Phosphorylation of CCAAT/Enhancer-binding Protein α Regulates GLUT4 Expression and Glucose Transport in Adipocytes (original) (raw)
Abstract
The transcription factor CCAAT/enhancer-binding protein α (C/EBPα) is required during adipogenesis for development of insulin-stimulated glucose uptake. Modes for regulating this function of C/EBPα have yet to be determined. Phosphorylation of C/EBPα on Ser-21 has been implicated in the regulation of granulopoiesis and hepatic gene expression. To explore the role of Ser-21 phosphorylation on C/EBPα function during adipogenesis, we developed constructs in which Ser-21 was mutated to alanine (S21A) to model dephosphorylation. In two cell culture models deficient in endogenous C/EBPα, enforced expression of S21A-C/EBPα resulted in normal lipid accumulation and expression of many adipogenic markers. However, S21A-C/EBPα had impaired ability to activate the Glut4 promoter specifically, and S21A-C/EBPα expression resulted in diminished GLUT4 and adiponectin expression, as well as reduced insulin-stimulated glucose uptake. No defects in insulin signaling or GLUT4 vesicle trafficking were identified with S21A-C/EBPα expression, and when exogenous GLUT4 expression was enforced to normalize expression in S21A-C/EBPα cells, insulin-responsive glucose transport was reconstituted, suggesting that the primary defect was a deficit in GLUT4 levels. Mice in which endogenous C/EBPα was replaced with S21A-C/EBPα displayed reduced GLUT4 and adiponectin protein expression in epididymal adipose tissue and increased blood glucose compared with wild-type littermates. These results suggest that phosphorylation of C/EBPα on Ser-21 may regulate adipocyte gene expression and whole body glucose homeostasis.
Although adipocytes were initially thought to be passive storage vessels for caloric excess, it is now known that adipose tissue acts as an important metabolic and endocrine organ (1–4). One of the major regulatory hormones for fat cell function is insulin, which regulates whole body energy balance by increasing glucose uptake into muscle and adipose tissue via translocation of the glucose transporter GLUT4 to the cell surface and by inhibiting hepatic gluconeogenesis (4–7). Several studies have identified C/EBPα2 as a critical factor for development of insulin-sensitive glucose uptake in developing adipocytes (8–11).
Adipogenesis is a coordinated transcriptional cascade of gene expression regulated by many transcription factors, including PPARγ and members of the C/EBP and KLF families of transcription factors (12–17). Mouse models suggest that C/EBPα and PPARγ are each required for complete development of adipose tissue in vivo (18–20).In vitro cell culture studies determined that although constitutive expression of either PPARγ or C/EBPα is sufficient to convert fibroblasts into fatladen, adipocyte-like cells (21), C/EBPα is required for the establishment of insulin-stimulated glucose uptake in adipocytes (10,11). In both C/EBPα-/- mouse embryonic fibroblasts (MEFs) and NIH-3T3 fibroblasts, which are also deficient in C/EBPα, overexpression of PPARγ is sufficient to induce lipid accumulation, but these “adipocytes” do not transport glucose in response to insulin (8,9). The mechanisms for this dysfunction differ in the two cell types.
C/EBPα-/- MEFs overexpressing PPARγ have diminished insulin receptor and insulin receptor substrate-1 expression as well as insulin-stimulated phosphorylation. Additional studies suggest that in the absence of C/EBPα, the fast basal exocytosis of GLUT4-associated vesicles is not suppressed in these cells, leading to an increase in basal, non-insulin stimulated glucose transport (11). Thus, in this model, C/EBPα is required both for early steps in the insulin signaling cascade and for basal cytoplasmic retention of GLUT4. In contrast, enforced expression of PPARγ in NIH-3T3 cells stimulates lipid accumulation and normal early insulin signaling but does not fully stimulate GLUT4 expression (9). With exogenous GLUT4 expression, these cells have normal GLUT4 vesicle trafficking, suggesting that it is only the deficiency in GLUT4 expression that precludes development of insulin-stimulated glucose uptake in the absence of C/EBPα in these cells (10).
Together, these studies indicate that although C/EBPα is required for acquisition of insulin sensitivity, PPARγ may be sufficient for lipid accumulation (19), indicating that each may be required for expression of a subset of adipocyte genes and that together these master regulators of adipogenesis stimulate the full complement of genes required for the adipocyte phenotype. How this aspect of C/EBPα function may be regulated has yet to be determined; however, recent insights into the effects of post-translational modifications of C/EBPα in modulating its function suggest a role for phosphorylation (25–34).
The N terminus of C/EBPα has multiple transactivation domains, including several highly conserved regions separated by strings of glycines and prolines (22), and a number of laboratories have further categorized and characterized these transactivation domains (20–23). Post-translational modifications are a common mechanism for regulating protein structure and protein-protein interactions, and modification of the N terminus of C/EBPα by both SUMOylation and phosphorylation has been described (23–25). Recent in vitro studies in hepatocytes suggest that phosphorylation at Ser-193 of C/EBPα may be an important mechanism for regulating protein-protein interactions involving C/EBPα (26–28). Ultimately, these studies indicate that phosphorylation at Ser-193 leads to protein-protein interactions that promote C/EBPα growth inhibitory activity, whereas C/EBPα that is dephosphorylated at Ser-193 promotes liver cell proliferation in vitro (26–28). Hematopoietic cell lines have also been a productive source for studying effects of phosphorylation on C/EBPα function, and phosphorylation of Ser-248 through protein kinase C was found to promote interaction between Ras and C/EBPα, leading to increased granulopoiesis (29). In addition, GSK-3 phosphorylation of C/EBPα on Thr-222 and Thr-226 has been explored (30,31). Studies indicate that phosphorylation at these sites is important for C/EBPα function in adipocytes and hepatocytes in both in vitro and in vivo models (30,31).
Ser-21 has recently been a site of interest for its role in regulating C/EBPα activity in both hematopoietic and hepatic cell lines (32–34). In granulopoietic cell lines, phosphorylation of Ser-21 by Erk1/2 produced significant structural changes in C/EBPα and ultimately resulted in diminished C/EBPα-induced granulopoiesis (32). Phosphorylation of Ser-21 by p38 MAPK in hepatoma cell lines led to increased transactivation of phosphoenolpyruvate kinase (34). This study also reported that in mouse models of diabetes, both p38 MAPK expression and C/EBPα phosphorylation on Ser-21 were increased, further connecting regulation of C/EBPα activity by Ser-21 phosphorylation and altered liver metabolism. To better understand the role of Ser-21 phosphorylation on C/EBPα function, we utilized two in vitro cell culture models, as well as mouse models, to determine whether phosphorylation at this site regulated adipocyte differentiation or metabolism.
Here we report that with enforced expression of a phosphorylation-dead S21A-C/EBPα mutant, lipid accumulation in both C/EBPα-/- MEFs and NIH-3T3 fibroblasts is observed; however, both cell lines have defects in glucose transport in response to insulin compared with wild-type C/EBPα-induced cells. Neither early insulin signaling nor GLUT4 vesicle trafficking are altered in cells expressing S21A-C/EBPα, but there is a significant reduction in expression of GLUT4 and adiponectin. Supporting these findings, S21A-C/EBPα has diminished transactivation ability specifically for the Glut4 promoter. Furthermore, mice in which the endogenous C/EBPα gene locus has been replaced with an S21A mutation have adipose tissue with reduced expression of GLUT4 and adiponectin protein, despite elevated expression of C/EBPα. In addition, male S21A mice have elevated fasted and fed blood glucose levels compared with wild-type littermates and are glucose-intolerant, suggesting that phosphorylation of Ser-21 may be required to maintain glucose homeostasis.
EXPERIMENTAL PROCEDURES
_Plasmid Vectors_—Plasmids expressing wild-type C/EBPα, serine 21 to alanine (S21A), and serine 21 to aspartate (S21D) were described previously (35). Those plasmids were used to construct NH2-C/EBPα, NH2-S21A, and NH2-S21D, as well as pLHCX-C/EBPα and pLHCX-S21A as follows. NH2 is a derivative of the neomycin-resistant retroviral vector pLXSN (22). NH2 and the relevant C/EBPα variant were cut with EcoRI and HindIII and then ligated. The hygromycin-resistant retroviral expression vector pLHCX was cut with HpaI and ligated with appropriate C/EBPα variant that had been excised with EcoRI and then filled in to produce blunt insert. The puromycin-resistant retroviral expression vector pBABE was cut with EcoRI and SalI and ligated to CIEN.A12-adiponectin excised with EcoRI and XhoI to produce pBABE-adiponectin. The original adiponectin construct was a gift from Philipp E. Scherer (Albert Einstein College of Medicine). The GLUT4 construct pS9A2 was a generous gift from Maureen Charron (Albert Einstein College of Medicine) (36). GLUT4 from this construct was excised using EcoRV and BamHI. M72, which is a laboratory derivative of pBABE in which multiple cloning sites were inserted between BamHI and EcoRI, was cut with HpaI and BclI and then ligated to the excised GLUT4 to produce pBABE-GLUT4. pcDNA3-GLUT4-EGFP was a kind gift from Jeffrey E. Pessin (State University of New York, Stony Brook). The promoter-luciferase reporter construct for GLUT4 was provided by Sven Enerback (Goteberg University, Sweden), whereas the C/EBPα and leptin promoter-luciferase reporter constructs were donated by M. Daniel Lane (The Johns Hopkins University).
_Retroviral Infection and Selection_—Retroviral infection and selection procedures were performed essentially as described (35). Briefly, 293T cells were transfected by calcium phosphate coprecipitation. About 16 h after transfection virus-containing media were collected, passed through a 0.45-μm syringe filter, and combined with Polybrene (hexadimethrine bromide; Sigma) to a final concentration of 8 μg/ml. The media were then applied to subconfluent (25–40% confluent) 3T3-L1 preadipocytes, NIH-3T3 fibroblasts, or C/EBPα-/- MEFs in 10-cm plates. The infection protocol was repeated every 8–16 h until cells were ∼80% confluent. Cells were then split 1:5 in Dulbecco's modified Eagle's medium (Invitrogen catalog number 11965) supplemented with 10% calf serum (or 10% fetal bovine serum for C/EBPα-/- MEFs) and appropriate antibiotics (400 μg/ml neomycin, 150 μg/ml hygromycin, or 2 μg/ml puromycin) for selection, as described (35). At 60–70% confluence, cells were split via treatment with trypsin and then replated. Following 2–3 more rounds of splitting and replating that occurred over a span of no less than 5 days to isolate only infected cells, cells were then used for appropriate assays as described below. Cells were maintained in antibiotic-containing media throughout the differentiation process as well.
_Cell Culture_—NIH-3T3 fibroblasts were purchased from the American Type Tissue Culture repository, whereas C/EBPα-/- MEFs and 3T3-L1 preadipocytes were kind gifts from Gretchen Darlington (Baylor University) and M. Daniel Lane, respectively. NIH-3T3 and 3T3-L1 cells were cultured in Dulbecco's modified Eagle's medium containing 10% calf serum at 37 °C and 10% CO2. Two days post-confluence, cells were differentiated as described (9). C/EBPα-/- MEFs were maintained in 10% fetal bovine serum and 5% CO2 and differentiated as described (8). On day 9–12 of the differentiation protocol, adipocytes were used for experiments as described below.
_Oil Red-O Staining_—Oil Red-O was used to stain neutral lipid as described previously (35).
_Cell Lysis and Immunoblots_—Whole cell lysates were prepared by washing cells with PBS, followed by lysis with Western buffer (1% SDS, 60 mm Tris, pH 6.8, and protease inhibitors). Lysates were incubated for 30 min at 50–70 °C, centrifuged at 16,000 × g for 10 min at 4 °C, and stored at -20 °C until analyzed. Protein from adipose tissue was derived by lysis in Western buffer, Dounce homogenization, and a 30-min incubation at 50–70 °C. Protein concentrations were determined using Protein Assay Solution (Bio-Rad). For immunoblot analysis, equal amounts of protein (20–40 μg, as indicated) were separated by SDS-PAGE and transferred onto nitrocellulose membranes. Immunoblotting was performed essentially as described (35) using the following antibodies: insulin receptor, C/EBPα, and PPARγ (Santa Cruz Biotechnology); laminin (Sigma); FABP4 (David Bernlohr, University of Minnesota, Minneapolis); polyclonal C/EBPα antibody raised against a synthetic peptide of amino acids 253–265 (37); ERK1/2, phospho-ERK1/2, Akt, phospho-Akt, and phospho-Ser-21 C/EBPα (Cell Signaling Technology Inc.); insulin receptor substrate-1 (Liangyou Rui, University of Michigan); phosphotyrosine (Upstate Biotechnology); GLUT4 and GLUT1 (Frank C. Brosius III, University of Michigan); GLUT4 (Alpha-Diagnostic); adiponectin (Mitchell Lazar, University of Pennsylvania; Philip Scherer, Albert Einstein College of Medicine). Immunoblotted proteins were detected using enhanced chemiluminescence or an Odyssey Infrared Imaging System (Li-Cor Biosciences). Immunoblots were quantified using Li-Cor Odyssey software and normalized for protein loading to laminin as indicated.
_Extraction of mRNA and Real Time PCR_—Total RNA from adipose tissue or cells in culture was extracted using RNA Stat60 (Tel-Test, Friendswood, TX), purified with RNeasy mini-kits (Qiagen, Valencia, CA), and treated with DNase I. For each sample, 1 μg of RNA was reverse-transcribed to cDNA using the TaqMan system (Applied Biosystems, Foster, CA) and random hexamer primers. Quantitative real time PCR was performed according to the manufacturer's protocol. SYBR Green I was used to monitor amplification of cDNA on the iCycler and IQ real time PCR detection system (Bio-Rad). Gene expression was normalized to cyclophilin mRNA or 18 S rRNA levels. All primers were validated as described (38), and primer sequences are available upon request.
_Transient Transfection and Luciferase Assay_—293T cells were transiently transfected by calcium phosphate coprecipitation. 3T3-L1 preadipocytes were transfected using Lipofectamine 2000 (Invitrogen). Cells were transfected with 500 ng of luciferase reporter gene, 125 ng of cytomegalovirus-β-galactosidase, and the indicated levels of expression plasmid as described (22). Samples were placed in Optocomp II luminometer (MGM Instruments, Hamden, CT) to measure relative light units emitted. These values were normalized against the relative light units measured when β-galactosidase activity was determined.
_Glucose Uptake Assay_—Adipocytes were fed with fresh medium, at most 24 h before assessing glucose uptake. Glucose transport assay was performed essentially as described previously (39,40), with slight modifications and with at least three plates used for each treatment. To assess glucose uptake, radioactivity in 200-μl aliquots were determined by scintillation counting, and results were normalized to total protein, as assessed by protein assay solution (Bio-Rad). Background glucose uptake was determined by treating with cytochalasin B during the last 10 min of insulin stimulation.
_GLUT4 Translocation Assays_—GLUT4-EGFP constructs were generously provided by Jeffrey Pessin (State University of New York, Stony Brook), and transfection was performed as described previously (41). EGFP fluorescence was analyzed by immunofluorescence microscopy with 2–3 pictures taken per slide. GLUT4 translocation was quantified by counting at least 90 cells per treatment group and by determining ratio of plasma membrane-ringed cells to total cells expressing GLUT-EGFP. Quantified data are averages from two separate experiments, and counts per experiment were averages of two independent and blinded tallies. Confocal microscope pictures were also taken for further visualization and to confirm observations.
Plasma membrane fractionation of cells was performed on day 9 or 11 after induction of adipogenesis, no more than 16 h after feeding. Cells were incubated for 30 min at 37 °C in PBS containing 0.7% bovine serum albumin with or without 100 nm insulin. Plasma membrane fraction was obtained using methods as described previously (42). Protein concentration of resuspended plasma membrane was determined using protein assay solution (Bio-Rad), and equal amounts of protein were used for immunoblot assays as described above.
_Animal Care_—All studies were approved by the University Committee on Use and Care of Animals, and mice were cared for by the Unit for Laboratory Animal Medicine (University of Michigan). Mice were housed with a regular 12-h light/dark cycle and were fed ad libitum with standard rodent chow diet (Laboratory Rodent Diet 5001, LabDiet, St. Louis). S21A and S21D knock-in mice were created from 129svev embryonic stem cells selected for the S21A or S21D mutation, which were then injected into C57Bl/6 blastocysts. Mice were genotyped for the presence of transgene, absence of wild-type C/EBPα, and the specific S21A or S21D mutation through PCRs and DNA sequencing. Between 9 a.m. and 12 p.m., mice were weighed and organs dissected for further analysis as described below.
_Blood Glucose Measurements and Glucose Tolerance Tests_—Tail blood was used to measure blood glucose with the OneTouch Ultra™ glucometer (Lifescan, Burnaby, British Columbia, Canada). Fasted blood glucose was measured after 12–16 h of fasting. Fed blood glucose measurements were taken between 9 a.m. and 12 p.m. to normalize feeding status. Mice were fasted for 16 h for the glucose tolerance test, after which mice were intraperitoneally injected with glucose (1.5 mg/g body weight). Blood glucose was measured from tail blood with the OneTouch Ultra™ glucometer at the times indicated.
RESULTS
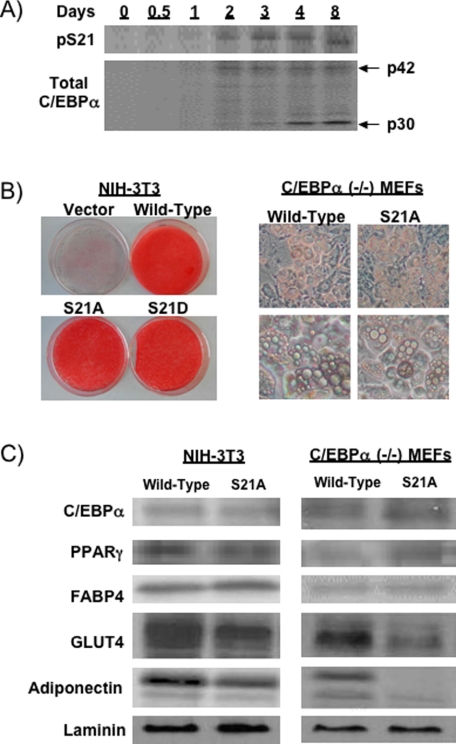
_C/EBP_α _Phosphorylation on Ser-21 Is Not Required for Adipogenic Lipid Accumulation but Is Necessary for Full Expression of GLUT4 and Adiponectin_—To determine whether C/EBPα is phosphorylated on Ser-21 during adipogenesis, we performed a time course in which whole cell lysates were collected throughout differentiation of 3T3-L1 preadipocytes. Immunoblot analyses with phospho-specific antisera revealed that phosphorylation on Ser-21 was detectable on day 2 and increased during adipogenesis proportional to total C/EBPα (Fig. 1_A_), consistent with a role for phosphorylation in modulating activity of C/EBPα during adipogenesis.
FIGURE 1.
C/EBPα phosphorylation on Ser-21 is not required for lipid accumulation but is necessary for a robust expression of GLUT4 and adiponectin. A, 3T3-L1 cells were lysed at the indicated times, and 20 μg of protein were separated by SDS-PAGE and subjected to immunoblot analysis using antibodies for total C/EBPα or for C/EBPα phosphorylated on Ser-21 (pS21). The arrows indicate p42- and p30-C/EBPα. B, NIH-3T3 cells and C/EBPα-/- MEFs were infected with control retrovirus or a retrovirus that expresses wild-type C/EBPα, S21A-C/EBPα, or S21D-C/EBPα. Nine days after induction of adipogenesis, NIH-3T3 cells were stained with Oil Red-O to visualize lipid accumulation. For C/EBPα-/- MEFs, phase contrast pictures at low (top panel) and high (lower panel) magnifications are presented. C, lysates from NIH-3T3 and C/EBPα-/- MEF adipocytes infected with wild-type or S21A-C/EBPα were analyzed by immunoblotting with antibodies for C/EBPα, PPARγ, FABP4, GLUT4, adiponectin, and laminin (control for protein loading).
To determine whether phosphorylation of Ser-21 influences the ability of C/EBPα to stimulate adipogenesis, we developed phosphorylation mutants in which Ser-21 is mutated either to alanine to mimic the unphosphorylated state, or to aspartate to mimic the phosphorylated state. In prior structural and functional studies (32,34), the S21D mutation mimicked phosphorylated C/EBPα, whereas the S21A mutation mimicked dephosphorylated C/EBPα. Enforced expression of wild-type, S21A-, or S21D-C/EBPα in two cell lines deficient in C/EBPα, NIH-3T3 fibroblasts, and C/EBPα-/- MEFs, did not reveal differences in ability to promote adipogenesis per se, as assessed by Oil Red-O staining and by visualization of lipid droplets (Fig. 1_B_). Immunoblot analyses revealed that as observed previously, mutation of Ser-21 does not affect steady state levels of ectopically expressed C/EBPα protein (Fig. 1_C_). In addition, whereas S21A-C/EBPα does not alter expression of adipocyte proteins such as FABP4 and PPARγ (Fig. 1_C_), reduced levels of GLUT4 and adiponectin suggest that phosphorylation of Ser-21 may specifically regulate expression of these genes. Although we have focused on NIH-3T3 cells and the S21A-C/EBPα mutation, where tested, similar results were observed in C/EBPα-/- MEFs, and S21D-C/EBPα behaved similarly to wild-type C/EBPα. However, differences we observed with C/EBPα-/- MEFs and/or S21D-C/EBPα will be noted.
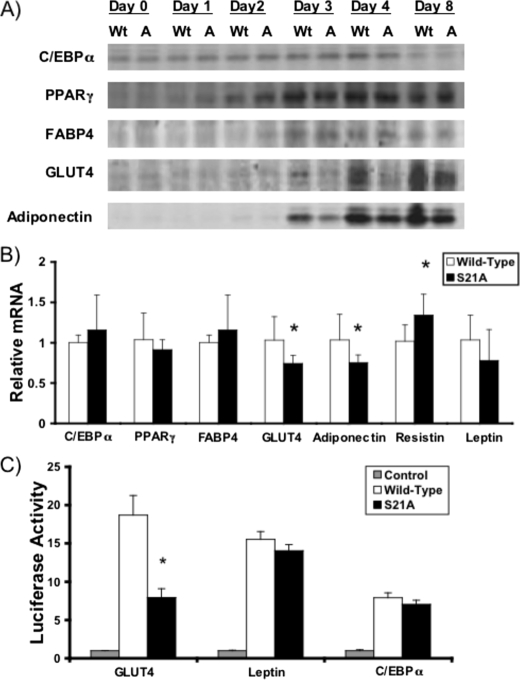
_Specific Impairment of GLUT4 Expression and Promoter Activity with S21A-C/EBP_α—To determine whether induction of adipocyte genes is delayed in S21A-C/EBPα cells during adipogenesis, lysates were prepared at the indicated times, and expression of adipocyte genes was evaluated by immunoblot (Fig. 2_A_). FABP4 and PPARγ were similarly increased by wild-type and S21A-C/EBPα during differentiation of NIH-3T3 cells (Fig. 2_A_). Although GLUT4 and adiponectin were first observed 3 days after induction of differentiation in both wild-type and S21A-C/EBPα cells, decreased expression with S21A-C/EBPα at days 3, 4, and 8 suggests that lack of Ser-21 phosphorylation impairs rather than delays expression of GLUT4 and adiponectin. Analyses of mRNA levels in these cells support these findings (Fig. 2_B_), with similar expression of ectopic C/EBPα and endogenous PPARγ, FABP4, and leptin mRNAs in wild-type and S21A-C/EBPα adipocytes but reduced expression of GLUT4 and adiponectin mRNAs in S21A-C/EBPα adipocytes. Interestingly, mRNA for resistin, a hormone that is associated with insulin resistance (43,44), was slightly increased in S21A-expressing NIH-3T3 cells (Fig. 2_B_); however, this was not observed in C/EBPα-/- MEFs (data not shown).
FIGURE 2.
Phosphorylation of C/EBPα regulates expression of specific adipocyte genes and activation of the Glut4 promoter. A, NIH-3T3 cells that express wild-type C/EBPα (Wt) or S21A-C/EBPα (A) were lysed on day 0, 1–4, or 8 of differentiation. Expression of C/EBPα, PPARγ, FABP4, GLUT4, and adiponectin was assessed by immunoblot. B, RNA was extracted from day 9 NIH-3T3 cells infected with wild-type or S21A-C/EBPα. Quantitative RT-PCR was utilized to assess relative expression of C/EBPα, PPARγ, FABP4, GLUT4, adiponectin, resistin, and leptin mRNAs. Expression of each gene was normalized to expression of 18 S rRNA, and gene expression is presented relative to that observed with wild-type C/EBPα. Differences were determined using Student's t test, and data are presented as mean ± S.D. p values <0.05 are depicted with an_asterisk. C,_ 3T3-L1 preadipocytes were transfected with luciferase reporter genes for Glut4, leptin, or C/EBPα promoters, along with 1 μg of empty vector (Control) or expression vector for wild-type or S21A-C/EBPα. Luciferase activity was normalized to β-galactosidase activity to correct for variations in transfection efficiency and is reported as units relative to the control values. Differences between wild-type and S21A-C/EBPα were determined using Student's t test and are indicated with an asterisk.
We next explored the mechanism for reduced expression of GLUT4 mRNA by evaluating activation of promoter-reporter gene constructs by wild-type C/EBPα and phosphorylation mutants. Although wild-type, S21A-C/EBPα, and S21D-C/EBPα activate the promoters for C/EBPα and leptin similarly in both 3T3-L1 (Fig. 2_C_) and 293T (data not shown) cell lines, the Glut4 promoter is less potently activated by S21A-C/EBPα. Similar expression of wild-type and S21A-C/EBPα in transfected cells was confirmed by immunoblot (data not shown). These data suggest that phosphorylation of Ser-21 is required for full activation of the Glut4 promoter by C/EBPα.
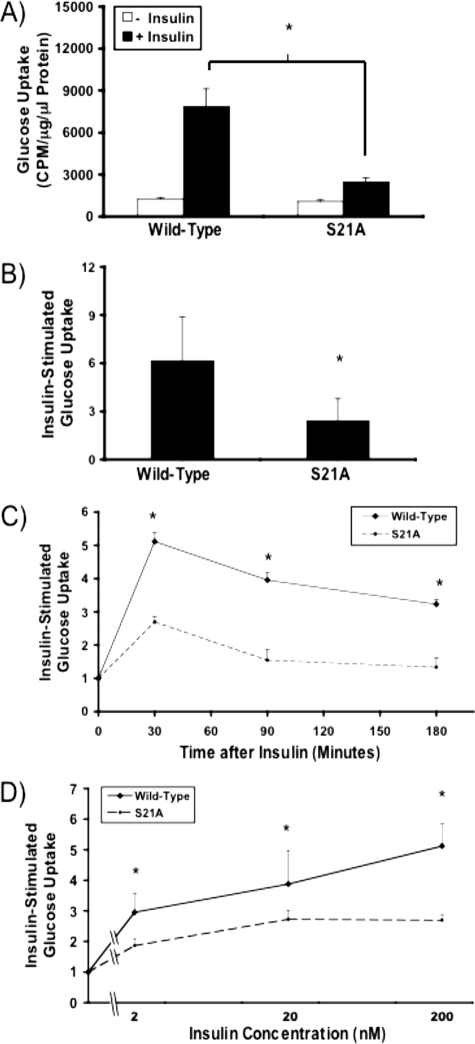
_Insulin-responsive Glucose Transport Is Reduced with S21A-C/EBP_α _Expression, but Timing of Glucose Uptake and Dependence on Insulin Concentration Are Unaltered_—Based on our findings that phosphorylation of Ser-21 is required for C/EBPα to activate full expression of both GLUT4, the insulin-regulated glucose transporter, and adiponectin, a protein associated with insulin sensitivity, in two cell models for adipogenesis, we hypothesized that adipocytes that express S21A-C/EBPα may have impaired response to insulin. Although basal glucose uptake was not altered, insulin-stimulated glucose uptake was decreased from ∼6-fold in adipocytes that express wild-type C/EBPα to ∼2.5-fold with S21A-C/EBPα (Fig. 3, A and_B_). Interestingly, whereas C/EBPα-/- MEFs expressing S21A-C/EBPα also have diminished glucose uptake in response to insulin, there is a slight (<50%) increase in basal glucose uptake, perhaps as a result of an increase in GLUT1 expression in C/EBPα-/- MEFs with enforced expression of S21A-C/EBPα (data not shown). Further studies reveal that adipocytes expressing S21A-C/EBPα do not have a temporal alteration in glucose uptake after insulin stimulation compared with control cells (Fig. 3_C_), and response to various concentrations of insulin is also similar in these cells (Fig. 3_D_). Furthermore, although not shown in Fig. 3_C_, more extensive studies suggest that in S21A-C/EBPα adipocytes, maximal glucose uptake in response to insulin is neither exhausted prior to 30 min of insulin stimulation nor delayed until after 30 min of insulin exposure (data not shown). Throughout these studies, the capacity for insulin-stimulated glucose uptake was quantitatively lower, suggesting that reduced expression of GLUT4 is the primary defect in S21A-C/EBPα adipocytes.
FIGURE 3.
Phosphorylation of C/EBPα on Ser-21 is required for complete insulin-stimulated glucose uptake. NIH-3T3 adipocytes infected with wild-type or S21A-C/EBPα were serum-starved and assessed for insulin-stimulated glucose uptake using 2-deoxy-d-[14C]glucose. A, representative experiment showing basal glucose uptake and glucose transport in response to 100 nm insulin is presented as mean ± S.D. Significance of_p_ < 0.05 is depicted with an asterisk. B, average fold glucose uptake after insulin stimulation from eight independent experiments. Data are presented as mean ± S.D. C, NIH-3T3 adipocytes expressing wild-type or S21A-C/EBPα were serum-starved for 3 h and stimulated with 100 nm insulin for the last 30, 90, or 180 min. Glucose uptake was assayed. D, fold glucose uptake with insulin concentrations of 2, 20, and 200 nm was determined. Data are presented as mean ± S.D. using a log scale on the_abscissa_.
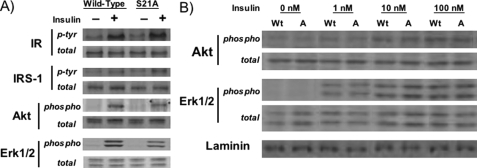
_S21A-C/EBP_α _Does Not Substantially Alter Proximal Insulin Signaling Events_—There are many steps between insulin binding to its receptor and the transport of glucose across the plasma membrane through GLUT4. The initial steps are the most well characterized, with binding of insulin to its receptor followed by receptor autophosphorylation and phosphorylation of various substrates. To determine whether the reduced insulin-stimulated glucose uptake found in S21A-expressing cells is a result of altered insulin signaling, we measured expression and insulin-stimulated phosphorylation of insulin receptor, insulin receptor substrate-1, and downstream target Akt, the phosphorylation of which is involved in insulin-stimulated glucose uptake, and Erk1/2, which is involved in cell growth and differentiation rather than metabolism (Fig. 4). Expression of S21A-C/EBPα in NIH-3T3 adipocytes did not substantially alter expression nor insulin-stimulated phosphorylation of any of these proximal components of the insulin signaling cascade, although phosphorylation of both Akt and Erk1/2 was reduced slightly in adipocytes stimulated with 100 nm insulin (Fig. 4_A_). Similar results were found in C/EBPα-/- MEFs (data not shown). Evaluation with lower concentrations of insulin supports the observation that expression of S21A-C/EBPα may lead to slightly reduced phosphorylation of Akt in response to insulin (Fig. 4_B_). However, because S21A-C/EBPα alters total glucose uptake rather than response to concentration of insulin (Fig. 3_D_), it is likely that this slight reduction in insulin-stimulated Akt phosphorylation is a minor contributor to impaired insulin-stimulated glucose uptake in S21A-C/EBPα adipocytes.
FIGURE 4.
Expression of S21A-C/EBPα does not substantially alter early insulin signaling events. A, after overnight serum deprivation, NIH-3T3 adipocytes expressing wild-type or S21A-C/EBPα were incubated with (+) or without (-) 100 nm insulin for 10 min. Lysates were separated by SDS-PAGE and subjected to immunoblot analysis for insulin receptor (IR), insulin receptor substrate-1 (IRS-1), Akt, or Erk1/2 total protein (total). Activation of these proteins was determined by phosphotyrosine (p-Tyr) antibodies for insulin receptor and insulin receptor substrate-1 or phospho-specific antibodies for Akt and Erk1/2 (phospho). B, phosphorylation of Akt and Erk1/2 in wild-type (Wt) or S21A-C/EBPα (A) expressing cells with 1, 10, or 100 nm insulin was assessed using antibodies specific for phosphorylated or total Akt and Erk1/2. Laminin was used as a loading control.
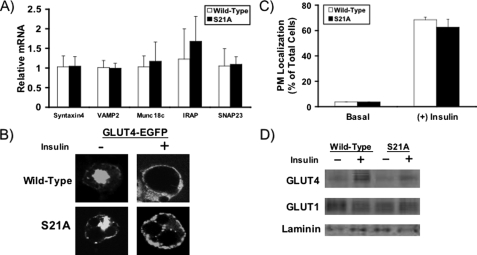
_GLUT4 Vesicle Translocation to Plasma Membrane Is Not Altered in S21A-C/EBP_α _Adipocytes_—We next sought to assess the integrity of GLUT4 vesicle trafficking in S21A-expressing cells. Through quantitative RT-PCR, we found that mRNA expression of Syntaxin-4, VAMP-2, Munc18c, IRAP, and SNAP23, molecules essential for proper GLUT4 vesicle trafficking, was not altered in NIH-3T3 adipocytes expressing S21A-C/EBPα (Fig. 5_A_). In contrast, Syntaxin-4 and Munc18c mRNA were both slightly but statistically reduced in C/EBPα-/- MEF adipocytes expressing S21A-C/EBPα (data not shown).
FIGURE 5.
GLUT4 vesicle transport is intact in S21A-C/EBPα adipocytes. A, RNA was extracted from NIH-3T3 adipocytes expressing wild-type or S21A-C/EBPα. Quantitative RT-PCR was utilized to assess relative expression of Syntaxin-4, VAMP2, Munc18, IRAP, and SNAP23 mRNAs. Expression of each gene was normalized to 18 S rRNA, and adipocyte gene expression in S21A cells is shown relative to that in wild-type C/EBPα adipocytes. Data are presented as mean ± S.D. (n = 6). B, on day 5 after induction of adipogenesis, NIH-3T3 adipocytes expressing wild-type or S21A-C/EBPα were electroporated with 100 μg of GLUT4-EGFP expression vector. Two days after transfection, cells were serum-starved for 2 h at 37 °C in PBS containing 0.7% bovine serum albumin and stimulated with (+) or without (-) 100 nm insulin for the last 30 min of incubation. Cells were then fixed and placed on glass slides. Representative confocal microscope pictures showing nuclear or membrane localization of GLUT4-EGFP are presented.C, trafficking of GLUT4 to the plasma membrane was quantified in wild-type or S21A-C/EBPα adipocytes under basal conditions and after stimulation with insulin for 30 min. This graph presents the average of two separate experiments in which percentage of cells with plasma membrane “ringing” of GLUT4-EGFP was assessed with fluorescent phase-contrast microscopy. D, NIH-3T3 adipocytes were serum-starved and then exposed to vehicle (-) or insulin (+) for 30 min. After lysis, plasma membrane fractions were separated using a sucrose gradient buffer and ultracentrifugation, and GLUT4 translocation was assessed by immunoblotting. GLUT1 and laminin were used as loading controls.
To test more directly whether GLUT4 vesicle trafficking is altered in adipocytes that express S21A-C/EBPα, we utilized two approaches. First, we transiently transfected NIH-3T3 adipocytes expressing wild-type or S21A-C/EBPα with a GLUT4-EGFP expression construct. After stimulation with insulin, we assessed frequency of cells in which GLUT4 translocated to the plasma membrane using fluorescent microscopy and observed no difference between cell types either qualitatively (Fig. 5_B_, confocal microscopy) or quantitatively (Fig. 5_C_, fluorescent phase-contrast microscopy). Similar results were seen in C/EBPα-/- MEFs (data not shown). Second, we fractionated plasma membranes and found by immunoblot analysis that in response to insulin, the localization of endogenous GLUT4 to the plasma membrane was similar relative to expression level in cells expressing wild-type or S21A-C/EBPα (Fig. 5_D_). Thus, phosphorylation of C/EBPα on Ser-21 does not appear to alter GLUT4 vesicle trafficking, and reduced plasma membrane-associated GLUT4 in S21A-C/EBPα adipocytes reflects the decrease in total expression of GLUT4.
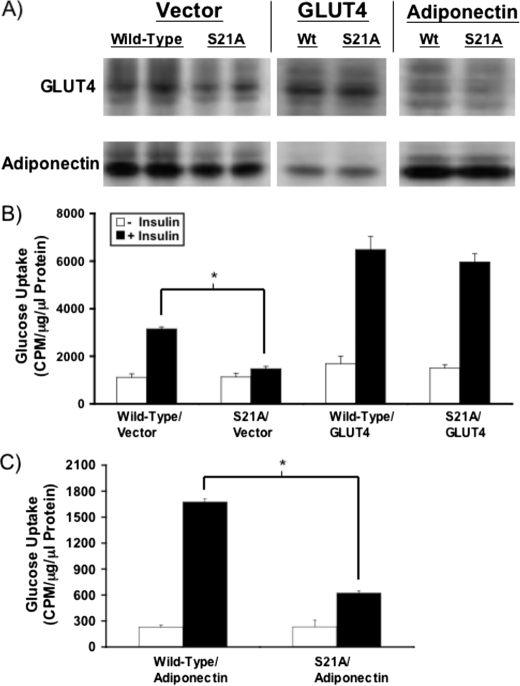
_Enforced Expression of GLUT4 but Not Adiponectin Rescues Insulin-stimulated Glucose Uptake in S21A-C/EBP_α_Adipocytes_—Based on these data, neither insulin signaling nor insulin-stimulated GLUT4 vesicle transport appears to be substantially altered in S21A-C/EBPα adipocytes. Consequently, we developed retroviral expression constructs for both GLUT4 and adiponectin to determine whether enforced expression of either molecule reconstituted full insulin-stimulated glucose uptake in S21A-C/EBPα adipocytes. Immunoblot analyses suggest that both constructs could normalize expression of GLUT4 and adiponectin in S21A-C/EBPα adipocytes to levels observed with wild-type C/EBPα (Fig. 6_A_). Importantly, enforced expression of GLUT4 in S21A-C/EBPα adipocytes rescued the impairment to insulin-stimulated glucose uptake (Fig. 6_B_). In contrast, S21A-C/EBPα adipocytes with ectopic expression of adiponectin still had reduced insulin-stimulated glucose uptake compared with wild-type C/EBPα adipocytes (Fig. 6_C_). Interestingly, reduction in adiponectin expression may be a secondary effect of decreased glucose transport, as enforced expression of GLUT4 virtually normalized expression of adiponectin in S21A-C/EBPα adipocytes (Fig. 6_A_). Taken together, these results further support the hypothesis that the primary defect leading to reduced insulin-stimulated glucose uptake in S21A-C/EBPα adipocytes is reduced GLUT4 expression.
FIGURE 6.
Rescue of insulin-stimulated glucose uptake with enforced expression of GLUT4 but not adiponectin. A, NIH-3T3 cells infected previously with retroviruses expressing wild-type or S21A-C/EBPα were infected again with control vector, GLUT4, or adiponectin. Adipocyte lysates were subjected to immunoblot analysis for GLUT4 and adiponectin to confirm that enforced expression of GLUT4 or adiponectin normalizes expression of these proteins in S21A-C/EBPα adipocytes. B, insulin-stimulated glucose uptake in wild-type and S21A-C/EBPα adipocytes after infection with ectopic GLUT4 or control vector was assessed. Data are presented as mean ± S.D. Asterisk indicates significance at p < 0.05. C, wild-type and S21A-C/EBPα expressing cells were infected with an adiponectin retrovirus, and insulin-stimulated glucose uptake was assessed on day 9 of differentiation. Data are presented as mean ± S.D. Asterisk indicates difference between wild-type/adiponectin and S21A/adiponectin after treatment with insulin (p < 0.05).
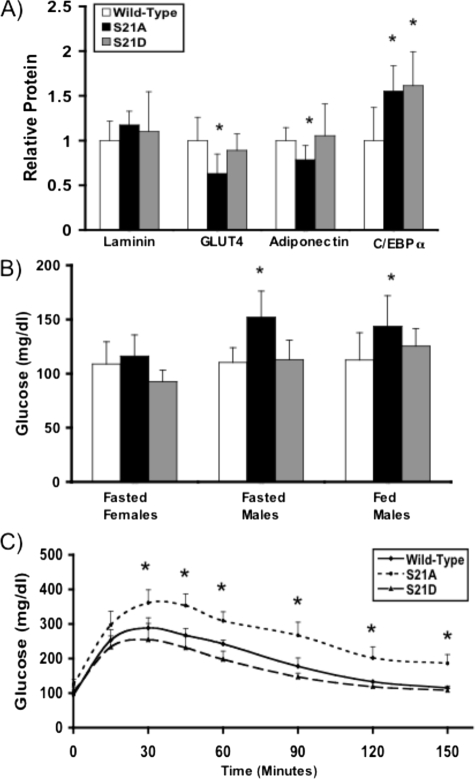
_S21A-C/EBP_α _Knock-in Mice Have Reduced Expression of GLUT4 and Adiponectin Protein Expression in Adipose Tissue and Are Hyperglycemic and Glucose-intolerant_—To determine whether phosphorylation of Ser-21 is important for ability of C/EBPα to direct development and gene expression of adipose tissue in vivo, we developed mice in which the endogenous C/EBPα locus is replaced with S21A-C/EBPα or S21D-C/EBPα. Although eight age-matched male homozygous knock-in and wild-type mice had no differences in total body weight or epididymal white adipose tissue weight (data not shown), we found that epididymal depots in eight homozygous S21A mice had an ∼40% reduction in GLUT4 protein expression and 25% reduction in adiponectin protein expression (Fig. 7_A_). Interestingly, this reduction in GLUT4 and adiponectin protein occurred despite a 55% increase in C/EBPα protein expression compared with wild-type mice (Fig. 7_A_). Although alterations in blood glucose were not observed in fasted female mice, the S21A mutation led to an increase in both fasted and fed blood glucose levels in male S21A mice (Fig. 7_B_), suggesting sexual dimorphism in effects of C/EBPα phosphorylation on blood glucose levels. Male S21A mice were also glucose-intolerant as assessed by the average response to glucose load (Fig. 7_C_), whereas S21D mice had similar glucose tolerance to wild-type mice. Our observations of reduced epididymal adipose tissue expression of GLUT4, increased blood glucose, and diminished glucose tolerance in S21A-C/EBPα mice suggest that phosphorylation of C/EBPα may contribute to maintenance of glucose homeostasis through a yet to be characterized mechanism.
FIGURE 7.
S21A-C/EBPα mice have impaired adipose expression of GLUT4 and adiponectin and are glucose-intolerant. A, mice in which the endogenous C/EBPα gene locus was replaced by S21A-C/EBPα or S21D-C/EBPα were generated. Heterozygous A/+ or D/+ mice were bred to produce homozygous knock-in mice or wild-type littermates. Eight male age-matched mice were selected for each genotype, and expression of laminin, GLUT4, adiponectin, and C/EBPα in E-WAT was assessed by immunoblot. Proteins were detected using an Odyssey Infrared Imaging System (Li-Cor Biosciences) and were quantified using Li-Cor Odyssey software. Data are presented as mean ± S.D., and significance is indicated with an_asterisk_ (p < 0.05). B, male homozygous wild-type, S21A, and S21D mice were assessed for fasted and fed blood glucose levels by measurement of tail blood with the OneTouch Ultra™ glucometer (Lifescan, Burnaby, British Columbia, Canada). Female mice were assessed for fasted blood glucose only. Fasted blood glucose was measured after 12–16 h of fasting in eight mice for each genotype. Fed blood glucose measurements were taken from 10 mice for each genotype between 9 a.m. and 12 p.m.C, for glucose tolerance tests, mice were fasted for 16 h, after which mice were intraperitoneally injected with glucose (1.5 mg/g body weight). Blood glucose was measured from tail blood with the OneTouch Ultra™ glucometer at 0, 15, 30, 45, 60, 90, 120, and 150 min. Shown graphically is the average of three separate experiments with n = 5 for each experiment. Data are presented as mean ± S.D.Asterisk represents difference between S21A and wild-type mice (p < 0.05).
DISCUSSION
C/EBPα and PPARγ are essential for producing many aspects of the adipocyte phenotype. Studies utilizing various preadipocyte and fibroblast cell lines have elucidated crucial aspects of the function for each factor. In MEFs deficient in PPARγ, enforced expression of C/EBPα is not sufficient to induce lipid accumulation, whereas in both NIH-3T3 fibroblasts and C/EBPα-/- MEFs, constitutive expression of PPARγ allows lipid accumulation and the morphological appearance of an adipocyte; however, these cells do not transport glucose in response to insulin stimulation (8–11,45). In the study presented here, we sought to determine whether the ability of C/EBPα to produce insulin-stimulated glucose transport in adipocytes is regulated by phosphorylation on Ser-21.
When phosphorylation of C/EBPα on Ser-21 was prevented by a codon mutation to alanine, both NIH-3T3 cells and C/EBPα-/- MEFs displayed a deficiency in insulin-responsive glucose uptake (Fig. 3). In addition, phosphorylation of C/EBPα on Ser-21 was required for full expression of GLUT4 and adiponectin, without alterations in lipid accumulation, gene expression, or insulin response, and this deficit in GLUT4 expression was largely responsible for reduced glucose uptake. Furthermore, we report that exclusive expression of S21A-C/EBPα does not confer a genetic advantage or disadvantage for survival in mice nor significant differences in total body or epididymal white adipose tissue (E-WAT) weights (data not shown). However, E-WAT expression of both GLUT4 and adiponectin was significantly reduced in S21A mice, and S21A mice displayed hyperglycemia and glucose intolerance (Fig. 7). A role for other tissues that affect glucose metabolism, such as liver and/or muscle, in contributing to these effects has not been excluded.
Erk1/2 is the kinase for Ser-21 phosphorylation in adipocytes (32), whereas in liver, p38 MAPK is thought to be responsible for phosphorylation at this site (34). Interestingly, although Ser-21 phosphorylation inhibits ability of C/EBPα to induce granulopoiesis, in hepatic cell lines, phosphorylation on Ser-21 enhances ability of C/EBPα to activate the phosphoenolpyruvate kinase promoter (32,34). These results suggest that effects of Ser-21 phosphorylation on C/EBPα function may be dependent on cell type and organ system. Also, because phosphorylation of C/EBPβ has been associated with increased expression of C/EBPα, but not PPARγ, FABP4, or fatty-acid synthase (47), modulation of a subset of adipocyte targets by C/EBP phosphorylation may be a common method of regulation in adipocytes. Furthermore, it is likely that the mechanism whereby phosphorylation of C/EBPα regulates specific promoters is dependent on the population of coactivators and corepressors present in each context. One potential partner is cAMP-response element-binding protein, which has been found to interact with C/EBPα as a cofactor for activation of the phosphoenolpyruvate kinase promoter following E1a-induced phosphorylation of C/EBPα (48), and C/EBPα interactions with NF-Y, CBP, and thyroid hormone receptor have been described (49–51). Interestingly, HNF6 and C/EBPα complex formation activates Foxa2 expression (52), an important regulator of gluconeogenic enzymes, and C/EBPα and p300 synergize to activate the leptin promoter (22). In addition, although phosphorylation of Ser-21 does not appear to alter DNA binding activity (32), phosphorylation at other sites on C/EBPα has been associated with changes in binding to DNA (53), and the possibility exists that phosphorylation on Ser-21 alters phosphorylation at other sites.
Because mechanisms for insulin-regulated glucose transport continue to be elucidated, unknown players in insulin-stimulated glucose uptake may be regulated by C/EBPα and contribute to the deficiencies found in the absence of C/EBPα phosphorylation on Ser-21. One of the proteins known to be important in the events between early insulin signaling and GLUT4 vesicle transport is AS160, which is required for basal intracellular retention and insulin-stimulated exocytosis of GLUT4 vesicles (54–56). However, we found no difference in expression of this molecule in either cell line with expression of S21A-C/EBPα (data not shown). In addition, cells expressing S21A-C/EBPα displayed no alteration in expression of the Cbl-activated protein (CAP), which is thought to be essential for the Cbl pathway for glucose transport and is known to have multiple C/EBP-binding sites (57) (data not shown). These results again point to the reduction in GLUT4 as the primary defect in insulin-stimulated glucose uptake in these cells.
Surprisingly, we found that C/EBPα protein expression is elevated in E-WAT of both S21A and S21D mice. Although this observation may be a result of altered transcriptional control of the C/EBPα gene with knock-in mutation, because there have been reports that C/EBPα that is phosphorylated (32) or dephosphorylated (34) on Ser-21 has deficient function in other systems, this observed increase in C/EBPα expression may be a result of compensatory mechanisms to replace decreased GLUT4 and/or adiponectin in S21A-expressing WAT. Compensation for deficits in other aspects of C/EBPα function because of constitutive absence or presence of phosphorylation on Ser-21 may also contribute to this effect.
The importance of GLUT4 expression in adipocyte and whole body metabolism has been highlighted by observations in human disease and by numerous animal models. In states of insulin resistance such as in obesity and type 2 diabetes, GLUT4 expression is decreased in adipose tissue but preserved in muscle (5,58). Based on the intimate interplay between insulin exposure, C/EBPα structure, and adipocyte function, it is possible that insulin may alter adipocyte function partially through regulation of C/EBPα phosphorylation. For instance, insulin was found to stimulate GLUT4 expression in brown adipocytes through increased C/EBPα expression (59), but because insulin activates Erk1/2, which is the kinase for Ser-21, perhaps this effect is amplified by increased phosphorylation on Ser-21 leading to enhanced C/EBPα-activated GLUT4 expression. In addition, multiple instances of regulation of GLUT4 expression through modulation of C/EBPα expression or activity in response to cell stress have been described (60–63). The importance of regulation of GLUT4 expression by transcription factors in producing alterations in glucose metabolism has also been reviewed (64).
Mice in which GLUT4 has been knocked out specifically in adipose tissue have normal growth and adipose mass, despite markedly impaired insulin-stimulated glucose uptake in adipocytes; however, these mice develop insulin resistance in muscle and liver, glucose intolerance, and hyperinsulinemia (65). In addition, although GLUT4 knock-out mice displayed significant reductions in IRAP and VAMP2 expression (66), we found no such difference in IRAP or VAMP2 expression in our cells (Fig. 5_A_), perhaps because enough GLUT4 was produced in S21A cells to allow normal expression of IRAP and VAMP2. There is also the potential that our study only detected defects during adipogenesis, as opposed to defects that may occur after differentiation in response to perturbed metabolic processes within adipocytes.
Interestingly, adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle (67), emphasizing the important interplay between insulin-responsive organs in maintenance of glucose homeostasis. Female S21A and S21D mice displayed no significant change in fasted blood glucose, but male S21A mice had markedly higher fasted and fed blood glucose levels (Fig. 7). Surprisingly, there were no significant differences in serum insulin or adiponectin levels (data not shown), despite decreased adiponectin expression in E-WAT of these hyperglycemic S21A mice. Whether these defects are primary defects as a result of enforced dephosphorylation of C/EBPα on Ser-21 or a result of alterations brought about by the effects of S21A expression in certain organs have yet to be determined. In addition, we reported that all of the C/EBPα-expressing organs tested displayed phosphorylation at Ser-21 (32), and it has been observed that both increased p38 MAPK activity in liver (34) and decreased GLUT4 expression in adipose tissue are associated with human type 2 diabetes (5,58), suggesting that the alterations in glucose homeostasis observed in S21A knock-in mice may be a result of altered C/EBPα function in multiple organs, particularly the liver, as well as adipose tissue. The central role of C/EBPα in adipocyte differentiation and metabolism, as well as adipokine gene expression (46), suggests that understanding the mechanisms by which C/EBPα function is modulated by phosphorylation may provide further insights into the regulation of whole body glucose homeostasis.
*
This work was supported, in whole or in part, by National Institutes of Health Grant DK62876 (to O. A. M.). This work was also supported by American Diabetes Association Grant 1-06-RA-88, by Fellowship 5-T32-HD007505 from the Center for Organogenesis (to H. C. C.), and by fellowships from the Horace Rackham School of Graduate Studies (to S. K.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “_advertisement_” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
2
The abbreviations used are: C/EBPα, CCAAT/enhancer-binding protein α; PPARγ, peroxisome proliferator-activated receptor γ; MEF, mouse embryonic fibroblast; PBS, phosphate-buffered saline; MAPK, mitogen-activated protein kinase; Erk, extracellular signal-regulated kinase; E-WAT, epididymal white adipose tissue; EGFP, enhanced green fluorescent protein; RT, reverse transcription.
References
- 1.Minokoshi, Y., Kahn, C. R., and Kahn, B. B. (2003) J. Biol. Chem. 278 33609-33612 [DOI] [PubMed] [Google Scholar]
- 2.Kahn, B. B., and Flier, J. S. (2000) J. Clin. Investig. 106 473-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mora, S., and Pessin, J. E. (2002) Diabetes Metab. Res. Rev. 18 345-356 [DOI] [PubMed] [Google Scholar]
- 4.Saltiel, A. R., and Kahn, C. R. (2001) Nature 414 799-806 [DOI] [PubMed] [Google Scholar]
- 5.Shepherd, P. R., and Kahn, B. B. (1999) N. Engl. J. Med. 341 248-257 [DOI] [PubMed] [Google Scholar]
- 6.Goodyear, L. J., and Kahn, B. B. (1998) Annu. Rev. Med. 49 235-261 [DOI] [PubMed] [Google Scholar]
- 7.Kanzaki, M., and Pessin, J. E. (2001) J. Biol. Chem. 276 42436-42444 [DOI] [PubMed] [Google Scholar]
- 8.Wu, Z., Rosen, E. D., Brun, R., Hauser, S., Adelmant, G., Troy, A. E., McKeon, C., Darlington, G. J., and Spiegelman, B. M. (1999) Mol. Cell 3 151-158 [DOI] [PubMed] [Google Scholar]
- 9.El-Jack, A. K., Hamm, J. K., and Farmer, S. R. (1999) J. Biol. Chem. 274 7946-7951 [DOI] [PubMed] [Google Scholar]
- 10.Gross, D. N., Farmer, S. R., and Pilch, P. F. (2004) Mol. Cell. Biol. 24 7151-7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wertheim, N., Cai, Z., and McGraw, T. E. (2004) J. Biol. Chem. 279 41468-41476 [DOI] [PubMed] [Google Scholar]
- 12.Darlington, G. J., Ross, S. E., and MacDougald, O. A. (1998) J. Biol. Chem. 273 30057-30060 [DOI] [PubMed] [Google Scholar]
- 13.Oishi, Y., Manabe, I., Tobe, K., Tsushima, K., Shindo, T., Fijiu, K., Nishimura, G., Maemura, K., Yamauchi, T., Kubota, N., Suzuki, R., Kitamura, T., Akira, S., Kadowaki, T., and Nagai, R. (2005) Cell Metab. 1 27-39 [DOI] [PubMed] [Google Scholar]
- 14.Li, D., Yea, S., Li, S., Chen, Z., Narla, G., Banck, M., Laborda, J., Tan, S., Friedman, J. M., Friedman, S. L., and Walsh, M. J. (2005) J. Biol. Chem. 280 26941-26952 [DOI] [PubMed] [Google Scholar]
- 15.Mori, T., Sakaue, H., Iguchi, H., Gomi, H., Okada, Y., Takashima, Y., Nakamura, K., Nakamura, T., Yamauchi, T., Kubota, N., Kadowaki, T., Matsuki, Y., Ogawa, W., Hiramatsu, R., and Kasuga, M. (2005) J. Biol. Chem. 280 12867-12875 [DOI] [PubMed] [Google Scholar]
- 16.Rosen, E. D., Walkey, C. J., Puigserver, P., and Spiegelman, B. M. (2000) Genes Dev. 14 1293-1307 [PubMed] [Google Scholar]
- 17.Rangwala, S. M., and Lazar, M. A. (2000) Annu. Rev. Nutr. 20 535-559 [DOI] [PubMed] [Google Scholar]
- 18.Barak, Y., Nelson, M. C., Ong, E. S., Jones, Y. Z., Ruiz-Lozano, P., Chien, K. P., Koder, A., and Evans, R. M. (1999) Mol. Cell 4 585-595 [DOI] [PubMed] [Google Scholar]
- 19.Rosen, E. D., Sarraf, P., Troy, A. E., Bradwin, G., Moore, K., Milstone, D. S., Spiegelman, B. M., and Mortensen, R. M. (1999) Mol. Cell 4 611-617 [DOI] [PubMed] [Google Scholar]
- 20.Wang, N. D., Finegold, M. J., Bradley, A., Ou, C. N., Abdeisayed, S. V., Wilde, M. D., Taylor, L. R., Wilson, D. R., and Darlington, G. J. (1995) Science 269 1108-1112 [DOI] [PubMed] [Google Scholar]
- 21.Freytag, S. O., Paielli, D. L., and Gilbert, J. D. (1994) Genes Dev. 8 1654-1663 [DOI] [PubMed] [Google Scholar]
- 22.Erickson, R. L., Hemati, N., Ross, S. E., and MacDougald, O. A. (2001) J. Biol. Chem. 276 16348-16355 [DOI] [PubMed] [Google Scholar]
- 23.Kim, J., Cantwell, C. A., Johnson, P. F., Pfarr, C. M., and Williams, S. C. (2002) J. Biol. Chem. 277 38037-38044 [DOI] [PubMed] [Google Scholar]
- 24.Subramanian, L., Benson, M. D., and Iniguez-Lluhi, J. A. (2003) J. Biol. Chem. 278 9134-9141 [DOI] [PubMed] [Google Scholar]
- 25.Hemati, N., Ross, S. E., Erickson, R. L., Groblewski, G. E., and MacDougald, O. A. (1997) J. Biol. Chem. 272 25913-25919 [DOI] [PubMed] [Google Scholar]
- 26.Wang, G. L., Shi, X., Salisbury, E., Sun, Y., Albrecht, J. H., Smith, R. C., and Timchenko, N. A. (2006) Mol. Cell. Biol. 26 2570-2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, G. L., and Timchenko, N. A. (2005) Mol. Cell. Biol. 25 1325-1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, G. L., Iakova, P., Wilde, M., Awad, S., and Timchenko, N. A. (2004) Genes Dev. 18 912-925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Behre, G., Singh, S. M., Liu, H., Bortolin, L. T., Christopeit, M., Radomska, H. S., Rangatia, J., Hiddemann, W., Friedman, A. D., and Tenen, D. G. (2002) J. Biol. Chem. 277 26293-26299 [DOI] [PubMed] [Google Scholar]
- 30.Liu, H. K., Perrier, S., Lipina, C., Finlay, D., McLauchlan, H., Hastie, C. J., Hundal, H. S., and Sutherland, C. (2006) BMC Mol. Biol. 7 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedersen, T. A., Bereshchenko, O., Garcia-Silva, S., Ermakova, O., Kurz, E., Mandrup, S., Porse, B. T., and Nerlov, C. (2007) EMBO J. 26 1081-1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross, S. E., Radomska, H. S., Wu, B., Zhang, P., Winnay, J. N., Bajnok, L., Wright, W. S., Schaufele, F., Tenen, D. G., and MacDougald, O. A. (2004) Mol. Cell. Biol. 24 675-686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radomska, H. S., Basseres, D. S., Zheng, R., Zhang, P., Dayaram, T., Yamamoto, Y., Sternberg, D. W., Lokker, N., Giese, N. A., Bohlander, S. K., Schnittger, S., Delmotte, M. H., Davis, R. J., Small, D., Hiddemann, W., Gilliland, D. G., and Tenen, D. G. (2006) J. Exp. Med. 203 371-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiao, L., MacDougald, O. A., and Shao, J. (2006) J. Biol. Chem. 281 24390-24397 [DOI] [PubMed] [Google Scholar]
- 35.Ross, S. E., Hemati, N., Longo, K. A., Bennett, C. N., Lucas, P. C., Erickson, R. L., and MacDougald, O. A. (2000) Science 289 950-953 [DOI] [PubMed] [Google Scholar]
- 36.Charron, M. J., Brosius, F. C., III, Alper, S. L., and Lodish, H. F. (1989) Proc. Natl. Acad. Sci. U. S. A. 86 2535-2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin, F.-T., MacDougald, O. A., and Lane, M. D. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 9606-9610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfaffl, M. W. (2001) Nucleic Acids Res. 29 e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross, S. E., Erickson, R. L., Gerin, I., DeRose, P. M., Bajnok, L., Longo, K. A., Misek, D. E., Kuick, R., Hanash, S. M., Atkins, K. B., Andersen, S. M., Nebb, H. I., Madsen, L., Kristiansen, K., and MacDougald, O. A. (2002) Mol. Cell. Biol. 22 5989-5999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baumann, C. A., Brady, M. J., and Saltiel, A. R. (2001) J. Biol. Chem. 276 6065-6068 [DOI] [PubMed] [Google Scholar]
- 41.Thurmond, D. C., Ceresa, B. P., Okada, S., Elmendorf, J. S., Coker, K., and Pessin, J. E. (1998) J. Biol. Chem. 273 33876-33883 [DOI] [PubMed] [Google Scholar]
- 42.Piper, R. C., Tai, C., Kulesza, P., Pang, S., Warnock, D., Baeniger, J., Slot, J. W., Geuze, H. J., Puri, C., and James, D. E. (1993) J. Cell Biol. 121 1221-1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steppan, C. M., Bailey, S. T., Bhat, S., Brown, E. J., Banerjee, R. R., Wright, C. M., Patel, H. R., Ahima, R. S., and Lazar, M. A. (2001) Nature 409 307-312 [DOI] [PubMed] [Google Scholar]
- 44.Steppan, C. M., Brown, E. J., Wright, C. M., Bhat, S., Banerjee, R. R., Dai, C. Y., Enders, G. H., Silberg, D. G., Wen, X., Wu, G. D., and Lazar, M. A. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 502-506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosen, E. D., Hsu, C. H., Wang, X., Sakai, S., Freeman, M. W., Gonzalez, F. J., and Spiegelman, B. M. (2002) Genes Dev. 16 22-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiao, L., Schaack, J., and Shao, J. (2006) Endocrinology 147 865-874 [DOI] [PubMed] [Google Scholar]
- 47.Park, B. H., Qiang, L., and Farmer, S. R. (2004) Mol. Cell. Biol. 24 8671-8680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Routes, J. M., Colton, L. A., Ryan, S., and Klemm, D. J. (2000) Biochem. J. 352 335-342 [PMC free article] [PubMed] [Google Scholar]
- 49.Xu, Y., Zhou, Y. L., Luo, W., Zhu, Q. S., Levy, D., MacDougald, O. A., and Snead, M. L. (2006) J. Biol. Chem. 281 16090-16098 [DOI] [PubMed] [Google Scholar]
- 50.Kovacs, K. A., Steinmann, M., Magistretti, P. J., Halfon, O., and Cardinaux, J. R. (2003) J. Biol. Chem. 278 36959-36965 [DOI] [PubMed] [Google Scholar]
- 51.Yin, L., Wang, Dridi, S., Vinson, C., and Hillgartner, F. B. (2005) Mol. Cell. Endocrinol. 245 43-52 [DOI] [PubMed] [Google Scholar]
- 52.Yoshida, Y., Hughes, D. E., Rausa, F. M., III, Kim, I. M., Tan, T., Darlington, G. J., and Costa, R. H. (2006) Hepatology 43 276-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahoney, C. W., Shuman, J., McKnight, S. L., Chen, H. C., and Huang, K. P. (1992) J. Biol. Chem. 267 19396-19403 [PubMed] [Google Scholar]
- 54.Kane, S., Sano, H., Liu, S. C., Asara, J. M., Lane, W. S., Garner, C. C., and Lienhard, G. E. (2002) J. Biol. Chem. 277 22115-22118 [DOI] [PubMed] [Google Scholar]
- 55.Larance, M., Ramm, G., Stockli, J., van Dam, E. M., Winata, S., Wasinger, V., Simpson, F., Graham, M., Junutula, J. R., Guilhaus, M., and James, D. E. (2005) J. Biol. Chem. 280 37803-37813 [DOI] [PubMed] [Google Scholar]
- 56.Eguez, L., Lee, A., Chavez, J. A., Miinea, C. P., Kane, S., Lienhard, G. E., and McGraw, T. E. (2005) Cell Metab. 2 263-272 [DOI] [PubMed] [Google Scholar]
- 57.Baumann, C. A., Ribon, V., Kanzaki, M., Thurmond, D. C., Mora, S., Shigematsu, S., Bickel, P. E., Pessin, J. E., and Saltiel, A. R. (2000) Nature 407 202-207 [DOI] [PubMed] [Google Scholar]
- 58.DeFronzo, R. A., Bonadonna, R. C., and Ferrannini, E. (1992) Diabetes Care 15 318-368 [DOI] [PubMed] [Google Scholar]
- 59.Hernandez, R., Teruel, T., and Lorenzo, M. (2003) Biochem. J. 372 617-624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernandez-Veledo, S., Hernandez, R., Teruel, T., Mas, J. A., Ros, M., and Lorenzo, M. (2006) Arch. Physiol. Biochem. 112 13-22 [DOI] [PubMed] [Google Scholar]
- 61.Miller, R. S., Diaczok, D., and Cooke, D. W. (2007) Biochem. Biophys. Res. Commun. 362 188-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pessler-Cohen, D., Pekala, P. H., Kovsan, J., Bloch-Damti, A., Rudich, A., and Bashan, N. (2006) Arch. Physiol. Biochem. 112 3-12 [DOI] [PubMed] [Google Scholar]
- 63.Liu, P. C., and Matsumura, F. (2006) J. Biochem. Mol. Toxicol. 20 79-87 [DOI] [PubMed] [Google Scholar]
- 64.Armoni, M., Harel, C., and Karnieli, E. (2007) Trends Endocrinol. Metab. 18 100-107 [DOI] [PubMed] [Google Scholar]
- 65.Abel, E. D., Peroni, O., Kim, J. K., Kim, Y.-B., Boss, O., Hadro, E., Minnemann, T., Shulman, G. I., and Kahn, B. B. (2001) Nature 409 729-733 [DOI] [PubMed] [Google Scholar]
- 66.Carvalho, E., Schellhorn, S. E., Zabolotny, J. M., Martin, S., Tozzo, E., Peroni, O. D., Houseknecht, K. L., Mundt, A., James, D. E., and Kahn, B. B. (2004) J. Biol. Chem. 279 21598-21605 [DOI] [PubMed] [Google Scholar]
- 67.Carvalho, E., Kotani, K., Peroni, O. D., and Kahn, B. B. (2005) Am. J. Physiol. 289 E551-E561 [DOI] [PubMed] [Google Scholar]