GEF-H1 Couples Nocodazole-induced Microtubule Disassembly to Cell Contractility via RhoA (original) (raw)
Abstract
The RhoA GTPase plays a vital role in assembly of contractile actin-myosin filaments (stress fibers) and of associated focal adhesion complexes of adherent monolayer cells in culture. GEF-H1 is a microtubule-associated guanine nucleotide exchange factor that activates RhoA upon release from microtubules. The overexpression of GEF-H1 deficient in microtubule binding or treatment of HeLa cells with nocodazole to induce microtubule depolymerization results in Rho-dependent actin stress fiber formation and contractile cell morphology. However, whether GEF-H1 is required and sufficient to mediate nocodazole-induced contractility remains unclear. We establish here that siRNA-mediated depletion of GEF-H1 in HeLa cells prevents nocodazole-induced cell contraction. Furthermore, the nocodazole-induced activation of RhoA and Rho-associated kinase (ROCK) that mediates phosphorylation of myosin regulatory light chain (MLC) is impaired in GEF-H1–depleted cells. Conversely, RhoA activation and contractility are rescued by reintroduction of siRNA-resistant GEF-H1. Our studies reveal a critical role for a GEF-H1/RhoA/ROCK/MLC signaling pathway in mediating nocodazole-induced cell contractility.
INTRODUCTION
Two major components of the cellular cytoskeleton, actomyosin fibers and microtubules, cooperate to regulate a variety of physiological and pathological cell functions, including polarity, motility, and epithelial barrier permeability (Rodriguez et al., 2003). Although microtubules themselves do not generate contractile forces, it has been noticed that microtubule depolymerization induces the formation of actin stress fibers and cell contraction (Danowski, 1989; Brown et al., 1996; Verin et al., 2001; Birukova et al., 2004b). However, in spite of the apparent universality of this phenomenon, the molecular basis by which microtubule disassembly stimulates cell contractility is not clearly established. One model suggests that microtubules function as rigid frames that resist actomyosin contraction in a cellular tensegrity array (Wang et al., 2001). An alternative mechanism is that microtubules control cell contractility by enhancing myosin light chain (MLC) phosphorylation (Kolodney and Elson, 1995; Verin et al., 2001; Birukova et al., 2004b), rather than simply removing an opposing structural element. The phosphorylation of MLC at Ser19 regulates the interaction of myosin with actin to control cellular contractility. Microtubules could be involved in the delivery, removal, or regulation of molecules that affect the MLC regulatory pathway and thus contractility.
Indeed, it has been shown that microtubule depolymerization induces the activation of RhoA in association with the contractile response (Enomoto, 1996). RhoA acts through the downstream effector Rho-associated kinase (ROCK) to induce the assembly of actin stress fibers and focal adhesions (Amano et al., 1997). In particular, phosphorylation targets of ROCK include the myosin phosphatase target subunit (MYPT) of MLC phosphatase and MLC itself (Amano et al., 1996; Kimura et al., 1996; Riento and Ridley, 2003). Phosphorylation of MYPT results in the inhibition of MLC phosphatase activity and a concomitant increase in phosphorylated MLC (pMLC). Thus, ROCK can promote phosphorylation of MLC both directly and indirectly to induce its interaction with actin and enhance contractility.
Among microtubule-associated molecules, GEF-H1, a guanine nucleotide exchange factor for Rho (Ren et al., 1998), is particularly interesting. The activity of GEF-H1 toward RhoA is suppressed when it binds to microtubules and is increased when it is released from microtubules in various cell types (Krendel et al., 2002; Matsuzawa et al., 2004; Birukova et al., 2005; Chang et al., 2006). Activation of GEF-H1 is accompanied by increased actin stress fiber formation and myosin II–dependent contraction (Krendel et al., 2002). In the present study, we show that depletion of endogenous GEF-H1 using small interfering RNA (siRNA) techniques abolishes the contractile response to nocodazole-induced microtubule disassembly. Consistent with the known role(s) of RhoA in contractility, RhoA activation and subsequent phosphorylation of MLC were also impaired in GEF-H1–depleted cells. Importantly, the contractile phenotype upon nocodazole stimulation was rescued by expression of an siRNA-resistant GEF-H1, thus proving that GEF-H1 is the key mediator of the influence of microtubule disassembly on contractility. This mechanism accounts for the activation of RhoA by microtubule disruption and the subsequent enhancement of cell contractility by a ROCK-dependent increase in MLC phosphorylation in several cell types, suggesting it to be a general regulatory paradigm linking microtubule assembly status to cell contractility via RhoA.
MATERIALS AND METHODS
Reagents and Antibodies
Nocodazole was purchased from Sigma Chemical (St. Louis, Mo.) and dissolved in dimethyl sulfoxide (DMSO). The ROCK inhibitor Y27632 (Calbiochem, San Diego, CA) was dissolved in DMSO. Generation of the affinity purified rabbit polyclonal anti-GEF-H1 antibody used in this study has been described previously (Zenke et al., 2004). Commercial antibodies were as follows: α-tubulin (05-829, mouse, Sigma), MHCIIA (M-8064, rabbit, Sigma), paxillin (610051, mouse, BD Biosciences, San Jose, CA), actin (691002, mouse, EMD Biosciences, San Diego, CA), green fluorescent protein (GFP;A6455, rabbit, Molecular Probes, Eugene, OR), Alexa 568 phalloidin (A12380, Molecular Probes), RhoA (26C4, sc-418, mouse, Santa Cruz Biotechnology, Santa Cruz, CA), MLC (MY-21, M4401, mouse, Sigma), and pMLC (sc-12896, Thr18/Ser19, goat, Santa Cruz).
siRNA
Nontargeting control and GEF-H1-specific-targeting double-stranded RNA (dsRNA) oligonucleotides were purchased from Dharmacon Research (Boulder, CO). Each siRNA was described as follows: negative control siRNA pool: si_CONTROL_ non-targeting siRNA pool D-001206-13 (Birkenfeld et al., 2007); GEF-H1–specific siRNA pool containing 4 oligos: oligo 6, siGENOME ON-TARGETplus J-009883-06 (5′-GAAUUAAGAUGGAGUUGCAUU-3′); oligo 7, siGENOME ON-TARGETplus J-009883-07 (5′-GUGCGGAGCAGAUGUGUAAUU-3′); oligo 8, siGENOME ON-TARGETplus J-009883-08 (5′-GAAGGUAGCAGCCGUCUGUUU-3′); and oligo 9, siGENOME ON-TARGETplus J-009883-09 (5′-CCACGGAACUGGCAUUACUUU-3′).
DNA Constructs
Enhanced GFP (EGFP)-GEF-H1WT and EGFP-GEF-H1(DHmut) constructs in the mammalian expression vector pCMV5-EGFP have been described previously (Zenke et al., 2004). For rescue experiments, siRNA-resistant EGFP-GEF-H18R, EGFP-GEF-H19R, and EGFP-GEF-H19R(DHmut) constructs were prepared by site-directed mutagenesis to replace the original nucleotide sequence targeted by oligos 8 and 9 GEF-H1–specific siRNA individually without changing the amino acid sequence. To do this, EGFP-GEF-H1WT or EGFP-GEF-H1(DHmut) plasmid were used as DNA template. Two silent mutations were introduced as indicated by underlined letters in the GEF-H1–specific siRNA 8 and 9 target sequence (8: 5′-GAAGGUAGUAGUCGUCUGUUU-3′; 9: 5′-CCACGGAACUAGCGUUACUUU-3′). The mutations were confirmed by sequence analysis.
Cell Culture and Transient Transfection
Human HeLa cells were maintained in DMEM (Invitrogen-BRL, Gaithersburg, MD) containing 2 mM l-glutamine, 100 U/ml penicillin G, 100 U/ml streptomycin, and 8% fetal bovine serum (FBS). For siRNA transient transfection experiments, HeLa cells were grown on six-well plate in complete medium overnight before transfection (day 0). Cells were transfected with 3 μl of the indicated siRNA (20 μM) and 6 μl of Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) overnight according to the manufacturer's instruction (day 1). At 24 h after transfection, cells were trypsinized and replated onto culture Petri dishes or on glass coverslips with appropriate dilution (day 2). To transfect DNA constructs of EGFP-GEF-H1, cells were then transfected on the next day of replating with each DNA construct and Lipofectamine 2000 reagent for 5 h and replaced with complete medium (day 3). At 72 h after siRNA transfection, cells were assayed as described (day 4).
Two hematopoietic D2 stable lines, control LacZshRNA, and GEF-H1–depleted GEF-H1217shRNA, were produced as described previously (Chang et al., 2006). D2 cells were maintained in RPMI 1640 (Invitrogen-BRL) supplemented with 10% heat-inactivated FBS (Hyclone, Logan, UT), 2 mM l-glutamine, 100 U/ml penicillin G, and 100 U/ml streptomycin.
Microscopic Observation and Immunofluorescence Staining
For HeLa live cell phase-contrast images, cells were filmed on a Nikon TE2000-U microscope)Melville, NY) with CCD digital camera (CoolSNAP HQ; Photometrics, Tucson, AZ) equipped with 20× objective lens. The temperature was maintained at 37°C by a microscope stage heater. For time-lapse imaging, images were recorded at 5-s intervals. Digital images were acquired by MetaMorph image processing software (Universal Imaging, Downingtown, PA).
For immunofluorescence staining, HeLa cells on coverslip were fixed with 4% paraformaldehyde in PBS at 37°C for 30 min and then permeabilized with 0.5% Triton X-100 in PBS at RT for 5 min. The cells were washed with PBS and blocked by incubation with 5% bovine serum albumin (BSA)/PBS for 1 h. Coverslips were then incubated with antibody against α-tubulin (1:1000 dilution), MHCIIA (1:100 dilution), paxillin (1:500 dilution), or GFP (1:500 dilution) in PBS containing 2% BSA for 2 h at RT. After PBS wash, the cells were incubated with Alexa 488–conjugated goat anti-mouse or -rabbit IgG antibody (Molecular Probes) and Alexa 568–conjugated goat anti-rabbit IgG antibody (Molecular Probes) or Alexa 568-phalloidin (1:500 dilution) in 2% BSA/PBS for 1 h at RT. Cells were then washed with PBS and mounted for analysis. Fluorescence images were obtained with a 60×/1.4 NA objective lens and processed by MetaMorph software.
For D2 morphological observations, the cells suspended in RPMI serum-free medium were treated with phorbol-12-myristate-13-acetate (PMA, 32 nM) and plated onto dishes for 2 h. PMA-induced differentiated cells were then treated with or without nocodazole (3.3 μM) for 1.5 h and observed by phase-contrast microscopy.
Rho GTPase Activity Assay
The glutathione _S_-transferase (GST)–RhoA-binding domain of Rhotekin (RBD) pulldown assay was used to detect cellular GTP bound RhoA (Ren et al., 1999). In brief, cells were washed and lysed in a buffer containing 50 mM Tris-HCl, pH 7.5, 1% (vol/vol) Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 500 mM NaCl, 10 mM MgCl2, 1 mM PMSF, and appropriate dilution of protease inhibitor leupeptin/aprotinin/pepstatin. After centrifugation at 13,000 × g for 10 min at 4°C, the supernatants of the lysates were incubated at 4°C for 1.5 h with GST-RBD–coupled glutathione-Sepharose beads. The beads were then washed four times with buffer containing 50 mM Tris-HCl, pH 7.5, 1% (vol/vol) Triton X-100, 150 mM NaCl, 10 mM MgCl2, 0.1 mM PMSF, and appropriate dilution of protease inhibitor leupeptin/aprotinin/pepstatin. The amounts of total and active GTP-bound Rho GTPases were detected by Western blotting with mAb against RhoA (1:500 dilution).
MLC Phosphorylation
After 72 h of siRNA treatment, transfected cell cultures in 60-mm-diameter dishes were pretreated with or without ROCK inhibitor Y27632 (10 μM) for 20 min and then treated with or without nocodazole (10 μM) for 40 min at 37°C. After treatment, the cells were rinsed with ice-cold PBS and scraped off into 100 μl of lysis buffer (50 mM Tris-HCl, pH 7.5, 50 mM NaF, 200× dilution of Ser/Thr phosphatase inhibitor cocktail 1 [Sigma], 1% [vol/vol] Triton X-100, 5 mM MgCl2, 150 mM NaCl, 1 mM DTT, 1 mM PMSF, and appropriate dilution of protease inhibitor leupeptin/aprotinin/pepstatin) for Western blotting with pMLC antibody (1:250 dilution).
RESULTS
GEF-H1 Mediates Nocodazole-induced Contractility
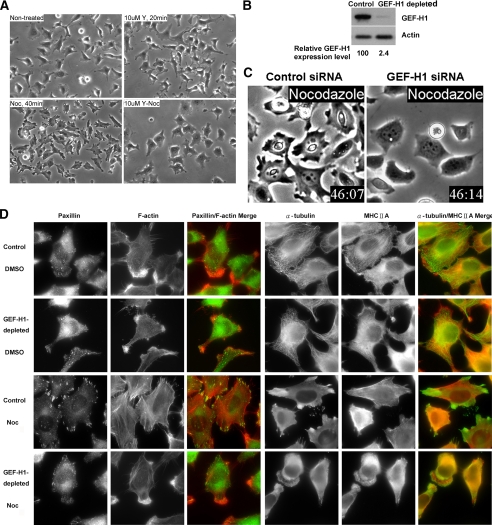
The formation of actomyosin filaments (stress fibers) and focal adhesions is associated with increased cellular contractility. Focal adhesions are sites where cells adhere strongly to the underlying extracellular matrix via specific members of the integrin family (Burridge et al., 1988). At their cytoplasmic face, focal adhesions provide attachment sites for F-actin stress fibers. One major RhoA downstream target involved in stimulating stress fiber assembly and contractility is the Ser/Thr kinase ROCK. Therefore, we first established that inhibition of ROCK with the specific Y27632 inhibitor completely prevented nocodazole-induced contraction of HeLa cells (Figure 1A). Strikingly, a similar inhibition of contractile morphology was also observed in cells depleted of GEF-H1 using a GEF-H1–specific siRNA pool (Figure 1, B and C; Videos 1 and 2).
Figure 1.
GEF-H1 depletion impairs nocodazole-induced contractility. (A) HeLa cells were pretreated with or without 10 μM of ROCK inhibitor Y27632 (Y) for 20 min and then treated with or without 10 μM nocodazole (Noc) for 40 min, as indicated. Representative phase-contrast images of the treated cells are shown. (B) HeLa cells treated with GEF-H1–specific siRNA pool or control siRNA for 72 h were harvested for Western blotting using anti-GEF-H1 antibody. Actin is shown as a loading control. (C) siRNA transfected cells as described in B were treated with nocodazole and imaged by time-lapse video phase-contrast microscopy. Note the morphological differences in the two cell populations: The control cells exhibited retraction of the cell edges and overall cell shrinkage. In contrast, the GEF-H1–depleted cells remained well spread and showed rounded edges with membrane ruffles (see Videos 1 and 2). (D) Control or GEF-H1–specific siRNA-transfected cells were treated with DMSO or nocodazole for 40 min and then fixed for immunostaining against α-tubulin/MHCIIA or paxillin/F-actin, as in Materials and Methods. Results shown are representative of three independent experiments.
GEF-H1 is a RhoA-specific guanine nucleotide exchange factor that links microtubule dynamics and RhoA GTPase regulation of the actin cytoskeleton (Krendel et al., 2002; Matsuzawa et al., 2004; Birukova et al., 2005; Chang and Lee, 2006). We have shown that overexpression of active GEF-H1 deficient in microtubule binding, or the depolymerization of microtubules with nocodazole to induce GEF-H1 release, activates RhoA and is accompanied by contraction of the cell body and edges (Krendel et al., 2002). This phenotype is mimicked by expression of constitutively active RhoAQ63L and is inhibitable by dominant negative RhoAT19N (Krendel et al., 2002).
To ascertain whether the contractile morphological changes observed in nocodazole-treated HeLa cells were due to the assembly of contractile stress fibers and associated focal adhesion complexes, we examined the cellular distribution of F-actin and the focal adhesion component paxillin. In both control and GEF-H1–depleted cells in the absence of nocodazole stimulation, modest actin stress fiber assembly was observed in the cell body, but F-actin was particularly enriched in prominent membrane ruffles (Figure 1D). Microtubules and myosin heavy chain IIA (MHCIIA) were well evident and organized and appeared similar in control cells and in cells depleted of GEF-H1. After nocodazole treatment in control cells, microtubule depolymerization was associated with the redistribution of tubulin throughout the cytosol and to areas surrounding stress fiber–associated adhesion sites. In addition, control nocodazole-treated cells exhibited retraction of the cell edges and often of the cell body (see Video 1), accompanied by enhanced stress fiber formation and robust paxillin-containing focal adhesions. By contrast, in GEF-H1–depleted cells tubulins were diffusely distributed in the cytosol and enriched at the anterior of membrane ruffles. In parallel, there were no stress fibers, nor did associated focal adhesions form in the GEF-H1–depleted cells stimulated with nocodazole. Furthermore, cells in the absence of GEF-H1 exhibited prominent and extensive membrane ruffles after nocodazole stimulation (27.4% of GEF-H1–depleted cells as opposed to 4.0% of control cells; data not shown). In some cells, even new protrusions with membrane ruffles were observed. To exclude the possibility of unspecific effects of the siRNA oligos in the pool for GEF-H1, we also tested each oligo individually. All four oligos produced a substantial increase in the number of cells with membrane ruffling (oligo 6: 14.3%, oligo 7: 27.8%, oligo 8: 28.9%, oligo 9: 44.4%). In contrast, siRNA-mediated depletion of another well-known Rho GEF, Ect2, had no effect on the contractile and/or ruffling phenotype induced by nocodazole addition (data not shown), suggesting there was a specific requirement for GEF-H1. Taken together, these data strongly suggest that nocodazole-initiated microtubule depolymerization requires GEF-H1 to stimulate RhoA/ROCK-mediated actomyosin contraction.
GEF-H1 Regulates RhoA Signaling During Nocodazole-induced Microtubule Depolymerization
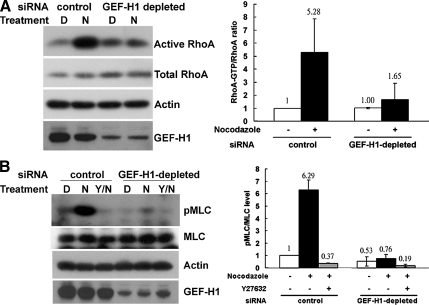
It has been shown that nocodazole-induced microtubule depolymerization can induce RhoA activation and contractility in several cell types, including HeLa cells (Krendel et al., 2002) and D2 cells (Chang et al., 2006). To determine if the nocodazole-induced changes in RhoA activity are mediated by GEF-H1 activation, we measured the amount of active RhoA present in cells exposed to either control or GEF-H1–specific siRNA oligonucleotides (Figure 2A). Although siRNA-mediated depletion of GEF-H1 had no detectable effect on the activity of RhoA under untreated conditions, the activation of RhoA upon nocodazole stimulation was effectively blocked in GEF-H1–depleted cells compared with control cells (Figure 2A). Quantification revealed that RhoA activation was decreased by more than 80% by the knockdown of GEF-H1 protein (Figure 2A, right panel).
Figure 2.
Depletion of GEF-H1 impairs nocodazole-induced RhoA/pMLC activation. (A) HeLa cells transfected with GEF-H1–specific siRNA pool or control siRNA were treated with DMSO (D) or 10 μM nocodazole (N) for 40 min. Cells were then harvested to quantify endogenous RhoA activity by GST-RBD pulldown assay. RhoA in GTP-bound form and in total lysates were analyzed by Western blotting with anti-RhoA antibody. By densitometric scanning, the intensity ratio of RhoA in GTP-bound form to total RhoA in lysate of control siRNA transfection with DMSO treatment was set to 1, and the relative ratio of active RhoA for each condition is shown in the right panel. Results are presented as means ± SD of three individual experiments. (B) HeLa cells transfected with siRNA as described above were pretreated with or without ROCK inhibitor Y27632 (Y) and then treated with DMSO (D) or 10 μM nocodazole (N) for 40 min, as indicated. Cells were then lysed in lysis buffer containing Ser/Thr phosphatase inhibitors and analyzed by Western blotting against pMLC, MLC, GEF-H1, and actin, as in Materials and Methods. By densitometric scanning, the intensity ratio of pMLC to total MLC in lysate of control siRNA transfection with DMSO treatment was set to 1, and the relative ratio of pMLC for each condition is shown in the right panel. Results are presented as means ± SD of three individual experiments.
The assembly of actomyosin complexes requires the phosphorylation of MLC. Nocodazole-stimulated cells transfected with control siRNA showed a significance increase in the levels of MLC phosphorylation. This increase in pMLC was abolished by pretreatment with the ROCK inhibitor Y27623 (Figure 2B). In contrast, there was no increase in MLC phosphorylation in GEF-H1–depleted cells upon nocodazole stimulation (>85% inhibition, Figure 2B, right panel). These data, combined with previous results, indicate an essential role of GEF-H1 in the regulation of RhoA activation and MLC-dependent contractility of HeLa cells in response to microtubule depolymerization by nocodazole.
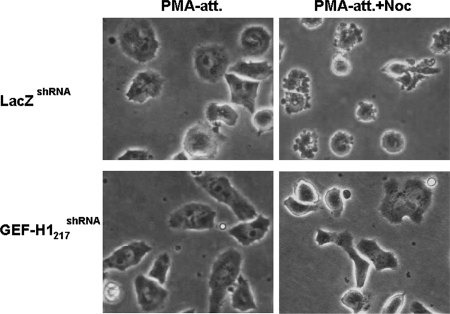
The requirement for GEF-H1 in mediating the nocodazole-induced increase in RhoA activation and contractility was not limited to HeLa cells. We observed that the knockdown of GEF-H1 also ablated both RhoA activation (Chang et al., 2006) and contraction of hematopoietic PMA-induced differentiated D2 cells (Figure 3). Thus, the microtubule depolymerization-induced activation of GEF-H1 appears to be a generally utilized mechanism to activate RhoA and cellular contractility.
Figure 3.
GEF-H1 depletion abolishes nocodazole-induced contraction in PMA-induced differentiated D2 cells. Two stable shRNA-expressing erythroblastoma D2 cell lines, control LacZshRNA, and GEF-H1–depleted GEF-H1217shRNA were treated with 32 nM PMA in serum-free RPMI medium for 2 h to induce adhesion and differentiation. Cells were then treated with or without 3.3 μM nocodazole for 1.5 h. Representative PMA-induced attached (PMA-att) and nocodazole-treated adherent (PMA-att+Noc) D2 cells were observed by phase-contrast microscopy and are shown as indicated.
siRNA-resistant GEF-H1 Restores Nocodazole-induced Contractility
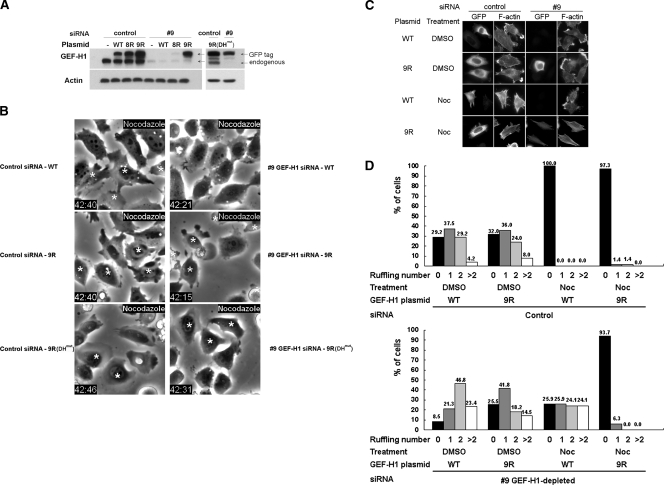
To validate that the ablated nocodazole-induced contractility was solely due to the loss of GEF-H1 in the siRNA-treated cells, we tested whether reintroduction of an siRNA-resistant GEF-H1 could rescue the normal phenotype. We first verified that we could knockdown GEF-H1 and replicate the observed depletion phenotype using individual oligonucleotides 8 and 9 in the siRNA pool (Figure 4A and data not shown). In the EGFP-GEF-H19R or EGFP-GEF-H19R(DHmut) construct, two silent mutations were generated within the target sequence of GEF-H1–specific siRNA 9 to confer resistance (see Materials and Methods). For comparison, cells were transfected with EGFP-GEF-H1WT, EGFP-GEF-H18R, EGFP-GEF-H19R, or EGFP-GEF-H19R(DHmut) plasmid 2 d after siRNA transfection. The potency of GEF-H1 depletion by the GEF-H1–specific siRNA 9 and the efficiency of the RNAi resistance of GEF-H19R were confirmed by Western blot against GEF-H1. As shown in Figure 4A, the expression of GEF-H1 was rescued to a level comparable to that in control siRNA cells when the cells were transfected with the GEF-H19R or GEF-H19R(DHmut) vector, but not with GEF-H1WT and GEF-H18R plasmids added after the GEF-H1–specific siRNA 9 treatment.
Figure 4.
Expression of siRNA-resistant GEF-H1 restores nocodazole-induced contractility. (A) HeLa cells treated with individual GEF-H1–specific siRNA oligo 9 or control siRNA oligo were then transfected with or without each EGFP-GEF-H1 construct as indicated. 8R, 9R, and 9R(DHmut) represent GEF-H1 constructs individually resistant to GEF-H1–specific siRNA oligo 8 and 9 (see Materials and Methods.). Cells were lysed and analyzed by Western blotting using anti-GEF-H1 antibody. Note that the transfected GEF-H1 migrated slower because of the attached EGFP tag. (B) HeLa cells transfected with siRNA and EGFP-GEF-H1 plasmid as indicated were treated with 10 μM nocodazole for 40 min and imaged by phase-contrast and fluorescence microscopy. EGFP-expressing cells are indicated by asterisks. (C and D) Cells transfected with siRNA and EGFP-GEF-H1 construct were treated with DMSO or 10 μM nocodazole for 40 min then fixed and stained for GFP and F-actin. Representative fluorescent images are shown in C to show the distribution of membrane ruffles and/or stress fibers. Quantitative analysis of ruffle number in each transfected cell was derived from two independent transfection and double-staining experiments with at least 50 cells counted for each condition. The percent of cells exhibiting the indicated number of ruffles for each siRNA/EGFP-GEF-H1 combination is shown in D.
Interestingly, GEF-H1–specific siRNA-treated cells transfected with plasmid GEF-H19R exhibited nocodazole-induced contraction and formation of stress fibers, as determined by time-lapse phase-contrast microscopy and fluorescent microscopy for the staining of GFP and F-actin (Figure 4, B and C; Videos 3–6). Our earlier study had shown that GEF-H1(DHmut) construct with an inactivating mutation (Y393A) in the Dbl domain showed no nucleotide exchange activity (Krendel et al., 2002). The dominant-negative effect of GEF-H1(DHmut) is specific for RhoA activation. Cells reintroduced with GEF-H19R(DHmut) were therefore used to determine whether the reversible effect of GEF-H19R on nocodazole-induced contraction requires its nucleotide exchange activity. Expression of GEF-H19R(DHmut) blocked the ability of GEF-H1 to rescue nocodazole-induced contractility (Figure 4B; Videos 7 and 8). We therefore conclude that the ability of GEF-H19R to restore nocodazole-stimulated contractility requires its nucleotide exchange activity toward RhoA.
In addition, because the actin stress fiber assembly induced by RhoA activity is associated with the inhibition of membrane ruffling in nocodazole-treated control cells (Figures 1D and 4C), we analyzed the number of membrane ruffles formed in each siRNA/EGFP-GEF-H1–transfected cell population. Statistical analysis showed that nocodazole stimulation resulted in significant inhibition of membrane ruffling, accompanied by stress fiber formation, in EGFP-GEF-H1WT– and EGFP-GEF-H19R–expressing cells treated with control siRNA (Figure 4, C and D). In contrast, depletion of GEF-H1WT with GEF-H1–specific siRNA 9 not only prevented the loss of membrane ruffles induced by nocodazole treatment, but actually resulted in an increase in the numbers of ruffles per cell compared with DMSO controls. The rescue of GEF-H1 expression using the siRNA-resistant EGFP-GEF-H19R restored the inhibition of ruffle formation by nocodazole. Overall, these results provide clear evidence that activation of GEF-H1 is essential and sufficient for the contractility induced by the microtubule-depolymerizing agent nocodazole.
DISCUSSION
The regulation of cell contractility by the cytoskeletal network is known to play important roles in both normal cell biology and in various pathological conditions (Dudek and Garcia, 2001). For example, during cell motility the retraction of the cell rear requires that it be pulled forward through contractile forces generated by the actomyosin cytoskeleton (Ridley et al., 2003). Similarly, microtubules have been shown to play critical roles in regulating the formation of the contractile actomyosin ring that generates the cleavage furrow in dividing mitotic cells (Burgess and Chang, 2005). The dysregulation of epithelial/endothelial permeability is implicated in the pathogenesis of many severe diseases, including bronchial asthma, atherosclerosis, and acute lung injury (Wettschureck and Offermanns, 2002). RhoA- and microtubule-mediated signaling pathways play an important role in controlling epithelial/endothelial barrier function by regulating actomyosin-mediated contraction and reorganization of the barrier cytoskeleton to control leakage (Verin et al., 2001; Birukova et al., 2004a,b). RhoA activation occurs in response to agonists that mediate breakdown of the endothelial microtubule network. Indeed, it has been shown that GEF-H1 is required for the increase in vascular endothelial cell permeability seen upon microtubule disassembly by thrombin or nocodazole (Birukova et al., 2005).
Although the microtubular network is not directly involved in the contractile machinery, it has been established that depolymerization of microtubules generally leads to an increase in cell contractility. This appears to be due to the coupling of the microtubule polymerization state to the activation state of RhoA, which has long been recognized as a key regulator of cell contractility (Hall, 1998). The Dbl family of GEFs are multifunctional molecules that transduce diverse intracellular signals leading to the activation of Rho GTPases (Zheng, 2001). Among them, GEF-H1 and p190RhoGEF have been identified as microtubule-associated Rho-GEFs for RhoA in humans. However, the activity of p190RhoGEF does not appear to be directly regulated by its interaction with microtubules (van Horck et al., 2001). In contrast, GEF-H1 is a RhoA-specific guanine nucleotide exchange factor whose activity is suppressed by microtubule binding, whereas GEF-H1 activity toward RhoA is enhanced by its release from microtubules upon depolymerization of the microtubule network. Thus, GEF-H1 has been shown to induce cell contractility and actin stress fibers formation upon nocodazole-induced microtubule disruption (Krendel et al., 2002; Birukova et al., 2005).
In this study, we provide direct evidence that nocodazole-induced contractility requires the action of GEF-H1 released upon microtubule depolymerization, which leads to activation of RhoA/ROCK/MLC signaling. Thus, the depletion of GEF-H1 protein using siRNA methods totally prevents the contractile phenotype observed upon nocodazole addition (Figure 1; Videos 1 and 2). Indeed, our data strongly indicate that microtubule depolymerization in itself is not sufficient to induce contractility. Reintroduction of siRNA-resistant wild-type GEF-H1 in GEF-H1–specific siRNA-treated cells was able to rescue the contractile phenotype observed upon nocodazole stimulation. However, expression of the catalytically inactive GEF-H19R(DHmut) was unable to restore contractility in response to microtubule depolymerization. Thus, microtubules sequester GEF-H1 under normal conditions and drug-induced or other conditions that induce microtubule disassembly initiate a GEF-H1/RhoA/ROCK/MLC signaling pathway to control cell contractility. This appears to be a general effect of GEF-H1, as it is observed in HeLa cells, PMA-induced differentiated D2 cells, vascular endothelial cells, and colonic epithelial cells (Krendel et al., 2002; Matsuzawa et al., 2004; Birukova et al., 2005; Chang et al., 2006). We note that siRNA-mediated depletion of another RhoA GEF, Ect2, does not affect nocodazole-induced HeLa cell contractility (data not shown), even though Ect2 is closely linked to RhoA activation and GEF-H1 action during mitotic cleavage furrow formation (Birkenfeld et al., 2007).
In conclusion, we establish that GEF-H1 serves as a critical linker between microtubule polymerization state and the resulting activation of a RhoA-mediated signaling pathway leading to cell contractility. The regulation of GEF-H1 has been shown to be complex, involving its phosphorylation on multiple sites by various kinases (Zenke et al., 2004; Callow et al., 2005; Birkenfeld et al., 2007), as well as its interaction with other proteins (Zenke et al., 2004; Aijaz et al., 2005). How these regulatory mechanisms might act to modulate the effects of GEF-H1 action during microtubule disassembly remains to be determined.
Supplementary Material
[Supplemental Materials]
ACKNOWLEDGMENTS
We thank Dr. David Schlaepfer, University of California-San Diego, for technical advice. This work was supported by National Institutes of Health Grant GM44428 to G.M.B. and Grant NSC096-2917-I-002-011 from the National Science Council, Taiwan, ROC to Y-C.C. This is manuscript 19358-IMM from the Scripps Research Institute.
Footnotes
REFERENCES
- Aijaz S., D'Atri F., Citi S., Balda M. S., Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev. Cell. 2005;8:777–786. doi: 10.1016/j.devcel.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Amano M., Chihara K., Kimura K., Fukata Y., Nakamura N., Matsuura Y., Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y., Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Birkenfeld J., Nalbant P., Bohl B. P., Pertz O., Hahn K. M., Bokoch G. M. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev. Cell. 2007;12:699–712. doi: 10.1016/j.devcel.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova A. A., Adyshev D., Gorshkov B., Bokoch G. M., Birukov K. G., Verin A. A. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;290:540–548. doi: 10.1152/ajplung.00259.2005. [DOI] [PubMed] [Google Scholar]
- Birukova A. A., Birukov K. G., Smurova K., Adyshev D., Kaibuchi K., Alieva I., Garcia J. G., Verin A. D. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J. 2004a;18:1879–1890. doi: 10.1096/fj.04-2328com. [DOI] [PubMed] [Google Scholar]
- Birukova A. A., et al. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: role of Rho-dependent mechanisms. J. Cell. Physiol. 2004b;201:55–70. doi: 10.1002/jcp.20055. [DOI] [PubMed] [Google Scholar]
- Brown R. A., Talas G., Porter R. A., McGrouther D. A., Eastwood M. Balanced mechanical forces and microtubule contribution to fibroblast contraction. J. Cell. Physiol. 1996;169:439–447. doi: 10.1002/(SICI)1097-4652(199612)169:3<439::AID-JCP4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Burgess D. R., Chang F. Site selection for the cleavage furrow at cytokinesis. Trends Cell Biol. 2005;15:156–162. doi: 10.1016/j.tcb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu. Rev. Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Callow M. G., Zozulya S., Gishizky M. L., Jallal B., Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J. Cell Sci. 2005;118:1861–1872. doi: 10.1242/jcs.02313. [DOI] [PubMed] [Google Scholar]
- Chang Y. C., Lee H. H., Chen Y. J., Bokoch G. M., Chang Z. F. Contribution of guanine exchange factor H1 in phorbol ester-induced apoptosis. Cell Death Differ. 2006;13:2023–2032. doi: 10.1038/sj.cdd.4401901. [DOI] [PubMed] [Google Scholar]
- Chang Z. F., Lee H. H. RhoA signaling in phorbol ester-induced apoptosis. J. Biomed. Sci. 2006;13:173–180. doi: 10.1007/s11373-005-9056-4. [DOI] [PubMed] [Google Scholar]
- Danowski B.A. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J. Cell Sci. 1989;93(Pt 2):255–266. doi: 10.1242/jcs.93.2.255. [DOI] [PubMed] [Google Scholar]
- Dudek S. M., Garcia J. G. Cytoskeletal regulation of pulmonary vascular permeability. J. Appl. Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- Enomoto T. Microtubule disruption induces the formation of actin stress fibers and focal adhesions in cultured cells: possible involvement of the rho signal cascade. Cell Struct. Funct. 1996;21:317–326. doi: 10.1247/csf.21.317. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Kimura K., et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kolodney M. S., Elson E. L. Contraction due to microtubule disruption is associated with increased phosphorylation of myosin regulatory light chain. Proc. Natl. Acad. Sci. USA. 1995;92:10252–10256. doi: 10.1073/pnas.92.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendel M., Zenke F. T., Bokoch G. M. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat. Cell Biol. 2002;4:294–301. doi: 10.1038/ncb773. [DOI] [PubMed] [Google Scholar]
- Matsuzawa T., Kuwae A., Yoshida S., Sasakawa C., Abe A. Enteropathogenic Escherichia coli activates the RhoA signaling pathway via the stimulation of GEF-H1. EMBO J. 2004;23:3570–3582. doi: 10.1038/sj.emboj.7600359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X. D., Kiosses W. B., Schwartz M. A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Li R., Zheng Y., Busch H. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J. Biol. Chem. 1998;273:34954–34960. doi: 10.1074/jbc.273.52.34954. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Riento K., Ridley A. J. Rocks: multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Rodriguez O. C., Schaefer A. W., Mandato C. A., Forscher P., Bement W. M., Waterman-Storer C. M. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat. Cell Biol. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- van Horck F. P., Ahmadian M. R., Haeusler L. C., Moolenaar W. H., Kranenburg O. Characterization of p190RhoGEF, a RhoA-specific guanine nucleotide exchange factor that interacts with microtubules. J. Biol. Chem. 2001;276:4948–4956. doi: 10.1074/jbc.M003839200. [DOI] [PubMed] [Google Scholar]
- Verin A. D., Birukova A., Wang P., Liu F., Becker P., Birukov K., Garcia J. G. Microtubule disassembly increases endothelial cell barrier dysfunction: role of MLC phosphorylation. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;281:L565–L574. doi: 10.1152/ajplung.2001.281.3.L565. [DOI] [PubMed] [Google Scholar]
- Wang N., Naruse K., Stamenovic D., Fredberg J. J., Mijailovich S. M., Tolic-Norrelykke I. M., Polte T., Mannix R., Ingber D. E. Mechanical behavior in living cells consistent with the tensegrity model. Proc. Natl. Acad. Sci. USA. 2001;98:7765–7770. doi: 10.1073/pnas.141199598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettschureck N., Offermanns S. Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J. Mol. Med. 2002;80:629–638. doi: 10.1007/s00109-002-0370-2. [DOI] [PubMed] [Google Scholar]
- Zenke F. T., Krendel M., DerMardirossian C., King C. C., Bohl B. P., Bokoch G. M. p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J. Biol. Chem. 2004;279:18392–18400. doi: 10.1074/jbc.M400084200. [DOI] [PubMed] [Google Scholar]
- Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem. Sci. 2001;26:724–732. doi: 10.1016/s0968-0004(01)01973-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Materials]