Long-Term Tolerance to Allogeneic Thymus Transplants in Complete DiGeorge Anomaly (original) (raw)
. Author manuscript; available in PMC: 2009 Mar 1.
Published in final edited form as: Clin Immunol. 2007 Dec 26;126(3):277–281. doi: 10.1016/j.clim.2007.11.009
Abstract
Thymus transplantation in subjects with complete DiGeorge anomaly using postnatal allogeneic HLA-nonmatched cultured thymus tissue provides immunoreconstitution. Tolerance of the newly developed T cells toward the donor thymus has not previously been studied. Mixed lymphocyte cultures were used to test 12 thymus transplant recipients for long-term tolerance toward their thymus allografts. Two subjects tested for responses toward thymus donor peripheral blood mononuclear cells showed significantly less reactivity toward the donors compared to responses against third-party allogeneic cells. Peripheral blood mononuclear cells from 10 other subjects were less responsive toward cryopreserved donor thymic cells than toward allogeneic cells (P = 0.00007). Adult control peripheral blood mononuclear cells proliferated strongly in response to the donor thymic cells. Both the subjects and controls showed similar proliferative responses against allogeneic cells and phytohemagglutinin. This study provides in vitro evidence for long-term tolerance of complete DiGeorge anomaly thymus transplantation recipients toward their HLA-nonmatched thymus grafts.
Keywords: DiGeorge anomaly, thymus transplantation, tolerance, allorecognition
INTRODUCTION
DiGeorge anomaly arises from abnormal embryologic development of the third and fourth pharyngeal pouches, although defects ranging from the first to sixth pharyngeal arches have been known to occur [1]. Abnormalities seen at birth involve to varying degrees the heart, parathyroid glands, and thymus [2–6]. Fewer than 1 in 100 infants born with DiGeorge anomaly demonstrates athymia (“complete DiGeorge anomaly”), leading to a congenital combined immunodeficiency that is usually fatal due to infection by 2 years of age.
Thymus transplantation using postnatal cultured allogeneic HLA-nonmatched thymus tissue provides immunoreconstitution [7, 8]. These thymus grafts provide an environment for recipient thymocyte precursors to enter, undergo positive and negative selection, and emerge in the peripheral circulation as functional naïve T cells. Immune evaluation of thymus recipients surviving beyond 1 year reveals the presence of naïve T cells with normal mitogen proliferative responses and diverse T cell receptor repertoires. These data suggest that the thymus grafts are both functioning and not being rejected after transplantation [8].
Because the thymus grafts are HLA-nonmatched, a potential risk for alloreactive rejection of the transplanted tissues remains present [9]. The assessment of these subjects for tolerance toward their thymus donors presents an important area of investigation in their long-term follow-up. Traditionally, mixed lymphocyte cultures (MLCs) have been used to detect the presence of alloreactive T cells. Because we have limited blood samples from the thymus donors, cryopreserved peripheral blood mononuclear cells (PBMCs) are generally unavailable after donor screening has been completed. For several subjects, significant numbers of donor thymic cells (DTCs) have been collected and cryopreserved during the thymus slicing process. Here we report the evaluation of complete DiGeorge anomaly subjects after thymus transplantion for long-term alloreactivity against their thymus donors using MLCs against either donor PBMCs or DTCs.
METHODS
Subjects
All subjects were enrolled in protocols approved by the Duke Institutional Review Board. Informed consent for these studies was obtained from the parents of all thymus donors and transplant recipients. A total of 49 infants with complete DiGeorge anomaly were given postnatal cultured allogeneic HLA-nonmatched thymus tissue transplantation at Duke between September 1993 and October 2007. Of these 49 recipients, 36 survive (73%). We identified 12 surviving subjects for testing when sufficient blood could be obtained and either cryopreserved donor PBMCs or DTCs were available. These subjects had naïve (CD45RA+CD62L+) T cells with normal mitogen proliferative responses and normal T cell receptor repertoires and were not receiving immunosuppression. All of these subjects have been described elsewhere [8, 10–12].
Immune Function Assays
Lymphocyte proliferative responses to mitogens were performed before and after transplantation according to standard protocols [8, 11]. The data from proliferative responses to phytohemagglutinin (PHA) (Sigma-Aldrich, St. Louis, MO) in studies performed either simultaneously with the experimental assays described below or at the date most proximal to those assays were used for comparative analyses. Proliferative responses to PHA were assessed by 3H-thymidine incorporation measured in counts per minute (cpm). Unused remaining PBMCs were cryopreserved for future testing in freezing media composed of RPMI 1640 with 2.5% 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 50% fetal bovine serum (Gibco, Grand Island, NY), and 10% dimethyl sulfoxide.
Donor Thymic Cells (DTCs)
Cells from donor thymus tissues were collected under aseptic conditions during the thymus culturing process, layered over Lymphoprep (Axis-Shield, Oslo, Norway) to remove dead thymocytes, red blood cells and debris, and cryopreserved in freezing media [7]. Samples were thawed at 37°C, washed, and resuspended in culture medium consisting of RPMI 1640 with HEPES, 10% human AB serum (Gemini Bio-Products, W. Sacramento, CA), and 1% penicillin/streptomycin. The cells were carefully again layered over Lymphoprep to remove dead thymocytes. After centrifugation, the DTCs at the interface were harvested, washed, and resuspended in culture medium. The median percentage of cells recovered after the second Lymphoprep separation was 8.5% [3–49%]. The viabilities of these cells were high (median 88%), with 2 outliers (26% and 30%).
Mixed Lymphocyte Cultures (MLCs)
Transplant recipient PBMCs were separated from whole blood over Lymphoprep and co-cultured in quadruplicate with stimulator cells that were irradiated at 40 Gy. Cryopreserved recipient PBMCs were thawed and rested overnight at 37°C in a 5% CO2 incubator in culture medium prior to plating. Irradiated stimulator cells included DTCs or thymus donor PBMCs, allogeneic healthy adult volunteer PBMCs, and autologous PBMCs. Cells were cultured for 6 days, and responder PBMC proliferation was assayed by 3H-thymidine incorporation. Irradiated stimulators in medium alone, including DTCs, had low median backgrounds (122 cpm). Subject DIG105 had PBMCs that were obtained on the same date tested at different times: the first assay used PBMCs immediately after isolation from whole blood, and the second assay used PBMCs that had been cryopreserved. Responder PBMCs from healthy adult volunteers served as controls. Four different control PBMC samples, including 3 that had been cryopreserved, were tested simultaneously for the MLC assay with subject DIG120.
Statistical Analysis
Acceptable negative and positive threshold levels were established for the MLC assays. The data obtained from recipient and control co-cultures with irradiated autologous PBMCs were used to determine an acceptable negative threshold for the MLCs. This threshold level was defined as 2 standard deviations above the geometric mean for all autologous responses. Responses below the negative threshold were considered non-reactive. Similarly, a threshold level for positivity was defined as 2 standard deviations below the geometric mean of all recipient and volunteer responses against irradiated allogeneic PBMCs. Responses to allogeneic cells had to exceed the positive threshold to be considered positive. Using these methods, a negative threshold of 6,070 cpm and a positive threshold of 11,630 cpm were derived for the MLCs.
Statistical analyses were used to compare subject and control responses. Log transformation was applied to the background-adjusted data for proliferation assays prior to data analysis. Paired t tests compared the responses of subjects toward DTCs and toward allogeneic PBMCs. Analysis of variance (ANOVA) was performed to compare the responses of the subjects against the volunteers, modeling both disease status (subjects vs. controls) and repeated measurements in each individual. Due to the small sample sizes in this study, we used nQuery Advisor 4.0 to assess the statistical power of each comparison based on the observed mean and variance of the outcomes. Except where specifically mentioned, all significant P values were determined to have from 77 to 99% statistical power.
Criteria for inclusion in data analysis
To be included in the data analysis, assays had to demonstrate that 1) the recipient PBMCs could respond to allogeneic cells, 2) the thymus donor PBMCs or DTCs were capable of eliciting a response from control PBMCs, and 3) the recipient PBMCs were non-reactive toward autologous cells. Individual positive responses within an experiment were rejected if variability exceeded 50% of the mean for any of the 4 replicates in a set of responders but did not invalidate the entire assay. These conditions led to the exclusion of the responses of 2 of the 4 control PBMC samples for DIG120 against irradiated DTCs only.
RESULTS
Recipient and donor characteristics
The 12 subjects tested for tolerance by MLCs and ELISPOT assays ranged from 10½ months to 6.4 years (median 4.2 years) after transplantation when studied (see Table 1). None of the subjects who were tested shared both alleles with the thymus donor at HLA-DR. Only 3 subjects had 22q11 hemizygosity by fluorescence in situ hybridization testing. Six recipients were enrolled in protocols without peri-transplantation immunosuppression; the other 6 received immunosuppression.
Table 1.
Characteristics of all subjects tested by mixed lymphocyte cultures and/or ELISPOT assays.
| Recipient | HLA Alleles Shared With Donor | MLC vs. Donor PBMCs | MLC vs. DTCs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Yearsa | Race | Sex | Donor Sex | Genetics | A | B | C | DRB1 | ISb | ||
| DIG001 | 1.7 | W | F | M | IDM | 1 | 0 | 1 | 0 | N | X | - |
| DIG007 | 5.8 | A | F | M | 22q11 | 0 | 0 | 0 | 1 | N | - | X |
| DIG010 | 5.6 | W | M | M | CHARGE | 0 | 0 | 0 | 1 | N | - | X |
| DIG012 | 5.1 | B | F | M | IDM | 0 | 0 | 1 | 0 | N | - | X |
| DIG019 | 5.0 | B | F | F | 22q11 | 1 | 0 | 0 | 1 | N | - | X |
| DIG020 | 4.2 | B | F | M | CHARGE | 0 | 0 | NT | 0 | N | - | X |
| DIG102 | 0.9 | W | M | M | 22q11 | 0 | 0 | 0 | 0 | Y | X | - |
| DIG105 | 4.2 | W | F | M | IDM | 1 | 0 | NT | 0 | Y | - | Xc |
| DIG106 | 3.9 | W | M | M | None | 1 | 1 | 1 | 0 | Y | - | X |
| DIG107 | 4.1 | W | M | M | CHARGE | 1 | 0 | NT | 0 | Y | - | X |
| DIG112 | 2.9 | W | M | M | CHARGE | 1 | 0 | 0 | 0 | Y | - | X |
| DIG120 | 1.3 | W | M | M | CHARGE | 1 | 1 | 1 | 1 | Y | - | X |
Tolerance to the thymus donor assessed by MLCs
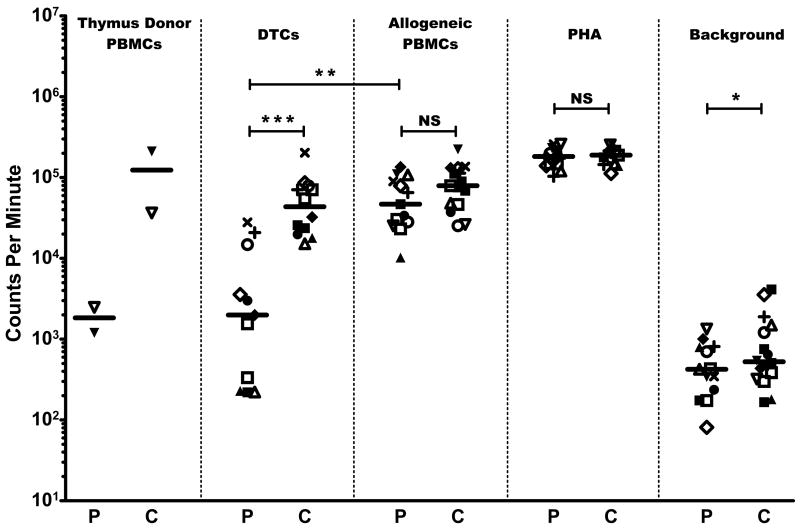
Subjects demonstrated hyporesponsiveness toward their thymus donors in MLCs. The 2 recipients who were tested against thymus donor PBMCs in MLCs showed responses against their thymus donors (2,474 and 1,201 cpm) that were 10-fold and 92-fold lower compared to their responses toward third-party allogeneic PBMCs (25,268 and 110,103 cpm, respectively). The 2 subjects’ PBMC responses toward the thymus donor PBMCs were also 15-fold and 200-fold lower than the responses of PBMCs from 2 adult controls (36,524 cpm and 210,610 cpm; see Figure 1). In 11 tests for reactivity against DTCs, the 10 subjects showed less reactivity against DTCs than did controls (P = 0.000007). The paired t tests showed that all 10 subjects consistently had lower responses toward DTCs than against allogeneic PBMCs (P = 0.00007).
Figure 1. Proliferation studies.
Patient results (P) are shown compared to healthy adult controls (C). Symbols denote individual subject and corresponding control responses toward the stimuli listed at the top of each column for a given experiment; median values are shown by the horizontal bars (—). DIG105 (□) had the same sample of PBMCs tested twice. DIG120 (■) had multiple control samples tested in the same assay (see text). Responses are measured by 3H-thymidine incorporation shown in counts per minute. (*) = P < 0.05, (**) = P < 0.005, (***) = P < 0.00001, NS = P ≥ 0.05.
Proliferative responses of subjects against allogeneic cells and PHA
The subjects and controls showed similar responses toward PHA and toward allogeneic cells (Figure 1). No significant difference was observed between the responses of recipient PBMCs and control PBMCs toward allogeneic PBMCs (median for subjects = 46,909 cpm vs. median for controls = 79,346 cpm). Subject and adult control PBMCs also showed no difference in PHA response (median for subjects = 182,551 cpm vs. median for controls = 189,099 cpm). A statistical difference was detected between the subject and adult PBMC background responses (P = 0.02 with 70% power), although the values used were low (median for subjects = 424 cpm vs. median for controls = 525 cpm).
DISCUSSION
This study examined the critical issue of whether or not long-term tolerance toward HLA-nonmatched thymus grafts exists in subjects with complete DiGeorge anomaly who have developed naïve T cells with normal mitogen proliferative responses and normal T cell receptor repertoires by 1 year after transplantation.
We report the results of testing 12 thymus recipients from 0.9 to 6.4 years after transplantation for alloreactivity toward their donors by MLCs. In 2 MLC experiments testing responses toward thymus donor PBMCs, the geometric mean of the subjects’ responses was significantly lower than both the subjects’ responses toward third-party allogeneic PBMCs and adult control responses to the thymus donor PBMCs. Similarly, in 11 separate MLC studies, cryopreserved donor thymic cells induced significantly less stimulation of recipients’ PBMCs than allogeneic third-party PBMCs and compared to control PBMCs’ responses toward the DTCs. This tolerance toward the thymus donor was observed regardless of whether or not the subjects had received peri-transplantation immunosuppression.
Our results compare similarly to assessments of tolerance after transplantation of allogeneic thymus tissues into congenitally athymic nude mice [13, 14]. These and multiple other studies have demonstrated that tolerance toward allogeneic thymus donors develops after immune reconstitution in these animal models. Although allogeneic thymus transplantation into nude (FOXN1 deficient) human subjects would therefore be predicted to show similar success, few of these subjects have been reported and the results of allogeneic thymus transplantation in these subjects has not yet been described. Our results show the induction of tolerance toward allogeneic thymus donors in complete DiGeorge anomaly, a more prevalent presentation of human congenital athymia.
Mechanisms for the observed tolerance include clonal deletion within the thymus graft, peripheral anergy of T cells with alloreactivity toward the thymus, and suppression of rejection by regulatory T cells. In the nude mice models, clonal deletion appeared to play a more significant role in tolerance toward the thymus donors, although peripheral suppression (presumably by regulatory T cells) could not be ruled out [13]. We expect future studies to clarify the roles of each of these mechanisms for the induction of tolerance toward allogeneic thymus transplantation in human subjects with complete DiGeorge anomaly.
Acknowledgments
This work was funded by NIH grants R01 AI 47040, M03 RR 30 (National Center for Research Resources), U54 RR 023469 (Duke Clinical and Translational Institute), and T32 AI 007062-28A2; FDA grant FD-R-002606 (Office of Orphan Products Development); and the American Academy of Allergy, Asthma, and Immunology 2006 Third-Year Fellow-in-Training Research Award. MLM is a member of the Duke Comprehensive Cancer Center.
We acknowledge the work of S. Gupton, E.A. McCarthy, M. Alexieff, J. Li, J. Lonon, and J. Cox for obtaining and processing specimens. We also thank the referring physicians for sending blood samples for these studies.
Abbreviations
cpm
counts per minute
DTCs
donor thymic cells
HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
MLC
mixed lymphocyte culture
PBMCs
peripheral blood mononuclear cells
PHA
phytohemagglutinin
Footnotes
Disclosures: None of the authors has any potential financial conflict of interest related to this manuscript.
References
- 1.Thomas RA, Landing BH, Wells TR. Embryologic and other developmental considerations of thirty-eight possible variants of the DiGeorge Anomaly (DGA) Am J Med Genetics Suppl. 1987;3:43–66. doi: 10.1002/ajmg.1320280508. [DOI] [PubMed] [Google Scholar]
- 2.DiGeorge AM. Discussion of Cooper MD, Peterson RDA, Good RA. A new concept of cellular basis of immunity. J Pediatrics. 1965;67:907. [Google Scholar]
- 3.Conley ME, Beckwith JB, Mancer JFK, Tenckhoff L. The Spectrum of the DiGeorge Syndrome. J Pediatrics. 1979;94:883–890. doi: 10.1016/s0022-3476(79)80207-3. [DOI] [PubMed] [Google Scholar]
- 4.Müller W, Peter HH, Wilken M, Juppner H, Kallfelz HC, Krohn HP, Miller K, Rieger CH. The DiGeorge syndrome. I. Clinical evaluation and course of partial and complete forms of the syndrome. Eur J Pediatrics. 1988;147:496–502. doi: 10.1007/BF00441974. [DOI] [PubMed] [Google Scholar]
- 5.Hong R. The DiGeorge Anomaly. Immunodefic Rev. 1991;3:1–14. [PubMed] [Google Scholar]
- 6.Markert ML, Hummell DS, Rosenblatt HM, Schiff SE, Harville TO, Williams LW, Schiff RI, Buckley RH. Complete DiGeorge Syndrome: Persistence of Profound Immunodeficiency. J Pediatrics. 1998;132:15–21. doi: 10.1016/s0022-3476(98)70478-0. [DOI] [PubMed] [Google Scholar]
- 7.Markert ML, Boeck A, Hale LP, Kloster AL, McLaughlin TM, Batchvarova MN, Douek DC, Koup RA, Kostyu DD, Ward FE, Rice HE, Mahaffey SM, Schiff SE, Buckley RH, Haynes BF. Thymus Transplantation in Complete DiGeorge Syndrome. N Engl J Med. 1999;341:1180–1189. doi: 10.1056/NEJM199910143411603. [DOI] [PubMed] [Google Scholar]
- 8.Markert ML, Devlin BH, Alexieff MJ, Li J, McCarthy EA, Gupton SE, Chinn IK, Hale LP, Kepler TB, He M, Sarzotti M, Skinner MA, Rice HE, Hoehner JC. Review of 54 patients with complete DiGeorge anomaly enrolled in protocols for thymus transplantation: outcome of 44 consecutive transplants. Blood. 2007;109:4539–4547. doi: 10.1182/blood-2006-10-048652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherman LA, Chattopadhyay S. The Molecular Basis of Allorecognition. Ann Rev Immunol. 1993;11:385–402. doi: 10.1146/annurev.iy.11.040193.002125. [DOI] [PubMed] [Google Scholar]
- 10.Markert ML, Sarzotti M, Ozaki DA, Sempowski GD, Rhein ME, Hale LP, Le Deist F, Alexieff MJ, Li J, Hauser ER, Haynes BF, Rice HE, Skinner MA, Mahaffey SM, Jaggers J, Stein LD, Mill MR. Thymus transplantation in complete DiGeorge syndrome: immunologic and safety evaluations in 12 patients. Blood. 2003;102:1121–1130. doi: 10.1182/blood-2002-08-2545. [DOI] [PubMed] [Google Scholar]
- 11.Markert ML, Alexieff MJ, Li J, Sarzotti M, Ozaki DA, Devlin BH, Sedlak DA, Sempowski GD, Hale LP, Rice HE, Mahaffey SM, Skinner MA. Postnatal thymus transplantation with immunosuppression as treatment for DiGeorge syndrome. Blood. 2004;104:2574–2581. doi: 10.1182/blood-2003-08-2984. [DOI] [PubMed] [Google Scholar]
- 12.Markert ML, Alexieff MJ, Li J, Sarzotti M, Ozaki DA, Devlin BH, Sempowski GD, Rhein ME, Szabolcs P, Hale LP, Buckley RH, Coyne KE, Rice HE, Mahaffey SM, Skinner MA. Complete DiGeorge syndrome: Development of rash, lymphadenopathy, and oligoclonal T cells in 5 cases. J Allergy Clin Immunol. 2004;113:734–741. doi: 10.1016/j.jaci.2004.01.766. [DOI] [PubMed] [Google Scholar]
- 13.Kindred B, Sordat B. Lymphocytes which differentiate in an allogeneic thymus. II. Evidence for both central and peripheral mechanisms in tolerance to donor strain tissues. Eur J Immunol. 1977;7:437–442. doi: 10.1002/eji.1830070707. [DOI] [PubMed] [Google Scholar]
- 14.Hong R. Transplantation of cultured thymus fragments. III. Induction of allotolerance. Thymus. 1982;4:91–106. [PubMed] [Google Scholar]