Generalized Metabolic Bone Disease in Neurofibromatosis Type I (original) (raw)
. Author manuscript; available in PMC: 2009 Jan 23.
Published in final edited form as: Mol Genet Metab. 2008 Mar 4;94(1):105–111. doi: 10.1016/j.ymgme.2007.12.004
Abstract
Skeletal abnormalities are a recognized component of Neurofibromatosis type I (NF1) but a generalized metabolic bone defect in NF1 has not been fully characterized thus far. The purpose of this study was to characterize at the densitometric, biochemical and pathological level the bone involvement in NF1 patients. Using dual energy X-ray absorptiometry (DXA) we analyzed bone status in 73 unselected NF1 subjects, 26 males and 47 females, mainly children and adolescents (mean age: 16.6 years). In a subgroup of subjects with low bone mass, we measured indices of calcium-phosphate metabolism, bone turnover, and bone density before and after vitamin D and calcium treatment. We found statistically significant and generalized reduction in bone mass with the mean lumbar bone mineral density (BMD) z-score being −1.38 ± 1.05 (CI 95% −1.62 to −1.13), and whole body bone mineral content (BMC) z-score −0.61 ± 1.19 (CI 95% −0.94 to −0.29), both significantly reduced compared to normal controls (p<.001). PTH was moderately elevated and after 4 months of supplemental therapy with calcium and vitamin D, it decreased to the normal range. However, BMD z-scores did not significantly improve after two years of follow-up. Histological analysis of bone samples from NF1 patients revealed substantial alteration of bone microarchitecture due mainly to reduced trabecular bone.
Our observations are consistent with a generalized bone metabolic defect due to loss of the function of neurofibromin. Early identification of patients with osteoporosis may permit more timely and aggressive treatments to prevent the likely substantial morbidity associated with increased fracture risk later in life.
Keywords: Neurofibromatosis type I, NF1, osteoporosis, osteopenia
INTRODUCTION
Neurofibromatosis type 1 (NF1) is the most common autosomal dominant disease and it presents with progressive clinical manifestations involving the cutaneous, nervous, vascular, and skeletal systems [1,2]. Skeletal problems are common in NF1 patients especially during childhood. Although highly variable, these problems can include scoliosis, pseudoarthrosis, and short stature. The osseous dysplasia result from disturbed bone growth, perhaps secondary to a mineralization disturbance [3–5]. While these findings indicate defects of localized skeletal segments, the ubiquitous expression and pleiotropic functions of neurofibromin raises the possibility of systemic skeletal or bone disease in individuals with NF1 [6].
Reduced bone mineral mass is emerging as an important complication of NF1. Reduced bone mineral density (BMD) in NF1 patients was initially recognized by Illes et al. [7]. Based on the intraoperative findings of poor vertebral bone quality, they measured the BMD of the lumbar spine in 12 young (mean age of 19.1 years) NF1 patients with severe scoliosis requiring surgical correction and found a significant reduction in the BMD (mean z-score = −2.5) at the lumbar spine which was inversely correlated with the severity of scoliosis. Following this initial report, recent studies have confirmed a decreased bone mass in NF1 patients including children [8–12]. Lammert et al. performed a cross-sectional study on 104 adults with NF1, using quantitative ultrasonometry (QUS) and found that BMD was significantly lower in NF1 patients than in the normal population and similarly to Illes et al. the decrease in BMD appeared to be more pronounced in patients with severe scoliosis [9]. Subsequently, Stevenson et al. using DXA in a group of 5–18 year-old NF1 patients found decreased localized and generalized reduction compared to a local control population of subjects without NF1 [10]. These findings have been further confirmed in small cohorts of pediatric patients using DXA [11,12].
Osteoporosis is a common disease with substantial morbidity and mortality. The densitometric criteria for osteoporosis are well established for the diagnosis in postmenopausal women as a predictor of fracture risk. While useful for evaluation of fracture risk in adults, T-scores are derived from reference populations of young-adult women and are not applicable to the diagnosis of osteoporosis in children. The diagnosis of osteoporosis in children is more difficult: densitometric data have been compared with age-matched control populations, i.e., z-scores. It is generally agreed that z-scores <−1.5 are indicative of low bone mass, and that osteoporosis is suspected strongly with z-scores <−2, especially with history of fracture. Recently z-score <− 2 have been proposed to define low bone density in children [13]. Analysis of both BMD and bone mineral content (BMC) in growing children may be the most accurate in the assessment of skeletal status and none of the previous studies have provided concurrent analyses of both these parameters.
In the present study, we investigated the bone mineral status, and the bone metabolic markers in a group of prospectively non-selected subjects with NF1. In addition, we evaluated at the histological and ultrastructural levels the alterations present in the NF1 bone.
METHODS
The study was approved by the Institutional Review Board for Human Subject Research at Baylor College of Medicine. Informed, written consent was obtained from all enrolled subjects or from parents as appropriate.
Subjects
The participants in the study were 73 NF1 subjects (26 males, 47 females) attending the Neurofibromatosis Clinic at Texas Children’s Hospital, Houston, Texas. Average age was 16.0 years (range: 2.8–58.9 years). Unlike most of the previous studies, these subjects were unselected for skeletal problems. This population included different ethnicities representative of the region and the diversity of the NF Clinic population: Caucasian (n = 46), African-American (n = 10), Hispanic (n = 15), Asiatic (n = 2). Among all subjects, the diagnosis was established clinically with the presence of two or more classic diagnostic criteria according to the NIH Consensus Conference [14]. Height was measured with a stadiometer to ± 0.5 cm, and weight (± 0.1 kg) was measured on a standard clinical balance. The body mass index (BMI) was calculated as weight/height2 (kg/m2). Dietary calcium intake was assessed by detailed food frequency questionnaire about dairy products [15]. Total calcium intake from all sources was categorized as <500 mg/d, 500–1000 mg/d, and >1000 mg/d. Habitual physical activity was measured by questionnaire [16]. Drug intake and fracture history were also included in the questionnaires administered to the subjects. Questionnaires were completed by 67 of the 73 participants of this study.
Densitometric analyses
BMC and areal density measurements were obtained with a Hologic Delphi-A instrument with fan-beam technology (Software version 11.2). Total body and regional measurements of bone were obtained. The following bone parameters were recorded: BMC, bone area, and BMD for whole body, lumbar spine, trochanter and femoral neck. Z-scores for BMC and BMD were calculated from the reference values provided by the Body Composition Laboratory of the Children’s Nutrition Research Center [17].
Bone pathology
Bone samples from three NF1 patients with reduced bone mass were obtained from the spine vertebrae during surgical correction of scoliosis. The histopathological examinations were compared to a bone specimen from the same site of healthy, age-, sex-, and gender-matched subject obtained at autopsy.
Bone samples were preserved in glutaraldehyde for 18–24 hours and small pieces of calcified bone were post-fixed in 1% osmium tetraoxide, dehydrated in graded ethyl alcohols, and embedded in epoxy resin (Epon 812; Polysciences). Thin sections were obtained with a diamond knife and collected on water containing one drop of bromthymol blue, with pH adjusted to 8.0. This prevented decalcification of the section while floating on water during sectioning. Sections approximately 80 nm in thickness were collected and stained with uranyl acetate in 70% alcohol and aqueous basic lead citrate.
Statistical methods
We used SPSS 11.5 (SPSS Inc., Chicago, Illinois, USA) for statistical analysis. Comparison of z-score mean with the zero mean reference value was made with the one sample t-test. 95th centile confidence intervals were calculated: results are shown as mean ± SD. Linear regression assessed the relationship between dependent variables (whole body BMC z-score, lumbar spine BMD z-score) and independent variables (calcium intake, physical activity).
RESULTS
Subjects
The anthropometric characteristics of our population are summarized in Table 1. Our population is composed of children, adolescents, and young adults (54 out of 73 participants were less than 20 years old); the mean age is 16.0 ± 12.2 years. As expected [18], the mean height is reduced, as indicated by height z-score which is on average ≈ −0.40. The mean BMI was 20.1 ± 5.0. The mean calcium intake was 1356 ± 736mg/day.
TABLE 1.
Description of NF1 subjects (mean ± SD)
| Height(cm) | Weight(kg) | BMI(kg/cm2) | ||
|---|---|---|---|---|
| Males | N = 26 | |||
| 0–4 yrs | n = 2 | 94.7 ± 0.42 | 14.7 ± 0.0 | 16.4 ± 0.1 |
| 5–9 yrs | n = 9 | 124.5 ± 9.1 | 26.4 ± 5.2 | 16.9 ± 1.5 |
| 10–14 yrs | n = 8 | 154.8 ± 7.3 | 47.6 ± 10.5 | 19.8 ± 4.1 |
| 15–20 yrs | n = 4 | 170.3 ± 12.3 | 73.8 ± 10.3 | 25.5 ± 3.2 |
| 21–30 yrs | n = 0 | - | - | - |
| 31–50 yrs | n = 3 | 172.1 ± 12.6 | 96.5 ± 31.2 | 32.1 ± 7.2 |
| Females | N = 47 | |||
| 0–4 yrs | n = 1 | 87.5 | 12.9 | 16.8 |
| 5–9 yrs | n = 14 | 116.7 ± 8.8 | 22.3 ± 4.2 | 16.3 ± 1.7 |
| 10–14 yrs | n = 16 | 148.3 ± 10.4 | 42.7 ± 10.4 | 19.2 ± 3.4 |
| 15–20 yrs | n = 1 | 159.9 | 49.9 | 19.5 |
| 21–30 yrs | n = 2 | 159.2 ± 10.5 | 65.6 ± 8.0 | 25.8 ± 0.3 |
| 31–50 yrs | n = 12 | 160.7 ± 7.3 | 60.8 ± 12.8 | 23.4 ± 4.0 |
| 50–70 yrs | n = 1 | 168.9 | 67.9 | 23.8 |
Total calcium intake was less than 500 mg/d in 8 subjects, 500–1000 mg/d in 15 subjects, and >1000 mg/d in 44 subjects. Linear regression assessed the relationship between dependent variables (whole body BMC z-score, lumbar spine BMD z-score) and independent variables (calcium intake, physical activity). When two extreme outliers (subjects with low whole body BMC z-score and high calcium intake) are excluded from analysis, no statistical relationship existed between calcium intake and whole body BMC or lumbar BMD z-scores. Likewise, the level of physical activity was not correlated with whole body BMC and lumbar spine BMD z-scores. None of the subjects enrolled were taking corticosteroids at the time of the study. In our cohort fractures occurred in 52% of the patients with 33.3% with more than one fracture.
Bone status assessment
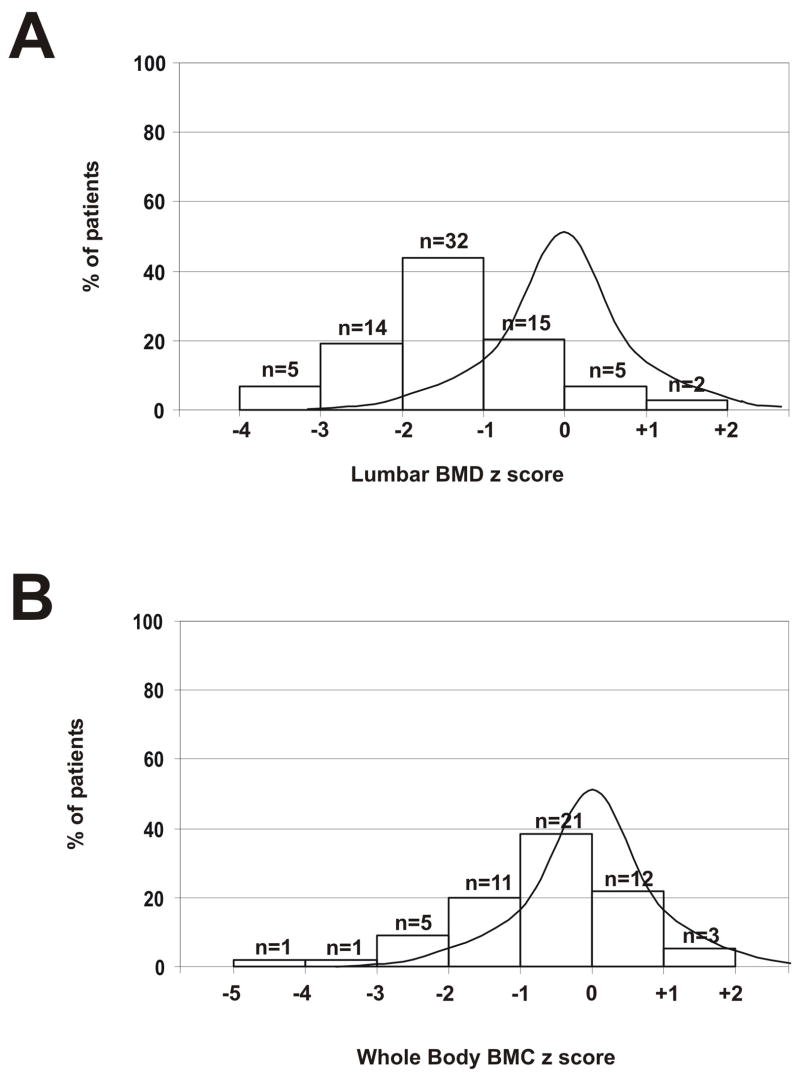
Whole body, lumbar spine, femoral, and trochanteric BMD z-scores are all significantly reduced; the spine is most severely affected. The mean lumbar spine BMD z-score is −1.38 ± 1.05, [95% CI −1.62; −1.13], which is significantly below normal (p<.001). The distribution of the lumbar spine BMD z-scores in our population is shown as Figure 1A. Mean femoral neck z-score was −0.77 ± 0.87 [95% CI −1.0; −0.5] and mean trochanteric BMD z-score was −0.73 ± 0.94 [95% CI −0.95; −0.49]. Fifty-seven percent of subjects with NF1 had at least one bone measurement in the osteopenic range (z-score <−1.5) with 33.3% in the osteoporosis range (z-score <−2.5).
Figure 1.
A. Lumbar spine BMD z-score. Distribution of BMD z-score in NF1 compared to a normal distribution. B. Whole body BMC z-score. Distribution of BMC z-score in NF1 compared to a normal distribution.
The y axis represents the percentage of patients and z-scores are shown on the x axis. Comparison of z-score mean with the zero mean reference value was made with the one sample t-test using SPSS 11.5 (SPSS Inc., Chicago, Illinois, USA). 95th centile confidence intervals were calculated: results are shown as mean ± SD.
The use of only age-matched z-score does not consider differences in body size or growth between NF1 and healthy children of the same age. We therefore used the prediction model developed by Ellis et al. [17] to recalculate a BMC z-score adjusted for age, gender, height, and ethnicity. The adjusted BMC z-score calculated for the 54 subjects less than 20 years old was significantly reduced (p<.001) (Figure 1B). Mean BMC z-score was −0.61 ± 1.19 [95% CI −0.94; −0.29].
Biochemical measurements
In a subgroup of 16 subjects from the cohort studied with DXA and with significant osteoporosis and osteopenia (mean lumbar z-score = −2.1, age range 6–38 yrs), we evaluated several markers of calcium and phosphate metabolism and bone turn-over (Table 2). All biochemical parameters measured were within the normal range except PTH, which was increased significantly (compared to age-matched controls) in 8/16 subjects of mean age of 13.5 yrs (range 7–18 yrs) (Table 2). In 2/8 subjects with higher PTH, serum osteocalcin levels were elevated as compared with age-matched normal values. The remaining 6 subjects with increased PTH presented with normal values of serum osteocalcin. In 10/16 subjects 25(OH)D was between 5–20 ng/ml, a level generally accepted as a state of vitamin D insufficiency. The range was 25–50 ng/ml in the remaining 6 subjects. Among the subjects with increased PTH, 6/8 also had 25(OH)D in the range of vitamin D insufficiency. However, in none of the subjects was the 25(OH)D level in the range of deficiency, and the calcium intake was adequate in all (mean daily calcium intake: 1626 ± 789 g). In the 8 subjects with elevated serum PTH levels, therapy with vitamin D (400 UI per day) and calcium supplementation (1000 mg elemental calcium) was initiated. After four months of therapy, PTH was reduced in 6 of the 8 subjects treated. The mean PTH decreased to 30.4 ± 22.6 ng/l (Figure 2). Seven of 8 treated subjects were compliant to therapy, while PTH increased from 73 to 81 ng/l after 4 months for the noncompliant patient (included in the Figure 2). Compared to baseline, serum AP, serum 25(OH)D and urinary NTx did not change. Likewise, after 24 months of therapy, BMD and BMC showed no significant improvement.
TABLE 2.
Biochemical markers of bone metabolism (mean ± SD)
| Value N = 16* | |
|---|---|
| Calcium (mg/dl) | 9.4 ± 0.4 |
| Phosphate (mg/dl) | 4.5 ± 0.7 |
| AP (U/l) | 176.9 ± 84.2 |
| PTH (ng/l) | 44.1 ± 24.8 |
| 25(OH)2D (ng/ml) | 20.6 ± 4.5 |
| Osteocalcin (ng/ml) | 39.2 ± 26.2 |
| NTx (nmoles/nmole of creatinine) | 273 ± 189.9 |
| TRP (%) | 87.4 ± 3.8 |
| TRCa (%) | 99.0 ± 0.52 |
Figure 2.
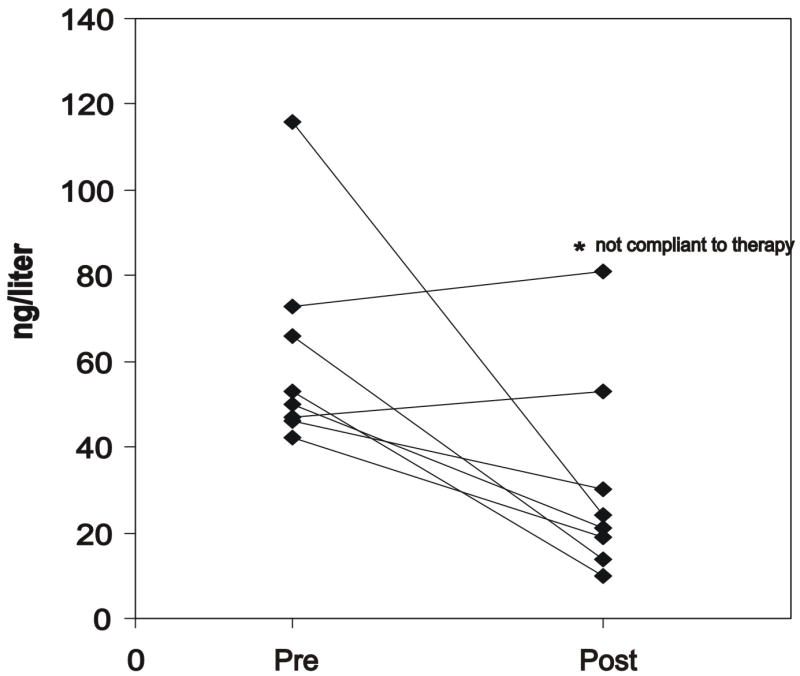
Serum level of PTH before (pre) and after (post) 4 months of vitamin D and calcium therapy.
Bone pathology analyses
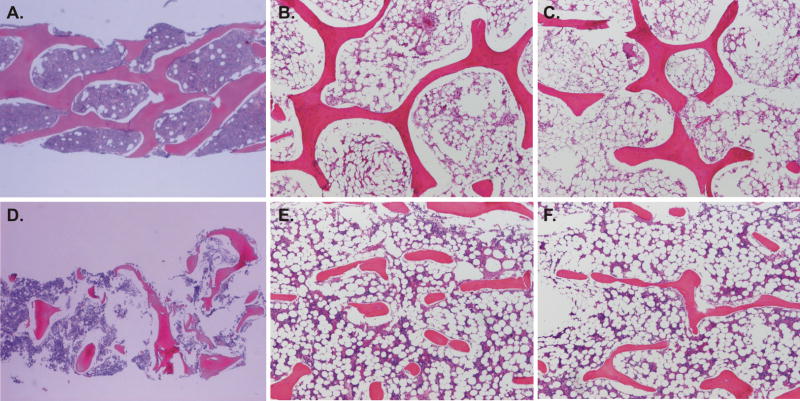
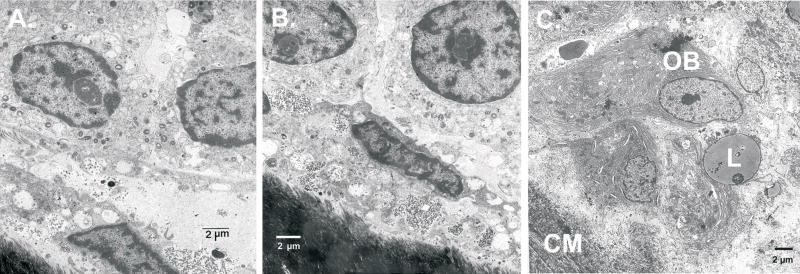
The NF1 bone had marked reduction in the number and thickness of the bony trabecula and appropriate cement lines and viable osteocytes (Figure 3D, E, F) as compared with bone specimen from the same site of healthy, age-, sex-, and gender-matched subject (Figure 3A, B, C). A Von Kossa stain showed normal mineralization of the bone (not shown). At the ultrastructural level, we observed on the bone surface of two bone samples from two distinct NF1 patients the presence of numerous lining cells which have replaced well differentiated osteoblasts (Figures 4A and B). As a control, we used a sample from a osteogenesis imperfecta (OI) patient (Figure 4C) which shows osteoblasts with abundant endoplasmic reticulum and well differentiated cytoplasmic structures. In contrast, the lining cells in the NF1 bone samples (Figures 4A and B) are not well differentiated and show few cytoplasmic structures that resemble active osteoblasts. The nature of these cells is unclear and they were not found in the OI bone sample. Further studies are needed to determine the identity of these cells. In addition, electron microscopy of the mineralized matrix in NF1 patients shows a severe reduction in mineral content.
Figure 3.
Representative section of the bone biopsies from controls (A, B, C) and from three subjects with NF1 (D, E, F) (H&E, decalcified section). The bony trabeculae are reduced in number and thickness. The control bone samples from healthy age-, and gender-matched child demonstrate the typical trabecular structure of a vertebral body and a marrow space occupied by typical bone marrow elements (H&E, decalcified section).
Figure 4.
Electron microscopy of the bone from two NF1 patients. Examples of the numerous round cells adjacent to the bone surface observed in two independent bone samples from two distinct NF1 patients are shown in A and B. These cells were not detected in the bone sample obtained from a patient with osteogenesis imperfecta used for comparison in C.
DISCUSSION
Increased risks for osteoporosis are emerging as complications among selected genetic disease populations. Early diagnosis in the pediatric population is especially important since the highest contribution to peak bone mass is attained in the first three decades. A primary goal of this study was to evaluate the bone mineral status and biochemical markers of bone metabolism in pediatric and young adult subjects affected by NF1, the most common dominantly inherited genetic disorders.
NF1 may have diverse skeletal abnormalities usually present in childhood [19]. They include scoliosis, pseudoarthrosis, limb length discrepancy, macrocephaly, and sphenoid wing dysplasia. Our study is the first to systematically investigate the bone status of NF1 subjects through a multi-level (DXA, biochemical and histological) analysis. For adults, clinical evaluations of bone status are usually based only on BMD. However, this approach may be insufficient in children because both BMC and bone area are changing during growth and their temporal rates of changes are not necessarily coupled [20]. When only BMD is used, the relative density of smaller bones can be systemically underestimated while that of larger bones is overestimated, thus confounding interpretation especially for values obtained during growth.
For children, height, age, gender, and ethnicity must be accounted. For the children included in this study, we have used the pediatric-based prediction model for whole BMC as reported by Ellis et al.[17], which is based on a convenient sample of over 2300 children and adolescents from the same geographic area as our NF1 subjects [17].
Our results indicate that the mean lumbar and whole body BMD z-scores were in the range of osteopenia and osteoporosis in 35/73 (48%) and 18/73 (25%) subjects, respectively. BMD was reduced at multiple bone sites, the lumbar spine being more severely affected. The lumbar spine contains mainly trabecular bone which is metabolically active and is sensitive to derangements of the mineralization process, particularly in young subjects [21,22]. Interestingly, the prevalence of this bone complication is higher compared to the other bone problems reported in NF1 patients. Bone histological analysis performed in patients confirmed the severe involvement of trabecular bone. However, these results need to be interpreted with caution since the three patients from whom the samples were obtained exhibited a more severe bone involvement.
In the subgroup of 16 subjects with significant osteopenia/osteoporosis, we found eight subjects with mild serum PTH elevation. Osteocalcin concentrations are elevated during skeletal growth and in various conditions characterized by increased bone turnover, including hyperparathyroidism. However, it was elevated in only two subjects with secondary hyperparathyroidism and levels were normal in the remaining subjects including six subjects with hyperparathyroidism. In the six subjects with higher levels of PTH, the normal levels of osteocalcin should be considered as inappropriately low and the absence of a compensatory increase of osteocalcin may suggest an insufficiency of osteoblastic activity. Serum 25(OH)D is the best indicator of vitamin D status, although it is still debated as to the threshold levels indicating vitamin D deficiency [23][24]. Therefore, we cannot rule out vitamin D insufficiency as contributing to the hyperparathyroidism in the subjects we analyzed. In the present study vitamin D treatment and calcium supplement resulted in the normalization of PTH in 6/8 subjects substantiating the secondary nature of the hyperparathyroidism. However, levels of 25(OH)D and both lumbar spine BMD and whole body BMC remained unchanged even after normalization of PTH levels.
In summary, we demonstrated that, on a population basis, young NF1 subjects have reduced bone mass; the lumbar spine having the most severe involvement. More than half the subjects of our NF1 population have at least one regional site in the osteopenic range, and one-third has at least one regional site in the osteoporotic range.
The finding of a mild PTH increase in the face of normal bone turnover indices suggests that NF1 may be associated with a disturbance in bone metabolism. The neurofibromin protein, independent of its RasGTPase-activating activity, can modulate adenyl cyclase activity and protein kinase A (PKA) [25–27]. Since cAMP and PKA are primary signaling pathways regulating osteoblast and osteoclast cell function in response to PTH, it is plausible that haploinsufficiency of NF1 in humans can result in both decreased bone formation (osteoblastic activity) and/or osteoclastic activity and the clinical picture of adynamic bone [28].
Recent studies investigating neurofibromin have suggested multiple essential roles in skeletal development and growth. Nf1 inactivation in murine undifferentiated mesenchymal cells leads in fact to bowing of the tibia and diminished growth associated with decreased stability of the cortical bone, high degree of porosity, decreased stiffness and reduction in the mineral content. At the cellular level, osteoblasts showed an increase in proliferation and a decreased ability to differentiate and mineralize in vitro [29]. In addition to an osteblastic defect, Nf1(+/−) mice were found to contain elevated numbers of osteoclasts with increased survival, proliferation, migration, adhesion, and resorptive activity. These gains of function were even more pronounced in ovariectomized mice [30].
Although the long-term consequence of reduced bone mass in childhood in NF1 patients is unknown, the findings of our study will have important implications for the follow-up and prevention of osteopenia/osteoporosis in this common genetic disease. As suggested by our results, intervention with vitamin D and calcium in NF1 patients may correct mild PTH increase, although long-term effects on bone accretion are unknown. At least in this subgroup of subjects, normalization of PTH levels did not result in improved BMD or BMC over a two year period. Prospective clinical trials to determine whether more aggressive interventions such as bisphosphonates will translate into increased bone mass in NF1 young adults are needed.
Acknowledgments
Dr. Lewis is a Senior Scientist of Research to Prevent Blindness, New York, New York. We are grateful to the individuals and families reported here for their willing and generous cooperation in these investigations. Part of this work was carried out at the Analytical Microscopy Core laboratory at Hospital for Special Surgery.
Abbreviations
NF1
Neurofibromatosis type 1
DXA
dual energy X-ray absorptiometry
BMD
bone mineral density
BMC
bone mineral content
GAP
GTPase-activating proteins
QUS
quantitative ultrasonometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedman JM. Epidemiology of neurofibromatosis type 1. Am J Med Genet. 1999;89:1–6. [PubMed] [Google Scholar]
- 2.Carey JC, Viskochil DH. Neurofibromatosis type 1: A model condition for the study of the molecular basis of variable expressivity in human disorders. Am J Med Genet. 1999;89:7–13. [PubMed] [Google Scholar]
- 3.Crawford AH, Jr, Bagamery N. Osseous manifestations of neurofibromatosis in childhood. J Pediatr Orthop. 1986;6:72–88. doi: 10.1097/01241398-198601000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Riccardi VM. Von Recklinghausen neurofibromatosis. N Engl J Med. 1981;305:1617–1627. doi: 10.1056/NEJM198112313052704. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson DA, Zhou H, Ashrafi S, Messiaen LM, Carey JC, D'Astous JL, Santora SD, Viskochil DH. Double inactivation of NF1 in tibial pseudarthrosis. Am J Hum Genet. 2006;79:143–148. doi: 10.1086/504441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alwan S, Tredwell SJ, Friedman JM. Is osseous dysplasia a primary feature of neurofibromatosis 1 (NF1)? Clin Genet. 2005;67:378–390. doi: 10.1111/j.1399-0004.2005.00410.x. [DOI] [PubMed] [Google Scholar]
- 7.Illes T, Halmai V, de Jonge T, Dubousset J. Decreased bone mineral density in neurofibromatosis-1 patients with spinal deformities. Osteoporos Int. 2001;12:823–827. doi: 10.1007/s001980170032. [DOI] [PubMed] [Google Scholar]
- 8.Yilmaz K, Ozmen M, Bora Goksan S, Eskiyurt N. Bone mineral density in children with neurofibromatosis 1. Acta Paediatr. 2007;96:1220–1222. doi: 10.1111/j.1651-2227.2007.00401.x. [DOI] [PubMed] [Google Scholar]
- 9.Lammert M, Kappler M, Mautner VF, Lammert K, Storkel S, Friedman JM, Atkins D. Decreased bone mineral density in patients with neurofibromatosis 1. Osteoporos Int. 2005;16:1161–1166. doi: 10.1007/s00198-005-1940-2. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson DA, Moyer-Mileur LJ, Murray M, Slater H, Sheng X, Carey JC, Dube B, Viskochil DH. Bone mineral density in children and adolescents with neurofibromatosis type 1. J Pediatr. 2007;150:83–88. doi: 10.1016/j.jpeds.2006.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuorilehto T, Poyhonen M, Bloigu R, Heikkinen J, Vaananen K, Peltonen J. Decreased bone mineral density and content in neurofibromatosis type 1: lowest local values are located in the load-carrying parts of the body. Osteoporos Int. 2005;16:928–936. doi: 10.1007/s00198-004-1801-4. [DOI] [PubMed] [Google Scholar]
- 12.Dulai S, Briody J, Schindeler A, North KN, Cowell CT, Little DG. Decreased bone mineral density in neurofibromatosis type 1: results from a pediatric cohort. J Pediatr Orthop. 2007;27:472–475. doi: 10.1097/01.bpb.0000271310.87997.ae. [DOI] [PubMed] [Google Scholar]
- 13.Writing Group for the ISCD Position Development Conference. Diagnosis of osteoporosis in men, premenopausal women, and children. J Clin Densitom. 2004;7:17–26. doi: 10.1385/jcd:7:1:17. [DOI] [PubMed] [Google Scholar]
- 14.Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988;45:575–578. [PubMed] [Google Scholar]
- 15.Angus RM, Sambrook PN, Pocock NA, Eisman JA. A simple method for assessing calcium intake in Caucasian women. J Am Diet Assoc. 1989;89:209–214. [PubMed] [Google Scholar]
- 16.Bertelloni S, Baroncelli GI, Battini R, Perri G, Saggese G. Short-term effect of testosterone treatment on reduced bone density in boys with constitutional delay of puberty. J Bone Miner Res. 1995;10:1488–1495. doi: 10.1002/jbmr.5650101009. [DOI] [PubMed] [Google Scholar]
- 17.Ellis KJ, Shypailo RJ, Hardin DS, Perez MD, Motil KJ, Wong WW, Abrams SA. Z score prediction model for assessment of bone mineral content in pediatric diseases. J Bone Miner Res. 2001;16:1658–1664. doi: 10.1359/jbmr.2001.16.9.1658. [DOI] [PubMed] [Google Scholar]
- 18.Szudek J, Birch P, Friedman JM. Growth charts for young children with neurofibromatosis 1 (NF1) Am J Med Genet. 2000;92:224–228. [PubMed] [Google Scholar]
- 19.Vitale MG, Guha A, Skaggs DL. Orthopaedic manifestations of neurofibromatosis in children: an update. Clin Orthop. 2002:107–118. doi: 10.1097/00003086-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Bass S, Delmas PD, Pearce G, Hendrich E, Tabensky A, Seeman E. The differing tempo of growth in bone size, mass, and density in girls is region-specific. J Clin Invest. 1999;104:795–804. doi: 10.1172/JCI7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnick S. Bone densitometry in clinical practice. Humana Press Inc; Totowa (NJ): 1998. pp. 31–64. [Google Scholar]
- 22.Gluer CC, Faulkner KG, Estilo MJ, Engelke K, Rosin J, Genant HK. Quality assurance for bone densitometry research studies: concept and impact. Osteoporos Int. 1993;3:227–235. doi: 10.1007/BF01623825. [DOI] [PubMed] [Google Scholar]
- 23.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 24.Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr. 2001;73:288–294. doi: 10.1093/ajcn/73.2.288. [DOI] [PubMed] [Google Scholar]
- 25.Andersen LB, Fountain JW, Gutmann DH, Tarle SA, Glover TW, Dracopoli NC, Housman DE, Collins FS. Mutations in the neurofibromatosis 1 gene in sporadic malignant melanoma cell lines. Nat Genet. 1993;3:118–121. doi: 10.1038/ng0293-118. [DOI] [PubMed] [Google Scholar]
- 26.Johnson MR, Look AT, DeClue JE, Valentine MB, Lowy DR. Inactivation of the NF1 gene in human melanoma and neuroblastoma cell lines without impaired regulation of GTP.Ras. Proc Natl Acad Sci U S A. 1993;90:5539–5543. doi: 10.1073/pnas.90.12.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The I, Murthy AE, Hannigan GE, Jacoby LB, Menon AG, Gusella JF, Bernards A. Neurofibromatosis type 1 gene mutations in neuroblastoma. Nat Genet. 1993;3:62–66. doi: 10.1038/ng0193-62. [DOI] [PubMed] [Google Scholar]
- 28.Coen G. Adynamic bone disease: an update and overview. J Nephrol. 2005;18:117–22. [PubMed] [Google Scholar]
- 29.Kolanczyk M, Kossler N, Kuhnisch J, Lavitas L, Stricker S, Wilkening U, Manjubala I, Fratzl P, Sporle R, Herrmann BG, Parada LF, Kornak U, Mundlos S. Multiple roles for neurofibromin in skeletal development and growth. Hum Mol Genet. 2007;16:874–886. doi: 10.1093/hmg/ddm032. [DOI] [PubMed] [Google Scholar]
- 30.Yang FC, Chen S, Robling AG, Yu X, Nebesio TD, Yan J, Morgan T, Li X, Yuan J, Hock J, Ingram DA, Clapp DW. Hyperactivation of p21ras and PI3K cooperate to alter murine and human neurofibromatosis type 1-haploinsufficient osteoclast functions. J Clin Invest. 2006;116:2880–28891. doi: 10.1172/JCI29092. [DOI] [PMC free article] [PubMed] [Google Scholar]