The zinc-binding domain of Nna1 is required to prevent retinal photoreceptor loss and cerebellar ataxia in Purkinje cell degeneration (pcd) mice (original) (raw)
. Author manuscript; available in PMC: 2008 Dec 15.
Abstract
The Purkinje cell degeneration (pcd) mouse undergoes retinal photoreceptor degeneration and Purkinje cell loss. Nna1 is postulated to be the causal gene for pcd. We show that a BAC containing the Nna1 gene rescues retinal photoreceptor loss and Purkinje cell degeneration, confirming that Nna1 loss-of-function is responsible for these phenotypes. Mutation of the zinc-binding domain within the transgene destroyed its ability to rescue neuronal loss in pcd5J homozygous mice. In conclusion, Nna1 is required for survival of retinal photoreceptors and other neuron populations that degenerate in pcd mice. A functional zinc-binding domain is crucial for Nna1 to support neuron survival.
Keywords: Retinal photoreceptor degeneration, Zinc-dependent carboxypeptidase, Genetic rescue, Purkinje cell degeneration
1. Introduction
A number of neurological disease mutations produce a combined cerebellar degeneration and retinal degeneration phenotype in affected human patients. An understanding of the genetic and molecular basis of such phenotypes has been aided by the study of spontaneous mouse mutants that exhibit similar phenotypes. The Purkinje cell degeneration (pcd) mouse is an autosomal recessive combined cerebellar—retinal degeneration mutant that arose spontaneously in 1976 in the C57BR/cdJ strain at the Jackson Laboratory (http://www.jax.org). The original spontaneous mouse mutant—known as _pcd1J_—displays moderate gait ataxia by the time of weaning, which progresses to a profound ataxia by adulthood. Histological studies have shown that pcd1J mice exhibit normal development of the cerebellum, as the cerebellum looks grossly normal at the light microscope level with the expected cytoarchitecture of Purkinje cells separating granule cells and the molecular layer at postnatal day 15 (P15), Mullen, Eicher, and Sidman (1976). However, soon thereafter, a sudden and dramatic Purkinje cell degeneration begins, yielding a progressive near-total loss of cerebellar Purkinje cells by P35. Although pcd1J mice are profoundly ataxic throughout their entire lives, they have normal life spans. The pcd locus has experienced a number of re-mutations, as six different pcd alleles have arisen (1J, 2J, 3J, 5J, 6J, and 7J), and one transgene insertion allele (pcdJWG) has occurred (http://www.informatics.jax.org/searches/allele_report.cgi?_Marker_key=79447). Most of the pcd alleles, including 1J, 3J, and 5J, yield interchangeably severe phenotypes, while certain pcd alleles, such as 2J, are hypo-morphs, preserve some expression, and thus result in milder phenotypes (http://www.informatics.jax.org/searches/allele_report.cgi?_Marker_key=79447).
The other hallmark feature described in pcd mutant mice are a progressive form of retinal degeneration, culminating in marked drop-out of photoreceptors and thinning of the outer segment region before 1 year of age, LaVail, Blanks, and Mullen (1982). Although the retinal degeneration unfolds over a much longer time frame than the rapid Purkinje cell degeneration from which pcd gets its name, signs of retinal degeneration do become apparent as early as P25, and frequent pyknotic photoreceptor nuclei together with notable outer segment thinning can be documented by P60, Blanks, Mullen, and LaVail (1982). Loss of photoreceptor nuclei and thinning of the outer nuclear layer of the retina are obvious by 6 months of age. While the outer plexiform layer exhibits thinning, neither the inner nuclear layer nor the inner plexiform layer are significantly affected, LaVail et al. (1982). Ultrastructural analysis of pcd retinal degeneration has indicated that photoreceptor degeneration is the principal feature of the retinal phenotype in pcd mice, and has noted membrane-associated vesicle formation involving the inner segments of photoreceptors3. In addition to retinal and cerebellar degeneration, pcd mice also exhibit degeneration of thalamic neurons from P50 and P60, gradual loss of mitral neurons in the olfactory bulb during the first year of life, and male infertility, Mullen et al. (1976), O’Gorman and Sidman (1985), O’Gorman (1985).
After mapping the pcd gene defect to a 1 cM region on mouse chromosome 13, screening of candidate genes yielded independent mutations in the Nna1 gene in two pcd strains (2J and 3J), Fernandez-Gonzalez et al. (2002).
Loss of function of the Nna1 gene as the cause of the pcd phenotype is further supported by a marked reduction in the level of Nna1 mRNA and protein expression in the original pcd1J mutant, Fernandez-Gonzalez et al. (2002), and the discovery of a single amino acid insertion in the Nna1 coding region in the pcd5J strain that destabilizes Nna1 protein Chakrabarti et al. (2006). The Nna1 gene encodes a putative protease—a zinc carboxypeptidase (ZnCP)—that is highly conserved in various species, ranging from worms to humans, Harris et al. (2000). In situ hybridization studies have shown that the pattern of Nna1 expression corresponds to the pattern of neurodegeneration observed in pcd mutant mice, Harris et al. (2000). However, Nna1’s ZnCP activity remains to be confirmed and its role in promoting neuronal survival in the cerebellum and retina is still unknown.
Although multiple independent mutant alleles within the Nna1 gene have been reported in three different pcd strains, Fernandez-Gonzalez et al. (2002), concomitant alteration of the function of another gene(s) could be contributing to the diverse neurodegenerative phenotypes observed in pcd mice. To address this question and to determine which of Nna1’s functional domains account for neuronal survival, we obtained a murine BAC that contains the entire Nna1 gene with generous DNA regions flanking the 5′ and 3′ ends of the Nna1 gene. We produced Nna1 BAC transgenic mice and demonstrated that restoration of Nna1 gene expression within the cerebellum and the retina is sufficient to rescue the Purkinje cell degeneration and retinal degeneration in pcd mice. We then derived an Nna1 BAC construct with mutations in the zinc-binding domain of the Nna1 carboxypeptidase region to abrogate putative enzymatic activity or any zinc-binding dependent function.
Expression of zinc-binding deficient Nna1 protein did not rescue pcd cerebellar or retinal degeneration, implicating ZnCP enzymatic activity or some other zinc-dependent protein activity in Nna1’s normal survival function in neurons.
2. Methods
2.1. Generation of BAC mice
A 190-kb BAC (clone RP-23-119N9) containing the Nna1 gene was released from the vector backbone by NotI digestion and injected into the pronuclei of fertilized eggs. A co-targeting method of recombineering was utilized to target the H912A–E915A mutations into this BAC 9A 499-bp genomic fragment was amplified by PCR (5′-ttggtcacgttacagactcctgca-3′; 5′-aaatcatcaaacccattatttgaattaac-3′), gel purified, and co-electroporated with a kanamyacin resistance gene flanked on both ends by 50 bp GalK targeting sequences. The 912/915 mutations (GCT-CCT-GGA-GCA) introduce a unique MwoI restriction site. Twenty-four kanamycin resistant clones were screened by PCR using primers that flanked the 499 bp targeting sequence (5′-ccaagtggtccctgtgctgtg-3′; 5′-aagagtctgacgcattacccac-3′) and one of the 24 clones analyzed yielded the expected MwoI polymorphism. The mutation was confirmed by sequence analysis and the integrity of the BAC clone was further verified by PFGE.
2.2. Bioinformatics
Functional domain prediction for Nna1 was carried out using Blastp at NCBI, http://www.ncbi.nlm.nih.gov/blast, and proSITE at ExPASy, http://au.expasy.org/tools.
2.3. Mouse genotyping
DNA was extracted from 1 cm tail snips and resuspended in 125 μl of low Tris/EDTA. Samples for the real-time PCR standard curve used DNA from a known pcd5J heterozygous animal. Master mixes for pcd5J analysis were made up as follows: 12.5 μl of 2× Taqman Universal PCR Master Mix (ABI), 0.225 μl of 100 μM forward primer, 5′-TACACCATCACCTTCACCGT-3′, 0.225 μl of 100 μM reverse primer. 5′-CACGTACTCGACTCTGCAGG, and 0.05 μl of 100 μm FAM-labeled probe (5′-caaggacgacgatgtctgct-3′) per reaction. For quantification, β-actin was measured using an Assay on Demand probe and primer set at the recommended dilution (ABI). For the assay, 5 μl of diluted DNA sample/standard was added to 20 μl of master mix in a 96-well optical reaction plate (ABI). We used an ABI 7500 thermal cycler with the following conditions: Stage 1; Reps 1; 50 °C; Time 2 min. Stage 2; Reps 1; 95 °C; Time 10 min. Stage 3; Reps 40; 950C; Time 15 s. 60 °C; Time 1 min. Data collection was set to occur at Stage 3–Step 2. Mean quantities were normalized against β-actin, and pcd5J mean quantity was normalized to β-actin with values obtained for the pcd5J heterozygous control set to 1. Unknowns were compared to C57BL/6J (wild-type), pcd5J heterozygous, and pcd5J homozygous controls.
2.4. Behavior and histology analysis
Mice were observed in their cages and were removed for visual gait analysis for signs of ataxia. For preparation of retinal and cerebellar tissue sections, mice under deep anesthetization were perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer (PBS, pH 7.4). For retinal histology, anterior chambers were removed and infiltrated with JB-4 methacrylate after washing and dehydration. Sections (5 μm thick) were cut and immersed in 5% Richardson’s stain. The brain was removed, placed in 4% paraformaldehyde for 4 h, and cryoprotected in 10% sucrose and then 30% sucrose in PBS. Parasagittal frozen sections were cut at 50 μm thickness on a cryostat. Immunohistochemistry was performed on free-floating sections as previously described, Chakrabarti et al. (2006). All experiments involving animals were approved by and performed in accordance with University of Washington IACUC guidelines.
2.5. Western blot analysis
Protein lysates were obtained by homogenizing tissues 1:10 (w/v) in PBS. Equal amounts of homogenate and sample buffer (62.5 mM Tris–HCl, pH 6.8, 4% SDS, 200 mM dithiothreitol, 10% glycerol, 0.001% Bromophenol blue) were boiled for 10 min. Protein samples were resolved by SDS–PAGE transferred to nitrocellulose, and probed with a polyclonal Nna1 antibody at 1:1000, Chakrabarti et al. (2006). Immunoblots were developed with HRP-coupled anti-rabbit antibodies at 1:2000 dilution and Enhanced Chemiluminescence (Amersham).
3. Results
3.1. Generation and characterization of Nna1 BAC transgenic mice
The existence of allelic mutations within the Nna1 gene in different strains of mice displaying the pcd phenotype has putatively established that Nna1 is the causal gene for pcd, Fernandez-Gonzalez et al. (2002), Chakrabarti et al. (2006). However, as mutations can affect gene expression and function at considerable distances, we deemed it necessary to test if transgenic expression of Nna1 is necessary and sufficient to rescue the principal neurodegenerative features of the pcd phenotype. As standard transgenic approaches relying upon heterologous promoters cannot accurately recapitulate proper temporal and spatial expression patterns, we chose to pursue a bacterial artificial chromosome (BAC) transgenic strategy. This strategy is especially warranted since the loss of function(s) of the Nna1 gene product that accounts for the full pcd phenotype is yet to be defined. The Nna1 gene spans ~109 kb on mouse chromosome 13. Our survey of available BAC’s yielded RP23-119N9 as the best candidate for Nna1 transgene expression, as RP23-119N9 is ~181 kb in length, with 45.5 kb of DNA 5′ to the Nna1 transcriptional start site and 28.5 kb of DNA 3′ to the last exon of the Nna1 gene (Supplementary Fig. 1). Based upon the current annotation of this region, no other genes are predicted within BAC RP23-119N9 http://genome.ucsc.edu/. The sequence preceding the transcriptional start site of Nna1 contains two predicted CpG islands http://genome.ucsc.edu/.
Of these two CpG islands, the CpG island adjacent to the start site of transcription is ~1 kb in length with 118 CpG dyads (Supplementary Fig. 1), suggesting that key regulatory sequences for Nna1 gene expression are contained within this BAC clone.
Using BAC isolation techniques commonly employed by our group, Hegde and Paulson (2004), Sopher and La Spada (2006), we generated two Nna1 transgenic founders, which were designated as Nna1-WT1 and Nna1-WT2. RT-PCR analysis confirmed increased expression of murine Nna1 at levels ~150–200% of non-transgenic control mice (data not shown). We thus selected the higher expressing Nna1-WT1 line for directed breeding experiments with pcd5J mice. To derive pcd5J homozygous mice carrying the Nna1-WT1 BAC transgene, we crossed Nna1-WT1 BAC mice with pcd5J homozygous female mice and then backcrossed pcd5J heterozygous—Nna1 WT1 transgene-positive males with pcd5J homozygous female mice. To identify pcd5J homozygous mice carrying the Nna1-WT1 BAC transgene, we developed a real-time PCR approach to quantify pcd5J allele dosage (Fig. 1), and combined this genotyping strategy with a standard PCR-based assay for BAC vector-specific sequences. In this way, we confirmed the derivation of Nna1-WT1 BAC transgene-positive, pcd5J homozygous mice.
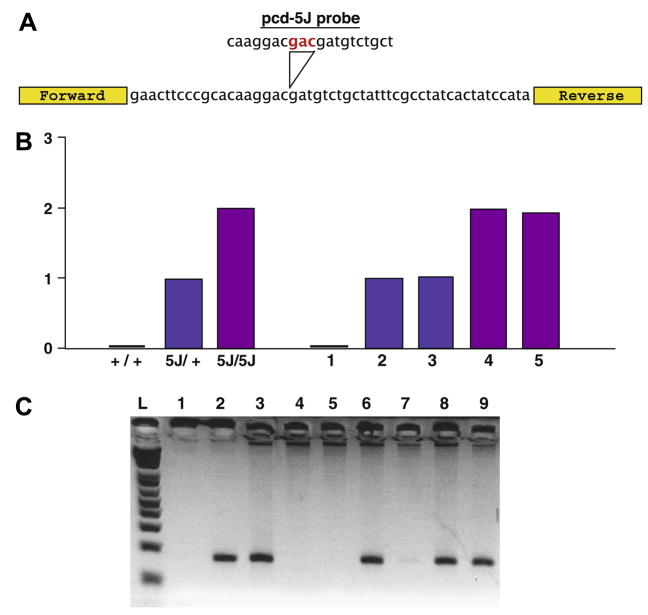
Fig. 1.
Genotyping strategy for detection of Nna1 BAC transgene-positive—pcd5J homozygous mice. (A) Quantitative PCR primer design. To differentiate pcd5J homozygous mice from pcd5J heterozygous mice (and non-pcd mutant mice), we performed quantitative DNA PCR for an amplicon containing the pcd5J mutation with a _pcd5J_-specific probe containing the pcd5J insertion mutation sequence (red text). (B) Representative quantitative DNA PCR genotyping results. Here we see the results of a quantitative real-time DNA PCR analysis on tail DNA’s from a set of controls (+/+; 5J/+; and 5J/5J) and a set of unknowns (1–5). 5J gene dosage was normalized to β-actin, and arbitrarily set to 1.0 for the known pcd5J heterozygous mouse. Based upon this analysis, we were able to assign wild-type status to mouse 1, heterozygous status to mice 2, and 3, and homozygous status to mice 4 and 5. (C) Determination of Nna1 BAC transgene status. Here we see a representative set of genotyping results for the Nna1 BAC transgene. PCR amplification of a BAC vector-specific amplicon was performed, and detection of a signal indicated that the mouse was Nna1 BAC transgene-positive. In this experiment, lane 1 contained no DNA template and served as the negative control, while lane 2 corresponds to BAC DNA template and served as the positive control. As shown here, individuals 6, 8, and 9 are Nna1 BAC transgene-positive.
3.2. The Nna1 BAC transgene rescues both Purkinje cell and retinal degeneration in pcd mice
Adult pcd5J homozygous mice undergo a profound degeneration and loss of Purkinje cell neurons in the cerebellum by seven weeks of age. To determine if expression of the Nna1-WT1 BAC was sufficient to rescue this dramatic Purkinje cell degeneration, we obtained cerebellar sections from 4-month-old littermate pcd5J homozygous mice that were either positive or negative for the Nna1-WT1 transgene. Calbindin immunostaining revealed a complete rescue of Purkinje cell degeneration in Nna1-WT1 BAC transgene-positive, pcd5J homozygous mice (Fig. 2A).
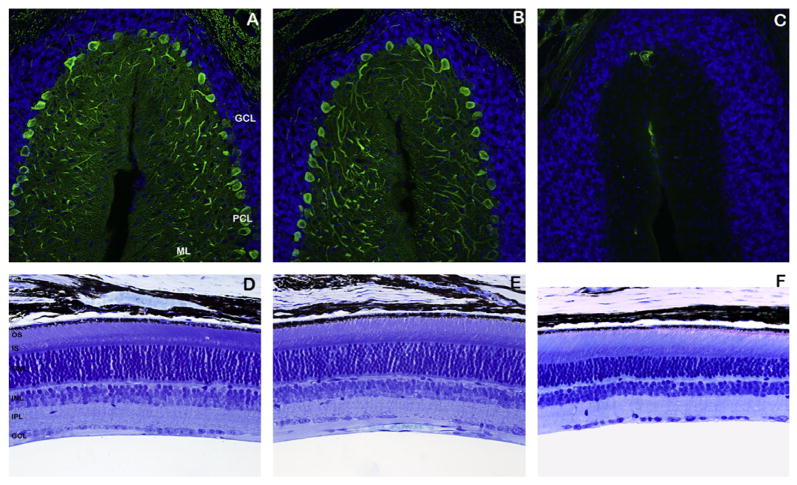
Fig. 2.
The Nna1-WT1 BAC transgene rescues both Purkinje cell degeneration and retinal photoreceptor cell degeneration. (A–C) Cerebellar sections from 4-month-old Nna1-WT1 BAC—pcd5J homozygous mice, pcd5J homozygous mice, and wild-type littermate mice were immunostained with an anti-calbindin antibody (green) and counter-stained with DAPI. Both wild-type mice (A) and Nna1-WT1 BAC—pcd5J homozygous mice (B) exhibited a normal number of Purkinje cell neurons, comparable molecular layer (ML) thickness, and prominent Purkinje cell dendritic arborization in the ML. Cerebellar sections from pcd5J homozygous mice (C), however, revealed a dramatic and total loss of Purkinje cell neurons. GCL, granule cell layer; PCL, Purkinje cell layer; ML, molecular layer (D–F) Retinal sections from identical regions of the retina were obtained from 8-month-old Nna1-WT1 BAC—pcd5J homozygous mice, pcd5J homozygous mice, and wild-type littermate mice, and were analyzed by Richardson’s staining. As shown here, wild-type littermate mice (D) and Nna1-WT1 BAC—pcd5J homozygous mouse (E) displayed normal retinal cytoarchitecture and normal photoreceptor nuclei number in the outer nuclear layer (ONL), with 10–12 photoreceptor nuclei across. Retinal sections from pcd5J homozygous mice (F) revealed a significant thinning of the ONL, with as few as five retinal photoreceptor nuclei adjacent in cross section. OS, outer segments; IS, inner segments; ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Analysis of home cage behavior similarly indicated that while all pcd5J homozygous mice lacking the Nna1-WT1 BAC transgene exhibited gait ataxia by the time of weaning, Nna1-WT1 BAC transgene-positive, pcd5J homozygous mice were indistinguishable from normal controls. Indeed, to date, we have generated over 30 litters of Nna1-WT1 BAC—pcd5J mice, and not a single Nna1-WT1 BAC transgene-positive, pcd5J homozygous mouse has ever displayed any visible signs of ataxia. Rescued pcd5J male mice also were able to successfully reproduce, siring normal-sized litters, though our breeding studies of the pcd5J strain found that male sterility is not fully penetrant for the 5J mutation (data not shown).
Although Nna1 transgenic expression directed to Purkinje cells has been shown to be capable of rescuing pcd cerebellar degeneration, Wang, Parris, Li, and Morgan (2006), the necessity and sufficiency of Nna1 expression for preventing pcd retinal degeneration is yet to be assessed. Our Nna1-WT1 BAC transgenic crosses with pcd5J mice allowed us to address this question. As pcd retinal degeneration is much more slowly progressive than the cerebellar degeneration, we examined the effect of Nna1 transgene expression in 8-month-old littermate pcd5J homozygous mice that were either positive or negative for the Nna1-WT1 BAC transgene. Staining of retinal sections from such litters revealed marked thinning of the outer nuclear photoreceptor layer as well as degenerating outer segments in pcd5J homozygous mice lacking the Nna1-WT1 BAC transgene (Fig. 2B). However, in all cases, Nna1-WT1 BAC transgene-positive, pcd5J homozygous mice exhibited normal retinal cytoarchitecture, comparable to the histological appearance of non-transgenic controls (Fig. 2B). Thus, Nna1 BAC transgenic expression does also rescue the pcd retinal degeneration phenotype.
3.3. Engineering a zinc-binding deficient Nna1 BAC transgene
After demonstrating that we could successfully rescue the key neurodegenerative phenotypes of the pcd5J mouse with an Nna1 BAC transgene, we next turned our attention to an evaluation of the role of Nna1’s postulated carboxypeptidase activity in the pcd phenotype. Alignment of the amino acid coding sequence of Nna1 with related proteins has shown that murine Nna1 possesses a highly-conserved zinc carboxypeptidase (ZnCP) domain, Harris et al. (2000). Analysis of the Nna1 protein sequence reveals the presence of multiple protein motifs and domains (Fig. 3). Indeed, Nna1 contains an aspartic acid rich domain starting at amino acid 314 and ending at amino acid 402, an ATP binding domain spanning amino acids 810–817, whence the gene gets its official name as ‘ATP and GTP binding protein 1 (Agtpbp1)′, a carboxy-terminal bipartite nuclear localization signal at amino acid position 1011–1015, and, finally, the ZnCP domain. Nna1’s region of peptidase sequence similarity extends from amino acid 851 to position 1075 (Fig. 4). Within this well-recognized motif, key conserved residues reside, including a HXXE consensus at position 912–915 that is required for zinc binding and catalytic activity, Abe, Abe, Aoki, Itoyama, and Tamai (1997). Given the absolute requirement for a histidine at position 912 and a glutamic acid at position 915 for putative zinc coordination and Nna1 ZnCP activity, we chose to introduce point mutations at these two residues to change both to alanine codons in the Nna1 BAC, using a standard BAC recombineering strategy Hegde and Paulson (2004). We designated the resulting BAC as the Nna1-MuZn BAC to denote its mutant zinc-binding status.
Fig. 3.
Nna1 functional domain analysis highlights key residues for zinc-binding and putative enzymatic function. Here we see the amino acid sequence for mouse Nna1. Green lettering denotes the aspartic acid rich domain starting at positions 314–402, pink lettering corresponds to the ATP binding domain. The highly conserved carboxypeptidase domain is underlined, while the entire enzymatic domain is indicated by bold lettering. Gray lettering demarcates the region of aspartoacylase sequence similarity, with various key residues for enzymatic activity indicated variously in red, blue, orange, or purple. The HXXE zinc-binding site is shown with the invariant ‘H’ and ‘E’ residues in red. These residues were selected for mutagenesis in the Nna1 BAC construct.
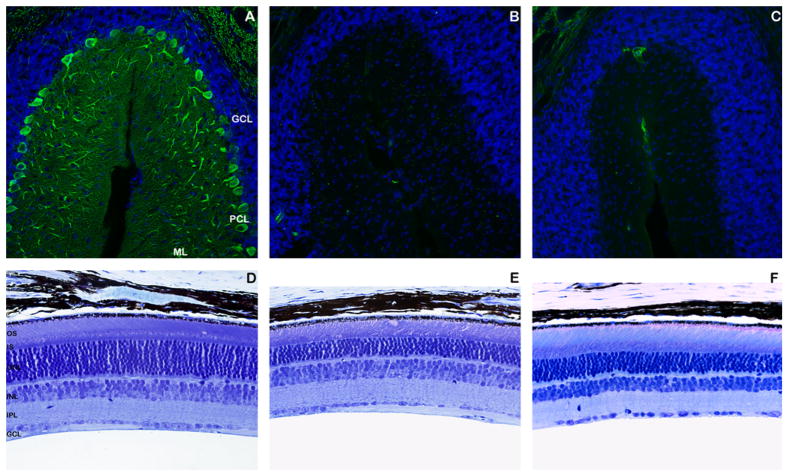
Fig. 4.
The Nna1-MuZn2 BAC transgene fails to prevent either Purkinje cell degeneration or retinal degeneration in pcd mice. (A–C) Cerebellar sections from 4-month-old Nna1-MuZn2 BAC—pcd5J homozygous mice, pcd5J homozygous mice, and wild-type littermate mice were immunostained with an anti-calbindin antibody (green) and counter-stained with DAPI. Wild-type mice (A) had a normal number of Purkinje cell neurons, comparable molecular layer (ML) thickness, and prominent Purkinje cell dendritic arborization in the ML. Cerebellar sections from Nna1-MuZn2 BAC—pcd5J homozygous mice (B) and pcd5J homozygous mice (C), however, both revealed a complete loss of Purkinje cell neurons. (D–F) Retinal sections from identical regions of the retina were obtained from 8-month-old Nna1-MuZn2 BAC—pcd5J homozygous mice, pcd5J homozygous mice, and wild-type littermate mice, and were analyzed by Richardson’s staining. As shown here, wild-type littermate mice (D) exhibited normal retinal cytoarchitecture and normal photoreceptor nuclei number in the outer nuclear layer (ONL), with 10–12 photoreceptor nuclei across. Retinal sections from Nna1-MuZn2 BAC—pcd5J homozygous mice (E) and pcd5J homozygous mice (F) revealed a significant thinning of the ONL. Abbreviations are as in Fig. 2.
3.4. Zinc-binding deficient Nna1 protein fails to rescue Purkinje cell or retinal degeneration in pcd mice
To determine if zinc coordination and presumably ZnCP enzymatic activity are required for -Nna1 genetic rescue of the pcd phenotype, we derived Nna1-MuZn BAC transgenic mice. RT-PCR analysis of two independent Nna1-MuZn BAC lines (Nna1-MuZn1 and Nna1-MuZn2) indicated that transgene-positive mice from these two lines, respectively, exhibit Nna1 expression levels at 140–180% of non-transgenic wild-type mice. We thus selected the Nna1-MuZn2 BAC transgenic line for cross-breeding experiments with pcd5J homozygous mice. Using the PCR genotyping strategy described above, we identified pcd5J homozygous mice that either lacked the Nna1-MuZn2 BAC transgene or were positive for the Nna1-MuZn2 BAC transgene. When we compared Nna1-MuZn2 BAC transgene-positive pcd5J homozygous mice with pcd5J homozygous mice littermates that did not possess the Nna1-MuZn2 BAC transgene, we noted that transgene-positive and transgene-negative pcd5J homozygous mice both displayed gait ataxia at the time of weaning. In agreement with our behavioral analysis, calbindin immunostaining of Nna1-MuZn2 BAC transgene-positive pcd5J homozygous mice yielded minimal immunoreactivity and a pattern of Purkinje cell loss that was indistinguishable from transgene-negative pcd5J homozygous mice (Fig. 4A). Furthermore, evaluation of retinal sections obtained from Nna1 MuZn2 BAC transgene-positive pcd5J homozygous mice revealed substantial thinning of the outer nuclear layer and outer segment disorganization, kin to the appearance of the retina in transgene-negative pcd5J homozygous mice (Fig. 4B). Thus, based upon behavioral and histological analysis, the presence of the Nna1-MuZn2 BAC transgene did not ameliorate pcd retinal and cerebellar degeneration. To ensure that the failure of the Nna1-MuZn2 BAC transgene to rescue the pcd5J phenotype could not be attributed to inadequate Nna1 protein expression from the BAC transgene, we performed Western blot analysis on cerebellar protein lysates from rescued Nna1-WT1 BAC pcd5J homozygous mice, Nna1-MuZn2 BAC transgene-positive pcd5J homozygous mice, pcd5J homozygous mice, and pcd5J heterozygous mice (Fig. 5). Nna1 immunoblotting confirmed that Nna1-MuZn2 BAC transgene-positive pcd5J homozygous mice express Nna1 protein at levels comparable to Nna1-pcd5J heterozygous mice. As pcd5J heterozygotes do not display any signs of retinal or cerebellar disease, the failure of the Nna1-MuZn2 BAC transgene to rescue the pcd5J phenotype indicates that a functional zinc coordination domain is required for Nna1 rescue.
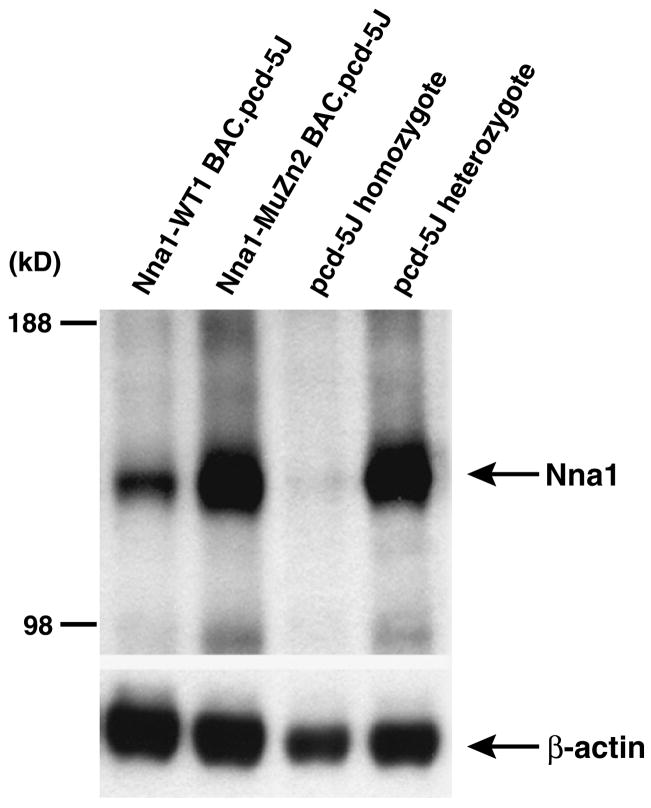
Fig. 5.
Failure of Nna1-MuZn2 BAC transgenic rescue of pcd phenotype can not be attributed to inadequate Nna1 transgene expression. Western blot analysis of cerebellar protein lysates immunoblotted with an anti-Nna1 antibody was performed on samples obtained from different types of transgenic/pcd5J mice as indicated above the four lanes. Nna1 protein expression in Nna1-MuZn2 BAC—pcd5J homozygous mice were comparable to the level of Nna1 protein expression in pcd5J heterozygous mice that are normal. Immunoblots were re-probed with an anti β-actin antibody as a loading control.
4. Discussion
Concomitant degeneration of the cerebellum and the retina is a feature of numerous human diseases, including spinocerebellar ataxia type 7, neuronal ceroid lipofuscinosis, and Joubert syndrome, Lindhout, Barth, Valk, and Boen-Tan (1980), Luiro, Kopra, Lehtovirta, and Jalanko (2001), Yvert et al. (2000). In mice, various spontaneous mutant strains similarly display progressive cerebellar and retinal degeneration, including nervous, harlequin, and Purkinje cell degeneration (pcd) Mullen et al. (1976), LaVail et al. (1993), Vaishnav et al. (2008). For this reason, fundamental processes of neuron development and neuron survival are likely to be shared between the cerebellum and retina, providing an opportunity to delineate such pathways by comparative analysis of the molecular basis of cerebellar and retinal degeneration involving such mutations. In this study, we considered the genetic and molecular basis of pcd, an autosomal recessive mouse mutant that exhibits a stereotypical phenotype of Purkinje cell degeneration and retinal degeneration, Mullen et al. (1976). Previous work identified mutations in a relatively novel gene, Nna1, and demonstrated that Nna1 loss-of-function accounts for the cerebellar degeneration, Wang et al. (2006), but an exclusive role for Nna1 loss-of-function in pcd cerebellar and retinal degeneration had not been established. Our results indicate that transgenic rescue of pcd5J mice with a BAC containing the Nna1 gene with generous 5′ and 3′ flanking regions is sufficient to rescue pcd retinal degeneration as well as the Purkinje cell loss and ataxia phenotype, and lend further credence to the view that Nna1 loss-of-function is alone responsible for the diverse phenotypes observed in pcd null mice.
One of the most intriguing questions stemming from our studies of pcd is the functional basis of Nna1 action in promoting neuron survival in cerebellar Purkinje cells and retinal photoreceptors. Nna1 is part of an extensive family of genes, all of which contain a highly conserved zinc-dependent carboxypeptidase (ZnCP) domain, Rodriguez de la Vega et al. (2007). Recently, five additional Nna1-like genes were identified in the mouse genome and designated cytosolic carboxypeptidases (CCP2—6), Rodriguez de la Vega et al. (2007).
Modeling algorithms indicated that the folding of these proteins resembles the M14 family of carboxypeptidases, with theoretically matching residues for activity and substrate specificity. All the CCPs are abundant in testis and are also expressed in the brain, pituitary, and eye, Rodriguez de la Vega et al. (2007). Whether or not these mammalian CCPs and Nna1 retain ZnCP enzymatic activity remains unclear, as biochemical studies in our lab and by another group are yet to document carboxypeptidase activity, even though a wide variety of carboxy-terminal residues were tested (data not shown), Wang et al. (2006). However, a recombinant form of one Nna1-like peptidase from Caenorhabditis elegans was shown to be a fully functional carboxypeptidase, Rodriguez de la Vega et al. (2007). The enzymatic activity of this protein required ATP/ADP and was inactivated by high salt concentrations, Rodriguez de la Vega et al. (2007). The amino acid sequence of mouse Nna1 has sufficient sequence similarity to theoretically assign it a functional asparto-acylase domain (BLASTP, NCBI). Nna1 also contains an ATP/GTP binding domain in the central portion of its predicted amino acid coding sequence, Harris et al. (2000). While these motif and enzymatic predictions suggest likely activities for Nna1, the true function(s) of Nna1 remains to be elucidated. Future studies aimed at understanding how Nna1 loss-of-function ultimately leads to cerebellar Purkinje cell degeneration and retinal photoreceptor degeneration will need to uncover the function of Nna1, before the mechanistic basis of pcd neuronal dysfunction can be determined. As Nna1 normal function appears crucial for both neuron survival and neuron regeneration, Harris et al. (2000), Wang and Morgan (2007), important insights into normal neuron function and injury recovery will undoubtedly emerge from such work.
Acknowledgments
The authors wish to thank S. Wang, M. Baker, and V. Damian. This work was supported by funds from the NIH: EY14997 to ARL; EY18106 to LC; EY01730 to UWMC (NEI research core); and P30 HD02274 (MRDDRC Center Grant) to UW.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.visres.2008.05.026.
References
- Abe T, Abe K, Aoki M, Itoyama Y, Tamai M. Ocular changes in patients with spinocerebellar degeneration and repeated trinucleotide expansion of spinocerebellar ataxia type 1 gene. Archives of Ophthalmology. 1997;115(2):231–236. doi: 10.1001/archopht.1997.01100150233013. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Mullen RJ, LaVail MM. Retinal degeneration in the pcd cerebellar mutant mouse. II. Electron microscopic analysis. Journal of Comparative Neurology. 1982;212(3):231–246. doi: 10.1002/cne.902120303. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L, Neal JT, Miles M, Martinez RA, Smith AC, Sopher BL, et al. The Purkinje cell degeneration 5J mutation is a single amino acid insertion that destabilizes Nna1 protein. Mammalian Genome. 2006;17(2):103–110. doi: 10.1007/s00335-005-0096-x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez A, La Spada AR, Treadaway J, Higdon JC, Harris BS, Sidman RL, et al. Purkinje cell degeneration (pcd) phenotypes caused by mutations in the axotomy-induced gene, Nna1. Science. 2002;295(5561):1904–1906. doi: 10.1126/science.1068912. [DOI] [PubMed] [Google Scholar]
- Harris A, Morgan JI, Pecot M, Soumare A, Osborne A, Soares HD. Regenerating motor neurons express Nna1, a novel ATP/GTP-binding protein related to zinc carboxypeptidases. Molecular and Cellular Neuroscience. 2000;16(5):578–596. doi: 10.1006/mcne.2000.0900. [DOI] [PubMed] [Google Scholar]
- Hegde S, Paulson RF. Co-targeting a selectable marker to the Escherichia coli chromosome improves the recovery rate for mutations induced in BAC clones by homologous recombination. Biotechniques. 2004;36(6):936–938. 940. doi: 10.2144/04366BM03. [DOI] [PubMed] [Google Scholar]
- LaVail MM, Blanks JC, Mullen RJ. Retinal degeneration in the pcd cerebellar mutant mouse. I. Light microscopic and autoradiographic analysis. Journal of Comparative Neurology. 1982;212(3):217–230. doi: 10.1002/cne.902120302. [DOI] [PubMed] [Google Scholar]
- LaVail MM, White MP, Gorrin GM, Yasumura D, Porrello KV, Mullen RJ. Retinal degeneration in the nervous mutant mouse. I. Light microscopic cytopathology and changes in the interphotoreceptor matrix. Journal of Comparative Neurology. 1993;333(2):168–181. doi: 10.1002/cne.903330204. [DOI] [PubMed] [Google Scholar]
- Lindhout D, Barth PG, Valk J, Boen-Tan TN. The Joubert syndrome associated with bilateral chorioretinal coloboma. European Journal of Pediatrics. 1980;134(2):173–176. doi: 10.1007/BF01846041. [DOI] [PubMed] [Google Scholar]
- Luiro K, Kopra O, Lehtovirta M, Jalanko A. CLN3 protein is targeted to neuronal synapses but excluded from synaptic vesicles: New clues to Batten disease. Human Molecular Genetics. 2001;10(19):2123–2131. doi: 10.1093/hmg/10.19.2123. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Eicher EM, Sidman RL. Purkinje cell degeneration, a new neurological mutation in the mouse. Proceedings of the National Academy of Sciences, USA. 1976;73(1):208–212. doi: 10.1073/pnas.73.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Gorman S. Degeneration of thalamic neurons in “Purkinje cell degeneration” mutant mice. II. Cytology of neuron loss. Journal of Comparative Neurology. 1985;234(3):298–316. doi: 10.1002/cne.902340303. [DOI] [PubMed] [Google Scholar]
- O’Gorman S, Sidman RL. Degeneration of thalamic neurons in “Purkinje cell degeneration” mutant mice. I. Distribution of neuron loss. Journal of Comparative Neurology. 1985;234(3):277–297. doi: 10.1002/cne.902340302. [DOI] [PubMed] [Google Scholar]
- Rodriguez de la Vega M, Sevilla RG, Hermoso A, Lorenzo J, Tanco S, Diez A, et al. Nna1-like proteins are active metallocarboxypeptidases of a new and diverse M14 subfamily. FASEB Journal. 2007;21(3):851–865. doi: 10.1096/fj.06-7330com. [DOI] [PubMed] [Google Scholar]
- Sopher BL, La Spada AR. Efficient recombination-based methods for bacterial artificial chromosome fusion and mutagenesis. Gene. 2006;371(1):136–143. doi: 10.1016/j.gene.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Vaishnav RA, Getchell ML, Huang L, Hersh MA, Stromberg AJ, Getchell TV. Cellular and molecular characterization of oxidative stress in olfactory epithelium of Harlequin mutant mouse. Journal of Neuroscience Research. 2008;86(1):165–182. doi: 10.1002/jnr.21464. [DOI] [PubMed] [Google Scholar]
- Wang T, Morgan JI. The Purkinje cell degeneration (pcd) mouse: An unexpected molecular link between neuronal degeneration and regeneration. Brain Research. 2007;1140:26–40. doi: 10.1016/j.brainres.2006.07.065. [DOI] [PubMed] [Google Scholar]
- Wang T, Parris J, Li L, Morgan JI. The carboxypeptidase-like substrate-binding site in Nna1 is essential for the rescue of the Purkinje cell degeneration (pcd) phenotype. Molecular and Cellular Neuroscience. 2006;33(2):200–213. doi: 10.1016/j.mcn.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Yvert G, Lindenberg KS, Picaud S, Landwehrmeyer GB, Sahel JA, Mandel JL. Expanded polyglutamines induce neurodegeneration and transneuronal alterations in cerebellum and retina of SCA7 transgenic mice. Human Molecular Genetics. 2000;9(17):2491–2506. doi: 10.1093/hmg/9.17.2491. [DOI] [PubMed] [Google Scholar]