Association of Intrauterine Exposure to Maternal Diabetes and Obesity With Type 2 Diabetes in Youth: The SEARCH Case-Control Study (original) (raw)
Abstract
OBJECTIVE—Limited data exist on the association between in utero exposure to maternal diabetes and obesity and type 2 diabetes in diverse youth. These associations were explored in African-American, Hispanic, and non-Hispanic white youth participating in the SEARCH Case-Control Study.
RESEARCH DESIGN AND METHODS—A total of 79 youth with type 2 diabetes and 190 nondiabetic control youth aged 10–22 years attended a research visit. In utero exposures to maternal diabetes and obesity were recalled by biological mothers.
RESULTS—Youth with type 2 diabetes were more likely to have been exposed to maternal diabetes or obesity in utero than were nondiabetic control youth (P < 0.0001 for each). After adjusting for offspring age, sex, and race/ethnicity, exposure to maternal diabetes (odds ratio [OR] 5.7 [95% CI 2.4–13.4]) and exposure to maternal obesity (2.8 [1.5–5.2]) were independently associated with type 2 diabetes. Adjustment for other perinatal and socioeconomic factors did not alter these associations. When offspring BMI was added, the OR for the association between in utero exposure to obesity and type 2 diabetes was attenuated toward the null (OR 1.1 [0.5–2.4]). Overall, 47.2% (95% CI 30.9–63.5) of type 2 diabetes in youth could be attributed to intrauterine exposure to maternal diabetes and obesity.
CONCLUSIONS—Intrauterine exposures to maternal diabetes and obesity are strongly associated with type 2 diabetes in youth. Prevention efforts may need to target, in addition to childhood obesity, the increasing number of pregnancies complicated by obesity and diabetes.
Type 2 diabetes has increasingly been reported in young adults and adolescents. The SEARCH for Diabetes in Youth Study found type 2 diabetes among youth of all major racial/ethnic groups, and high rates were noted among minority adolescents aged 15–19 years (1).
A maternal diabetic intrauterine environment has consequences for future type 2 diabetes risk in the offspring (2). Among the Pima Indians, exposure to diabetes in utero was the strongest risk factor for development of type 2 diabetes in young offspring (3). This association was independent of maternal obesity, father's diabetes, age at onset of diabetes in either parent, and offspring's birth weight and later obesity (2,3).
Recent studies have shown an association between maternal prepregnancy obesity and excessive neonatal growth and adiposity (4), independent of diabetes in pregnancy. There is increasing interest in the hypothesis that maternal obesity during pregnancy, even in the absence of frank diabetes, is also associated with lifelong metabolic abnormalities in offspring, such as the presence of obesity (5) and features of the metabolic syndrome (6). However, no study has specifically explored an association between exposure to maternal prepregnancy obesity and type 2 diabetes in youth.
In the last decade an increase in the prevalence of overweight among obstetric populations has been reported (7). There is also an increasing incidence and a younger age at onset of type 2 diabetes in adults (8), and an increasing prevalence of gestational diabetes mellitus (GDM) in all major racial/ethnic groups (9). In this context, the Fifth International Workshop on Gestational Diabetes Mellitus (10) has recommended studies of in utero exposure to maternal diabetes and obesity and type 2 diabetes in youth in populations other than American Indians.
Using data from the multiethnic (non-Hispanic white, African-American, and Hispanic) SEARCH Case-Control (SEARCH CC) Study, we hypothesized that youth with type 2 diabetes would be more likely to have been exposed to a diabetic and obese intrauterine environment compared with nondiabetic control youth. We also hypothesized that the association between exposure to maternal diabetes and offspring type 2 diabetes will be independent of other perinatal, early life, and familial socioeconomic factors; however, the association between maternal prepregnancy obesity and offspring type 2 diabetes will be, at least in part, accounted for by offspring BMI.
RESEARCH DESIGN AND METHODS
SEARCH CC is an ancillary study conducted at two of the six SEARCH for Diabetes in Youth Study clinical sites. The SEARCH for Diabetes in Youth Study is a multicenter study conducting population-based ascertainment of diabetes in youth with onset at <20 years of age (1).
SEARCH CC case inclusion
In Colorado and South Carolina, diabetes cases were identified using a network of health care providers. Type of diabetes was based on provider diagnosis and further confirmed with biochemical data, including diabetes autoantibody measurements (1). Between July 2003 and March 2006, the SEARCH for Diabetes in Youth patients with type 2 diabetes of non-Hispanic white, African-American, and Hispanic origin aged 10−22 years at study visit were invited to participate in SEARCH CC. Data collection unique to SEARCH CC included a perinatal questionnaire, completed by the biological mother. Overall, 53% of those invited participated in SEARCH CC.
SEARCH CC control inclusion
Because all SEARCH cases arose from health care provider offices, we recruited control youth from primary care offices in the same geographic areas. Within clinical sites, control recruitment oversampled youth based on the distribution of age, sex, and racial/ethnic background of cases. Overall, 49% of those invited participated in SEARCH CC. All control youth were confirmed to be nondiabetic by fasting glucose values.
Measurements
Maternal diabetes during pregnancy, including both pregestational diabetes and GDM, was reported by the biological mother. Exposure to diabetes in utero was considered present if the mother had diabetes diagnosed before delivery and absent if the mother did not have diabetes during pregnancy or if diabetes was diagnosed after delivery (3). When the mother had diabetes diagnosed before delivery, it was GDM in >90% of cases (i.e., diabetes first diagnosed during pregnancy). Exposure to diabetes in utero was validated in a sample of 64 Colorado participants with birth certificate data collected after 1990 and maintained by the Colorado Department of Public Health and Environment (CDPHE). Consistent with other studies (11), self-reported exposure to diabetes in utero demonstrated good agreement with the CDPHE data for both case patients (κ = 0.71) and control subjects (κ = 0.79).
Self-reported maternal prepregnancy weight (kilograms) and height (meters) were used to compute maternal prepregnancy BMI. Self-reported weight correlates well with measured weight (12), and self-reported prepregnancy weight was recently validated against measured weight 3 months before the last menstrual cycle in a sample of multiethnic women (13). Exposure to maternal overweight/obesity was defined as prepregnancy BMI ≥25 kg/m2. The biological mother also reported maternal smoking and alcohol use during pregnancy, history of breastfeeding the offspring, paternal diabetes status, offspring birth weight, and gestational age. Birth weight was validated in the Colorado sample through CDPHE birth certificates. The Spearman correlation between recalled and recorded birth weight was r = 0.95 for case patients and r = 0.94 for control subjects. Childhood height and weight were measured using a stadiometer and an electronic scale, respectively. Age- and sex-specific BMI Z scores were derived on the basis of the Centers for Disease Control and Prevention national standards (14). The study was reviewed and approved by the local institutional review boards.
Statistical analyses
Logistic regression was used to generate odds ratios (ORs) and 95% CIs for the association of exposure to maternal diabetes in utero and exposure to maternal obesity in utero with type 2 diabetes in the offspring. An interaction term between each in utero exposure and race/ethnicity was used to evaluate whether the association differed according to the race/ethnicity. A series of multivariate logistic regression models were developed for each in utero exposure of interest: 1) model 1, adjusted for offspring age, sex, and race/ethnicity to minimize the potential for residual confounding; 2) for model 2, in addition to model 1 variables, the two in utero exposures were adjusted for each other; 3) model 3, markers of other early life exposures (maternal age and behavior during pregnancy, child birth weight, and breastfeeding status) and shared familial factors (household income and maternal education) were added to model 2 variables; 4) model 4, offspring's current weight status (BMI Z score) was added to model 2.
The risk of type 2 diabetes in the children attributable to exposure to diabetes and overweight/obesity in utero was also estimated. The population-attributable fraction (PAF) is the percentage of a disease in a population that is due to a specific exposure (15). Although PAFs are usually derived for single risk factors, they also can be estimated for groups of simultaneous factors. In this situation, a PAF estimates the proportional amount by which disease risk would be reduced if all of the factors were to be simultaneously eliminated from the population (16). For this analysis, mutually exclusive exposure categories were derived (i.e., exposure to maternal diabetes only, to maternal overweight/obesity only, to both, and to neither) and category-specific attributable fractions were computed, as described by Miettinen (15) Pi [(ORi − 1)/ORi], where Pi is the proportion of cases falling into each exposure category and ORi is the adjusted odds ratio (for offspring age, sex, and race/ethnicity) comparing each exposed group with the unexposed category (i = 0). This formula produces internally valid estimates when confounding exists and, as a result, adjusted ORs must be used (15,16). By summing the category-specific fractions, a summary PAF was derived, representing the overall proportion of type 2 diabetes in youth attributable to these exposures.
RESULTS
Analyses included 79 youth with type 2 diabetes and 190 nondiabetic control youth with completed data on variables of interest. As shown in Table 1, youth with type 2 diabetes were older, were more likely to be of African-American background, had higher BMI, had families with lower socioeconomic indicators, and had more paternal history of diabetes. Of note, 30.4% of youth with type 2 diabetes were exposed to maternal diabetes and 57% to maternal overweight/obesity in utero, compared with 6.3 and 27.4%, respectively, of nondiabetic control youth (P < 0.0001 for each).
Table 1—
Characteristics of youth with type 2 diabetes and nondiabetic control youth
| Variables | Type 2 diabetes | Control | P value |
|---|---|---|---|
| n | 79 | 79 | |
| Age (years) | 15.7 ± 2.8 | 14.4 ± 2.8 | 0.003 |
| Sex (% male) | 29.1 | 38.9 | 0.1 |
| Race/Ethnicity (%) | <0.0001 | ||
| Non-Hispanic white | 27.9 | 55.8 | |
| African-American | 54.4 | 27.9 | |
| Hispanic | 17.7 | 16.3 | |
| BMI (kg/m2) | 34.7 ± 7.5 | 24.0 ± 6.8 | <0.0001 |
| BMI Z score | 2.1 ± 0.7 | 0.8 ± 1.1 | <0.0001 |
| Exposure to maternal diabetes in utero (% yes) | 30.4 | 6.3 | <0.0001 |
| Exposure to maternal obesity (BMI ≥25 kg/m2) in utero (% yes) | 57.0 | 27.4 | <0.0001 |
| Maternal education (%) | 0.009 | ||
| Less than high school | 13.9 | 4.7 | |
| High school and more | 86.1 | 94.2 | |
| Household income (%) | <0.0001 | ||
| <$25,000 | 49.4 | 21.6 | |
| >$25,000 | 50.6 | 77.9 | |
| Maternal smoking during pregnancy (% yes) | 11.4 | 11.1 | 0.9 |
| Maternal alcohol consumption during pregnancy (% yes) | 1.27 | 14.7 | 0.001 |
| Birth weight (g) | 3,218 ± 654 | 3,288 ± 620 | 0.4 |
| Breast-feeding (% yes) | 30.4 | 65.8 | <0.0001 |
| Paternal history of diabetes (% yes) | 29.1 | 6.3 | <0.0001 |
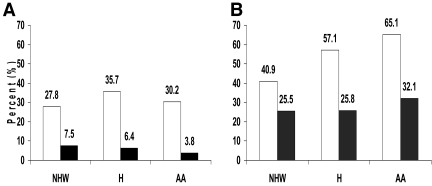
Figure 1_A_ shows the percentage of youth exposed to maternal diabetes in utero according to case-control status and race/ethnicity. Type 2 diabetic youth were more likely to have been exposed to maternal diabetes in utero than control youth (OR 7.3 [95% CI 3.2–16.8]; P < 0.0001, adjusted for age, sex, and race). A similar pattern was observed in all racial/ethnic groups (non-Hispanic white OR 5.5 [1.5–18.9]; Hispanic OR 4.7 [0.7–32.2]; and African-American OR 10.6 [2.1–51.5], adjusted for age and sex). No difference in the association of exposure to diabetes in utero and case-control status was observed according to race/ethnicity (P value for interaction = 0.7).
Figure 1—
Percentage of youth exposed in utero to maternal diabetes (A) and maternal overweight/obesity (B) by case-control status and race/ethnicity. □, case patients; ▪, control youth. A: Non-Hispanic white (NHW) P = 0.01; Hispanic (H) P = 0.02; African-American (AA) P = 0.02. B: NHW P = 0.1; H P = 0.04; AA P = 0.001.
Figure 1_B_ shows the percentage of youth exposed to maternal obesity in utero, according to case-control status and race/ethnicity. Type 2 diabetic youth were more likely to have been exposed to maternal obesity in utero than control youth (OR 3.6 [95% CI 1.9–6.4]; P < 0.0001, adjusted for age, sex, and race). A similar pattern was observed in all racial/ethnic groups (non-Hispanic white OR 2.2 [0.9–5.8]; Hispanic OR 13.4 [1.9–95.2]; and African-American OR 4.2 [1.7–10.2]; adjusted for age and sex). There was no difference in the association of exposure to obesity in utero and case-control status according to race/ethnicity (P value for interaction = 0.8).
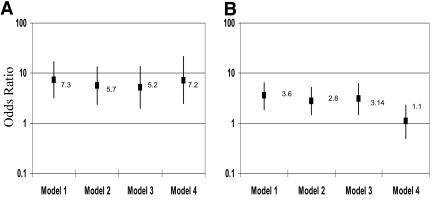
Figure 2 shows the association of offspring type 2 diabetes status with exposure to maternal diabetes in utero (Fig. 2_A_) and exposure to maternal overweight/obesity in utero (Fig. 2_B_), in sequentially adjusted models. Model 1 presents the OR and 95% CI for the associations of interest, when adjustment is made for offspring age, sex, and race/ethnicity. Additional adjustment for paternal diabetes (yes/no) made no difference. In model 2, each intrauterine exposure was adjusted for the other, resulting in some attenuation of the OR of interest; however, both exposures remained independently associated with offspring type 2 diabetes status. In model 3, additional adjustment for other perinatal exposures and markers of shared socioeconomic factors had no substantial influence. Finally, the addition of the subjects' current BMI Z score in model 4 made no difference to the association between maternal diabetes and offspring type 2 diabetes status (Fig. 2_A_), but substantially attenuated toward the null (OR 1.1 [95% CI 0.5–2.3]) the OR for the association between maternal obesity and offspring type 2 diabetes (Fig. 2_B_). The association between maternal prepregnancy BMI and offspring type 2 diabetes was graded across categories of maternal overweight (BMI 25–29 kg/m2; OR 2.6 [95% CI 1.2–5.5], adjusted for age, sex, and race) and obesity (BMI ≥30 kg/m2; 4.6 [2.2–9.5]), compared with normal prepregnancy BMI (<25 kg/m2). On adjustment for offspring BMI Z score, these associations became nonsignificant. Similar results were obtained when maternal prepregnancy BMI was modeled as a continuous variable.
Figure 2—
The association between offspring type 2 diabetes status with exposure to maternal diabetes in utero (A) and exposure to maternal overweight/obesity in utero (B) in sequentially adjusted multiple logistic regression models. Model 1: adjusted for offspring age, sex, race/ethnicity; model 2: model 1 + maternal obesity in utero (A) or maternal diabetes in utero (B); model 3: model 2 + maternal alcohol and smoking in pregnancy, maternal current age, parity, offspring's birth weight, breast-feeding, maternal education, household income; and model 4: model 2 + current offspring BMI Z score.
Table 2 shows the proportion of case patients and control subjects exposed to maternal diabetes only, maternal overweight/obesity only (BMI ≥25 kg/m2), and both; the ORs for the association between each exposure and type 2 diabetes status, adjusted for offspring age, sex, and race/ethnicity; and the proportion of type 2 diabetes in youth attributable to each exposure. Exposure to maternal diabetes in utero in the absence of obesity was infrequent and, although associated with type 2 diabetes in the offspring (OR 3.9 [95% CI 1.1–14.5]), resulted in an attributable risk of only 4.7%. Exposure to maternal overweight/obesity in utero in the absence of diabetes was frequent and, given an OR for type 2 diabetes of 2.5 (95% CI 1.3–5.0), contributed to an additional 19.7%. Finally, exposure to both maternal diabetes and maternal overweight/obesity in utero was frequent in case patients (24.1%) and rare in control youth (2.6%) but was most strongly associated with type 2 diabetes (19.2 [95% CI 6.1–60.8]) and therefore contributed to an additional 22.8% of type 2 diabetes in the offspring. Overall, 47.2% (95% CI 30.9–63.5%) of early-onset type 2 diabetes could be attributed to intrauterine exposure to maternal diabetes and maternal obesity.
Table 2—
Proportion of type 2 diabetes in youth attributable to intrauterine exposure to maternal diabetes and overweight/obesity
| Exposure category | Case patients | Control youth | OR (95% CI)* | PAF† |
|---|---|---|---|---|
| Not exposed to either maternal diabetes or maternal obesity | 36.7 | 68.9 | 1 | Unexposed |
| Exposed to maternal diabetes only | 6.3 | 3.7 | 3.9 (1.1–14.5) | 4.7 |
| Exposed to maternal obesity only | 32.9 | 24.7 | 2.5 (1.3–5.0) | 19.7 |
| Exposed to both maternal diabetes and maternal obesity | 24.1 | 2.6 | 19.2 (6.1–60.8) | 22.8 |
| Overall proportion of type 2 diabetes in youth attributable to in utero exposure to maternal diabetes and obesity | 47.2 (30.9–63.5)‡ |
CONCLUSIONS
We found that intrauterine exposure to maternal diabetes and overweight/obesity is strongly associated with type 2 diabetes in youth. Our study provides novel evidence that these exposures are important determinants of type 2 diabetes in youth of racial/ethnic groups other than American Indians, together contributing to 47% of type 2 diabetes in the offspring.
The association between exposure to maternal diabetes in utero and type 2 diabetes in youth of non-Hispanic, Hispanic, and African-American race/ethnicity is of a magnitude (OR 7.3 [95% CI 3.2–16.8]) similar to that reported in Pima Indians (10.4 [4.4–25.1]) (3). Several mechanisms that are not mutually exclusive may explain this association. They include genetic predisposition and shared familial factors, as well as specific intrauterine effects. Work with the Pima Indians (17) and other populations (18,19) strongly suggests that the effect of exposure to maternal diabetes in utero on offspring type 2 diabetes risk is in addition to genetic susceptibility. Within the same Pima family, siblings born after their mother's diagnosis of diabetes have a threefold higher risk of developing type 2 diabetes at an early age than siblings born before the diagnosis (17). Our findings that the association is independent of exposure to maternal obesity and other prenatal, early life, and familial factors support the previous evidence. Moreover, in our sample of 65 youth with a maternal history of diabetes, the odds for type 2 diabetes was 2.5-fold higher (95% CI 0.9–7.3) when the diabetes was diagnosed before versus after pregnancy. This finding suggests that, even in the selected group of offspring at high genetic risk, exposure to diabetes in utero is associated with a further increase in type 2 diabetes risk.
We found that the association between exposure to maternal diabetes in utero and type 2 diabetes in youth is not accounted for by childhood BMI. This finding is consistent with animal (20) and human (21,22) data suggesting that the effect of exposure to diabetes in utero on the offspring's future risk for type 2 diabetes is not completely explained by development of obesity; it is also mediated through subsequent β-cell dysfunction in the offspring.
Our study provides novel evidence that exposure to maternal obesity in utero is associated with type 2 diabetes in youth independent of diabetes during pregnancy. However, adjustment for childhood BMI attenuates the association toward the null. This result is consistent with a causal pathway in which exposure to maternal obesity increases the risk for childhood overweight, which may increase the risk for type 2 diabetes. The pathway is supported by other studies, suggesting that the risk that a child would be overweight increases with maternal prepregnancy BMI (5) and that adolescents exposed to maternal obesity have an increased risk of developing the metabolic syndrome (6).
The above associations may be due to specific intrauterine effects (“fuel-mediated teratogenesis”). For example, excess maternal pregestational obesity may increase lipid availability, modulate delivery of lipid substrates to the fetus, and have programming consequences (23). Data in rats (24) demonstrate that preconception obesity brought about by overfeeding leads to obesity, metabolic alterations, and increased adipose tissue cellularity in the offspring. Importantly, in rats, this process is in addition to maternal and paternal genetic influences (24). However, these associations may also be due in part to increased genetic susceptibility to obesity, coupled with postnatal availability of excess calories. More research is needed in this area because distinguishing between specific intrauterine mechanisms and general familial (genetic and nongenetic) factors is important for the development of randomized trials aimed at testing effective interventions.
Our study has several limitations. Recall bias is a potential concern. However, exposure to diabetes in utero was validated in a sample of participants, with very good agreement coefficients. Another concern is the potential for selection bias. However, the prevalences of intrauterine exposures within race/ethnic control groups (Fig. 1) are similar to those reported from the general population (7,9). We had limited data on paternal diabetes and were not able to explore how timing of exposure to paternal diabetes and obesity may be associated with an increased risk of type 2 diabetes in the offspring. To derive PAFs, we used ORs as measures of risk associations. When the outcome or exposure of interest is common, the adjusted ORs may exaggerate a risk association. However, even after correcting the ORs to better represent the true relative risks (25), the overall PAF was still 42.7%, well within the estimated 95% CI (30.9–63.5).
In summary, intrauterine exposures to maternal diabetes and obesity are strongly associated with type 2 diabetes in youth. In our multiethnic population, 47% of type 2 diabetes in youth could be attributed to the combined effect of these exposures. Our data suggest that for prevention of type 2 diabetes in youth we may need to take a life course approach, targeting, in addition to childhood obesity, the increasing number of women with pregnancies complicated by obesity and diabetes.
Acknowledgments
The SEARCH Case-Control study was funded by the National Institute of Diabetes, Digestive and Kidney Diseases (R01 DK59184).
The writing group thanks David J. Pettitt, MD (Sansum Diabetes Research Institute, Santa Barbara, CA) for his thoughtful comments on this manuscript.
Published ahead of print at http://care.diabetesjournals.org on 28 March 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Dabelea D, Bell RA, D'Agostino RB Jr, et al.: Incidence of diabetes in youth in the United States. JAMA 297:2716–2724, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Pettitt DJ, Bennett PH, Saad MF, et al.: Abnormal glucose tolerance during pregnancy in Pima Indian women: long-term effects on offspring. Diabetes 40(Suppl. 2):126–130, 1991 [DOI] [PubMed] [Google Scholar]
- 3.Dabelea D, Hanson RL, Bennett PH, et al.: Increasing prevalence of type II diabetes in American Indian children. Diabetologia 41:904–910, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Sebire NJ, Jolly M, Harris JP, et al.: Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes _Relat Metab Disord_25:1175–1182, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Salsberry PJ, Reagan PB: Dynamics of early childhood overweight. Pediatrics 116:1329–1338, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boney CM, Verma A, Tucker R, et al.: Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115:290–296, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Yeh J, Shelton JA: Increasing prepregnancy body mass index: analysis of trends and contributing variables. AmJ Obstet Gynecol 193:1994–1998, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Mokdad AH, Ford ES, Bowman BA, et al.: Diabetes trends in the U.S.: 1990–1998. Diabetes Care 23:1278–1283, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Dabelea D, Snell-Bergeon JK, Hartsfield CL, et al.: Increasing prevalence of gestational diabetes mellitus over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care 28:579–584, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Dabelea D: The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care 30(Suppl. 2):169–175, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Solomon CG, Willett WC, Carey VJ, et al.: A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 278:1078–1083, 1997 [PubMed] [Google Scholar]
- 12.Kuczmarski MF, Kuczmarski RJ, Najjar M: Effects of age on validity of self-reported height, weight, and body mass index: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. J Am Diet Assoc 101:28–34, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Oken E, Taveras EM, Kleinman KP, et al.: Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol 196:322–328, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Center for Health Statistics. CDC Growth Charts: United States. Atlanta, Centers for Disease Control and Prevention, 2006
- 15.Miettinen OS: Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol 99:325–332, 1974 [DOI] [PubMed] [Google Scholar]
- 16.Rockhill B, Newman B, Weinberg C: Use and misuse of population attributable fractions. Am J Public Health 88:15–19, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dabelea D, Hanson RL, Lindsay RS, et al.: Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 49:2208–2211, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Clausen TD, Mathiesen ER, Hansen T, et al.: High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 31:340–346, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Sobngwi E, Boudou P, Mauvais-Jarvis F, et al.: Effect of a diabetic environment in utero on predisposition to type 2 diabetes. Lancet 361:1861–1865, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Aerts L, Sodoyez-Goffaux F, Sodoyez JC, et al.: The diabetic intrauterine milieu has a long-lasting effect on insulin secretion by B cells and on insulin uptake by target tissues. Am J Obstet Gynecol 159:1287–1292, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Hunter WA, Cundy T, Rabone D, et al.: Insulin sensitivity in the offspring of women with type 1 and type 2 diabetes. Diabetes Care 27:1148–1152, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Gautier JF, Wilson C, Weyer C, et al.: Low acute insulin secretory responses in adult offspring of people with early onset type 2 diabetes. Diabetes 50:1828–1833, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Kitajima M, Oka S, Yasuhi I, Fukuda M, et al.: Maternal serum triglyceride at 24–32 weeks' gestation and newborn weight in nondiabetic women with positive diabetic screens. Obstet Gynecol 97:776–780, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Kartik S, Harrell A, Liu X, et al.: Maternal obesity at conception programs obesity in the offspring. Am J Physiol 291:528–538, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Yu KF: What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 280:1690–1691, 1998 [DOI] [PubMed] [Google Scholar]