Sustained Effect of Intensive Treatment of Type 1 Diabetes Mellitus on Development and Progression of Diabetic Nephropathy: The Epidemiology of Diabetes Interventions and Complications (EDIC) Study (original) (raw)
. Author manuscript; available in PMC: 2009 Jan 13.
Published in final edited form as: JAMA. 2003 Oct 22;290(16):2159–2167. doi: 10.1001/jama.290.16.2159
Abstract
Context
The Diabetes Control and Complications Trial (DCCT) demonstrated the benefits of intensive treatment of diabetes in reducing glycemic levels and slowing the progression of diabetic nephropathy. The DCCT cohort has been examined annually for another 8 years as part of the follow-up Epidemiology of Diabetes Interventions and Complications (EDIC) study, During the EDIC study, glycemic levels no longer differed substantially between the 2 original treatment groups.
Objective
To determine the long-term effects of intensive vs conventional diabetes treatment during the DCCT on kidney function during the EDIC study.
Design, Setting, and Participants
Observational study begun in 1993 (following DCCT closeout) in 28 medical centers in the United States and Canada. Participants were 1349 (of 1375) EDIC volunteers who had kidney evaluation at years 7 or 8.
Main Outcome Measures
Development of microalbuminuria, clinical-grade albuminuria, hypertension, or increase in serum creatinine level.
Results
Results were analyzed by intention-to-treat analyses, comparing the 2 original DCCT treatment groups. New cases of microalbuminuria occurred during the EDIC study in 39 (6.8%) of the participants originally assigned to the intensive-treatment group vs 87 (15.8%) of those assigned to the conventional-treatment group, for a 59% (95% confidence interval [CI], 39% -73%) reduction in odds, adjusted for baseline values, compared with a 59% (95% CI, 36%-74%) reduction at the end of the DCCT (P<.001 for both comparisons). New cases of clinical albuminuria occurred in 9 (1.4%) of the participants in the original intensive-treatment group vs 59 (9.4%) of those in the original conventional-treatment group, representing an 84% reduction in odds (95% Cl, 67%-92%), compared with a reduction of 57% (95% CI, -1 % to +81%) at the end of the DCCT. Fewer cases of hypertension (prevalence at year 8, 29.9% vs 40.3%; P<.001) developed in the original intensive-treatment group. Significantly fewer participants reached a serum creatinine level of 2 mg/dL or greater in the intensive-treatment vs the conventional-treatment group (5 vs 19, _P_=.004), but there were no differences in mean log clearance values. Although small numbers of patients required dialysis and/or transplantation, fewer patients experienced either of these outcomes in the intensive group (4 vs 7, _P_=.36).
Conclusions
The persistent beneficial effects on albumin excretion and the reduced incidence of hypertension 7 to 8 years after the end of the DCCT suggest that previous intensive treatment of diabetes with near-normal glycemia during the DCCT has an extended benefit in delaying progression of diabetic nephropathy.
The Diabetes Control and Complications Trial (DCCT) demonstrated the benefits of intensive treatment of diabetes in preventing the development of retinopathy and albuminuria and reducing their progression in patients with type 1 diabetes mellitus.1,2 Previous studies have documented a long pathologic process, presumably reflecting the effects of hyperglycemia on renal cell and matrix, which culminates in decreasing glomerular filtration rate (GFR) and end-stage renal disease. The periods during which these processes may be prevented, slowed, or even reversed, and the amount of the therapeutic exposure necessary to effect such changes, are poorly understood.
The intensive- and conventional-treatment groups in the DCCT, enrolled with either no clinically evident complications or with only early microvascular complications, were exposed to 2 different levels of glycemic control over an average of 6.5 years. At the end of the DCCT, the group receiving intensive treatment was encouraged to continue, and the group receiving conventional treatment was encouraged to initiate intensive treatment. Diabetes care was subsequently supervised by the patients’ own clinicians.
The 2 original treatment groups have now been followed up for an average of 8 more years, but at similar levels of glycemic control, during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study.3 The EDIC study provides the opportunity to ask how long the effects of intensive vs conventional treatment of type 1 diabetes mellitus are sustained. We have recently shown sustained efficacy of DCCT intensive treatment in reducing retinopathy during 4 years of the EDIC study after closeout of the DCCT.4 The data reported herein evaluate the sustained effect of intensive therapy and of previous differences in glycemia on the development and progression of functional measures of diabetic nephropathy after 8 years in the EDIC study.
METHODS
Patients
Detailed descriptions of the eligibility criteria and intensive and conventional treatment procedures for participants entering the DCCT, their DCCT baseline kidney function, and measures of their kidney function during the DCCT have been published.1,2,5 In brief, at DCCT baseline, all participants were aged 13 through 39 years and had a duration of type 1 diabetes mellitus of 1 to 15 years. They were free of advanced microvascular or macrovascular complications of diabetes, had normal GFRs (defined as serum creatinine levels ≤1.2 mg/dL [106.1 μmol/L] and/or creatinine clearance ≥100 mL/min [1.7 mL/s] per 1.73 m2), and were normotensive (blood pressure, ≤140/90 mm Hg). At DCCT baseline, albumin excretion rate (AER) was less than 28 μg/min (40 mg/24 h) for the primary prevention cohort (1-5 years duration and no retinopathy) and 140 μg/min (200 mg/24 h) or less for the secondary intervention cohort (1-15 years’ duration and at least 1 microaneurysm). In addition, all participants were free of severe neuropathy (ie, that requiring symptomatic treatment) and had calculated low-density lipoprotein cholesterol levels of less than 190 mg/dL (4.9 mmol/L). Complications of diabetes mellitus developed in many participants during the DCCT.2,6-9 Herein, the cumulative incidences of the nephropathic complications during the DCCT, and other factors at the end of the DCCT (the beginning of the EDIC study), will be presented as starting points from which to show changes that subsequently occurred during the first 8 years of the EDIC study.
Of the 1428 surviving members of the original DCCT cohort, 1375 participants, including 688 patients in the former conventional-treatment group and 687 in the former intensive-treatment group, volunteered to participate in the EDIC study in 1993, following DCCT closeout. A detailed description of the EDIC study procedures and baseline characteristics has been published.3 The report herein describes 1349 participants: 1337 who underwent 4-hour urine collections or had serum creatinine levels measured in years 7 or 8, and 12 who died after entering EDlC follow-up but prior to year 7.
These 1349 EDIC participants were comparable to the 79 surviving participants who did not contribute EDIC data, with the exception that these 1349 EDIC participants were significantly older (33.0 v 30.7 years, _P_= .003) and had lower mean levels of glycosylated hemoglobin (HbA1c) during the DCCT (8.1% v 8.6%, P<.00l).
Assessment of Glycemic Control, Renal Function, and Blood Pressure
Details regarding blood pressure measurement and assays for levels of HbA1c, creatinine, and albumin have been reported for the DCCT,1,2 and the procedures remained identical during the EDIC study.3 Annual measurements of blood pressure by sphygmomanometer and of HbA1c level by ionexchange high-performance liquid chromatography were performed in the EDIC study. The current mean HbA1c level during the EDIC study is the mean of the current and prior annual measures. The mean HbA1c level during the DCCT is the mean of all quarterly measures. The combined mean HbA1c level is computed as the average of the DCCT and current EDIC mean values, weighted by the time in the DCCT and the time in EDIC follow-up.
The 4-hour urine collections for albuminuria and creatinine clearance were performed every other year, with approximately half of the EDIC participants evaluated at odd EDIC study years and half at even years. Participants were not asked to discontinue any medications, including angiotensinconverting enzyme inhibitors, angiotensin II receptor blockers, or other antihypertensive medications, at the time of their annual assessments. Results are combined for those participants evaluated during years 1 and 2, years 3 and 4, years 5 and 6, and years 7 and 8. Creatinine levels in serum and urine were measured by a variation of the Jaffe method. Urine albumin level was measured by a fluoroimmunoassay.2 During the EDIC study, coefficients of variation and coefficients of reliability were, respectively, 0.7% and 100% for HbA1c level; 2.3% and 94% for serum creatinine concentration; 2.3% and 100% for urine creatinine concentration; 9.4% and 94% for urine albumin concentration; and 14% and 95% for the 4-hour excretion rate of albumin. Glomerular filtration rates were determined by timed clearance of 125I-iothalamate at DCCT closeout10 and adjusted for body surface area.
Statistical Analysis
Major nephropathic outcomes of interest were defined prior to the end of the DCCT and included microalbuminuria (defined as AER ≥28 μ/min [40 mg/24 h]); albuminuria (AER > 208 μg/min [>300 mg/24 h]); hypertension (blood pressure > 140/90 mm Hg or treatment with antihypertensive medication); doubling of the serum creatinine concentration since DCCT baseline; and serum creatinine concentration of 2.0 mg/dL (176.8 μmol/L) or greater; and the need for dialysis and/or renal transplantation.2,3 Frequency of events in each treatment group is expressed as the percentages of the participants at risk who experienced the events (cases). The Wilcoxon rank-sum test was used to compare the treatment groups with respect to the distributions of quantitative variables; the contingency χ2 test was used for categorical variables.11 Estimated reductions in the odds of progression past some threshold (eg, AER>300 mg/d) were obtained from logistic regression models12 that adjusted for the initial values of the measurement. Thus, DCCT results were adjusted for values measured at DCCT baseline, while EDIC results were adjusted for values observed at DCCT close-out (EDIC baseline). Aggregate group differences in the prevalence (odds) of a characteristic over time during the EDIC study were assessed using logit generalized estimating equations.l3
The cumulative incidence of new events during the EDIC study was computed using a modified Kaplan-Meier estimate allowing for scheduled examinations over time and the difference between groups was tested by the Mantel log-rank test.12 The discrete proportional hazards model, stratified by odd- vs even-year schedule of visits, assessed the relative risk of new events associated with factors, including timevarying covariates during the EDIC study.12 All outcomes were analyzed on the basis of the original DCCT treatment assignments. The percentage of an effect explained by another covariate is computed as the percentage reduction in the χ2 test value for the effect from a model without vs with the covariate.
Fixed-effects normal-errors models were used to assess aggregate differences between groups in a quantitative characteristic over time during the EDlC study, adjusted for baseline characteristics.14 Generalized estimating equations13 with a constant variance assumption were used when the assumptions of the normal-errors model did not apply.
For each outcome with multiple measurements over time, a single aggregate test was conducted of the average mean differences, prevalence odds ratio, or hazard ratio over time. Statistical analyses were carried out using SAS version 8.2 (SAS Institute Inc, Cary,NC), and p<.05 was used to determine statistical significance.
RESULTS
EDIC Baseline
At the closeout of the DCCT (ie, the EDIC baseline), the 1349 EDIC participants included herein had a mean age of 33 years and a mean duration of diabetes of 12 years (Table 1). At EDIC baseline, the DCCT treatment groups differed significantly in the median levels of AER (p<.001) and the prevalence of microalbuminuria (P<.001), but not albuminuria, reflecting the effects of DCCT therapy on these outcomes. The groups also differed significantly in levels of HbA1c (P<.001).
Table 1.
Participant Characteristics at EDIC Study Baseline
| Characteristic | Originai DCCT Treatment Group | P Value* | |
|---|---|---|---|
| Intensive(n=676) | Conventional(n=673) | ||
| Age, mean (SD), Y | 34 (7) | 33 (7) | .11 |
| Women, No. (%) | 330 (49) | 313(46) | .40 |
| Diabetes duration, mean (SD), y | 12 (5) | 12(5) | >.99 |
| HbAlc, mean (SD), % | 7,4 (1,1) | 9.1(1.6) | <.001 |
| Body mass index, mean (SD)† | 26.5(4) | 25.0(3) | >.001 |
| Smoking, No. (%) | 155(23) | 145(22) | .54 |
| LDL-C, mean (SD), mg/dL | 113(27) | 115(32) | .39 |
| Albumin excretion rate‡ Median (IQR), mg/24 h | 8.6 (5.8-14.4) | 10.1 (5.8-20.2) | <.001 |
| >28 μg/min, No. (%) | 50(7.4) | 87(12.9) | <.001 |
| >208 μg/min, No. (%) | 10(1.5) | 20(3.0) | .06 |
| Serum creatinine, mean (SD),mg/dl | 0.85(0.17) | 0.84(0.15) | .12 |
| GFR by 125I-iothalamate clearance, mean (SD),mL/min per 1.73 m2 | 125(20) | 126(21) | .26 |
| <70 mL/min per 1.73 m2, No.(%) | 2(0.4) | 3(0.6) | .68 |
| Standard creatinine clearance, mean (SD), mL/min per 1.73 m2 | 122(26) | 122(26) | .57 |
| <70 mL/min per 1.73 m2, No. (%) | 10(1.5) | 10(1.5) | .99 |
| Blood pressure, mm Hg >140/90, confirmed, No. (%) | 74(11) | 71(11) | .81 |
| >130/80, unconfirmed, No. (%) | 260(39) | 242(36) | .35 |
| Arterial pressure, mean (SD), mm Hg§ | 89(9) | 88(9) | .26 |
| Heart rate, mean (SD),beatdmin | 75(10) | 75(10) | .19 |
There were no differences in blood pressure or prevalence of hypertension at the end of the DCCT. The body mass index was significantly higher in the former intensive group at the end of the DCCT (P<.001). There were no differences in clearance of 125I-iothalamate, levels of serum creatinine, or standard creatinine clearance.
HbA1c Level
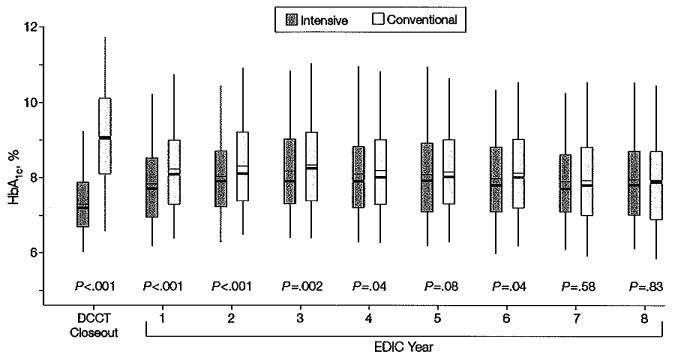
The difference in mean HbA1c level between the intensive- treatment and conventional-treatment groups maintained throughout the DCCT (7.2% vs 9.1%, respectively, for the EDIC participants reported herein; P<.001) began to narrow after DCCT closeout (Figure 1). The mean values of HbA1c throughout the 8-year period of the EDIC study were 8.0% in the group that received intensive treatment during the DCCT and 8.2% in the group that received conventional treatment (P =.002 by Wilcoxon rank-sum test).
Figure 1.
Distribution of HbA1c Concentration by Randomized Treatment Group at the End of the DCCT and in Each Year of the EDlC Study
DCCT indicates Diabetes Control and Complications Trial; EDIC, Epidemiology of Diabetes Interventions and Complications; HbA1c, glycosylated hemoglobin. Boxes indicate 25th and 75th percentiles of HbA1c, level; whiskers, 5th and 95th percentiles; heavy horizontal lines, medians; thin horizontal lines, means.
Development of Microalbuminuria
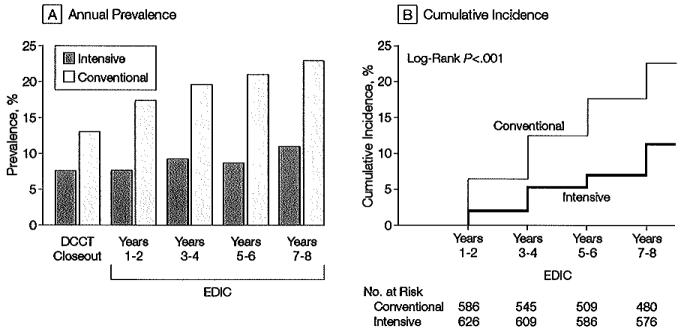
Of 572 participants originally assigned to receive intensive treatment for diabetes and whose AERs were normal (<28 μg/min) at both the beginning andat the end of the DCCT, 39 (6.8%) of those at risk exhibited microalbuminuria at the EDIC Years 7 or 8 evaluation. Of 550 participants originally assigned to conventional treatment and with normoalbuminuria at both the beginning and at the end of the DCCT, 87 (15.8%) of those at risk had microalbuminuria at the year 7 or 8 evaluation. Intensive therapy carried out during the DCCT continued to reduce the odds of microalburminuria by 59% (95% confidence interval [CI], 39%-73%; P<.001) at the EDIC year 7 or 8 evaluation compared with 59% (95% CI, 36%-74%; P<.001) at the end of the DCCT, after adjusting for the corresponding baseline values. Microalbuminuria was consistently more prevalent in the former conventional-treatment group and the difference between the 2 treatment groups in the cumulative incidences of new cases of microalbuminuria after the end of DCCT also was significant (P<.001) (Figure 2). Over the 8 years, the adjusted risk (hazard) reduction was 49% (95% CI, 32%-62%), likewise adjusted for EDIC baseline AER and odd vs even schedule of visits, compared with 39% (95% CI, 2 1%-52%; P<.001) during the 9 years of DCCT follow-up. This effect increased to a 57% risk reduction after adjustment for the presence of hypertension, level of body mass index, mean arterial pressure, and level of low-density lipoprotein cholesterol at DCCT closeout.
Figure 2.
Prevalence and Cumulative Incidence of Microalbuminuria
Microalbuminuria defined as albumin excretion rate ≥28 μg/min, equivalent to 40 mg/24 h. A, Prevalence at the end of the Diabetes Control and Complications Trial (DCCT) and during the Epidemiology of Diabetes Interventions and Complications (EDIC) study. The differences between the 2 treatment groups are significant at each time point after DCCT closeout (P<.001). B, Cumulative incidence of new cases in the EDIC study for those participants in the intensive- and conventional-treatment groups with normal albuminuria at the beginning and end of the DCCT. The difference in cumulative incidences is significant by the log-rank test (P<.001).
Development of Clinical Albuminuria
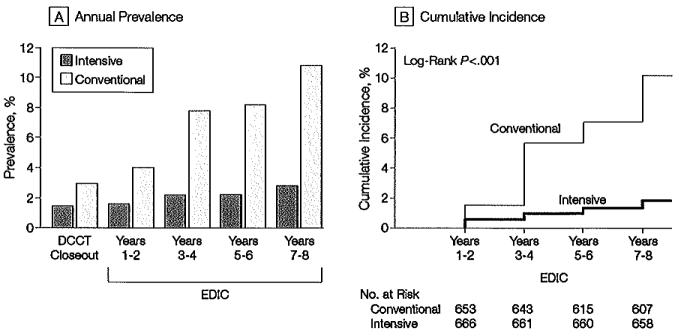
Of 632 participants originally assigned to receive intensive therapy and who did not exhibit clinical alburminuria at the end of the DCCT, 9 (1.4%) of those at risk had clinical albuminuria at the EDIC year 7 or 8 evaluation compared with 59 of 630 participantsin the group receiving conventional treatment (9.4% of those at risk). After adjustment for the respective EDIC baseline values, intensive treatment in the DCCT continued to reduce the odds of clinical alburninuria: 84% (95% CI, 67%-92%; P<.001) at the year 7 or 8 evaluation in the EDlC study compared with 57% (95% CI, -1% to +81%; _P_=.05) at the end of the DCCT. As with microalbuminuria, the prevalence of alburminuria and the cumulative incidence of new cases after DCCT closeout between the 2 treatment groups differed significantly (Figure 3), with an adjusted risk (hazard) reduction of 78% (95% CI, 58%-88%; P<.00l) compared with 54% (95% CI, 19%-74%; P<.001) during the 9 years of DCCT follow-up. This effect likewise increased to an 84% (95% CI, 68%-92%; P<.001) risk reduction after adjustment for other factors.
Figure 3.
Prevalence and Incidence of Albuminuria
Albuminuria defined as albumin excretion rate ≥208 μg/min, equivalent to 300 mg/24 h. A, Prevalence of clinical albuminuria at the end of the Diabetes Control and Complications Trial (DCCT) and during the Epidemiology of Diabetes Interventions and Complications (EDIC) study. The differences between the treatment groups are significant at each time point after DCCT close-out (P<.01). B, Cumulative incidence of new cases in the EDIC study for those participants in the intensive- and conventional-treatment groups with either normoalburninuria or microalbuminuria at the end of the DCCT. The difference in cumulative incidences is significant by the tog-rank-test. (P<.001).
The continued salutary effects of DCCT intensive treatment on the development of albuminuria were present for different subsets, defined a priori. For example, in participants with normoalbuminuria at the end of the DCCT, after 8 years in the EDIC study there was a significant reduced odds of clinical albuminuria in the intensive-treatment group vs the conventional-treatment group (87% reduction; 95% CI, 65%-95%, P<.001). In participants with microalbuminuria at the end of the DCCT, after 8 years in the EDIC study there was also a significant reduced odds of clinical albuminuria in the intensive-treatment group (77% reduction; 95% CI, 25%-93%; _P_=.01).
Other Kidney Outcomes
At EDIC baseline there was no difference between treatment groups in the distribution of the GFR as assessed by 125I-iothalamate clearance, and very few participants had actual or estimated values less than 70 mL/min [1.2 mL/s] per 1.73 m2. There was no difference between groups in serum creatinine concentrations or standard creatinine clearance. During the EDIC study, slight but nominally significant differences in serum creatinine concentration emerged at years 7 or 8. By a generalized estimating equation analysis (due to skewness of the residuals) the mean serum creatinine concentration over EDIC years 1 through 8 was significantly lower in the former intensive-treatment group vs the conventional-treatment group (0.89 vs 0.92 mg/dL [78.7 vs 81.3 μmol/L], respectively; P<.001).
During the EDIC study, there were isolated, nominally significant differences between groups in the standard creatinine clearance values at years 2 through 8. By a generalized estimating equation analysis of the log clearance values, the geometric mean over the 8 years of the EDIC study approached significance (114.0 mL/min [l.9 mL/s] per 1.73 m2 with intensive treatment vs 112.7 mL/min [1.9 mL/s] per 1.73 m2 with conventional treatment, _P_=.07). After the 8 years of EDIC follow-up, the prevalence of a measured creatinine clearance less than 70 mL/min per 1.73 m2 in the intensive-treatment group was below 1%, compared with 4% in the conventional-treatment group (P<.001).
Twenty-seven patients doubled their serum creatinine concentration since DCCT baselie (Table 2), with 15 of these reaching a creatinine concentration of 2 mg/dL [176.8μm ol/L] or greater, with no significant difference between groups. In 16 patients this occurred during the average 6.5 years of DCCT follow-up. The number of those reaching a creatinine concentration of 2 mg/dL or greater was significantly lower in the intensive-treatment group vs the conventional-treatment group (5 vs 19, _P_=.004). Of these, 11 required dialysis or transplant (4 vs 7, _P_=.14) (Table 2).
Table 2.
Patients With Kidney Outcomes Through Year 8 in the EDIC Study*
| Outcome | Original DCCT Treatment Group, No. (%) | _P_Value† | ||
|---|---|---|---|---|
| Total(N=1349) | Intensive(n=676) | Conventional(n=673) | ||
| Doubling of serum creatinine level | 27(2.0) | 10(1.5) | 17(2.5) | .17 |
| since DCCT baseline | ||||
| Serum creatinine >2 mg/dL | 15(1.1) | 4(0.6) | 11(1.6) | .21 |
| Dialysis or kidney transplant | 4(0.3) | 3(0,4) | 1(0.1) | 13 |
| Serum creatinine >2 mg/dL | 24(1.8) | 5(O.7) | 19(2.8) | .004 |
| Dialysis or kidney transdant | 11(0.8) | 4(0.6) | 7(1.O) | .14 |
| Dialysis or kidney transplant | 11(0.8) | 4(0.6) | 7(1.O) | .36 |
| Dialysis | 9(0.7) | 4(0.6) | 5(0.7) | .49 |
| Kidney transplant | 7(0.5) | 2(0.3) | 5(0.7) | .56 |
Blood Pressure/Hypertension
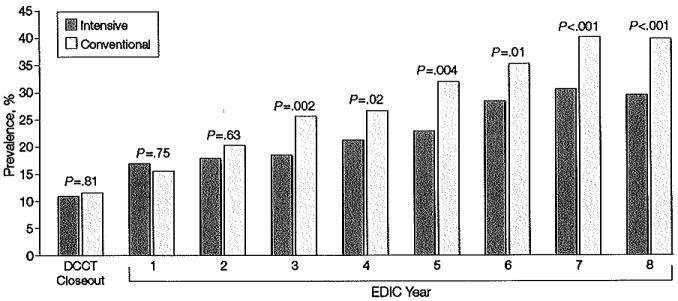
While there was no difference between groups in levels of blood pressure during the DCCT, a difference between groups has emerged during the EDIC study. On average over the 8 years of follow-up, and adjusted for the level at the close of the DCCT, the mean level of systolic blood pressure was significantly lower in the participants in the former intensive-treatment group vs those in the conventional-treatment group (117.7 vs 119.0 mm Hg, _P_=.003), as was the mean arterial pressure (89.3 vs 90.0 mm Hg, _P_=.02). The slight difference in diastolic blood pressure was not significant (75.1 vs 75.6 mm Hg, _P_=.16). Likewise, although the prevalence of hypertension did not differ between treatment groups at the end of DCCT (11% in both groups, _P_=.81), more participants in the original conventional-treatment group developed hypertension over time, with the difference becoming significant during years 3 through 8 of the EDIC study (Figure 4). By year 8 in the EDIC study, the prevalence of hypertension in the conventional-treatment group was 40.3% compared to 29.9% in the intensive-treatment group (P<.001) and hypertension was reduced by 40% with intensive vs conventional treatment (P.001). Among those participants who were normotensive at the end of the DCCT, former DCCT intensive therapy reduced the risk (hazard) of onset of hypertension by 32% (95% CI, 18%-44%, P<.00l) during the 8 years of EDEC follow-up, adjusted for DCCT and EDIC baseline covariates.
Figure 4.
Prevalence of Hypertension at Each Year of the EDlC Study
Prevalence of hypertension (defined as blood pressure >140/90 mm Hg) at the end of the Diabetes Control and Complications Trial (DCCT) and during the Epidemiology of Diabetes Interventions and Comptications (EDIC) study for participants in the intensive- vs conventional-treatment groups. The aggregate odds reduction with intensive vs conventional therapy of emergent hypertension during the EDIC study, adjusted for DCCT mean arterial pressure, was 40.4% (95% confidence interval, 33.7%-46.5%; P<.001).
These differences are not explained by differential use of antihypertensive medications. Beginning in year 1 of the EDIC study, 5.6% of the intensive-treatment group and 6.7% of the conventional- treatment group reported using angiotensin-converting enzyme inhibitors for any reason (eg, for hypertension, microalbuminuria, or both). By year 8 of the EDIC study, 21.6% of the intensive-treatment group and 29.0% of the conventional-treatment group reported using angiotensin-converting enzyme inhibitors.
Adjustment for EDIC Covariates
In additional proportional hazardsards models, the differences between DCCT treatment group in the risk of micro-albuminuria or clinical albuminuria remained highly significant after adjusting for the levels of median arterial pressure and incidence of hypertension during the EDIC study. These differences likewise remained significant after adjusting for the incidence of hyperlipidemia (low-density lipoprotein cholesterol level > 160 mg/dL [4.1 mmol/L] or use of antihyperlipidemic medication), body mass index, or GFR.
Among these covariates, the current prevalence of hypertension was highly significantly associated with increased risk of microalbuminuria and of albuminuria, but independently of the effect of DCCT intensive vs conventional therapy. The current prevalence of hypertension increased the risk of microalbuminuria by 68% (95% CI, 19%-138%; _P_=.004), yet DCCT group differences in hypertension explained only 2.9% of the effect of the DCCT treatment group on the risk of microalbuminuria. Hypertension increase the risk of albuminuria by 290% (95% C1, 126%-574%; P<.001) and explained 22.l% of the effect of DCCT treatment group. In each case the DCCT group effect remained significant at P<.001 after adjustment for hypertension during the EDIC study.
The DCCT group differences in risk of microalbuminuria and albuminuria also remained after adjustment for use of intensive vs conventional therapy during the EDIC study.
Effect of Glycemia
Proportional hazards regression models, adjusted for other factors, assessed the effect of the DCCT and EDIC combined mean HbA1c, level on the risk of new kidney events during the EDIC study among those at risk for such events during the study (ie, those event free during the DCCT). There was a 50.2% (95% CI, 42.2%-57.1%; P<.00l) reduction in the risk (incidence, hazard) of microalbuminuria per 10% reduction in the current combined mean HbA1c, level that explains 7.25% of the variation in risk. There was a 56.4% (95% CI, 43.4%-66.4% P<.001) reduction in the risk of clinical albuminuria per 10% reduction in the current combined mean HbA1c level that explains 3.31% of the variation in risk. The gradient is slightly steeper for clinical albuminuria than for microalbuminuria, but the strength of the effect is smaller (χ2 values of 42.2 vs 86.8, respectively), yielding a smaller proportion of variation explained. In part this may be due to the smaller number of events of clinical albuminuria vs microalbuminuria (73 vs 207, respectively).
In additional models that included both the DCCT and EDIC mean HbA1c levels separately, the EDIC HbA1c level over the 8 years of follow-up had a greater effect than the DCCT HbA1c level, each considered separately. For the analysis of microalbuminuria during the ED1C study, the DCCT mean HbA1c effect had a χ2 test value of 43.09 while the current EDIC mean HbA1c level had a value of 87.12 (P<.001 for both). For the analysis of albuminuria, the DCCT and EDIC HbA1c χ2 test values were 25.6 and 35.2, respectively. In each case, however, a model with separate DCCT and EDIC effects did not provide better fit than the model using the combined mean HbA1c level.
Since the DCCT mean HbA1c level continues to affect risk of onset of albuminuria during the EDIC study, and since the groups differed substantially with respect to DCCT HbA1c level but only slightly with respect to EDIC HbA1c level, it follows that the prolonged effect of DCCT intensive therapy is almost completely explained by the differences in the mean HbA1c level in the DCCT but not in the EDIC study. The χ2 test value for the effect of treatment group on risk of microalbuminuria was 21.45. Adjustment for the DCCT mean HbA1c level explains 91% of this group effect, while adjustment for the EDIC mean HbA1c level (in a separate model) explains 23%. Likewise, the group effect on risk of albuminuria was χ2= 20.98, of which 99% is explained by the mean DCCT HbA1c level and 16% by the EDIC level (separately). The fact that the ED1 C HbA1c level explains some of the group difference may be more a reflection of its correlation with the DCCT level than an independent effect.
COMMENT
Follow-up of the DCCT cohort for 8 additional years in the EDlC study has shown persistent differences in nephropathic outcomes between the former intensive-treatment and conventional-treatment groups. During the EDIC study, only 6.8% of participants in the previous intensive-treatment group developed microalbuminuria and 1.4% developed clinical albuminuria, compared with 15.8% and 9.4% of participants in the previous conventional-treatment group. Thus, the DCCT period of intensive vs conventional treatment, characterized by a 1.8% absolute difference in HbA1c levels over an average of 6.5 years, has continued to produce benefit with regard to nephropathy for at least 7 to 8 years, even though the difference in mean HbA1c level between the 2 former treatment groups diminished and averaged only 0.2% during the EDIC study. Similar results have previously been reported for diabetic retinopathy in the same patient cohort after 4 and 7 years of EDIC follow-up.4,15
The DCCT prespecified that microalbuminuria and albuminuria based on AER were the major nephropathic outcomes because other outcomes of impaired kidney function (reduced GFR and increasing blood pressure) were expected to occur too infrequently to provide adequate power to detect treatment-group effects. Thus far in the EDIC study, the numbers of events have not yet yielded significant differences in the prevalence of diabetic nephropathy requiring dialysis or transplant, although the total number of severe kidney events (kidney insufficiency) is more than Hold greater in the conventional-treatment group (Table 2).
Since microalbuminuria and clinical-grade albuminuria are understood to be precursors of end-stage renal disease, these effects may be expected to result in a future decrease in the development of severe stages of nephropathy in the intensive-treatment group. This is supported by the observation of emerging differences between groups in serum creatinine levels and the prevalence of abnormal standard clearance. However, longitudinal measures of GFR were not obtained during the EDIC study, and there is no reliable method that can be used to estimate GFR based on creatinine values in the normal range. Thus it is premature to reach any conclusion as to the effect of intensive treatment during the DCCT on chronic kidney failure.
In addition, for the first time we have demonstrated clear benefits of intensive diabetes treatment on the development of hypertension, a finding expected to yield long-term benefits regarding nephropathy and retinopathy.16-19 The development of hypertension also was highly significantly associated with increased risk of microalbuminuria and albuminuria, and these effects persisted independently of those of the initial DCCT treatment group. Thus, the long-term benefit of previous intensive therapy on kidney outcomes was not mediated by differences in blood pressure, since the intemive-treatment effect persisted when blood pressure was included in the regression models.
While the total exposure to glycemia is the dominant determinant of risk of progression during the EDIC study, the long-term differences between the original DCCT intensive-treatment vs conventional-treatment groups are virtually all explained by the differences in glycemia established during the DCCT. This lends support to the hypothesis that a “metabolic memory” effect has occurred. This carryover or imprinting phenomenon related to previous levels of glycemia may have accounted for the 3-to 4-year lag time between the initiation of intensive therapy in the DCCT and a demonstrated benefit with regard to nephropathy and retinopathy.1,2
The mechanism of this enduring effect of DCCT treatment during EDIC follow-up is unknown. Given the quantitative relationships between retinopathy and nephropathy and HbA1c level20,21 it is reasonable to posit that different degrees of hyperglycemic tissue damage occurred in the kidneys of participants in the 2 treatment groups during the DCCT. Moreover, quantitatively or even qualitatively different pathogenic processes may have been set in motion by sharply different degrees of hyperglycemia during the DCCT. These pathogenic differences could have had persistent effects, even though HbA1c level rose about 1% in the former intensive-treatment group and fell about 1%in the former conventional-treatment group to nearly equalize during the EDIC study at a midpoint of about 8.0% A number pathogenic pathways initiated by elevated plasma glucose levels have been described that lead to retinopathy, nephropathy, and neuropathy in amimal and human studies.22 At least 1 such pathway, the advanced glycation end product (AGE) pathway, is capable of producing tissue changes that could possibly out-last a particular level of hyperglycemia.23 Since intensive treatment was associated with lower long-lived skin collagen AGE levels than conventional treatment in the DCCT cohort,24 perhaps tissue AGES could play a role in explaining the carryover effect of hyperglycemia we have consistently observed.
Natural history studies have documented a decade-plus exposure to hyperglycemia before the first manifestations of diabetic nephropathy occur.25,26 Thus, the intensively treated participants had few manifestations of diabetic nephropathy during the DCCT due to the relatively low level of HbA1c achieved. However, the near-normal glycemic control for 6.5 years may have simply delayed the development of indicators of diabetic nephropathy during 8 more years of follow-up. Whether diabetic nephropathy will eventually increase and “catch up” in the former intensive-treatment patients if they continue with their currently higher mean levels of HbA1c is unknown.
In conclusion, the current results reaffirm that intensive treatment of type 1 deabetes should be initiated as early as is safely possible in order to provide strong and durable protection from the development and progression of diabetic microvascular disease.4 The protection initiated by intensive treatment appears to outlast the intensive treatment itself, although the duration of the effect remains to determined.
Acknowledgments
Funding/Support: This study was supported by contracts with the Division of Diabetes, Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, and the General Clinical Research Centers Program, National Center for Research Resources.
Footnotes
Financial Disclosure: Dr Molitch has received research support from Eli Lilly and Co, a manufacturer of insulin.
The DCCT/EDIC Research Group: Study Chairmen: S. Genuth, D. Nathan; Albert Einstein College of Medicine: S. Engel, H. Martinez, H. Shamoon, H. Engel; Case Western Reserve University: W. Dahms, L. Mayer, S. Pendegeras. H. Zegarra, D. Miller, L. Singerman, S. Smith-Brewer, S. Genuth (past); cornell-university Medical Center: D. Brillion, M. Lackaye, M. Heinemann, V. Reppuci, T. Lee; Henry Ford Health System: F. Whitehouse, D. Kruger, A. Galpern, J. D. Carey; International Diabetes Center: R. Bergenstal, M. Johnson, D. Kendall, M. Spencer, D. Noller, K. Morgan, D. Etzwiler (deceased); Joslin Diabetes Center:A, Jacobson, E. Golden, G. Sharuk, Paul Arrigg, R. Baeser, O. Ganda, J. Rosenzweig, H. Wolpert, P. Economides, O. Handy, L. Rand (past); Massachusetts General Hospital: D. Nathan, S. Fritz, J. Godine, C. McKitrick, P. Lou; Mayo Foundation: F. J. Service, G. Ziegler, J. Pach, J. Lindsey; Medical University of South Carolina: J. Colwell, D. Wood, R. Mayfietd, K. Hermayer, M. Szpiech, T. Lyons, J. Parker, A. Farr, S. Elsing, T. Thompson, J. Selby, M. Bracey; Northwestern University: M. Molitch, B. Schaefer, L. lampol, D. Weinberg, A. Lyon, Z. Strugula, 1. Shankle, P. Astlesford; University of California, San Diego: O. Kolterman, G. Lorenzi, M. Goldbaum; University of Iowa: W. Sivitz, M. Bayless, R. Zeither (past), T. Weingeist, E. Stone, H. Culver Boldt, K. Gehres, S. Russell; University of Maryland School of Medicine: D. Counts, A. Kowarski (past), D. Ostrowski, T. Donner, S. Steidl, B. Jones; University of Michigan: W. Herman, D. Greene (past), C. Martin, M. J. Stevens, A. K. Vine, S. Elner; University of Minnesota: J. Bantle, B. Rogness, T. Olsen, E, Steuer; University of Missouri: D. Gold-stein, s. Hitt. J. Giangiacormo, D. Hainsworth; University of New Mexico: D. Schade, M. Burge, J. Canady, M. Schluter, A. Das, D. Hornbeck (past); University of Pennsylvania: S. Schwartz, P. A. Bourne. B. J. Maschak-Carey (past), L. Baker, (deceased), S. Braunstein, A. Brucker; University of Pittsburgh: T. Orchard, N. Silvers. T. Songer, B. Doft, S. blson. R. L. Bergren, L. Lobes, M. Fineman, A. Drash (past); University of South Florida: J. Malone, J. Vaccaro-Kish, C. Berger, R. Gstalder, P.R. Pavan, A. Morrison; University of Tennessee: S. Dagogo-Jack, S. Schussler, A. Kitabchi, H. Lambeth, M. B. Murphy, S. Moser, D. Meyer, A. lannacone, M. Bryer-Ash (past); University of Texas Southwestern University Medical Center: P. Raskin, S. Strowig, A. Edwards, J. Alappatt (past), C. Wilson (past), S. Park (past), Y. He; University of Toronto: B. Zinman, A. Barnie, S. MacLean, R. Devenyi, M. Mandelcorn, M. Brent; University of Washington: J. Palmer, S. Catton, J. Kinyoun, L. Van Ottingham (past), J. Ginsberg (past); University of Western Ontario: J. Dupre, J. Harth, C. Canny (past), D. Nicolle; Vanderbilt University: M. May, R. Lorenz (past), J. Lipps, L. Survant, S. Feman (past), K. Tawansy, A. Agarwal, T. Adkins; Washington University, St Louis: N. White, J. Santiago (deceased), L. Levandoski, I. Boniuk, G. Grand, M. Thomas, D. Burgess, D. Joseph, K. Blinder, G. Shah; Yale University School of Medicine: W. Tamborlane, P. Gatcomb, K. Stoessel, K. Taylor; Clinical Coordinating Center (Case Western Reserve University): B. Dahms, R. Trail, J. Quin; Data Coordinating Center(The George Washington University, Biostatistics Center): J . Lachin, P. Cleary, D. Kenny, J. Backlund, L. Diminick, A. Determan, K. Klump, M. Hawkins; National lnstitute of Diabetes and Digestive and Kidney Disease Program Office: C. Cowie, J. Fradkin, C. Siebert (past), R. Eastman (past); Central Fundus Photograph Reading Center (University of Wisconsin): M. Davis, L. Hubbard, P. Geithman, L. Kastorff, M. Neider, D. Badal, B. Esser, K. Miner, H. Wabers, K. Glander, J. Joyce, N. Robinson, C. Hurtenbach, C. Hannon; Central Biochemistry Laboratory (University of Minnesota): M. Steffes, J. Bucksa, 3. Chavers; Central Carotid Ultrasound Unit ((New England Medical Center): D. O’Leary, L. Funk, J. Polak; Central ECG Reading Unit (University of Minnesota): R. Crow, C. O’Donnell (past), 3. Gloeb, S. Thomas; Computed Tomography Reading Center (Harbor UCLA Research and Education Institute): R. Detrano, N. Wong, M. Fox, L. Kim, R. Oudiz; External Advisory Committee: G. Weir (Chairman), C. Clark, R. D’Agostino, M. Espeland, B. Klein, T. Manolio, L. Rand, D. Singer, M. Stern; Molecular Risk Factors Program Project (Medical University of South Carolina): W. T. Garvey, T. J. Lyons, A, Jenkins, R. Klein, M. Lopes-Virella, G. Virella, A. A. laffa, D. Zheng, D. Lackland, D. McGee, R. K. Mayfield, M. Brabham; Genetic Studies Group (Hospital for Sick Children): A. Boright, A. Paterson, 5. Scherer, B. Zinman; Lipoprotein Distribution/Obesify Group (University of Washington): J. Brunzell, J. Hokanson, S. Marcovina, J. Purnell, S. Sibley, S. Deeb, K. Edwards; Editor, EDIC Publications: D. Nathan.
REFERENCES
- 1.Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabets on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial (DCCT) Research Group Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720. doi: 10.1038/ki.1995.236. [DOI] [PubMed] [Google Scholar]
- 3.Epidemiology of Dibetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy [published correction appears in N Engl J Med. 2000;342:1376] N Engl J Med. 2000;342:381–389. [Google Scholar]
- 5.Molitch ME, Steffes MW, Cleary PA, Nathan DM, the Diabetes Control and Complications Trial Research Group Baseline analysis of renal function in the Diabetes Control and Complications Trial [published correction appears in Kidney Int. 1993;43:1196] Kidney Int. 1993;43:668–674. doi: 10.1038/ki.1993.96. [DOI] [PubMed] [Google Scholar]
- 6.Diabetes Control and Complications Trial The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus. Arch Ophthalmol. 1995;113:36–51. doi: 10.1001/archopht.1995.01100010038019. [DOI] [PubMed] [Google Scholar]
- 7.Diabetes Control and Complications Trial Research Group The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann Intern Med. 1995;122:561–568. doi: 10.7326/0003-4819-122-8-199504150-00001. [DOI] [PubMed] [Google Scholar]
- 8.Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol. 1995;75:894–903. doi: 10.1016/s0002-9149(99)80683-3. [DOI] [PubMed] [Google Scholar]
- 9.Diabetes Control and Complications Trial Research Group Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46:271–286. [PubMed] [Google Scholar]
- 10.Levey AS, Greene T, Schluchter MD, et al. Modification of Diet in Renal Disease Study Group and the Diabetes Control and Complications Trial Research Group Glomerular filtration rate measurements in clinical trials. J Am Soc Nephrol. 1993;4:1159–1171. doi: 10.1681/asn.v451159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snedecor GW, Cochran WG. Statistical Methods. 7th Iowa State University Press; Ames: 1980. [Google Scholar]
- 12.Lachin JM. Biostatistical Methods: The Assessment of Relative Risks. John Wiley & Sons; New York, NY: 2000. [Google Scholar]
- 13.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 14.Diggle PJ, Liang K-Y, Zegter SL. Anlysis of Longitudinal Data. Oxford University Press; New York, NY: 1994. [Google Scholar]
- 15.Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications Trial (EDIC) Research Group Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–2569. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgensen CE. Long-term antihypertensive treatment inhibiting progression of diabetic nephropathy. BMJ. 1982;285:685–688. doi: 10.1136/bmj.285.6343.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parving HH, Andersen AR, Smidt UM, Svendsen PA. Early aggressive antihypertensive treatment reduces the rate of decline in kidney function in diabetic nephropathy. Lancet. 1983;1:1175–1179. doi: 10.1016/s0140-6736(83)92462-5. [DOI] [PubMed] [Google Scholar]
- 18.Klein R, Klein BE, Moss SE, Davis MD, DeMet DL. Wisconsin Epidemiologic Study of Diabetic Retinopathy, III: risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102:520–526. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 19.Hovind P, Tarnow L, Kasper R, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003;26:1258–1264. doi: 10.2337/diacare.26.4.1258. [DOI] [PubMed] [Google Scholar]
- 20.The relationship of glycemia exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes. 1995;44:968–983. [PubMed] [Google Scholar]
- 21.The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial (DCCT) Diabetes. 1996;45:1289–1298. [PubMed] [Google Scholar]
- 22.King GL, Brownlee M. The cellular and molecular mechanisms of diabetic complications. Endocrinol Metab Clin North Am. 1996;25:255–270. doi: 10.1016/s0889-8529(05)70324-8. [DOI] [PubMed] [Google Scholar]
- 23.Cerami A, Vlassara H, Brownlee M. Role of advancerd glycosylation products in complications of diabetes. Diabetes Care. 1988;11(suppl 1):73–79. [PubMed] [Google Scholar]
- 24.Monnier VM, Bautista O, Kenny D, et al. the DCCT Skin Collagene Ancillary Study Group Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. Diabetes. 1999;48:870–880. doi: 10.2337/diabetes.48.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borch Johnsen K, Nissen H, Henriksen E, et al. The natural history of insulin-dependent diabetes mellitus in Denmark, I: long-term survival with and without late diabetic complications. Diabet Med. 1987;4:201–210. doi: 10.1111/j.1464-5491.1987.tb00863.x. [DOI] [PubMed] [Google Scholar]
- 26.Borch Johnsen K, Andersen PK, Deckert T. The effect of proteinuria on relative mortality in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1985;28:590–596. doi: 10.1007/BF00281993. [DOI] [PubMed] [Google Scholar]