells induced exacerbated disease in WT recipients. Ultrasensitive immunohistochemical staining visualized sparse IRBP-positive cells, undetectable by conventional assays, in thymi of WT (but not of KO) mice. IRBP message was PCR amplified from these cells after microdissection. Thymus transplantation between KO and WT hosts demonstrated that this level of expression is functionally relevant and sets the threshold of immune (and autoimmune) reactivity. Namely, KO recipients of WT thymi generated reduced IRBP-specific responses, and WT recipients of KO thymi developed enhanced responses and a highly exacerbated disease. Repertoire culling and thymus-dependent CD25+ T cells were implicated in this effect. Thus, uveitis-susceptible individuals display a detectable and functionally significant tolerance to their target antigen, in which central mechanisms play a prominent role.
Keywords: gene knockout mice, interphotoreceptor retinoid-binding protein, thymic selection, uveitis, autoimmunity
Introduction
Experimental autoimmune uveitis (EAU) is an autoimmune disease that targets the neural retina. It can be induced in susceptible animals by immunization with retinal antigens and serves as a model for human autoimmune uveitis (1). Uveitogenic antigens are typically derived from the retinal photoreceptor cells, function in the visual cycle, and are highly evolutionarily conserved. Examples are arrestin (retinal soluble antigen [S-Ag]), interphotoreceptor retinoid-binding protein (IRBP), rhodopsin and its illuminated form opsin, recoverin, and phosducin (2). Susceptibility to uveitis induced with these antigens varies among species and among strains (3). Some strains of mice are susceptible to IRBP, but all are relatively resistant to S-Ag (3, 4).
The question of tolerance to immunologically privileged retinal antigens remains elusive. Evidence for peripheral tolerance is sparse and inconclusive. As part of the immune privilege of the eye, retinal antigens are largely sequestered from the immune system by an efficient blood-retinal barrier and by lack of direct lymphatic drainage of the interior of the globe. Retina-specific antigens are not found outside the eye with the exception of the pineal gland (“third eye”), whose size is very small and whose contribution as a peripheral source of antigen is unclear. The phenomenon known as anterior chamber–associated immune deviation (ACAID), where antigens introduced into the eye elicit a deviant response involving regulatory cells and suppression of delayed hypersensitivity, has been well studied in relation to injected foreign antigens (5, 6). Although induction of ACAID to IRBP by its injection into the anterior chamber can protect from EAU (7), it is not at all clear whether ACAID exists naturally to native retinal antigens present in the intact eye. Indeed, “unsequestering” IRBP or S-Ag by expressing them in the periphery induces profound resistance to EAU (8–10), supporting the notion that peripheral tolerance to antigens residing in the eye is minimal.
On the other hand, recent studies demonstrated expression in the thymus of some retinal antigens and suggested an association with EAU resistance. Egwuagu et al. (11) showed that IRBP is expressed by IRBP-resistant, but not by susceptible, mouse strains, whereas S-Ag (which does not elicit EAU in mice) was expressed by all examined strains. Conversely, AIRE-deficient mice were shown to lack thymic expression of S-Ag (12). These mice developed antiretinal antibodies and retinal inflammation as they aged. Although the antigen specificity of that response has not been examined, in the aggregate, these studies support the notion that some retinal antigens can be expressed in the thymus at levels sufficient to elicit central tolerance. However, a confounding factor is the multiplicity of retinal antigens, each of which can potentially serve as a target for uveitis. What follows is that the antigen(s) with the poorest thymic expression will determine the functional outcome in terms of antiretinal autoimmunity. In uveitis-susceptible individuals, the extent of thymic expression and of central tolerance to their particular target antigen is unclear.
In the present study, we address this issue directly by analyzing the consequences of genetic IRBP deficiency versus sufficiency on immunological responses to this antigen and its ability to induce uveitis. Using highly sensitive methods and thymic transplantation, we demonstrate that EAU-susceptible WT mice express IRBP in the thymus and display a detectable and functionally significant level of tolerance to this antigen in which central selection mechanisms play a major role. These data provide direct evidence that, in uveitis-susceptible individuals, central tolerance sets the threshold of autoimmune reactivity to their target antigen and provides new insights into self-tolerance to immunologically privileged retinal antigens.
Materials and Methods
Mice.
The highly EAU-susceptible B10.RIII mice (H-2r) were purchased from Jackson Labs. IRBP-knockout (KO) mice, lacking the promoter region and 81% of the IRBP coding sequence, were generated as described (13) and were backcrossed onto the B10.RIII background for 10 generations. Mice were used between the ages of 8 and 12 wk. No difference in disease susceptibility between males and females was noted. Treatment of animals was in compliance with Institutional Guidelines and all animal study protocols were approved by an Institutional Review Board.
Antigens, Antibodies, and Reagents.
Native bovine IRBP and recombinant repeat 1 of human IRBP (rIRBP) were prepared as described (14–16). Overlapping 20-aa peptides representing rIRBP and murine peptide 161–180 were synthesized as reported (17, 18). Polyclonal rabbit and mAbs to IRBP (clone H3B5) were described (19, 20). Mycobacterium tuberculosis (strain H37RA) was from Difco. Bordetella pertussis toxin and complete Freund's adjuvant (CFA) were from Sigma-Aldrich. RPMI 1640 medium was from BioWhittaker and was supplemented as described (21). The monoclonal anti-CD25 antibodies 7D4 and PC61 (FITC labeled) and anti-CD4 (PE labeled) for flow cytometry were from BD Biosciences. PC61 and 7D4 for in vivo use were (respectively) purchased from Serotec or produced in house using an Integra CL 1000 culture system (Integra Biosciences) following manufacturer's protocol.
Immunocytochemistry.
Frozen sections of eyes were immunostained for IRBP using the Vectastain Elite ABC (peroxidase) kit from Vector Laboratories and polyclonal anti–monkey IRBP (1:5,000). Frozen sections of thymus from 8–10-wk-old mice were stained using H3B5 mAbs (1:200) and the mouse-on-mouse Innogenex Iso-IHC kit with the following modifications: slides were air dried (30 min), fixed in acetone (7 min), saturated with H2O2 (0.3% in PBS), and washed with PBS instead of water. Incubation with the substrate buffer was for 20 s. Dehydration was through graded ethanol series and then dipping through three changes of xylene. Tissue sections were incubated with the antibody for 60 min before visualization of the antigen per manufacturers' instructions.
RT-PCR Analysis of IRBP Expression.
Areas of 5–10 cells containing IRBP-positive or adjacent negative cells were microdissected from thymus sections immunostained as described under microscopic visualization. Total RNA was isolated from 10–20 microdissected samples using the PicoPure RNA isolation kit (Arcturus). First strand cDNA synthesis was done with 1 μg of total RNA using the Advantage RT for PCR kit from CLONTECH Laboratories. For PCR, 2–5 μl of the cDNA synthesis reaction was used as template. IRBP cDNA transcripts were amplified using the forward and reverse primers as described (11). Control 18S ribosomal RNA transcripts were amplified using the primer kit from Ambion.
Thymectomy, Thymus Grafting, Immunoablation, and Reconstitution.
Mice were thymectomized at 4–6 wk of age by aspiration of both thymic lobes through a small incision in the skin just above the sternum. Lack of thymic remnant was confirmed by autopsy. Thymus grafting was performed 2–4 wk later by placing two to three lobes of neonatal (less than 72 h old) thymus under the left kidney capsule. Mice were then gamma irradiated (950 rads from a cesium source) and injected i.v. with syngeneic BM cells (15–20 × 106 per animal). Recipient mice were given oxytetracycline (0.4 mg/ml) in drinking water for 4 wk after irradiation and BM infusion and were allowed to reconstitute for 8–16 wk. Alternatively, recipients were thymectomized at 3 wk of age, implanted with neonatal thymus without immunoablation, and used 2–3 mo later.
Depletion of CD25+ Cells.
Depletion of CD25+ cells was performed essentially as described (22). Briefly, mice were given two i.p. injections, 3 d apart, of 0.5 mg of the anti-CD25 mAb 7D4 (IgM isotype). This treatment reduced CD4+CD25+ T cells in the spleen from ∼10% to less than 0.1% as assayed by flow cytometry on gated CD4+ cells with anti-CD25 mAb PC61, which is specific to a different epitope of the IL-2 receptor. In an alternative protocol, mice were depleted of CD25+ cells by three injections of 1 mg PC61 antibody every other day (23), and efficiency of depletion was confirmed by flow cytometry with 7D4.
Immunizations, EAU Induction, and EAU Scoring.
Mice were immunized subcutaneously in the thighs and base of the tail with 25–100 μg of IRBP or 25–50 μg of peptide 161–180 (24). Native bovine IRBP and rIRBP were used interchangeably as specified. Bovine and human IRBPs are highly homologous, immunologically crossreactive with each other, and induce similar disease when injected into mice. rIRBP was preferred in experiments where responses were to be recalled subsequently by overlapping peptides, which are derived from the human sequence. The antigen was emulsified in 0.2 ml emulsion in CFA supplemented with M. tuberculosis strain H37RA to 2.5 mg/ml. Mice immunized with peptide also received 0.5 μg Bordetella pertussis toxin by i.p. injection. In some experiments, EAU was induced by adoptive transfer of cells collected from IRBP-primed donors 12 d after immunization and stimulated in culture with IRBP or with its component peptides essentially as described (24). Clinical disease was evaluated by fundoscopy. Eyes were harvested for histopathology 21 d after immunization or 14 d after adoptive transfer. Disease was scored in a masked fashion by an ophthalmic pathologist (C.-C. Chan), on a scale of 0 (no disease) to 4 (maximum disease) in half-point increments, according to a semiquantitative system described previously (24, 25).
Determination of Immunological Responses.
Delayed type hypersensitivity (DTH) to IRBP was evaluated by the ear swelling assay (18). Serum antibodies to IRBP were measured by ELISA (26) 14 d after immunization. For antigen-specific lymphocyte proliferation and cytokine production in primary cultures, the spleen and draining LNs (inguinal and iliac) of individual mice were collected 2 wk after immunization and were cultured with antigen as described (21). Short term T cell lines from IRBP-primed mice were derived by three cycles of stimulation with 2 μg/ml peptide and IL-2–driven expansion, essentially as described (18), before being tested in a proliferation assay. Proliferation was determined by 3H-thymidine uptake. Cytokines were quantitated in 48-h antigen-stimulated supernatants using the Pierce Chemical Co. multiplex SearchLight™ Arrays technology (27) (http://www.SearchLightOnline.com). In some of the experiments, IFN-γ production was determined by conventional ELISA using antibody pairs from Endogen (21, 28). Antigen-specific cell frequency was assayed by ELISPOT for IL-2 and IFN-γ–producing cells essentially as described (29). Spots were counted using the ImmunoSpot series 1.0 analyzer (CTL).
Reproducibility and Statistical Analyses.
Statistical analysis of EAU scores was done using the Snedecor and Cochran z test for linear trend in proportions (nonparametric, frequency based) (30). Each mouse (average of both eyes) was treated as a statistical event. DTH, lymphocyte proliferation, and cytokine data were analyzed by independent t test. Probability values ≤0.05 were considered significant.
Results
IRBP KO Mice Lack IRBP Expression in the Retina and Are Resistant to IRBP-induced Uveitis
IRBP KO mice lack the promoter region and exon 1 of IRBP but retain 19% of the IRBP coding sequence and transcribe mRNA from this sequence (13). We examined whether any IRBP-crossreactive material is detectable in the retina of the KO mice using polyclonal antibodies to IRBP. Although strong immunoreactivity was present in the WT, no staining was seen in KO retina (Fig. 1) . To address the possibility that a putative IRBP fragment, undetectable by antibodies, might be present and serve as a target for EAU, IRBP KO mice and WT controls were immunized with an aggressive uveitogenic regimen of IRBP—up to 100 μg of IRBP or 50 μg of peptide 161–180, the major T cell epitope associated with EAU in B10.RIII (H-2r) mice (18). This regimen is considerably more intense than normally required to induce EAU in the highly susceptible B10.RIII strain. WT B10.RIII mice developed uveitis within 8–9 d as reported previously (28). In contrast, IRBP KO mice were completely healthy when the experiment was terminated on day 21 (Fig. 1). These data confirm that neither IRBP nor any other crossreactive protein that could serve as a target for EAU is present in IRBP KO mice.
Figure 1.
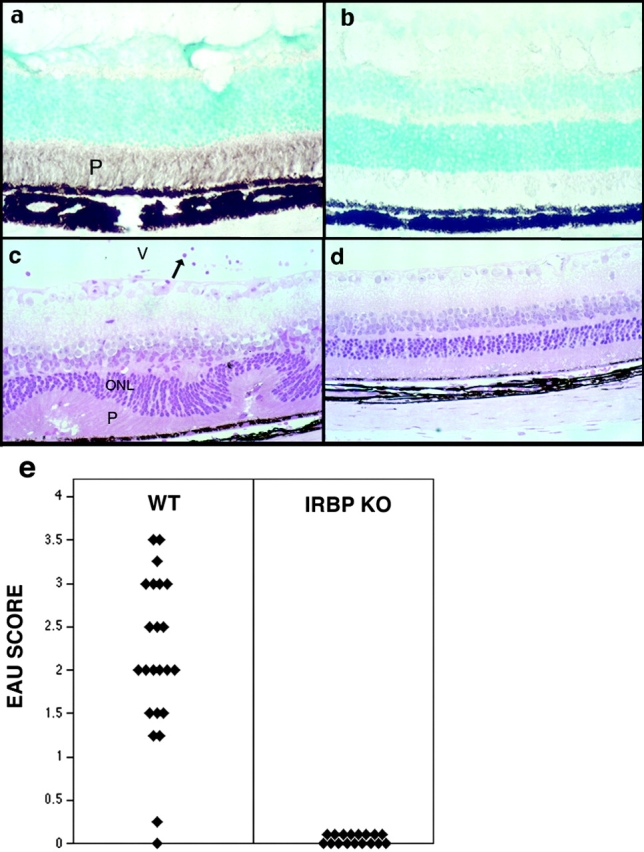
IRBP KO mice fail to express residual IRBP in the eye and are resistant to EAU. (a and b) Retinal sections of WT and KO mice, respectively, stained with polyclonal anti-IRBP antibody. Only the WT retina stains positive. (c and d) Histology of WT and KO eyes, respectively, after immunization of mice with IRBP. Only the WT (c) develop disease. Note inflammatory cells in the retina and vitreous (V), and damage of the photoreceptor layer (P) and of the outer nuclear layer (ONL). Hematoxylin and eosin stained. Original magnification, ×400. (e) EAU scores of IRBP-immunized WT and KO mice. Each point is 1 mouse (average of both eyes). Data are compiled from three separate experiments.
IRBP KO Mice Have Strongly Enhanced Immunological Responses and an Expanded T Cell Repertoire to IRBP
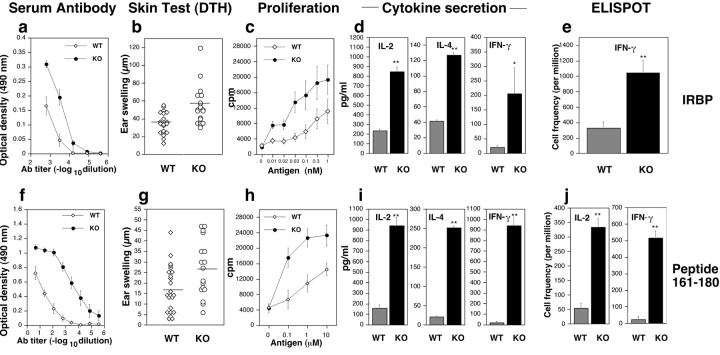
IRBP KO and WT mice were immunized with IRBP or peptide 161–180 and were examined for antigen-specific humoral and cellular responses to the immunizing antigen. Serum antibodies, DTH, proliferation, and production of cytokines to IRBP and the 161–180 epitope were all strongly enhanced in the KO mice (Fig. 2) . IFN-γ and IL-4, representing the Th1 and Th2 arms of the immune response, respectively, were elevated to a similar extent. Production of IL-5, IL-6, IL-10, IL-13, and TNF-α was also similarly enhanced (not depicted).
Figure 2.
Humoral and cellular responses are increased in IRBP KO mice. Mice were immunized with IRBP (a–e) or with peptide 161–180 (f–j). Spleens were used for proliferation and cytokine production, whereas LNs were used for ELISPOT. All data represent individual mice or averages of individual mice ± SE. (a and f) Antibody titers (ELISA, OD 490 nm) are averaged of four to five individual mice ± SE corrected for background in normal mouse sera. (b and g) DTH is the difference in microns between the antigen- and the PBS-injected ears. Responses are significantly higher in KO (P < 0.025). Horizontal bars represent the mean of each group. (c and h) Proliferation to the immunizing antigen was significantly elevated in the KO compared with WT (P < 0.001). Data are mean counts per minute (CPM) ± SE of triplicate cultures. Results are averaged from three separate experiments including nine WT and seven KO mice. (d and i) Cytokines produced in response to the immunizing antigen were tested using the multiplex Pierce SearchLight™ array technology. IL-2, IL-4, and IFN-γ are elevated in KO over WT (**P < 0.001; *P < 0.02). Data are composites of three separate experiments. (e and j) Frequency of IFN-γ and IL-2–producing cells by ELISPOT. Shown are spots per million cells, averaged from triplicate cultures of individual mice (at least three mice per group; P < 0.001).
Frequency of antigen-specific cells was evaluated by ELISPOT (Fig. 2, e and j). IRBP KO mice immunized and recalled with IRBP had a threefold higher frequency of IFN-γ–producing cells than WT (1,048 versus 330). Responses to IRBP are expected to include a background directed against nonconserved epitopes foreign to the mouse. In keeping with this, mice immunized with peptide 161–180 had a 6–20-fold higher frequency of antigen-specific cells (516 versus 25 per million for IFN-γ and 334 versus 55 per million for IL-2).
In an attempt to discern whether the TCR avidity of the 161–180-specific T cell repertoire in the KO was also increased, we quantitated the antigen sensitivity of 161–180-specific T cells in WT and KO mice. Short term T cell lines specific to peptide 161–180 were derived (to circumvent the different frequency of T cells having this specificity in the two genotypes), and their proliferative response to graded doses of the antigen presented on irradiated syngeneic splenocytes was tested. The dose–response of the KO cells was down-shifted by an order of magnitude compared with WT, such that they required a 10× lower dose of antigen to mount an equivalent proliferative response (not depicted). Although this is not a direct measurement of TCR avidity, it nevertheless strongly suggests that the average TCR avidity of the KO-derived T cell population was higher.
The ability of KO cells to induce EAU was evaluated after adoptive transfer into IRBP-sufficient hosts. WT or IRBP KO donors were immunized with IRBP. Pooled spleen and LN cells were restimulated in culture with IRBP, and a fixed number of cells were infused into naive WT recipients. Recipients of cells from WT mice developed a moderate form of EAU, with a later onset (day 6–7) and lower scores (Fig. 3) . In contrast, recipients of cells from KO mice developed an exacerbated form of EAU characterized by early onset (day 4), elevated scores, and high incidence. This enhanced pathogenicity could be due to a greater frequency of effector cells in the IRBP KO as indicated by ELISPOT data, but it is also possible that on a per cell basis KO effector T cells could be more potent than those of WT, owing to a higher apparent TCR avidity.
Figure 3.
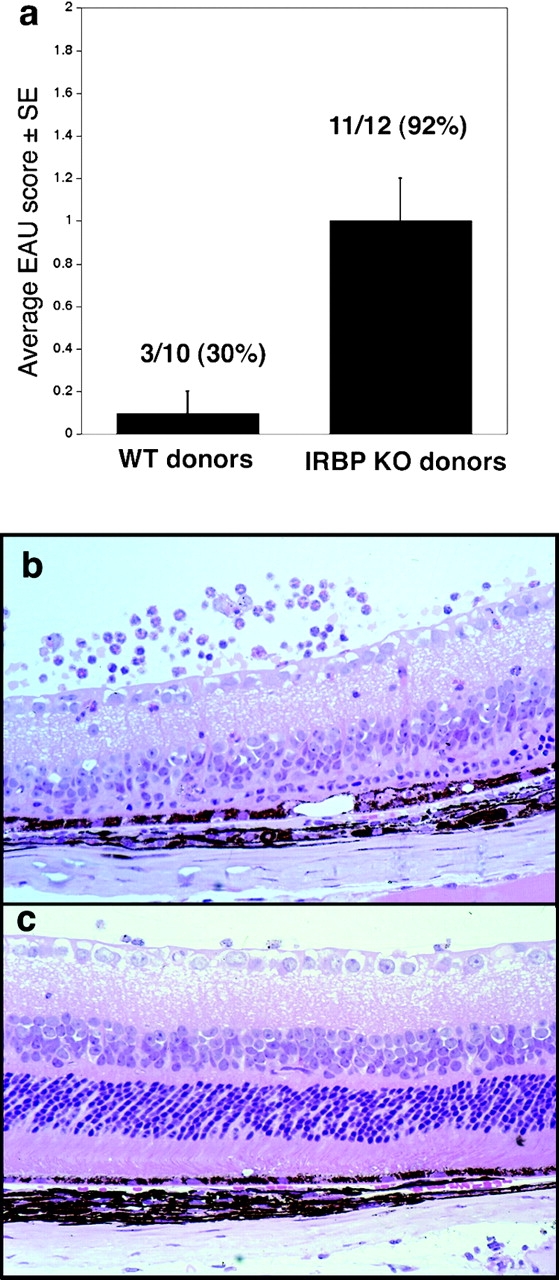
Primed lymphoid cells of IRBP KO mice induce exacerbated EAU in IRBP-sufficient recipients. Lymphocytes from IRBP-immunized KO or WT mice were cultured with IRBP and were infused into naive WT recipients (30 × 106 per mouse). Eyes collected 14 d after adoptive transfer were scored by histopathology. (a) EAU scores in recipients of cells from WT or KO donors. (b and c) Histopathology of eyes revealed severe retinal damage in recipients of KO cells (b) and only mild damage in recipients of WT cells (c). Hematoxylin and eosin stained. Original magnification, ×400.
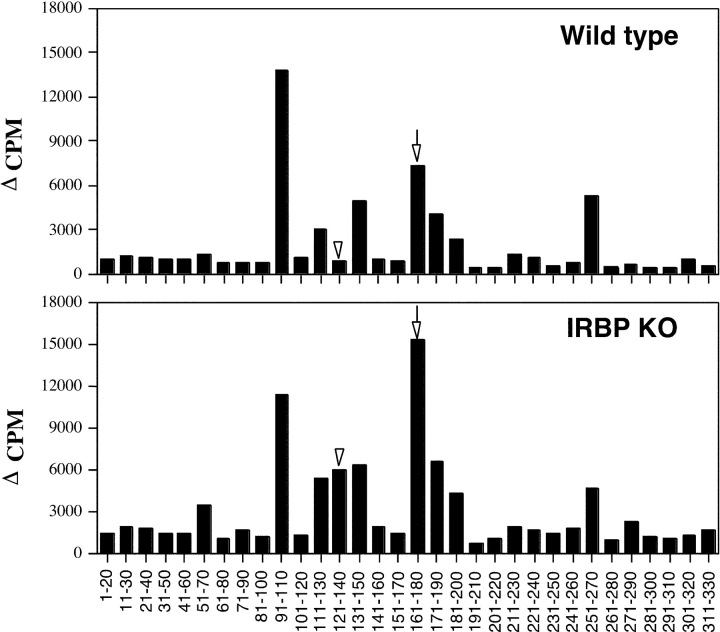
To assess whether KO and WT mice have differences in recognition of IRBP epitopes, mice were immunized with rIRBP and primed spleen cells were restimulated in culture with overlapping peptides spanning the entire sequence of rIRBP. Responses in KO mice appeared slightly higher across the entire panel with significant changes apparent in responses to peptide 161–180, the major pathogenic epitope recognized by WT mice, and peptide 121–140, which elicited a response in KO but not in WT splenocytes (Fig. 4) . To evaluate whether 121–140 represented a pathogenic specificity that is eliminated in the WT, we infused peptide 121–140-restimulated cells taken from IRBP-primed KO donors into WT recipients. All WT recipients (n = 6 in two experiments) developed bilateral EAU, with 40 × 106 cells eliciting mild to moderate disease (scores up to 1) and 80 × 106 cells eliciting severe disease (scores up to 3). Enhanced responsiveness to IRBP, enhanced uveitogenicity, and an altered IRBP epitope recognition were also observed in T cells of IRBP KO mice on the C57BL/6 background (31).
Figure 4.
T cell epitope recognition by WT and IRBP KO mice. Spleen cells of mice immunized with rIRBP (25 μg in CFA) were harvested on day 14 and were stimulated in culture with overlapping 20-aa peptides of rIRBP (10 μM). Data are presented as Δ CPM ± SE of triplicate cultures for each peptide tested (averaged results are from five individual mice tested in each experimental group). Highly significant changes (P < 0.001) were seen in responses to peptide 161–180 (arrows) and peptide 121–140 (arrowheads).
EAU-susceptible WT Mice Express IRBP in the Thymus
A previous study was unable to detect IRBP in thymic extracts of EAU-susceptible B10.RIII mice using PCR and Western blotting, although under the same conditions clear signals were detected in resistant strains (11). We reexamined this issue here using a sensitive and highly optimized immunohistochemical staining. Frozen sections from thymi of naive WT or KO mice were stained using the monoclonal anti-IRBP antibody H3B5 (20). Sparse positively staining cells were detected in WT thymi but were absent in KO thymi (Fig. 5 , a and b). The IRBP-positive cells were rare and appeared unevenly distributed: only 17 out of the 50 WT sections examined contained positively staining cells. They were usually seen in the thymic medulla or at the cortico–medullary junction and often appeared in clusters of several cells. Discrete dark striations in the cytoplasm could often be discerned (Fig. 5, a, insets). Positively staining cells and neighboring negative areas were carefully microdissected, and pools of 10–20 picks were subjected to RT-PCR using IRBP-specific primers. IRBP message was detectable only in the immunopositive areas (Fig. 5 c). No IRBP signal was detectable in microdissected cells from IRBP KO thymi (not depicted).
Figure 5.
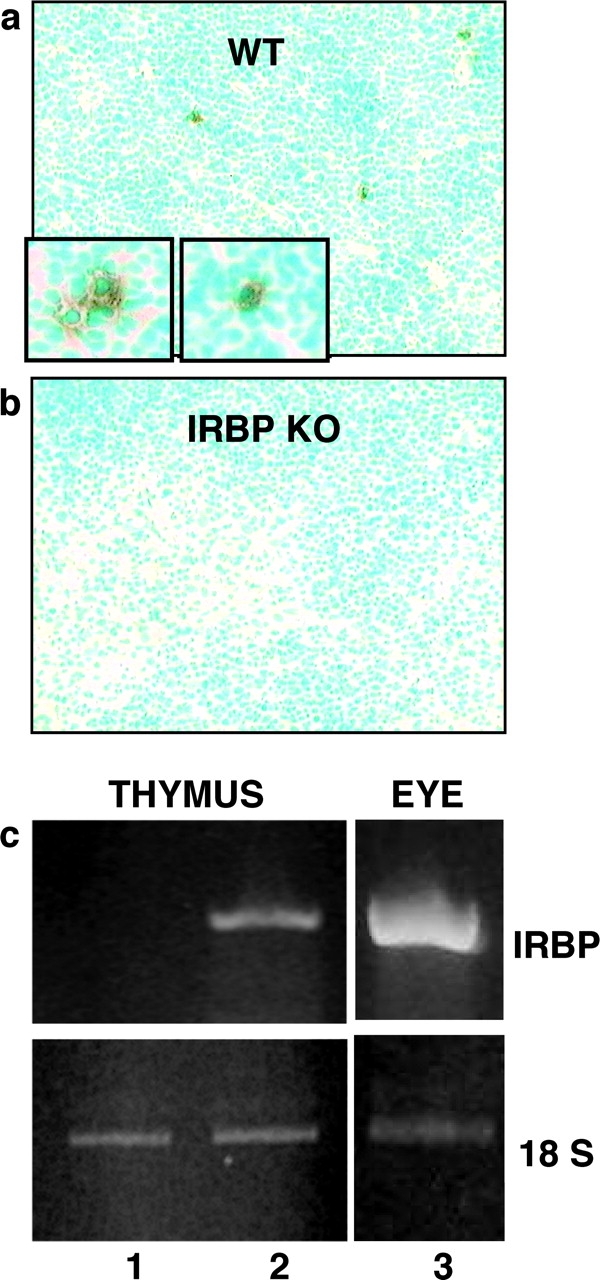
WT B10.RIII mice express IRBP mRNA and protein in the thymus. Frozen thymic sections from unmanipulated WT or KO mice were immunostained with monoclonal anti-IRBP antibody. Sparse positive cells (enlarged in insets) were detectable in WT thymi (a) but were absent in KO thymi (b). IRBP-positive and negative areas microdissected from WT thymic sections were amplified by RT-PCR using IRBP and 18S ribosomal RNA primers. IRBP message was detectable in the immunopositive areas (c, lane 2) but not in the immunonegative areas (c, lane 1). Ocular tissue served as a positive control (c, lane 3).
Thymic Expression of IRBP Confers Immunological Tolerance and Raises the Threshold of Susceptibility to Retinal Autoimmune Disease
To address the functional significance of thymic IRBP expression, we performed thymus transplantation between WT and KO mice. Thus, paired sets of mice were prepared that had an identical periphery but differed by thymic expression of IRBP: KO recipients of either WT or KO thymus (KOWT versus KOKO) and WT recipients of either WT or KO thymus (WTWT versus WTKO). Although both types of recipients could be used to analyze immunological responses, WT recipients also provided the opportunity to examine the impact of thymic IRBP expression on the ability to develop EAU.
KO Recipients.
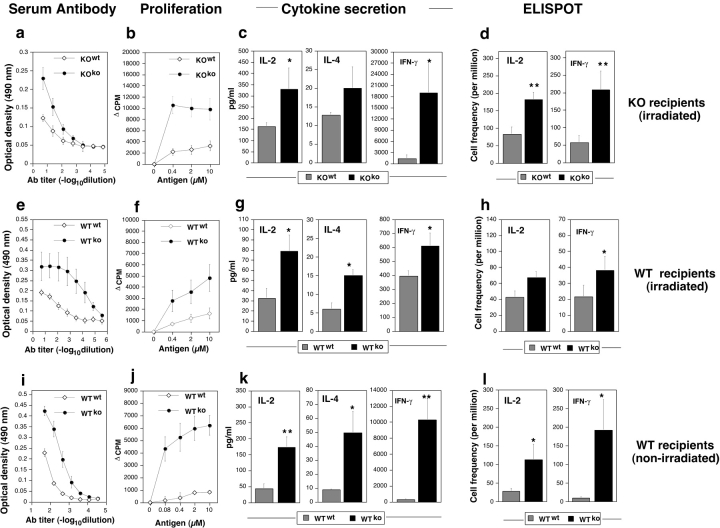
KO mice were adult thymectomized, transplanted with either WT or KO thymus under the kidney capsule, lethally irradiated, and reconstituted with matching (KO) BM cells. The mice were immunized 2–4 mo after immunoablation and BM reconstitution with rIRBP. Responses were recalled in vitro with the whole protein (Fig. 6 a) or with peptide 161–180 (Fig. 6, b–d). KO recipients of WT (KOWT) thymus had reduced immunological responses compared with their KOKO counterparts. As in the unmanipulated mice, tolerance affected the pathogenic 161–180 T cell epitope, as judged by proliferation and ELISPOT assay for IFN-γ– and IL-2–producing cells (Fig. 6 d). Responses of splenocytes from these mice to overlapping peptides representing the entire length of rIRBP were lower across the board, with a particularly prominent reduction of the response to peptide 161–180 (not depicted). Thus, the pattern of immunological responses to IRBP followed the donor of the thymus.
Figure 6.
WT recipients of KO thymus grafts have enhanced immunological responses compared to recipients of WT grafts. Adult thymectomized KO (a–d) or WT (e–h) recipients grafted with a mismatched or homologous thymus were immunoablated by irradiation and reconstituted with homologous BM and were subsequently immunized with rIRBP. Alternatively, WT mice thymectomized at weaning (i–l) were grafted with a mismatched or homologous thymus without immunoablation and were immunized with peptide 161–180. Mice were tested for immunological responses essentially as described in Fig. 2, except for IFN-γ secretion, which was determined by conventional ELISA. Note that in all panels the grafted thymus genotype is denoted as a superscript. Both humoral responses (a, e, and i) and cellular responses (b–d, f–h, and j–l) were upregulated in all the recipients of KO thymi compared with recipients of WT thymi, irrespective of the IRBP sufficiency status of the recipient. (**P < 0.005; *P < 0.05; ⋄, P < 0.1).
WT Recipients.
Conversely, WT recipients of KO thymi (immunoablated, reconstituted, and challenged with rIRBP as above) developed strongly enhanced immunological responses to IRBP (Fig. 6 e). This enhancement specifically included peptide 161–180 as evidenced by lymphocyte proliferation and by frequency of IL-2– and IFN-γ–producing cells (Fig. 6, f–h). Splenocyte responses of these mice to overlapping rIRBP peptides were elevated across the board and to peptide 161–180 in particular (not depicted). Notably, WT recipients of KO thymus developed highly exacerbated EAU characterized by early onset and high disease scores (Table I). Disease in recipients of KO thymus was so severe that the experiments had to be terminated a week early, 14 d after immunization.
Table I.
WT Recipients of IRBP KO Thymi Develop More Severe EAU than Recipients of WT Grafts
| Graft origin | Individual EAU scoresa | Median | Mean ± SE | P valueb |
|---|---|---|---|---|
| Immunization with: | ||||
| Peptide 161–180c | ||||
| WT | (0, 0) (0, 0.5) (0, 0.5) | 0.25 | 0.4 ± 0.2 | |
| (0, 0.5) (0, 0.5) (1, 2) | ||||
| KO | (2, 2) (2, 2.5) (2.5, 2.5) | 2.5 | 2.9 ± 0.3 | <0.0005 |
| (2.5, 2.5) (2.5, 3) (3, 3) | ||||
| (4, 4) (4, 4) | ||||
| IRBPd | ||||
| WT | (0.5, 0.5) (2, 2) | 1.25 | 1.25 ± 0.75 | |
| KO | (3, 3.5) (4, 4) | 3.75 | 3.6 ± 0.4 | <0.012 |
| rIRBPe | ||||
| WT | (0.5, 0.5) (0.5, 0.5) (0.5, 0.5) | 0.5 | 0.5 | |
| KO | (0.5, 0.5) (1.5, 2) (2, 2) (2, 2) | 2.0 | 1.6 ± 0.4 |
Because the high dose of irradiation may damage the blood-retinal barrier and affect the ability of the mice to develop EAU, it was important to rigorously confirm these data in WT mice that had not been irradiated (Fig. 6, i–l). Therefore, WT mice thymectomized at weaning were transplanted with a neonatal thymus and were immunized with peptide 161–180 2 mo later. These early thymectomized mice, which had not been immunoablated, possessed a mixed WT plus KO T cell compartment. Nevertheless, even such a partial KO repertoire was sufficient to cause a profound enhancement of immunological responses (Fig. 6, i–l). These mice also developed an exacerbated disease after immunization with peptide 161–180 or with whole IRBP (Fig. 7) . Their individual disease scores are included in Table I.
Figure 7.
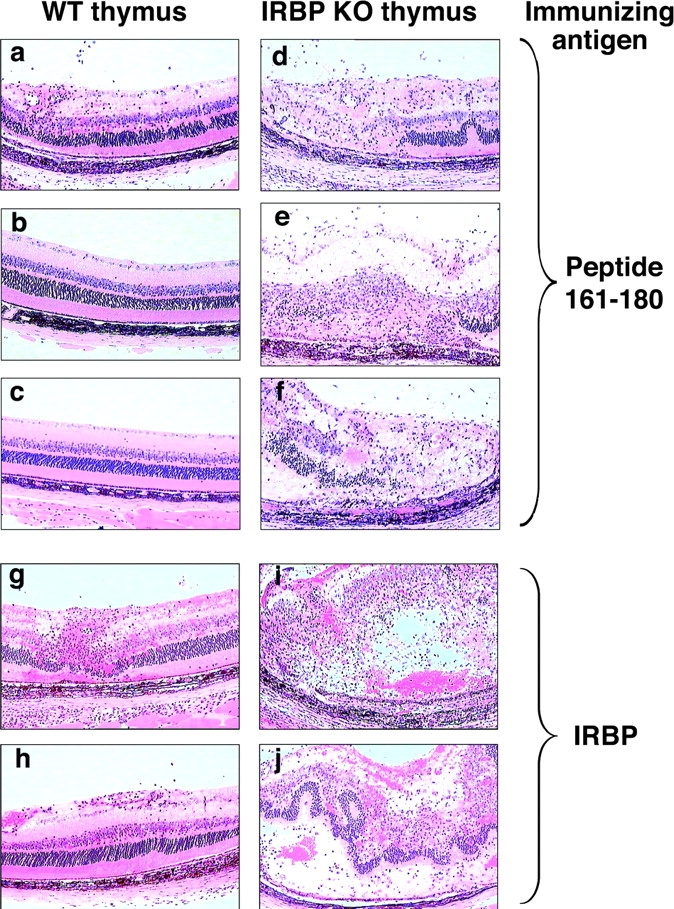
EAU in WT recipients of IRBP KO thymi is greatly enhanced compared with mice grafted with WT thymi. Early thymectomized mice were immunized with IRBP or 161–180 peptide 4 wk after transplantation with WT (a–c, g, and h) or KO (d–f, i, and j) thymus. Histology was performed 21 d after immunization. Hematoxylin and eosin stained. Original magnification, ×400.
Thymic Control of Susceptibility to IRBP-induced EAU Includes a Role for CD4+CD25+ Regulatory T Cells
The thymus controls autoimmune responses not only by culling high-affinity self-reactive precursor-effector cells but also by releasing CD4+CD25+ T regulatory cells (32, 33). Our early thymectomized nonimmunoablated WT recipients are expected to have functional CD25+ T regulatory cells, since such cells are released by the thymus during the first week of life. Nevertheless, these mice developed greatly enhanced disease after receiving a KO thymus transplant, equivalent to mice that had been immunoablated before transplant. This might be interpreted as evidence against a role for CD4+CD25+ T cells in this model. However, it is also possible that potent effector-precursor cells released by the KO thymus might have overwhelmed the regulatory potential of these cells.
To examine a possible contribution of CD4+CD25+ cells in setting the threshold of susceptibility to EAU, WT mice were depleted of CD25+ cells and were challenged for EAU by immunization with 5 μg of IRBP. At this low IRBP dose, the untreated control mice developed only moderate or no disease. In contrast, the CD25-depleted mice developed 100% disease incidence and severe EAU scores (Table II). To address the possibility that the CD25+ cells in this experiment were not thymically derived, WT mice were thymectomized at 4 wk of age, were depleted of CD25+ cells, and then were rested for 4 wk to allow regeneration of any CD25+ regulators that might not be thymically derived. When challenged for EAU, the CD25-depleted mice again developed significantly more disease than their nondepleted counterparts, supporting a thymic origin for the regulatory CD25+ cells (Table II).
Table II.
CD25 Depletion Results in Enhancement of EAU Scores
| Experiment no. | Treatment | Individual EAU scoresa | Median | Mean ± SE | P valueb |
|---|---|---|---|---|---|
| 1 | Nonthymectomizedc | ||||
| CD25 depleted | (2.5, 2.5) (2.5, 2.5) | 2.5 | 2.7 ± 0.1 | <0.009 | |
| (3, 3) (3, 2.5) | |||||
| Untreated control | (0, 0) (0.5, 0.75) | 0.3 | 0.5 ± 0.4 | ||
| (0, 0) (1.5, 1.5) | |||||
| 2 | Thymectomizedd | ||||
| CD25 depleted | (1.5, 2) (2, 2) | 2.0 | 2.5 ± 0.4 | <0.027 | |
| (3, 3) (3.5, 3) | |||||
| Untreated control | (0.5, 0.75) (0, 0.75) | 0.6 | 0.7 ± 0.5 | ||
| (2.5, 2.5) (0, 0) (0, 0) |
Discussion
Tolerance to immunologically privileged antigens that reside behind blood-tissue barriers, such as the eye and the brain, is still not understood completely. Studies in “shiverer” mice that lack myelin basic protein revealed that endogenous myelin basic protein expression is tolerogenic and alters the T cell repertoire selection (29, 34). The present study was undertaken to directly address the question whether tolerance to antigens residing in the retina of the eye can be demonstrated in mice that are susceptible to retinal autoimmunity. Previous data pointed to an association between presence of a particular retinal antigen in the thymus and resistance to EAU induced by immunization with that antigen (11). However, the questions regarding uveitogenic antigens whose thymic expression is below the sensitivity of conventional assays could not be addressed until susceptible mice deficient for the target antigen became available.
It was first necessary to ascertain that the IRBP KO mice, which retain part of the IRBP gene, indeed fail to express antigenic protein transcribed from this sequence. This appeared to be the case, both by lack of immunoreactive material in the retina and by the resistance of these mice to an aggressive regimen of immunization with IRBP. Additionally, the immunological responses to IRBP of IRBP KO mice were elevated across the board, implying lack of systemic tolerance to this molecule. Thus, EAU susceptible WT mice displayed a detectable level of tolerance not present in the KO.
We then decided to examine the thymus of B10.RIII WT and KO mice for IRBP expression. PCR analysis of thymic extracts, under the conditions used by Egwuagu et al. (11) to detect IRBP in resistant but not susceptible mouse strains failed to detect an IRBP signal, confirming what had been published. However, looking at individual cells by immunohistochemistry and microdissection, positive IRBP signals at the protein and the mRNA levels were detected in scattered cells in the medulla and cortico–medullary junction of WT thymus that were absent in KO thymus. Although we did not identify the cell type expressing IRBP in the thymus, it is likely that those were thymic medullary epithelial cells, as reported by others (35–37).
Thymus transplantation between WT and KO mice provided direct evidence that this level of thymic expression is functionally significant in terms of setting the threshold of responsiveness to IRBP in general and the threshold of susceptibility to EAU in particular. Importantly, this included control of the response to the disease-relevant peptide 161–180, which is the single most dominant uveitogenic IRBP epitope for WT B10.RIII mice and accounts for much of the uveitogenicity of whole IRBP in that strain (10). The frequency of p161–180-specific cells in WT mice and their apparent avidity was markedly reduced compared with the KO. It appears, therefore, that the 161–180-specific response that drives IRBP-induced EAU in WT mice represents a residuum of the original T cell repertoire generated to this epitope. The IRBP KO thymus might also permit retention of pathogenic repertoires to other IRBP epitope(s) eliminated in the WT, such as peptide 121–140.
Thymic control of T cell responses in the periphery involves not only the direct elimination of pathogenic cells but also the release of regulatory cells that control tissue-specific autoimmunity (for review see 32). It is thought that the relevant tissue antigen must be expressed in the thymus to permit generation of specific CD4+CD25+ regulatory T cells (33). Enhanced responses to IRBP in mice bearing an IRBP KO thymus might therefore be attributable not only to culling of IRBP-reactive precursor-effector cells but also to lack of generation of CD25+ regulatory T cells specific to IRBP. It is important to point out in this context that although the protective role of CD25+ regulatory cells is well established in spontaneous autoimmunity models, it has been more difficult to demonstrate in induced models of autoimmunity and has not thus far been demonstrated in EAU. Furthermore, the eye elicits its own, highly specialized, immunoregulatory circuits that could make thymically derived CD25+ regulatory cells redundant (5–7, 38). Our finding that immunocompetent WT mice, deprived of thymus-dependent CD25+ regulatory cells, develop enhanced EAU scores, strongly supports a contributing role for these cells in setting the threshold of EAU susceptibility.
A role for peripheral mechanisms in self-tolerance to uveitogenic retinal antigens is still in question. Expression of retina-specific proteins is largely restricted to the eye, where blood-tissue barriers prevent free traffic of lymphocytes. Thus, peripheral tolerance by continuous exposure of potentially autoreactive T cells to tissue antigens in a noninflammatory milieu is unlikely to operate efficiently for retinal antigens. Indeed, we and others have demonstrated that responses to retina-specific antigens are dramatically reduced if their immune-privileged status is revoked by expressing them extraocularly (8–10). It is still unresolved whether the ACAID phenomenon, which is well studied with relation to injected proteins (5, 6), is elicited by native retinal antigens residing in the intact eye. Gregerson et al. have proposed this to be the case based on reduced responsiveness to β-galactosidase in mice expressing this protein as a neo-antigen on a retina-specific promoter (39), but they have not performed thymus transplants or CD25+ cell depletion to rigorously exclude central tolerance. Our results in mice immunoablated by irradiation clearly showed that WT mice given a KO thymus (whose only source of IRBP is the periphery), develop significant tolerance to IRBP compared with KO mice given a KO thymus (compare WTKO to KOKO mice in Fig. 6, a–h). However, although this demonstrates that peripheral tolerance can be induced in mice that have no thymic source of IRBP, it does not prove that the intact eye elicits such tolerance, since we cannot exclude the possibility that changes in the blood-retinal barrier resulting from irradiation have altered the accessibility of the ocular compartment. The question of peripheral tolerance by the intact eye is being addressed in a separate study.
Our current data complement and extend recent studies on thymic tolerance to proteolipid protein (PLP), one of the antigens driving disease in the experimental autoimmune encephalomyelitis model. Seminal studies by Klein et al. (40) and by Anderson et al. (41) revealed predominant expression in the thymus of DM20, a shortened splice variant of PLP message that lacks the region encoding the 139–151 epitope, instead of the full-length molecule that is expressed in the brain. This thwarts deletion of 139–151-specific T cells and explains the susceptibility of SJL/J mice to experimental autoimmune encephalomyelitis induced by this epitope but not by other PLP epitopes that are represented in the thymus. Unlike PLP, in the case of IRBP there is no evidence, either by PCR or by Western blotting, that the molecule expressed in the thymus represents a different splice variant than the one expressed in the eye (11). Therefore, despite presence of full-length IRBP demonstrable at the protein level, elimination of pathogenic specificities is inadequate, although at the same time apparently permitting positive selection of CD25+ regulatory cells. These results raise a concept that the balance of autoimmune effectors versus regulators generated by the thymus may represent a sliding scale that is influenced by quantitative aspects of thymic autoantigen expression.
In summary, the present study for the first time provides direct evidence that uveitis-susceptible WT mice express low levels of their target antigen in the thymus. This level of expression, although undetectable by conventional assays, is immunologically relevant and serves to raise the threshold of immunological responsiveness and of susceptibility to disease. The mechanism appears to involve culling of precursor effector cells and generation of CD4+CD25+ regulatory cells by the thymus. These data provide new insights into regulation of responsiveness and autoimmune susceptibility to immunologically privileged self-antigens.
Acknowledgments
The authors wish to thank Drs. Polly Matzinger and Colin C. Anderson from National Institute of Allergy and Infectious Diseases/NIH for assistance with adult thymectomy and thymus grafting. We are grateful to Dr. Defen Shen for help with RT-PCR. We thank the staff of the National Eye Institute Histology Core Facility for preparation of high quality histological specimens.
Generation of IRBP KO mice (G.I. Liou) was supported by an unrestricted departmental award from Research to Prevent Blindness Inc., New York, NY, and by grant EY03829 from the NIH.
Abbreviations used in this paper: ACAID, anterior chamber–associated immune deviation; CFA, complete Freund's adjuvant; DTH, delayed type hypersensitivity; EAU, experimental autoimmune uveoretinitis; IRBP, interphotoreceptor retinoid-binding protein; KO, knockout; PLP, proteolipid protein; rIRBP, recombinant repeat 1 of human IRBP; S-Ag, retinal soluble antigen.
References
- 1.Caspi, R.R. 1999. Immune mechanisms in uveitis. Springer Semin. Immunopathol. 21:113–124. [DOI] [PubMed] [Google Scholar]
- 2.Gery, I., and J.W. Streilein. 1994. Autoimmunity in the eye and its regulation. Curr. Opin. Immunol. 6:938–945. [DOI] [PubMed] [Google Scholar]
- 3.Caspi, R.R., C.C. Chan, B. Wiggert, and G.J. Chader. 1990. The mouse as a model of experimental autoimmune uveoretinitis (EAU). Curr. Eye Res. 9(Suppl.):169–174. [DOI] [PubMed] [Google Scholar]
- 4.Caspi, R.R., F.G. Roberge, C.C. Chan, B. Wiggert, G.J. Chader, L.A. Rozenszajn, Z. Lando, and R.B. Nussenblatt. 1988. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J. Immunol. 140:1490–1495. [PubMed] [Google Scholar]
- 5.Streilein, J.W., S. Masli, M. Takeuchi, and T. Kezuka. 2002. The eye's view of antigen presentation. Hum. Immunol. 63:435–443. [DOI] [PubMed] [Google Scholar]
- 6.Stein-Streilein, J., and J.W. Streilein. 2002. Anterior chamber associated immune deviation (ACAID): regulation, biological relevance, and implications for therapy. Int. Rev. Immunol. 21:123–152. [DOI] [PubMed] [Google Scholar]
- 7.Hara, Y., R.R. Caspi, B. Wiggert, C.C. Chan, G.A. Wilbanks, and J.W. Streilein. 1992. Suppression of experimental autoimmune uveitis in mice by induction of anterior chamber-associated immune deviation with interphotoreceptor retinoid-binding protein. J. Immunol. 148:1685–1692. [PubMed] [Google Scholar]
- 8.Xu, H., E.F. Wawrousek, T.M. Redmond, J.M. Nickerson, B. Wiggert, C.C. Chan, and R.R. Caspi. 2000. Transgenic expression of an immunologically privileged retinal antigen extraocularly enhances self tolerance and abrogates susceptibility to autoimmune uveitis. Eur. J. Immunol. 30:272–278. [DOI] [PubMed] [Google Scholar]
- 9.McPherson, S.W., J.P. Roberts, and D.S. Gregerson. 1999. Systemic expression of rat soluble retinal antigen induces resistance to experimental autoimmune uveoretinitis. J. Immunol. 163:4269–4276. [PubMed] [Google Scholar]
- 10.Agarwal, R.K., Y. Kang, E. Zambidis, D.W. Scott, C.C. Chan, and R.R. Caspi. 2000. Retroviral gene therapy with an immunoglobulin-antigen fusion construct protects from experimental autoimmune uveitis. J. Clin. Invest. 106:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egwuagu, C.E., P. Charukamnoetkanok, and I. Gery. 1997. Thymic expression of autoantigens correlates with resistance to autoimmune disease. J. Immunol. 159:3109–3112. [PubMed] [Google Scholar]
- 12.Anderson, M.S., E.S. Venanzi, L. Klein, Z. Chen, S.P. Berzins, S.J. Turley, H. von Boehmer, R. Bronson, A. Dierich, C. Benoist, and D. Mathis. 2002. Projection of an immunological self shadow within the thymus by the aire protein. Science. 298:1395–1401. [DOI] [PubMed] [Google Scholar]
- 13.Liou, G.I., Y. Fei, N.S. Peachey, S. Matragoon, S. Wei, W.S. Blaner, Y. Wang, C. Liu, M.E. Gottesman, and H. Ripps. 1998. Early onset photoreceptor abnormalities induced by targeted disruption of the interphotoreceptor retinoid-binding protein gene. J. Neurosci. 18:4511–4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pepperberg, D.R., T.L. Okajima, H. Ripps, G.J. Chader, and B. Wiggert. 1991. Functional properties of interphotoreceptor retinoid-binding protein. Photochem. Photobiol. 54:1057–1060. [DOI] [PubMed] [Google Scholar]
- 15.Pepperberg, D.R., T.L. Okajima, B. Wiggert, H. Ripps, R.K. Crouch, and G.J. Chader. 1993. Interphotoreceptor retinoid-binding protein (IRBP). Molecular biology and physiological role in the visual cycle of rhodopsin. Mol. Neurobiol. 7:61–85. [DOI] [PubMed] [Google Scholar]
- 16.Lin, Z.Y., G.R. Li, N. Takizawa, J.S. Si, E.A. Gross, K. Richardson, and J.M. Nickerson. 1997. Structure-function relationships in interphotoreceptor retinoid-binding protein (IRBP). Mol. Vis. 3:17–34. [PubMed] [Google Scholar]
- 17.Donoso, L.A., C.F. Merryman, T. Sery, R. Sanders, T. Vrabec, and S.L. Fong. 1989. Human interstitial retinoid binding protein. A potent uveitopathogenic agent for the induction of experimental autoimmune uveitis. J. Immunol. 143:79–83. [PubMed] [Google Scholar]
- 18.Silver, P.B., L.V. Rizzo, C.C. Chan, L.A. Donoso, B. Wiggert, and R.R. Caspi. 1995. Identification of a major pathogenic epitope in the human IRBP molecule recognized by mice of the H-2r haplotype. Invest. Ophthalmol. Vis. Sci. 36:946–954. [PubMed] [Google Scholar]
- 19.Redmond, T.M., B. Wiggert, F.A. Robey, N.Y. Nguyen, M.S. Lewis, L. Lee, and G.J. Chader. 1985. Isolation and characterization of monkey interphotoreceptor retinoid-binding protein, a unique extracellular matrix component of the retina. Biochemistry. 24:787–793. [DOI] [PubMed] [Google Scholar]
- 20.Donoso, L.A., M. Rodrigues, T.R. Vrabec, T.W. Sery, and S.L. Fong. 1990. IRBP: preparation and characterization of site-specific monoclonal antibodies. Curr. Eye Res. 9:357–362. [DOI] [PubMed] [Google Scholar]
- 21.Avichezer, D., P.B. Silver, C.C. Chan, B. Wiggert, and R.R. Caspi. 2000. Identification of a new epitope of human IRBP that induces autoimmune uveoretinitis in mice of the H-2b haplotype. Invest. Ophthalmol. Vis. Sci. 41:127–131. [PubMed] [Google Scholar]
- 22.Kohm, A.P., P.A. Carpentier, H.A. Anger, and S.D. Miller. 2002. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 169:4712–4716. [DOI] [PubMed] [Google Scholar]
- 23.McHugh, R.S., and E.M. Shevach. 2002. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J. Immunol. 168:5979–5983. [DOI] [PubMed] [Google Scholar]
- 24.Caspi, R.R. 2003. Experimental autoimmune uveoretinitis in the rat and mouse. Current Protocols in Immunology. J.E. Coligan, A.M. Kruisbeek, D.H. Margulies, E.M. Shevech, and W. Strober, editors. John Wiley and Sons, Inc., New York. Unit 15.6. [DOI] [PubMed]
- 25.Chan, C.C., R.R. Caspi, M. Ni, W.C. Leake, B. Wiggert, G.J. Chader, and R.B. Nussenblatt. 1990. Pathology of experimental autoimmune uveoretinitis in mice. J. Autoimmun. 3:247–255. [DOI] [PubMed] [Google Scholar]
- 26.Rizzo, L.V., R.H. DeKruyff, D.T. Umetsu, and R.R. Caspi. 1995. Regulation of the interaction between Th1 and Th2 T cell clones to provide help for antibody production in vivo. Eur. J. Immunol. 25:708–716. [DOI] [PubMed] [Google Scholar]
- 27.Moody, M.D., S.W. Van Arsdell, K.P. Murphy, S.F. Orencole, and C. Burns. 2001. Array-based ELISAs for high-throughput analysis of human cytokines. Biotechniques. 31:186–190, 192–184. [DOI] [PubMed]
- 28.Silver, P.B., C.C. Chan, B. Wiggert, and R.R. Caspi. 1999. The requirement for pertussis to induce EAU is strain-dependent: B10.RIII, but not B10.A mice, develop EAU and Th1 responses to IRBP without pertussis treatment. Invest. Ophthalmol. Vis. Sci. 40:2898–2905. [PubMed] [Google Scholar]
- 29.Targoni, O.S., and P.V. Lehmann. 1998. Endogenous myelin basic protein inactivates the high avidity T cell repertoire. J. Exp. Med. 187:2055–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snedecor, G.W., and W.G. Cochran. 1967. Statistical Methods, 6th edition. Iowa State University Press, Ames, IA.
- 31.Avichezer, D., G.I. Liou, C.-C. Chan, G.M. Lewis, B. Wiggert, L.A. Donoso, J.M. Nickerson, M.A. Crawford, and R.R. Caspi. Interphotoreceptor retinoid-binding protein (IRBP)-deficient C57BL/6 mice have enhanced immunological and immunopathogenic responses to IRBP and an altered recognition of IRBP epitopes. J. Autoimmun. 21:185–194. [DOI] [PubMed]
- 32.Shevach, E.M., R.S. McHugh, C.A. Piccirillo, and A.M. Thornton. 2001. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol. Rev. 182:58–67. [DOI] [PubMed] [Google Scholar]
- 33.Apostolou, I., A. Sarukhan, L. Klein, and H. von Boehmer. 2002. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 3:756–763. [DOI] [PubMed] [Google Scholar]
- 34.Harrington, C.J., A. Paez, T. Hunkapiller, V. Mannikko, T. Brabb, M. Ahearn, C. Beeson, and J. Goverman. 1998. Differential tolerance is induced in T cells recognizing distinct epitopes of myelin basic protein. Immunity. 8:571–580. [DOI] [PubMed] [Google Scholar]
- 35.Klein, L., B. Roettinger, and B. Kyewski. 2001. Sampling of complementing self-antigen pools by thymic stromal cells maximizes the scope of central T cell tolerance. Eur. J. Immunol. 31:2476–2486. [DOI] [PubMed] [Google Scholar]
- 36.Derbinski, J., A. Schulte, B. Kyewski, and L. Klein. 2001. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat. Immunol. 2:1032–1039. [DOI] [PubMed] [Google Scholar]
- 37.Kyewski, B., J. Derbinski, J. Gotter, and L. Klein. 2002. Promiscuous gene expression and central T-cell tolerance: more than meets the eye. Trends Immunol. 23:364–371. [DOI] [PubMed] [Google Scholar]
- 38.Hara, Y., R.R. Caspi, B. Wiggert, C.C. Chan, and J.W. Streilein. 1992. Use of ACAID to suppress interphotoreceptor retinoid binding protein-induced experimental autoimmune uveitis. Curr. Eye Res. 11(Suppl.):97–100. [DOI] [PubMed] [Google Scholar]
- 39.Gregerson, D.S., and C. Dou. 2002. Spontaneous induction of immunoregulation by an endogenous retinal antigen. Invest. Ophthalmol. Vis. Sci. 43:2984–2991. [PubMed] [Google Scholar]
- 40.Klein, L., M. Klugmann, K.A. Nave, V.K. Tuohy, and B. Kyewski. 2000. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nat. Med. 6:56–61. [DOI] [PubMed] [Google Scholar]
- 41.Anderson, A.C., L.B. Nicholson, K.L. Legge, V. Turchin, H. Zaghouani, and V.K. Kuchroo. 2000. High frequency of autoreactive myelin proteolipid protein-specific T cells in the periphery of naive mice: mechanisms of selection of the self-reactive repertoire. J. Exp. Med. 191:761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]