CA1 pyramidal to basket and bistratified cell EPSPs: dual intracellular recordings in rat hippocampal slices (original) (raw)
Abstract
- Dual intracellular recordings in the CA1 region of adult rat hippocampal slices and biocytin filling of synaptically connected cells were used to study the excitatory postsynaptic potentials (EPSPs) elicited in basket (_n_= 7) and bistratified interneurones (_n_= 7) by action potentials activated in simultaneously recorded pyramidal cells.
- Interneurones could be subdivided according to their electrophysiological properties into classical fast spiking, burst firing, regular spiking and fast spiking cells with a rounded spike after-hyperpolarization. These physiological classes did not, however, correlate with morphological type. EPSPs were not recorded in regular spiking cells.
- Average EPSP amplitudes were larger in bistratified cells (range, 0.5–9 mV) than in basket cells (range, 0.15–3.6 mV) and the probability of obtaining a pyramidal cell-interneurone EPSP was also higher for the bistratified cells (1:7) than for the basket cells (1:22). EPSP 10–90% rise times in bistratified cells (0.7–2 ms) and their widths at half-amplitude (3.9–11.2 ms) were slightly longer than in basket cells (rise times, 0.4–1.6 ms; half-widths, 2.2–9.7 ms).
- The majority of these EPSPs (6 of 8 tested) increased in amplitude and duration with postsynaptic depolarization, although in two (of 4) basket cells the voltage relation was conventional.
- All EPSPs tested in both basket (_n_= 7) and bistratified cells (_n_= 5) decreased in amplitude with repetitive presynaptic firing. The average amplitudes of second EPSPs elicited within 15 ms of the first were between 34 and 94% of the average amplitude of the first EPSP. Third and fourth EPSPs in brief trains were further depressed. This depression was associated with an increase in the incidence of apparent failures of transmission indicating a presynaptic locus.
In the CA1 region of the rat hippocampus non-pyramidal cells (also known as interneurones) constitute between 6 % (Aika, Ren, Kosaka & Kosaka, 1994) and 11 % (Woodson, Nitecka & Ben-Ari, 1989) of neurones. These interneurones form a heterogeneous group which can be subdivided in various ways (see Freund & Buzsáki, 1996 for review). One method of classification is based on the observation that particular interneurone types innervate specific postsynaptic domains of pyramidal neurones. For instance, of the interneurones whose somata are located in the cell body layer, those that innervate almost exclusively the axon initial segments of pyramidal cells are called axo-axonic (or chandelier) cells (Somogyi, 1977). Basket cells are those that contact predominantly the somata and proximal dendrites of pyramidal neurones (e.g. Gulyás, Miles, Hájos & Freund, 1993_a_;Buhl, Halasy & Somogyi, 1994_a_;Buhl, Szilágyi, Halasy & Somogyi, 1996) while bistratified interneurones innervate pyramidal dendrites adjacent to stratum pyramidale, in stratum radiatum and oriens (Buhl et al. 1994_a_;Halasy, Buhl, Lorinczi, Tamas & Somogyi, 1996).
The physiological properties of the outputs of some of these morphological classes of interneurones have been studied by simultaneous intracellular recordings from both the non-pyramidal cell and a postsynaptic target cell (Knowles & Schwartzkroin, 1981; Miles & Wong, 1984; Buhl et al. 1994_a_;Buhl, Cobb, Halasy & Somogyi, 1995; Cobb, Buhl, Halasy, Paulsen & Somogyi, 1995; Miles, Tóth, Gulyás, Hájos & Freund, 1996; Miles & Poncer, 1997 for review). Combining intracellular recording with biocytin filling of recorded neurones and correlated light and electron microscopy has identified the classes of non-pyramidal cells involved (Buhl et al. 1994_a_, 1995; Miles et al. 1996). These methods have shown that basket (Buhl et al. 1994_a_, 1995; Miles et al. 1996), bistratified (Buhl et al. 1994_a_) and axo-axonic cells (Buhl et al. 1994_a_) all generate GABAA receptor-mediated inhibitory postsynaptic potentials (IPSPs) in postsynaptic pyramidal cells. In the case of basket and axo-axonic cells the IPSPs elicited by firing in a single presynaptic cell can be powerful and can entrain the firing of a postsynaptic pyramidal cell (Cobb et al. 1995). Since a single basket cell is estimated to contact more than a thousand pyramidal cells (Sík, Penttonen, Ylinen & Buzsáki, 1995) it has been suggested that a single basket cell may synchronize the firing of these cells (Cobb et al. 1995; Sík et al. 1995). These IPSPs might therefore contribute to the generation of synchronous discharge as, for example, in hippocampal theta rhythm (Traub, Whittington, Stanford & Jefferys, 1996).
Since the outputs of interneurones appear to be so important it follows that the influences upon them are equally significant. Some local circuit inputs to non-pyramidal cells from closely neighbouring pyramidal cells have been examined using dual intracellular recordings (Knowles & Schwartzkroin, 1981; Miles, 1990) and in each of two studies combining dual intracellular recordings with light and electron microscopy, one in CA3 (Gulyás, Miles, Sík, Tóth, Tamamaki & Freund, 1993_b_;Poncer & Miles, 1994), one in CA1 (Buhl et al. 1994a), basket cells were found to receive only one synaptic bouton from each presynaptic pyramidal cell. These findings were confirmed when a larger population of immunocytochemically identified, parvalbumin-positive interneurones were found to receive typically one synaptic contact per presynaptic pyramidal axon (Sík, Tamamaki & Freund, 1993). These rather weak connections resulted in relatively small excitatory postsynaptic potentials (EPSPs), but in these earlier dual recording studies only responses to single action potentials (APs) were studied in a relatively small number of connections. The inputs to bistratified interneurones have never been investigated in this way. It is clear from studies in the neocortex (Thomson & Deuchars, 1997) that more complex patterns of presynaptic spikes in a single presynaptic neurone can elicit very different patterns of responses depending on the identity of the postsynaptic neurone. For instance, pyramidal cell to pyramidal cell connections in the hippocampus (Deuchars & Thomson, 1996; see Debanne, Guerineau, Gähwiler & Thompson, 1996, for similar findings in slice cultures) and neocortex (Thomson & West, 1993; Thomson, Deuchars & West, 1993_b_;Deuchars, West & Thomson, 1994; Markram & Tsodyks, 1996) exhibit paired pulse depression while neocortical pyramidal cell-interneurone connections can display either pronounced paired pulse facilitation (Thomson, Deuchars & West, 1993_a_;Thomson, West & Deuchars, 1995; Thomson, 1997) or depression (Thomson, 1997). Whether facilitation or depression dominates apparently depends on the postsynaptic target, although in neocortex it has not yet been possible to identify the class(es) of interneurones receiving depressing connections.
Thus both the presynaptic firing pattern and the type of postsynaptic target play a role in determining the efficacy of a synaptic connection. To examine these properties at pyramidal cell-interneurone synapses in the CA1 region of the rat hippocampus, dual intracellular recordings, from pre- and postsynaptic neurones, were made and recorded neurones filled with biocytin to identify the morphological classes of cells involved.
A preliminary report of this work has appeared (Ali & Thomson, 1997).
METHODS
The methods are described in detail elsewhere (Deuchars & Thomson, 1996; Thomson, 1997). Male rats, 90-180 g in body weight, were anaesthetized with Fluothane and sodium pentobarbitone (Sagatal, 60 mg kg−1 intraperitoneally) and perfused transcardially with an ice-cold artificial cerebrospinal fluid (ACSF) in which NaCl had been replaced with 248 mM sucrose. The rat was then killed by decapitation. Following removal of the brain, 450-500 μm thick coronal slices were cut (Vibroslice) and maintained in an interface chamber at 34-35°C. After 1 h in the sucrose-containing ACSF, this was replaced with standard ACSF in which all recordings were made (mM): 124 NaCl, 25.5 NaHCO3, 3.3 KCl, 1.2 KH2PO4, 1.0 MgSO4, 2.5 CaCl2 and 15 D-glucose, equilibrated with 95 % O2-5 % CO2. Connections from pyramidal cells to interneurones in stratum pyramidale of the CA1 region of the hippocampus were studied and the response of the postsynaptic interneurone to single and/or multiple presynaptic pyramidal cell spikes recorded. Following recording and biocytin filling of recorded cells, slices were fixed (see below for histological processing).
Electrophysiological recordings
Paired recordings were performed using conventional sharp electrodes (resistance, 80-160 MΩ) containing 2 M potassium methylsulphate and 2 % biocytin (w/v) under current clamp conditions, using an Axoprobe amplifier (Axon Instruments). The search strategy for connected pairs involved first obtaining a stable intracellular recording from one pyramidal neurone located in the cell body layer. Then a search was made for an interneurone within or very close to stratum pyramidale. Single spikes or brief trains of action potentials were elicited in the pyramidal cell by injection of square wave current pulses delivered once every 3 s, and any voltage change in the interneurones recorded. Any possible connection from the interneurone to the pyramidal cell was also tested. If both tests were negative, a second pyramidal cell was penetrated and tested and so on. With connected pairs, continuous analog recordings from both neurones were made on magnetic tape (Racal Store 4). To ensure that any one data set included data collected only at one postsynaptic membrane potential (± 1 mV), postsynaptic membrane potential was held constant by continuous manual current clamp and then changed to another potential after sufficient data had been collected. Electrode balance was continuously monitored by observing voltage responses to small brief current pulses injected into the postsynaptic cell prior to each activation of presynaptic spikes. The electrophysiological characteristics of recorded cells were obtained from their voltage responses to 500 ms current pulses between -2 and +1 nA in amplitude (delivered from membrane potentials in the range -65 to -85 mV) using pCLAMP software (Axon Instruments).
Data analysis
Data that had been collected on analog tape were digitized, stored on optical disc and analysed off-line (using in-house software). Individual sweeps were observed and either accepted, edited, or rejected according to the trigger points that would trigger measurements and averaging of the EPSPs during subsequent data analysis. Averaging of EPSPs was triggered by the rising phase of the first presynaptic spike for the first EPSP, the rising phase of the second presynaptic spike for the second EPSP and so on. For the averages illustrated and measurements given, individual records in which second, third, fourth and fifth spikes fell within a narrow time window (e.g. 14-20 or 35-40 ms) following the first spike were selected into data subsets and averaged. Average EPSP amplitude was measured between the baseline and the peak of the EPSP. The 10-90 % rise time was measured as the time taken for the EPSP to rise from 10 to 90 % of its peak amplitude. The width at half-amplitude was measured as the time interval between the EPSP rising to 50 % and falling to 50 % of its peak amplitude. Numbers given in the text are means ±s.d.
Spontaneous EPSPs that were at least twice the baseline noise in amplitude and readily distinguishable by eye were counted and their amplitudes measured in 50 to 100 randomly selected sweeps each of 200 ms duration (equivalent to 10-20 s of recording). These measurements were not found to change significantly when longer analysis periods were used.
Histological processing
Following recording, cell pairs were filled with biocytin by passing 0.5 nA depolarizing current pulses in a 50 % duty cycle at 1 Hz for 5-15 min. The slices were incubated in the recording chamber for a further 5-15 min and were then fixed for at least 1 h in a solution of 0.1 M phosphate buffer containing 1.25 % glutaraldehyde-2.5 % paraformaldehyde or 3 % paraformaldehyde-0.5 % glutaraldehyde. After embedding the sections in gelatin, they were sectioned at 60 μm on a Vibratome. Injected biocytin was localized using the Vector stain ABC Elite kit (Vector Laboratories, Peterborough, UK), incubated with 0.1 % Triton X-100 overnight. The injected biocytin was then visualized using 3,3′5,5′-diaminobenzidine (DAB; Sigma) and the sections dehydrated and embedded in Durcupan resin (Fluka) on slides. Filled and recorded interneurones were fully reconstructed under a ×100 objective using a drawing tube or the Neurolucida neuron tracing system. Neurones were classified by their dendritic and axonal architecture.
RESULTS
In 119 experiments involving > 700 hippocampal slices, 371 dual recordings in which a cell body layer interneurone was recorded simultaneously with a pyramidal cell were obtained. In twenty-three of these recordings an EPSP was elicited in the interneurone by APs in the pyramidal cell. Classification of interneurones according to axonal and dendritic morphology indicated that in nine cases the postsynaptic interneurone was a basket cell (e.g. Figs 4 and 6) and in eight cases it was a bistratified cell (Figs 2, 3 and 5). The remaining six postsynaptic interneurones were insufficiently well recovered histologically to be identified. The properties of sixteen of these connections are reported in detail here. Results from the other pairs were similar, but recordings were too brief for confident assessment of properties. Probabilities of obtaining such a connection with randomly selected pyramidal cell-interneurone pairs were 23:371 tested pairs, i.e. an average probability of approximately 1:16 for all stratum pyramidale interneurones. For identified basket cells this probability was 9:195 (approximately 1:22) and higher for identified bistratified cells; 8:53 (1:7).
Figure 4. EPSPs recorded in basket cells in response to APs in simultaneously recorded pyramids either increase, or decrease in amplitude with postsynaptic depolarization.
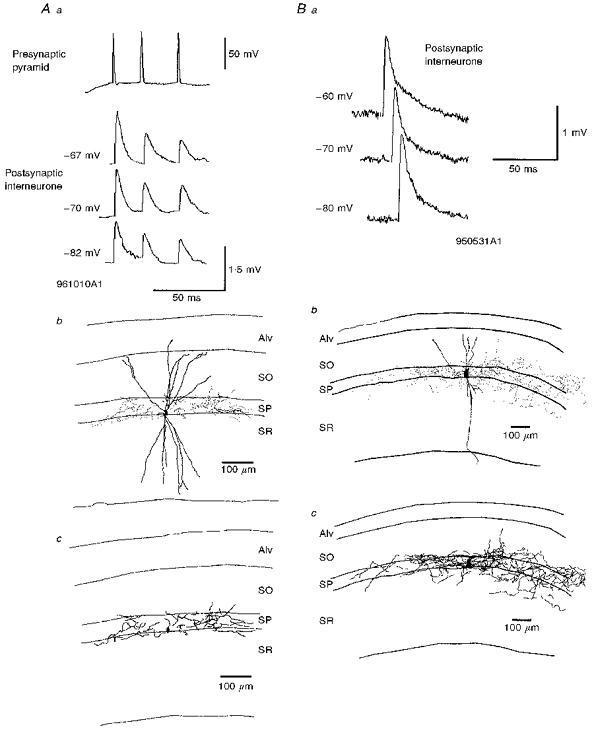
Aa, averaged EPSPs recorded in basket cell at three different membrane potentials and in response to trains of three presynaptic spikes in a pyramidal neurone. As the postsynaptic cell was depolarized the EPSP increased in amplitude and duration. The averaged EPSPs following the second and third spikes were smaller than following the first. The postsynaptic basket cell soma and dendrites are indicated by the continuous lines in Ab and the boutons by the dots. Only the axon from one 60 μm section is included in this drawing but the distribution was similar in all other sections. The complete axon from that one section is illustrated in Ac. The axon and boutons ramified mostly within SP and proximal SO and SR, indicating that it was a basket cell (cell code 961010A1). Ba, averaged EPSPs recorded in response to APs in a presynaptic pyramid at three different membrane potentials. As the postsynaptic basket cell was depolarized the EPSP average amplitude decreased. The soma/dendrites (continuous lines) and boutons (dots) of this cell are illustrated in Bb. Only some dendrites could be drawn due to light labelling. The axon remained well labelled (2 of 4 sections reconstructed) and was mostly confined to stratum pyramidale, as can also be seen in Bc where the complete axonal arborization in these two sections is shown (code 950531A1).
Figure 6. Brief train depression in EPSPs recorded in basket cells in response to brief trains of APs in presynaptic pyramids.
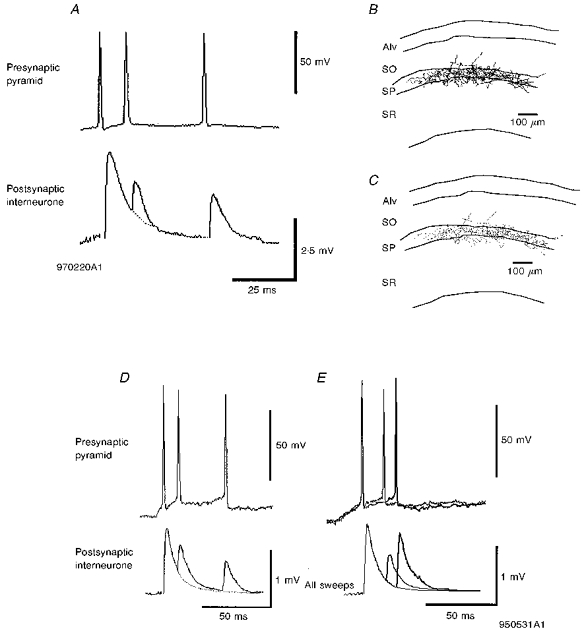
A, the averaged 2nd and 3rd EPSPs recorded in a basket cell in response to a brief train of three presynaptic pyramidal cell spikes were depressed when compared with the first EPSP. The axon of the cell was restricted to the cell body layer, i.e. this is a basket cell. The axonal distribution is shown in B and the boutons in C (cell code 970220A1). D, depression in a second postsynaptic basket cell in response to a brief train of APs in a presynaptic pyramid. See also Fig. 4_Ba_ and the reconstruction of this cell in 4_Bb_ and 4_Bc_ (code 950531A1). E, paired pulse depression was dependent on interspike interval. Averaged responses to pairs of APs at two different interspike intervals are illustrated. Stronger depression was apparent at the briefer interval (normal trace) than at the longer interval (bold trace).
Figure 2. The largest (A) and one of the smaller (B) average pyramid-bistratified cell EPSPs recorded in this study with the reconstructions of the postsynaptic interneurones.
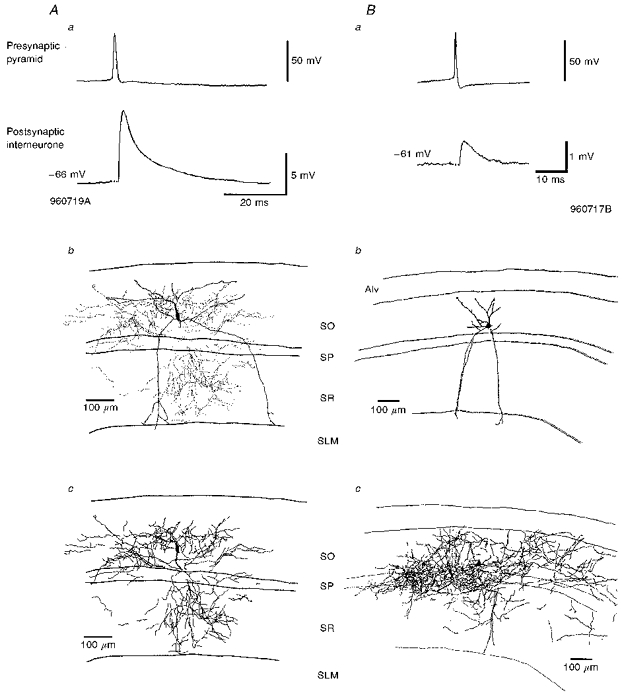
In Aa the average EPSP was the largest recorded in this study and at -66 mV was 9.06 mV (cell code 960719A). The interneurone soma was located in stratum oriens (SO) and dendrites traversed SO and stratum pyramidale (SP) into stratum radiatum (SR), but did not enter stratum lacunosum-moleculare (SLM) (Ab). This cell was reconstructed using the Neurolucida system which allows the boutons to be shown separately from the rest of the axon (Ab). The dots in Ab illustrate the distribution of the boutons while the axonal arborization in full is shown in Ac with the position of the interneurone soma shown for comparison with Ab. The axon of this cell ramified mostly in SO and SR, with only a few branches in SP. Ba illustrates one of the smaller average EPSPs recorded, which had an average amplitude of 0.72 mV at -61 mV (cell code 960717B). The interneurone was reconstructed by hand using a drawing tube. Bb illustrates the soma and dendrites (Alv, alveus). The axon of this cell (Bc) ramified mostly in SO, but there was also a large amount in SP, with some in SR. As stated in the text this cell may be a radially oriented trilaminar cell. Here and in all figures, averaged EPSPs include between 50 and 300 sweeps.
Figure 3. Pyramid to bistratified cell EPSPs increase in amplitude and duration with postsynaptic depolarization.
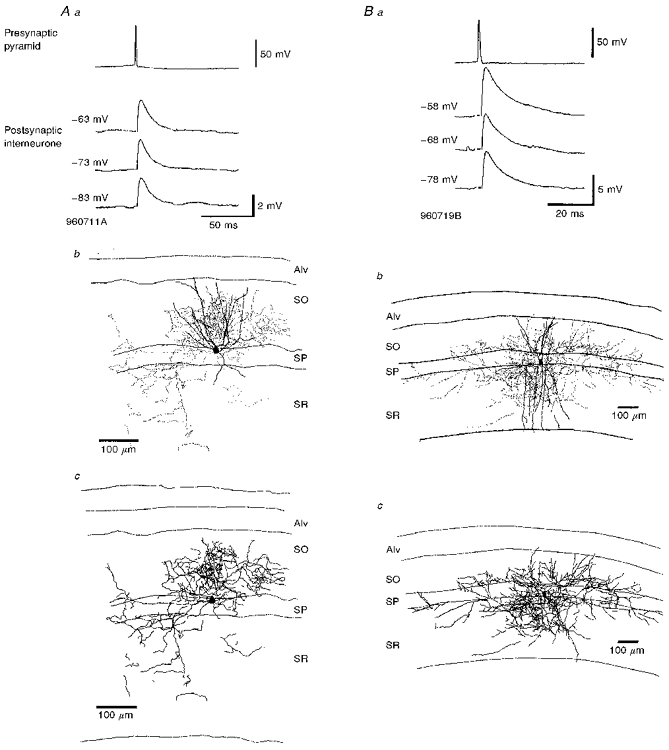
Aa and Ba, averaged EPSPs recorded in two different bistratified cells each at three different membrane potentials in response to single spikes in presynaptic pyramids. These EPSPs increased in average amplitude and duration with postsynaptic depolarization (cell codes 960711A and 960719B). Ab and Bb, in each case the reconstruction indicates the soma and dendrites of the postsynaptic bistratified cells and the dots represent the distribution of the terminal boutons as established at the light microscopic level. In Ab the axon terminated mostly in SO with some also evident in SR. The dendrites of this cell in SR are drawn truncated as their identification in one section was ambiguous. In Bb the boutons were located mostly in SO and SR, with only a few in SP. The dendrites of this cell extended throughout SR but did not enter SLM. Ac and Bc, the distribution of the complete axon of the same cells and only the soma of the interneurones is illustrated. The distribution of the axon was largely similar to the distribution of the boutons (Ab and Bb).
Figure 5. EPSPs recorded in bistratified cells in response to trains of spikes in single presynaptic pyramidal neurones exhibit brief train depression.
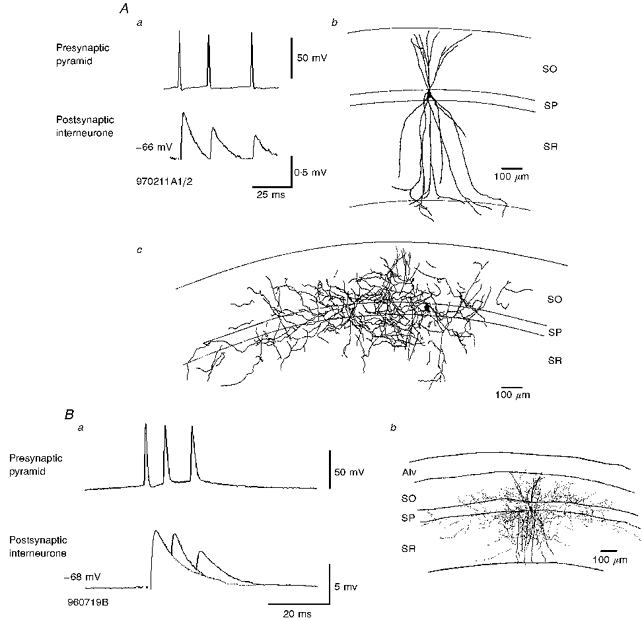
Aa, averaged postsynaptic responses recorded in a bistratified cell to a brief train of three APs in a presynaptic pyramid. With each successive AP in the train, the EPSP decreased in average amplitude. The interneurone soma and dendrites are shown in the camera lucida reconstruction in Ab and the axon separately in Ac. The axon arborized mostly in SO and SR, but also, to a lesser extent, in SP. The dendrites extended throughout SO and SR and entered only the very proximal portion of SLM (cell code 970211A1/2). Ba, brief train depression of an EPSP in another bistratified cell (drawn in Bb and earlier in more detail in Fig. 2_B_ c). The averaged EPSPs in this cell exhibited stronger brief train depression than in Aa, with the briefer interspike intervals recorded here (cell code 960719B).
Electrophysiological properties of CA1 cell body layer interneurones
Interneurones recorded in stratum pyramidale of CA1 could be subdivided into burst firing (BF), regular spiking (RS), classical fast spiking (CFS) interneurones and fast spiking interneurones with a rounded spike after-hyperpolarization (R-AHP) (see Table 1 for details and Fig. 1 for examples). However, interneurones in any one of three of these electrophysiologically defined groups could belong to either of the two main morphological cell types discussed here.
Table 1.
Electrophysiological properties of CA1 cell body layer interneurones
| AP | AP-AHP | Train AHP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cell | Threshold (mV) | Amplitude (mV) | Half-width (ms) | Amplitude (mV) | Half-width (ms) | Input resistance (MΩ) | Time constant | Amplitude (mV) | Half-width (ms) | |
| RS cells | ||||||||||
| 960725A1 | BistratI | −57 | 70 | 1 | 10 | 66 | 30 | 14 | 2.5 | 73 |
| 970514H | BistratI | −55 | 80 | 0.7 | 8.5 | 122 | 35 | 14.5 | 2 | 73 |
| 970425B | BasketI | −52 | 78 | 0.8 | 2.5 | 35 | 17.5 | 12.5 | 4 | 120 |
| 970430A3 | BasketI | −52 | 55 | 0.35 | 15 | 19.6 | 42.5 | 6.4 | 3 | 131 |
| 960805B | BistratI | — | 65 | 1 | 7 | 12 | 25 | 11 | 4.5 | 200 |
| 960624A | BasketI | −50 | 79 | 1.1 | 7.5 | 7.6 | 50 | 9.8 | 4 | 241 |
| Mean ±s.d. | 0.82 ± 0.25 | 8.4 ± 3.7 | 43.7 ± 40 | 33.3 ± 10.8 | 11.4 ± 2.7 | 3.3 ± 0.9 | 140 ± 62 | |||
| RS/BF cells | ||||||||||
| 960808D | BasketI | — | 60 | 0.5 | 8 | 23 | 32.5 | 9.4 | 3 | >200 |
| 960807A1 | BasketI | −51 | 58 | 0.3 | 9.5 | 8 | 19 | 10.2 | 2 | 31 |
| BF cells | ||||||||||
| 970403B1 | UnconE | −51 | 65 | 0.35 | 3 | 4 | 17 | 12 | 1.5 | 160 |
| 970319A1 | BasketI | −55 | 80 | 0.6 | 1.5 | 3.5 | 33 | 10 | 15 | >200 |
| Mean ±s.d. | 0.44 ± 0.12 | 5.5 ± 3.3 | 9.6 ± 7.9 | 25.4 ± 7.4 | 10.4 ± 1.0 | 5.37 ± 5.58 | 147 ± 69 | |||
| R-AHP cells | ||||||||||
| 960624B | BasketE | — | 50 | 0.8 | 20 | 25 | 87.5 | 5 | 6 | 137 |
| 970211A1/2 | BistratE/I | −54 | 75 | 0.3 | 12 | 40 | 86 | 8 | 5 | >200 |
| 960805A | BistratI | — | 60 | 0.5 | 12 | 13 | 35 | 5.6 | 2 | — |
| 960711A | BistratE | 50 | 53 | 0.5 | 19 | 70 | 90 | 7.3 | 1.5 | 37 |
| Mean ±s.d. | 0.52 ± 0.18 | 15.7 ± 3.77 | 37 ± 21.3 | 74.6 ± 22.9 | 6.5 ± 1.2 | 3.6 ± 1.9 | 125 ± 67 | |||
| CFS cells | ||||||||||
| 961010A1 | BasketE | — | 50 | 0.4 | 14.5 | 12 | 24 | 4.8 | 2.5 | 81 |
| 931012D1 | BasketE | −57 | 63 | 0.3 | 9.7 | 9 | 25 | 9 | 1 | 200 |
| 950831A1 | BasketE | −55 | 74 | 0.3 | 7 | 2 | 35 | 3 | 9 | 50 |
| 950824 | Uncon | −56 | 73 | 0.3 | 9.5 | 2 | 15 | 3 | 1 | — |
| 950817 | Uncon | −56 | 72 | 0.3 | 10 | 2 | 24 | 4 | 1 | — |
| Mean ±s.d. | 0.32 ± 0.04 | 10.1 ± 2.4 | 5.4 ± 4.3 | 24.6 ± 6.3 | 4.8 ± 2.2 | 2.9 ± 3.1 | 110 ± 65 |
Figure 1. Basket and bistratified cells displayed diverse electrophysiological characteristics.
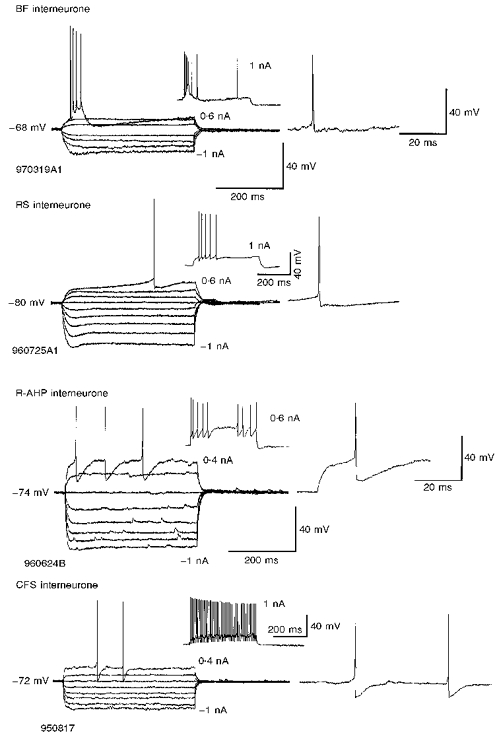
Interneurones of different morphological classes could display similar electrophysiological characteristics and those of the same class different electrophysiological features. They are classified here according to the shape and pattern of their discharge in response to depolarizing current injection into burst firing (BF), regular spiking (RS), classical fast spiking (CFS) or interneurones with a rounded spike after-hyperpolarization (R-AHP); see Table 1 for details of properties of each cell type. Burst firing interneurones responded to threshold depolarizations with a burst of high frequency action potentials (APs). Regular spiking interneurones were often difficult to distinguish from pyramidal neurones and were confirmed as interneurones only by morphological features, or by the IPSPs they elicited in other neurones. The example shown here resembles a neocortical late spiking cell (see Kawaguchi & Kubota, 1997). R-AHP interneurones were recognizable by the deep, rounded spike AHPs at threshold and non-accommodating fast spiking behaviour at more depolarized potentials (not illustrated). CFS interneurones had simple, fast spike AHPs and spike trains showed little accommodation or adaptation. Responses to subthreshold pulses are averages of between two and five sweeps.
Burst firing interneurones responded to depolarizing current injections applied from membrane potentials negative to -60 to -65 mV with a high frequency burst of APs (Fig. 1). If held depolarized for a longer period they could fire tonically, but the threshold response was always a burst. One of the BF cells was identified as a basket cell (Table 1). The other was not fully recovered histologically. Another two basket cells (as well as two putative radial trilaminar interneurones that are not reported in any detail here) displayed properties intermediate between BF and RS cells. They could fire a single AP at threshold, but larger depolarizing pulses elicited a burst of APs superimposed upon a depolarizing envelope. These four cells had fast APs, relatively low input resistances and long time constants. Regular spiking interneurones were often difficult to distinguish from pyramidal neurones and could be either basket or bistratified cells according to their morphological features. They had the broadest APs and the longest spike AHPs, but similar input resistances and time constants to the BF cells. This broad group of RS cells also includes cells reminiscent of neocortical late spiking interneurones (Kawaguchi & Kubota, 1997; see Fig. 1 for an example). Four interneurones (1 basket and 3 bistratified cells) were classified as R-AHP interneurones by the rounded shape of their spike after-hyperpolarizations, which were longer lasting than the spike AHPs of CFS cells near spike threshold. R-AHP cells had the highest input resistances, but with their brief time constants, deep spike AHPs and relative lack of spike accommodation or frequency adaptation (during large depolarizing pulses, not illustrated) they resembled CFS cells. Two of the interneurones defined as CFS were basket cells. The other two were not recovered sufficiently for identification. However, one interneurone (not included in Table 1 because its AP did not overshoot 0 mV) resembled other CFS cells and was identified as a bistratified cell (cell 960719A, shown in Fig. 2_A_). The APs of CFS cells had simple, fast spike AHPs and trains of spikes showed little accommodation or adaptation. CFS cells had relatively low input resistances, brief time constants and a linear current-voltage response negative to resting potential (Table 1, Fig. 1).
Morphology of postsynaptic interneurones
The recorded, filled and recovered postsynaptic interneurones could be divided into two broad classes, basket and bistratified cells, based on their axonal and dendritic morphology. These classifications were based on those described previously (Buhl et al. 1994_a_;Sík et al. 1995; Buhl et al. 1996; Halasy et al. 1996).
Bistratified cells
Interneurones were classified as bistratified cells when their axons ramified mainly in the proximal half of stratum radiatum and throughout stratum oriens (Figs 2_Ac_, 2_Bc_, 3_Ac_, 3_Bc_ and 5_Ac_). Their dendrites were generally less beaded than those of the basket cells and rarely extended significantly into stratum lacunosum-moleculare (Fig. 2_Ab_, 2_Bb_, 3_Ab_, 3_Bb_ and 5_Ab_). In the present study the cells classified as bistratified cells displayed some, if not all of these features. However, one example also had substantial axonal arborization in stratum pyramidale (Fig. 2_Bc_). As the tissue was not prepared for electron microscopy the postsynaptic targets of this axon could not be ascertained and it is not certain whether this is a bistratified cell or a trilaminar interneurone (Sík et al. 1995) with radially orientated dendrites. For the purpose of this report it will be considered within the bistratified group since the EPSP recorded in this neurone displayed properties similar to those of others reported here (cell 960717B, Fig. 2_B_).
Basket cells
Cells were classified as basket cells by the following main features: their axons covered the entire depth of stratum pyramidale and only very proximal regions of stratum radiatum and stratum oriens and were arranged in dense networks of fibres criss-crossing the cell body layer (Figs 4_Ac_, 4_Bc_, 6_B_ and 6_C_). Their dendrites were usually beaded and extended into stratum lacunosum-moleculare when fully labelled. In some cases not all of these features were apparent. For example, in a few cases only the axon was recovered histologically (Fig. 6_B_) but here the cell was identifiable as a basket cell by its axonal distribution. They are unlikely to be axo-axonic cells whose axons occupy only half of stratum pyramidale and the most proximal region of stratum oriens (Buhl et al. 1994_b_). We did not record and fill any such cell in the present study.
Properties of pyramidal cell to bistratified cell EPSPs
The properties of spontaneous EPSPs recorded in postsynaptic bistratified cells are summarized for each cell in Table 2. The rate of spontaneous EPSPs in these cells was relatively high and ranged from 2.8 to 22 s−1 and their average amplitude from 1.40 to 4.45 mV (mean, 2 ± 1.1 mV). There appeared to be no relationship between frequency and size of spontaneous EPSPs. The properties of EPSPs in response to single APs, or the first of a train of APs, are also summarized in Table 2. The average amplitude of these EPSPs recorded in seven pairs varied widely and could be as large as 9 mV in average amplitude (Fig. 2_Aa_ and 2_Ba_). These EPSPs were relatively brief with rise times < 2 ms and widths at half-amplitude between 4 and 11 ms (Figs 2_Aa_, 2_Ba_, 3_Aa_, 3_Ba_ and 5_A_). The apparent failure rate in response to the first presynaptic AP ranged from 0 (no failures) to 36 % (mean, 6.4 ± 12.2 %). The EPSPs displayed non-conventional voltage relations with an average increase in amplitude of 23.7 ± 14.9 % with a 10 mV depolarization of the postsynaptic interneurone from rest (_n_= 4; Fig. 3_Aa_ and Ba).
Table 2.
Properties of spontaneous EPSPs and EPSPs elicited in bistratified cells by single spikes in presynaptic pyramidal cells
| Spontaneous EPSPs | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cell no. | No. per second | Average amplitude (mV) | EPSP average ampitude (mV) | Membrane potential (mV) | EPSP 10–90 % rise time (ms) | EPSP half-width (ms) | Failure rate 1st EPSP | Voltage relation change in ampl. with 10 mV depol. (%) | |
| 960227A | 12.2 | 1.40 | 2.56 | −66 | 2.0 | 11.2 | 0/200 (0 %) | — | |
| 960711A | R-AHP | 16.2 | 1.49 | 2.88 | −73 | 1.6 | 10.8 | 12/772 (1.6 %) | +7 |
| 960717B | 6.1 | 1.62 | 0.72 | −61 | 0.7 | 5.5 | 35/96 (36 %) | — | |
| 960719A | CFS | 2.8 | 4.45 | 9.06 | −66 | 0.8 | 5.4 | 16/259 (6 %) | — |
| 960719B | R-AHP | 6.6 | 2.23 | 7.29 | −68 | 0.9 | 7.7 | 15/1361 (1.1 %) | +19 |
| 960604B | R-AHP | 22 | 1.55 | 1.13 | −72 | 0.8 | 8.4 | 0/300 (0 %) | +21 |
| 970211A1/2 | R-AHP | 15 | 1 | 0.5 | −76 | 1.6 | 3.9 | 0/695 (0 %) | +48 |
| Mean ±s.d. | 11.6 ± 6.2 | 2 ± 1.1 | 3.4 ± 3.1 | — | 1.2 ± 0.5 | 7.6 ± 2.6 | 6.4 ± 12.2 % | 24 ± 15 |
The properties of the EPSPs recorded in bistratified cells in response to second and third APs delivered in brief trains are shown in Table 3. Second EPSPs were the same shape as first EPSPs, but generally smaller and third and fourth EPSPs were smaller than second EPSPs (_n_= 5; Fig. 5_Aa_ and Ba). With an interspike interval of < 15 ms the second EPSP amplitude was on average 72 ± 27 % of the first EPSP (_n_= 3). Failures were observed in response to second APs where no failures had been apparent in responses to first APs (11 of 800 and 30 of 695, respectively). Failure rates in response to third APs were higher than following second APs in the two pairs so tested.
Table 3.
Properties of EPSPs elicited in bistratified cells by second and third action potentials in brief bursts
| Cell no. | 2nd EPSP as % 1st <15 ms | 2nd EPSP as % 1st 15–30 ms | 2nd EPSP failure rate <15 ms | 2nd EPSP failure rate 15–30 ms | 3rd EPSP as % 1st | 3rd EPSP failure rate | |
|---|---|---|---|---|---|---|---|
| 960227A | — | 100 | — | (0 %) | — | — | |
| 960711A | R-AHP | — | 65 (48–64 ms) | — | 6/118 (5.1 %) | — | — |
| 960719B | R-AHP | 34 | — | — | — | 16 | — |
| 960604B | R-AHP | 94 | — | 11/800 (1.4 %) | — | 90 | 40/800 (5 %) |
| 970211A1/2 | R-AHP | 88 | — | 30/695 (4.3 %) | — | 42 | 140/695 (20 %) |
| Mean ±s.d. | 72 ± 27 | — | — | — | 49 ± 31 | — |
Properties of EPSPs recorded in pyramidal cell to basket cell connections
The properties of spontaneous and AP-elicited EPSPs recorded in basket cells are shown in Table 4. Spontaneous EPSPs occurred at a similar frequency to those observed in bistratified neurones, but were typically of smaller amplitude. EPSPs elicited by the first AP in a train were smaller and a little briefer than those recorded in bistratified cells, although with small samples these differences do not reach significance. Typical failure rates were higher than for bistratified cell EPSPs, possibly reflecting the lower average amplitudes. However, the depression observed during responses to trains of presynaptic APs was similar (Table 5 and Figs 4, 6 and 7). Basket cell EPSPs did not exhibit a consistent response to changes in membrane potential. Two EPSPs so tested increased in amplitude and two decreased with postsynaptic depolarization (Fig. 4).
Table 4.
Properties of spontaneous EPSPs and EPSPs elicited in basket cells by single spikes in presynaptic pyramidal cells
| Spontaneous EPSPs | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cell no. | No. per second | Average amplitude (mV) | EPSP average (mV) | Membrane potential (mV) | EPSP 10–90 % rise time (ms) | EPSP Half-width (ms) | Failure rate 1st EPSP | Voltage realtion change in ampl. with 10 mV depol. (%) | |
| 970220A1 | 13 | 0.6 | 3.57 | −76 | 0.6 | 5.2 | 0/77 | — | |
| 970317A1 | 10 | 1.6 | 1.88 | −76 | 0.4 | 2.2 | 0/100 | — | |
| 961010A | CFS | 20 | 0.7 | 1.66 | −70 | 0.4 | 4.2 | 0/600 | +15 |
| 931012D1 | CFS | 6.2 | 0.5 | 0.5 | −69 | 1.1 | 9.7 | 23/143 (16 %) | −18 |
| 950531A1 | 2.7 | 0.9 | 0.83 | −70 | 1.6 | 5.8 | 361/754 (48 %) | −17 | |
| 960624B | R-AHP | 11 | 0.6 | 1.13 | −70 | 1.4 | 6.6 | 0 | +10 |
| 950831A1 | CFS | 0.5 | 0.7 | 0.15 | −60 | 0.7 | 4 | 41/84 (49 %) | — |
| Mean ±s.d. | 9 ± 6.1 | 0.8 ± 0.34 | 1.4 ± 1.05 | — | 0.88 ± 0.44 | 5.4 ± 2.2 | 16 ± 21 % | −2.5 ± 15 | |
| Properties of EPSPs elicited in interneurones with unconfirmed morphology | |||||||||
| 961128B | 20 | 0.85 | 1.65 | −70 | 0.4 | 3.4 | 0 | +22 | |
| 970403B1 | BF | 35 | 1.75 | 1.83 | −75 | 1.6 | 5.6 | 0/88 (0 %) | — |
Table 5.
Properties of EPSPs elicited in basket cells by second and third action potentials in brief bursts
| Cell no. | 2nd EPSP as % 1st < 15 ms | 2nd EPSP as % 1st 15–30 ms | 2nd EPSP failure rate < 15 ms | 2nd EPSP failure rate 15–30 ms | 3rd EPSP as % 1st | 3rd EPSP failure rate | |
|---|---|---|---|---|---|---|---|
| 970220A1 | 75 | — | 15/77 (19.5 %) | — | 56 | 15/77 (19.5 %) | |
| 970317A1 | 59 | — | 4/100 (4 %) | — | 53 | 10/100 (10 %) | |
| 961010A1 | CFS | 48 | — | 20/1600 (1.25 %) | — | 87 | 135/1600 (8.4 %) |
| 931012D1 | CFS | — | 80 | — | 38/99 (38 %) | — | — |
| 950531A1 | 54 | 90 | 127/256 (49.6 %) | — | 46 | — | |
| 960624B | R-AHP | 60 | 73 | 9/350 (2.5 %) | — | 80 | 30/350 (8.5 %) |
| 950831A1 | CFS | — | 100 | 40/80 (50 %) | — | 100 | 44/80 (55 %) |
| Mean ±s.d. | 59 ± 9 % | 86 ± 10 % | 21 ± 21 % | 70 ± 20 % | 20 ± 18 % | ||
| Properties of EPSPs elicited in cells with unconfirmed morphology by 2nd and 3rd spikes in a presynaptic pyramidal cell | |||||||
| 961128B | 54 | — | 9/280 (3.2 %) | — | 42 | 34/280 (12 %) | |
| 970403B1 | BF | 51 | 59 | 2/48 (4 %) | — | 31 | 3/12 (25 %) |
Figure 7. Frequency-dependent depression in two pyramid to basket cell connections.
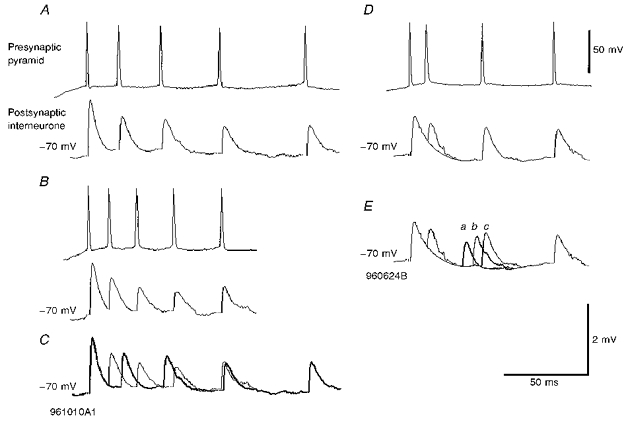
In A and B averaged responses to two trains each of five presynaptic APs are shown. The first EPSP for both trains was the same. In A the interspike intervals were longer than in B and averaged 3rd, 4th and 5th EPSPs were less strongly depressed than in B. See comparison in C in which the bold trace repeats the averaged EPSPs in A superimposed on the averaged response shown in B (cell code 961010A1). D and E illustrate averaged EPSPs elicited by three types of trains, each of four presynaptic APs, in which the 3rd AP occurred at three different interspike intervals. Responses to the 1st, 2nd and 4th APs are the same for all three trains. One example is shown in D and in E, the three 3rd EPSPs are superimposed for comparison (a, b and c). Some recovery from the strong 3rd EPSP depression apparent at the briefest interval can be seen at the longer intervals (cell code 960624B).
Figure 8. Schematic of the excitatory connections made by CA1 pyramidal cells with three classes of interneurones.
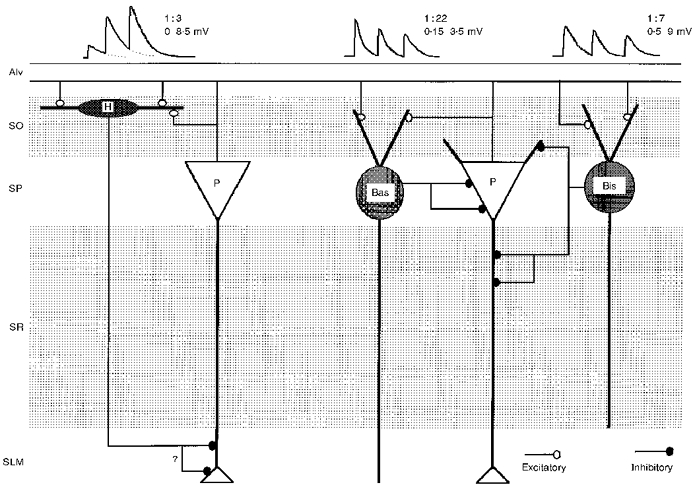
The EPSPs elicited in basket cells (Bas) and bistratified cells (Bis) exhibit frequency-dependent depression. In contrast, the EPSPs elicited in horizontally oriented oriens/alveus interneurones (H) display frequency-dependent facilitation (see Ali & Thomson, 1997, 1998). The probabilities of finding connections in randomly selected pyramid-interneurone pairs in these parallel studies are indicated and the ranges of average EPSP amplitudes recorded. For bistratified and basket cells, the largest EPSP amplitudes are for the first EPSP and for horizontally oriented O/A interneurones for the 4th or 5th EPSPs.
The third group of interneurones were recorded with the electrode placed in the stratum pyramidale but were not sufficiently well recovered histologically for morphological classification. However, their properties and those of their EPSPs are similar to those of the other two groups and are therefore included in Tables 4 and 5 (_n_= 2).
DISCUSSION
This paper describes the properties of pyramidal cell to bistratified cell connections for the first time and details further the properties of pyramidal cell to basket cell connections in the CA1 region of the adult rat hippocampus in vitro. The data presented demonstrate that these connections are not uncommon (there was a probability of 1:22 for basket cells and 1:7 for bistratified cells) and the resultant EPSPs can be large, especially in bistratified cells, but that they exhibit paired pulse and brief train depression when pyramidal cells fire repetitively. With an estimated 1000 pyramidal cells in the CA1 region of a typical transverse slice, each basket cell could receive excitatory inputs (with average EPSP amplitudes between 0.1 and 3.5 mV, mean 1.4 mV) from fifty and each bistratified cell inputs (with average EPSP amplitudes between 0.5 and 9 mV, mean 3.4 mV) from 140 CA1 pyramidal cells.
Cells of the same morphological class could display different electrophysiological properties. Bistratified cells could be classified electrophysiologically as RS cells or R-AHP cells (Table 1), or possibly as CFS cells and basket cells could display any one of the four behaviours observed. In addition, morphologically different interneurones could share the same electrophysiological features, e.g. both basket and bistratified cells could be RS or R-AHP cells (Table 1). To date, the only RS/BF or BF cells recovered histologically have been basket cells (or putative radial trilaminar interneurones, not reported here), but with a small sample it would be premature to claim a distinction. A similar variation has been described in the electrophysiological properties within the axo-axonic cell class (Buhl, Han, Lorinczi, Stezhka, Karnup & Somogyi, 1994_b_). The results of the present study and those of Buhl et al. (1994_b_) indicate the necessity for morphological identification of interneurones. This is particularly important with regular spiking interneurones since they are easily confused electrophysiologically with pyramidal cells. It also suggests that there may be distinct functional sub-classes within morphological classes. In the neocortex the firing patterns of presynaptic interneurones were found to correlate with the duration of the IPSPs they elicited in postsynaptic pyramidal cells. Even within a broad class of interneurones with basket-like terminations, fast spiking interneurones elicited briefer IPSPs than interneurones displaying regular spiking behaviour (Thomson, West, Hahn & Deuchars, 1996).
We could not therefore, distinguish electrophysiologically between basket and bistratified cells, although others have reported significant differences between the two groups (Buhl et al. 1996). It may be more appropriate to consider EPSP parameters, such as time course, in relation to the electrophysiological properties of the postsynaptic neurone, rather than, or in addition to, its morphological class. In the present study, most of the well characterized postsynaptic interneurones were either CFS or R-AHP cells, possessing relatively brief time constants and EPSPs that are typically briefer than EPSPs recorded using similar protocols in CA1 pyramidal cells. In the present study, no well characterized RS interneurones were recorded as postsynaptic partners of pyramidal cells. It remains to be determined therefore whether these cells are less densely innervated by CA1 pyramidal cells, or whether, being less readily identified, they were simply less rigorously studied.
EPSPs elicited in interneurones by action potentials in pyramidal cells
Voltage relations of EPSPs
EPSPs elicited in basket cells and bistratified cells frequently displayed a non-conventional voltage relation, i.e. an increase in EPSP amplitude with depolarization of the postsynaptic cell. In neocortex (Thomson et al. 1993_a_, 1995; Buhl, Tamas, Szilagyi, Stricker, Paulsen & Somogyi, 1997) and in CA3 (Miles, 1990) pyramidal cell-interneurone EPSPs were found to decrease in amplitude with depolarization, despite partial mediation of at least some of these EPSPs by NMDA receptors (e.g. Thomson, 1997). NMDA receptors have been shown to mediate, in part, the EPSPs elicited in CA1 pyramidal cells by neighbouring pyramidal cells and to contribute to their non-conventional voltage relations (Deuchars & Thomson, 1996). These receptors may also be involved in the present pyramidal cell-interneurone synapses.
Amplitude and time course of EPSPs
The average amplitudes of the EPSPs elicited by single presynaptic APs in the present study were generally larger than those previously reported for pyramidal cell-interneurone connections in hippocampus. Indeed for one pyramidal cell-bistratified interneurone connection, the average EPSP was 9.06 mV in amplitude at -66 mV. Such large average EPSP amplitudes would suggest that at least some of these connections are mediated by multiple release sites and together with the moderately high probability of connectivity, that the excitatory drive to stratum pyramidale interneurones from neighbouring CA1 pyramidal cells can be very powerful, particularly that to bistratified cells.
However, the relatively fast time courses of these EPSPs reduces the opportunity for temporal summation of EPSPs, compared with EPSPs in CA1 pyramidal cells. Summation of inputs from several presynaptic pyramidal cells would therefore require tighter synchrony for effective recruitment of these interneurones than would recruitment of other pyramidal cells.
Depression at pyramidal cell to basket and bistratified cell connections
In this study of the paired pulse and brief train effects at pyramidal cell-interneurone synapses in CA1, depression was found to be prevalent at synapses onto stratum pyramidale interneurones. This is in marked contrast to the profound facilitation observed using the same protocols at certain classes of pyramidal cell-interneurone connection in the neocortex (Thomson et al. 1993_a_, 1995; Deuchars & Thomson, 1995; Thomson, 1997). However, pyramidal cell to horizontally orientated oriens/alveus interneurone connections in the hippocampus also display marked facilitation (Ali & Thomson, 1997) and some, as yet incompletely characterized, neocortical pyramidal cell- interneurone connections also exhibit paired pulse depression (Thomson & Deuchars, 1997). Since the paired pulse effects that have been observed to date are consistent across morphological subclasses within any one region, the effect seems to be dependent on the identity of the postsynaptic cell. However, the factor(s) that determine(s) how the postsynaptic target influences the behaviour of the presynaptic terminal have yet to be established.
The mechanisms involved in presynaptically mediated synaptic depression have been studied more extensively at pyramidal cell-pyramidal cell connections in the neocortex of adult (Thomson, 1997 for discussion of the time course of depression) and juvenile rats (Markram & Tsodyks, 1996; Markram, 1997). There are several putative mechanisms (see Markram, 1997) but a current hypothesis is that such synaptic depression is due to refractoriness at release sites, i.e. once a release site has released, it is refractory for tens of milliseconds (Thies, 1965; Betz, 1970; Koerber & Mendel, 1991; Thomson et al. 1993_b_;Stevens & Wang, 1995; Thomson, 1997). The larger the proportion of available release sites that has released, the smaller will be the proportion available for the next release.
Functional importance of synaptic depression in hippocampal local circuits
The functional consequences of synapses exhibiting paired pulse and brief train depression have been studied and modelled (Markram & Tsodyks, 1996; Abbott, Varela, Sen & Nelson, 1997). These models propose that small changes in presynaptic firing rate (especially changes from low to high rates) will generate a phasic response, but that these connections convey little information about static firing rates. Once a population of pyramidal cells has been active for even a few tens of milliseconds and their synapses onto basket and bistratified cells have depressed, little additional activation of these interneurones will result from an increase in firing in these pyramidal cells. Only recruitment of additional pyramidal cells will enhance their activation. This is in marked contrast to the patterns of activity that most effectively excite horizontally oriented oriens/alveus interneurones (Ali & Thomson, 1997, 1998). These interneurones will be more effectively recruited by a small population of pyramidal cells firing at high frequency than by a large population firing slowly and tonically.
The dynamic behaviour of CA1 pyramidal inputs onto three types of interneurones and onto other pyramidal cells in the CA1 region have therefore been studied in some detail. Relatively few, rather sparse axonal branches of pyramidal cells have been seen to cross stratum pyramidale and to enter stratum radiatum in these studies, the majority of their axonal arbour being confined to stratum oriens and the alveus. The innervation that basket and bistratified cells can receive from CA1 pyramidal cells, therefore, may largely be confined to the oriens. It is perhaps surprising that basket and particularly bistratified cells with dendrites that also span stratum radiatum receive such a strong input from a relatively discrete portion of their dendritic tree. The properties of the inputs that bistratified cells receive in stratum radiatum and that basket cells receive in radiatum and lacunosum-moleculare (where entorhinal cortex input to CA1 occurs), and the interactions amongst these several inputs, remain to be determined.
Acknowledgments
This work was supported by Novartis Pharma (Basel), The MRC and The Wellcome Trust. A.B.A is a Novartis Pharma (Basel) funded student.
References
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- Aika Y, Ren JQ, Kosaka K, Kosaka T. Quantitative analysis of GABA-like-immunoreactive and parvalbumin-containing neurons in the CA1 region of the rat hippocampus using a stereological method, the dissector. Experimental Brain Research. 1994;99:267–276. doi: 10.1007/BF00239593. [DOI] [PubMed] [Google Scholar]
- Ali AB, Thomson AM. Brief train depression and facilitation at pyramid-interneurone connections in slices of rat hippocampus; paired recordings with biocytin filling. The Journal of Physiology. 1997;501.P:9. P. [Google Scholar]
- Ali AB, Thomson AM. Facilitating pyramid to horizontal oriens-alveus interneurone inputs: dual intracellular recordings in slices of rat hippocampus. The Journal of Physiology. 1998;507:185–199. doi: 10.1111/j.1469-7793.1998.185bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz WJ. Depression of transmitter release at the neuromuscular junction of the frog. The Journal of Physiology. 1970;206:620–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl EH, Cobb SR, Halasy K, Somogyi P. Properties of unitary IPSPs evoked by anatomically identified basket cells in the rat hippocampus. European Journal of Neuroscience. 1995;7:1989–2004. doi: 10.1111/j.1460-9568.1995.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994a;368:823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Han Z-S, Lorinczi Z, Stezhka VV, Karnup SV, Somogyi P. Physiological properties of anatomically identified axo-axonic cells in the rat hippocampus. Journal of Neurophysiology. 1994b;71:1289–1307. doi: 10.1152/jn.1994.71.4.1289. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Szilágyi T, Halasy K, Somogyi P. Physiological properties of anatomically identified basket and bistratified cells in the CA1 area of the rat hippocampus in vitro. Hippocampus. 1996;6:294–305. doi: 10.1002/(SICI)1098-1063(1996)6:3<294::AID-HIPO7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Támas G, Szilágyi T, Stricker C, Paulsen O, Somogyi P. Effect, number and location of synapses made by single pyramidal cells onto aspiny interneurones of cat visual cortex. The Journal of Physiology. 1997;500:689–713. doi: 10.1113/jphysiol.1997.sp022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gähwiler BH, Thompson SM. Paired pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. The Journal of Physiology. 1996;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuchars J, Thomson AM. Innervation of burst firing spiny interneurons by pyramidal cells in deep layers of rat somatomotor cortex - paired intracellular recordings with biocytin filling. Neuroscience. 1995;69:739–755. doi: 10.1016/0306-4522(95)00288-t. 10.1016/0306-4522(95)00288-T. [DOI] [PubMed] [Google Scholar]
- Deuchars J, Thomson AM. CA1 pyramid-pyramid connections in rat hippocampus in vitro: dual intracellular recordings with biocytin filling. Neuroscience. 1996;74:1009–1018. doi: 10.1016/0306-4522(96)00251-5. [DOI] [PubMed] [Google Scholar]
- Deuchars J, West DC, Thomson AM. Relationships between morphology and physiology of pyramid-pyramid single axon connections in rat neocortex in vitro. The Journal of Physiology. 1994;478:423–435. doi: 10.1113/jphysiol.1994.sp020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:345–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Miles R, Hájos N, Freund TF. Precision and variability in postsynaptic target selection of inhibitory cells in the hippocampal CA3 region. European Journal of Neuroscience. 1993a;5:1729–1751. doi: 10.1111/j.1460-9568.1993.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Miles R, Sík A, Tóth K, Tamamaki N, Freund TF. Hippocampal pyramidal cells excite inhibitory neurons through a single release site. Nature. 1993b;366:683–687. doi: 10.1038/366683a0. 10.1038/366683a0. [DOI] [PubMed] [Google Scholar]
- Halasy K, Buhl EH, Lorinczi Z, Tamas G, Somogyi P. Synaptic target selectivity and input of GABAergic basket and bistratified interneurones in the CA1 area of the rat hippocampus. Hippocampus. 1996;6:306–329. doi: 10.1002/(SICI)1098-1063(1996)6:3<306::AID-HIPO8>3.0.CO;2-K. 10.1002/(SICI)1098-1063(1996)6:3<306::AID-HIPO8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cerebral Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Knowles WD, Schwartzkroin PA. Local circuit synaptic interactions in hippocampal brain slices. Journal of Neuroscience. 1981;1:318–322. doi: 10.1523/JNEUROSCI.01-03-00318.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber HR, Mendel LM. Modulation of synaptic transmission at Ia-afferent fiber connections on motoneurons during high frequency stimulation: role of postsynaptic target. Journal of Neurophysiology. 1991;65:590–597. doi: 10.1152/jn.1991.65.3.590. [DOI] [PubMed] [Google Scholar]
- Markram H. A network of thick tufted layer V pyramidal neurons. Cerebral Cortex. 1997;7:523–533. doi: 10.1093/cercor/7.6.523. 10.1093/cercor/7.6.523. [DOI] [PubMed] [Google Scholar]
- Markram H, Tsodyks M. Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature. 1996;382:807–810. doi: 10.1038/382807a0. 10.1038/382807a0. [DOI] [PubMed] [Google Scholar]
- Miles R. Synaptic excitation of inhibitory cells by single CA3 hippopcampal pyramidal cells of the guinea pig in vitro. The Journal of Physiology. 1990;428:61–77. doi: 10.1113/jphysiol.1990.sp018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R, Poncer J-C. Paired recordings from neurones. Current Opinion in Neurobiology. 1997;6:387–394. doi: 10.1016/s0959-4388(96)80124-3. 10.1016/S0959-4388(96)80124-3. [DOI] [PubMed] [Google Scholar]
- Miles R, Tóth K, Gulyás AI, Hájos N, Freund TF. Differences bewteen somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. 10.1016/S0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RKS. Unitary inhibitory synaptic potentials in the guinea-pig hippocampus in vitro. The Journal of Physiology. 1984;356:97–113. doi: 10.1113/jphysiol.1984.sp015455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncer J-C, Miles R. Fast and slow excitation of inhibitory cells in the CA3 region of the hippocampus. Journal of Neurobiology. 1994;26:386–395. doi: 10.1002/neu.480260310. [DOI] [PubMed] [Google Scholar]
- Sík A, Penttonen M, Ylinen A, Buzsáki G. Hippocampal CA1 interneurons: an in vivo intracellular labelling study. Journal of Neuroscience. 1995;15:6651–6665. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sík A, Tamamaki N, Freund TF. Complete axonal arborization of a single CA3 pyramidal cell in the rat hippocampus, and its relationship with postsynaptic parvalbumin-containing interneurons. European Journal of Neuroscience. 1993;5:1719–1728. doi: 10.1111/j.1460-9568.1993.tb00239.x. [DOI] [PubMed] [Google Scholar]
- Somogyi P. A specific ‘axo-axonal’ interneuron in the visual cortex of the rat. Brain Research. 1977;136:345–350. doi: 10.1016/0006-8993(77)90808-3. 10.1016/0006-8993(77)90808-3. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Wang Y. Facilitation and depression at single central synapses. Neuron. 1995;14:795–802. doi: 10.1016/0896-6273(95)90223-6. 10.1016/0896-6273(95)90223-6. [DOI] [PubMed] [Google Scholar]
- Thies RE. Neuromuscular depression and the apparent depletion of transmitter in mammalian muscle. Journal of Neurophysiology. 1965;28:427–442. doi: 10.1152/jn.1965.28.3.427. [DOI] [PubMed] [Google Scholar]
- Thomson AM. Activity-dependent properties of synaptic transmission at two classes of connections made by rat neocortical pyramidal axons in vitro. The Journal of Physiology. 1997;502:131–147. doi: 10.1111/j.1469-7793.1997.131bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J. Synaptic interactions in neocortical local circuits: dual intracellular recordings in vitro. Cerebral Cortex. 1997;7:510–522. doi: 10.1093/cercor/7.6.510. 10.1093/cercor/7.6.510. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J, West DC. Single axon excitatory postsynaptic potentials in neocortical interneurons exhibit pronounced paired pulse facilitation. Neuroscience. 1993a;54:347–360. doi: 10.1016/0306-4522(93)90257-g. 10.1016/0306-4522(93)90257-G. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J, West DC. Large, deep layer pyramid-pyramid single axon EPSPs in slices of rat motor cortex display paired pulse and frequency-dependent depression, mediated presynaptically and self-facilitation, mediated postsynaptically. Journal of Neurophysiology. 1993b;70:2354–2369. doi: 10.1152/jn.1993.70.6.2354. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC. Fluctuations in pyramid- pyramid excitatory postsynaptic potentials modified by presynaptic firing pattern and postsynaptic membrane potential using paired intracellular recordings in rat neocortex. Neuroscience. 1993;54:329–346. doi: 10.1016/0306-4522(93)90256-f. 10.1016/0306-4522(93)90256-F. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Deuchars J. Properties of single axon excitatory postsynaptic potentials elicited in spiny interneurons by action potentials in pyramidal neurones in slices of rat neocortex. Neuroscience. 1995;69:727–738. doi: 10.1016/0306-4522(95)00287-s. 10.1016/0306-4522(95)00287-S. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Hahn J, Deuchars J. Single axon IPSPs elicited in pyramidal cells by three classes of interneurones in slices of rat neocortex. The Journal of Physiology. 1996;496:81–102. doi: 10.1113/jphysiol.1996.sp021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Stanford IM, Jefferys JGR. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature. 1996;383:621–624. doi: 10.1038/383621a0. 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- Woodson W, Nitecka L, Ben-Ari Y. Organisation of the GABAergic system in the rat hippocampal formation: a quantitative immunocytochemical study. Journal of Comparative Neurology. 1989;280:254–271. doi: 10.1002/cne.902800207. [DOI] [PubMed] [Google Scholar]